Synthesis of Metakaolin Based Alkali Activated Materials as an Adsorbent at Different Na2SiO3/NaOH Ratios and Exposing Temperatures for Cu2+ Removal
Abstract
:1. Introduction
2. Methodology
2.1. Materials
2.1.1. Metakaolin
2.1.2. Sodium Hydroxide
2.1.3. Sodium Silicate
2.1.4. Foaming Agent
2.2. Methods
2.2.1. Preparation of Metakaolin Geopolymer Adsorbents
2.2.2. Water Absorption Test
2.2.3. Density Test
2.2.4. Compressive Strength Test
2.2.5. Characterization of Metakaolin Based Alkali Activated Materials
2.2.6. Adsorption of Copper Ions Test
3. Results and Discussion
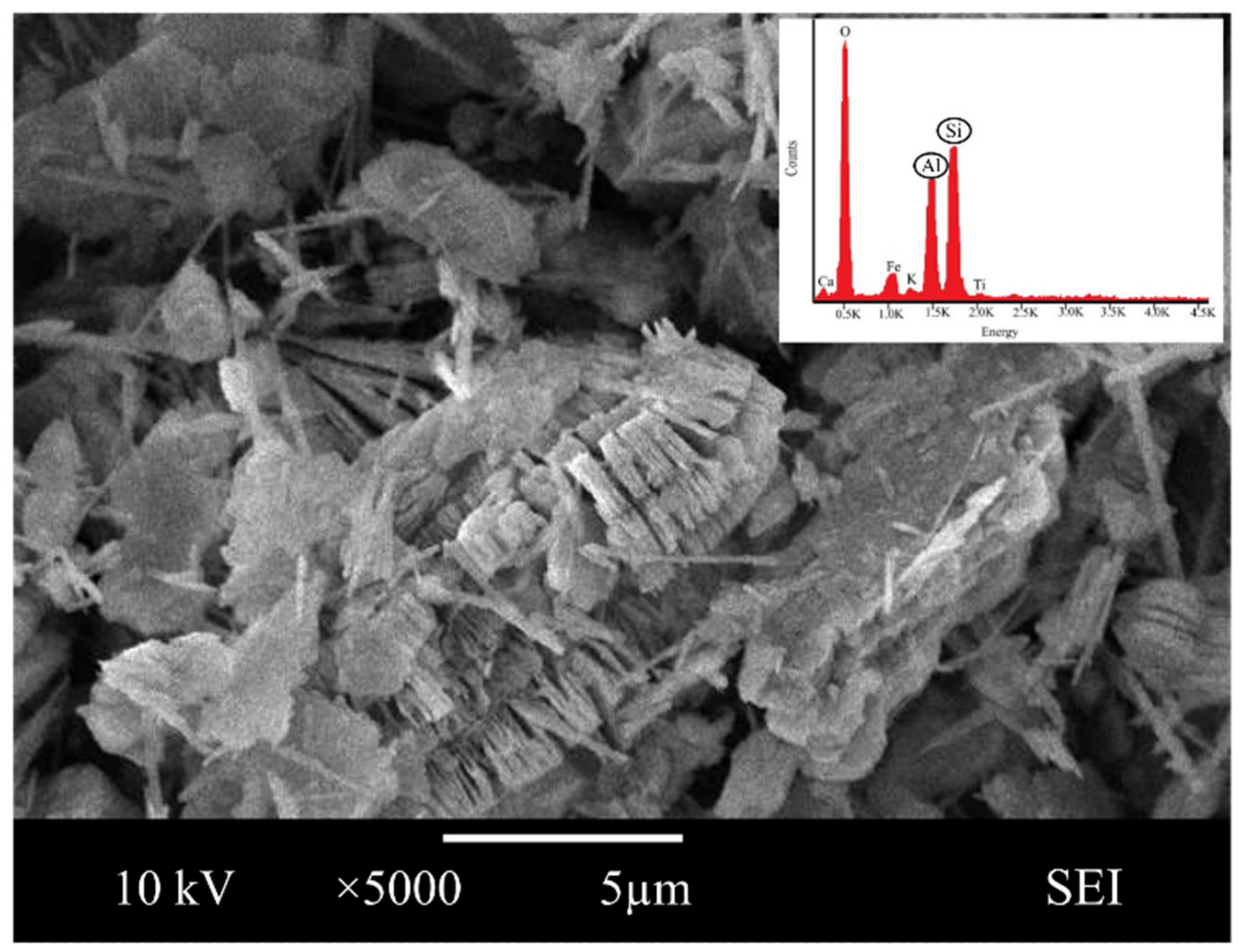
3.1. Raw Material Characterization
3.2. Effect on Na2SiO3/NaOH Ratio to Metakaolin Based Alkali Activated Materials
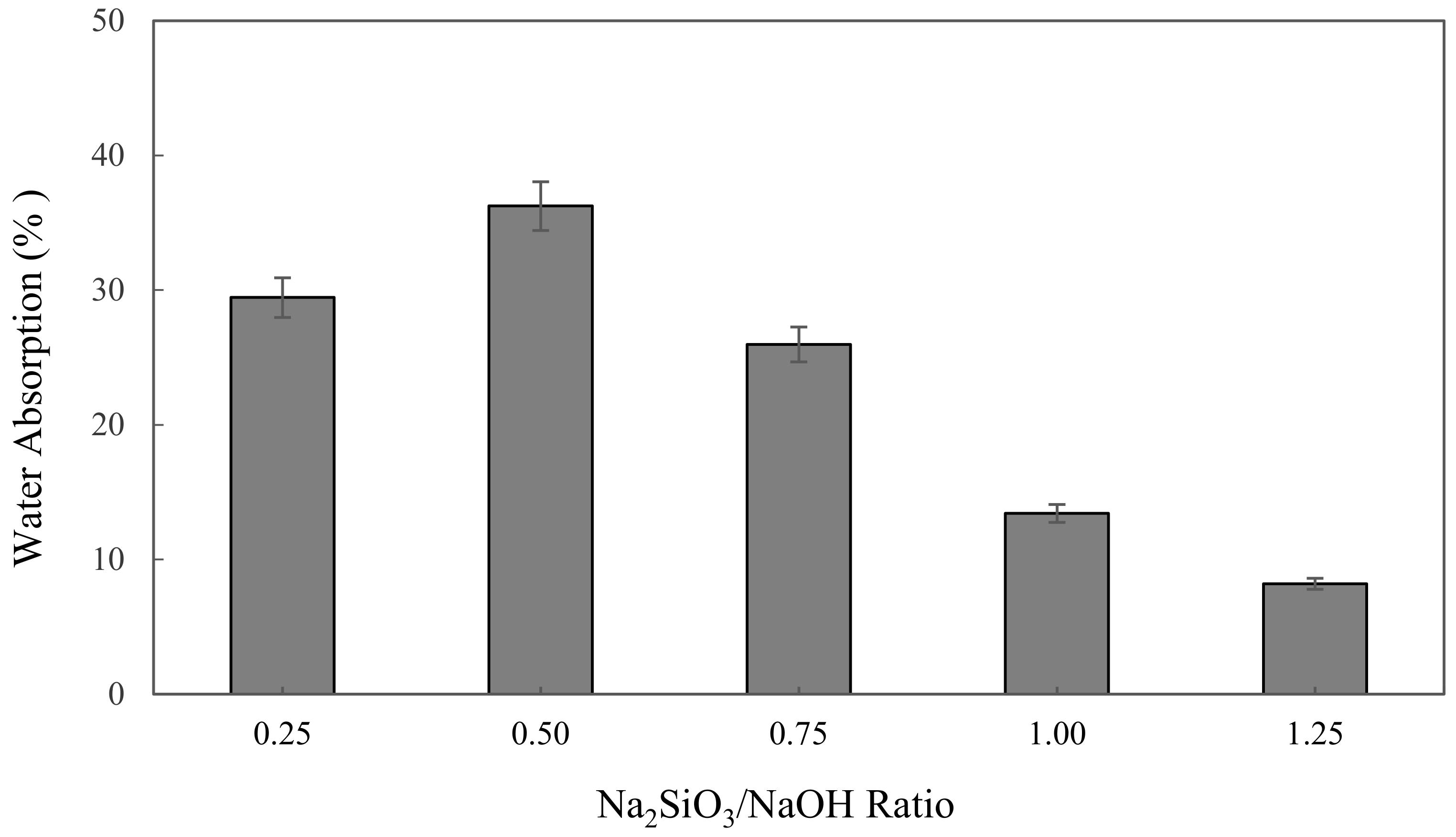
3.2.1. Water Absorption Analysis
3.2.2. Density and Compressive Strength Analysis
3.2.3. Microstructure Analysis
3.3. Effect on Different Exposing Temperature to Metakaolin Based Alkali Activated Materials
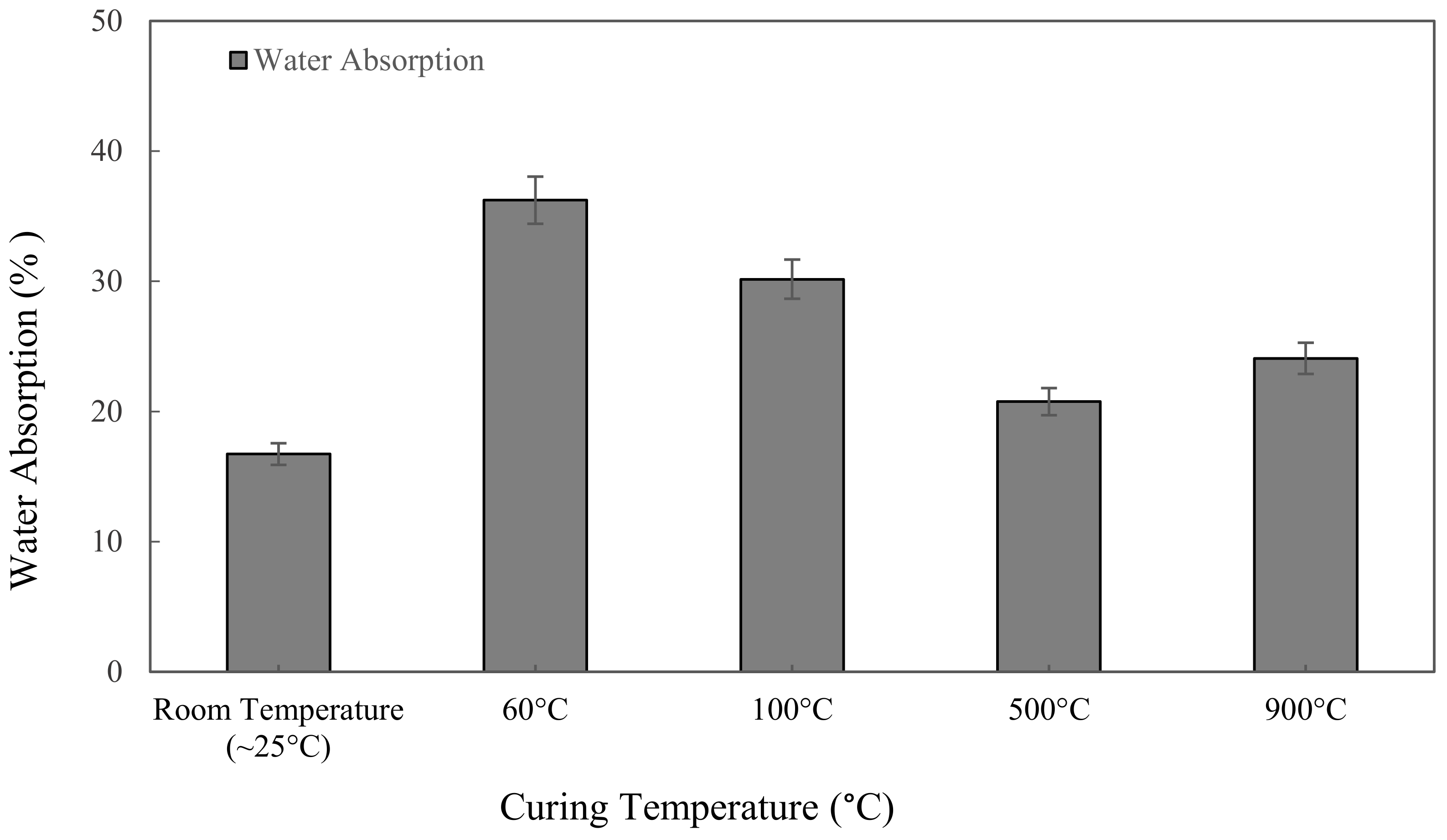
3.3.1. Water Absorption Analysis
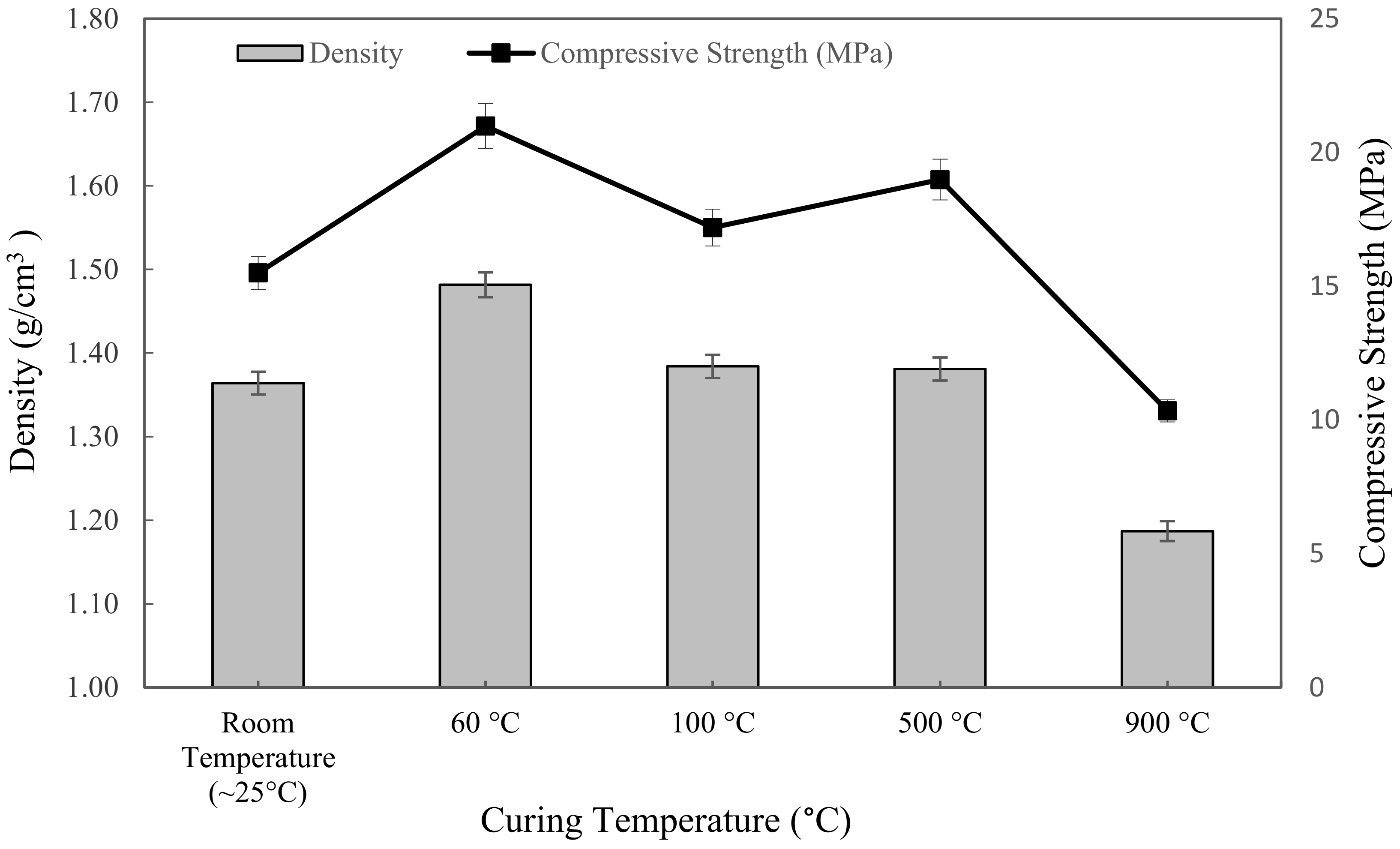
3.3.2. Density and Compressive Strength Analysis
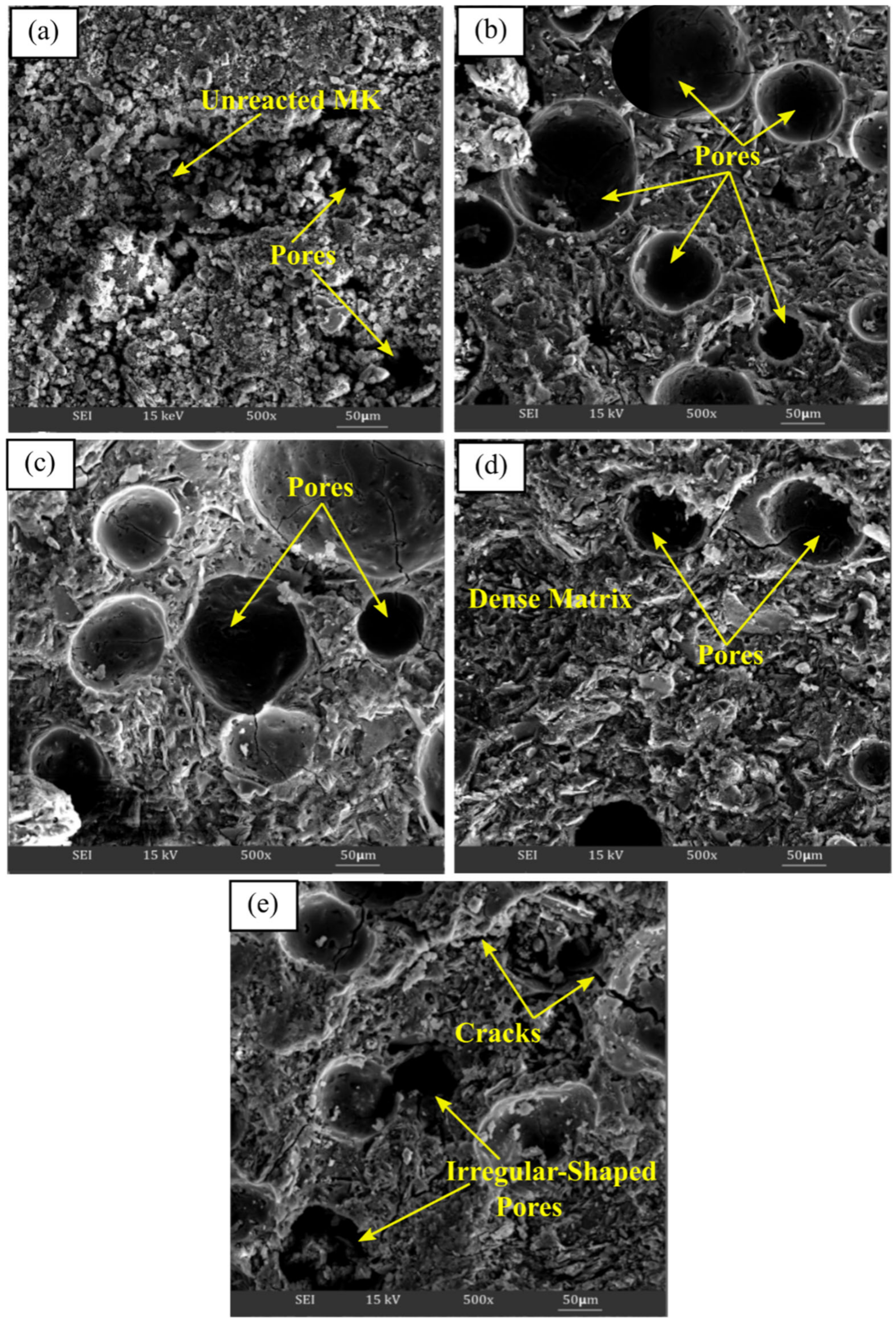
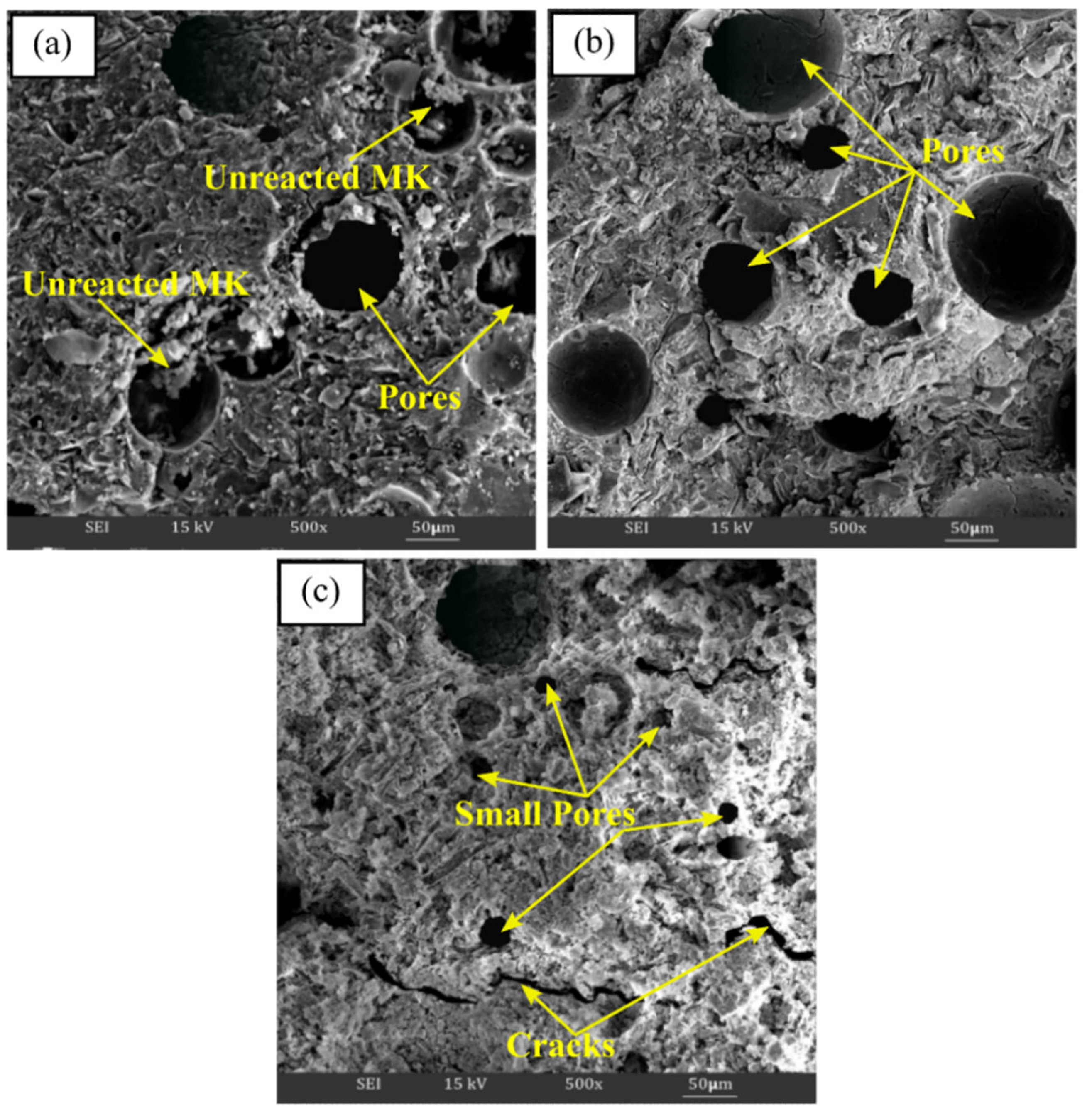
3.3.3. Microstructure Analysis
3.3.4. Phase Analysis
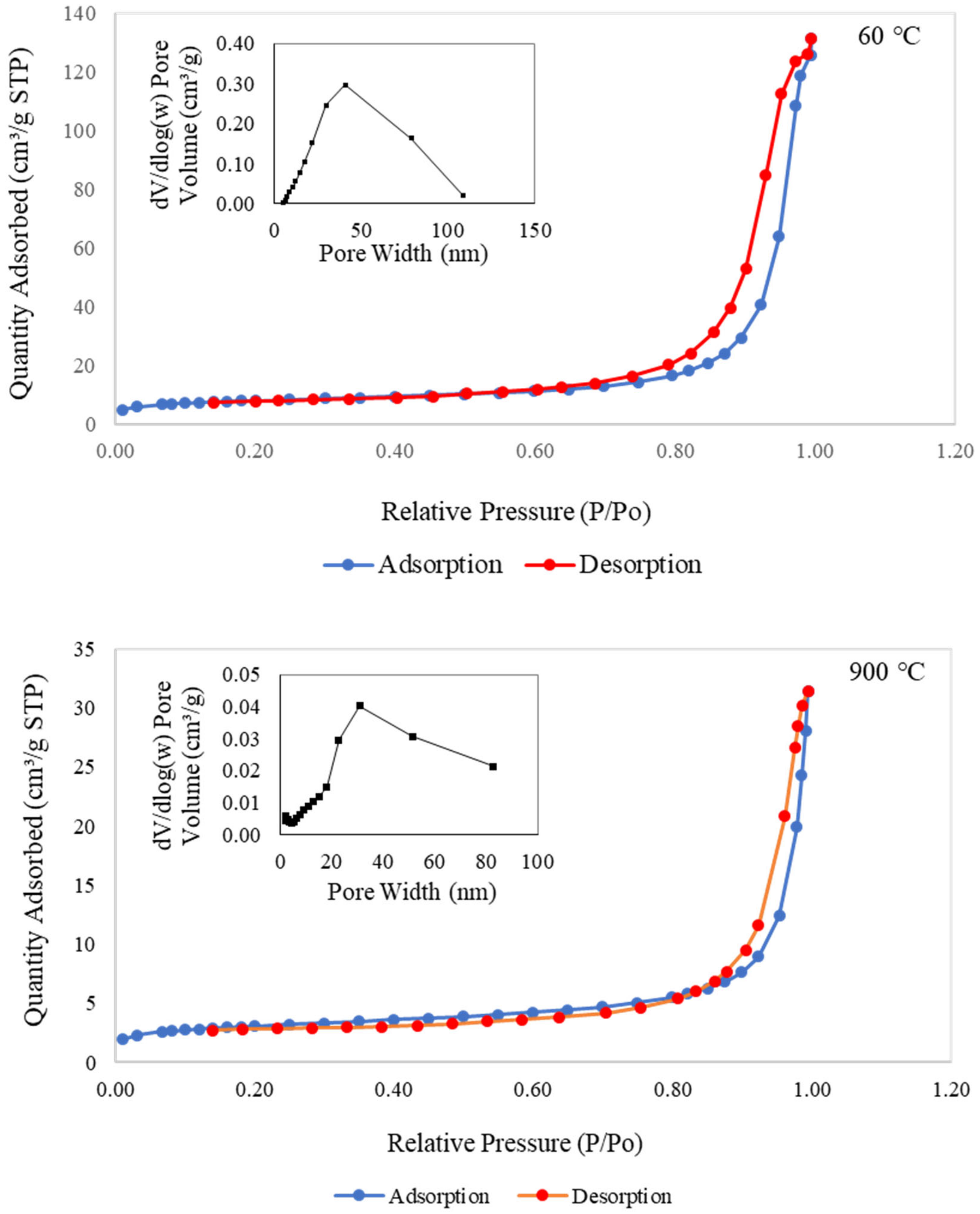
3.3.5. Nitrogen Adsorption–Desorption Isotherms Analysis
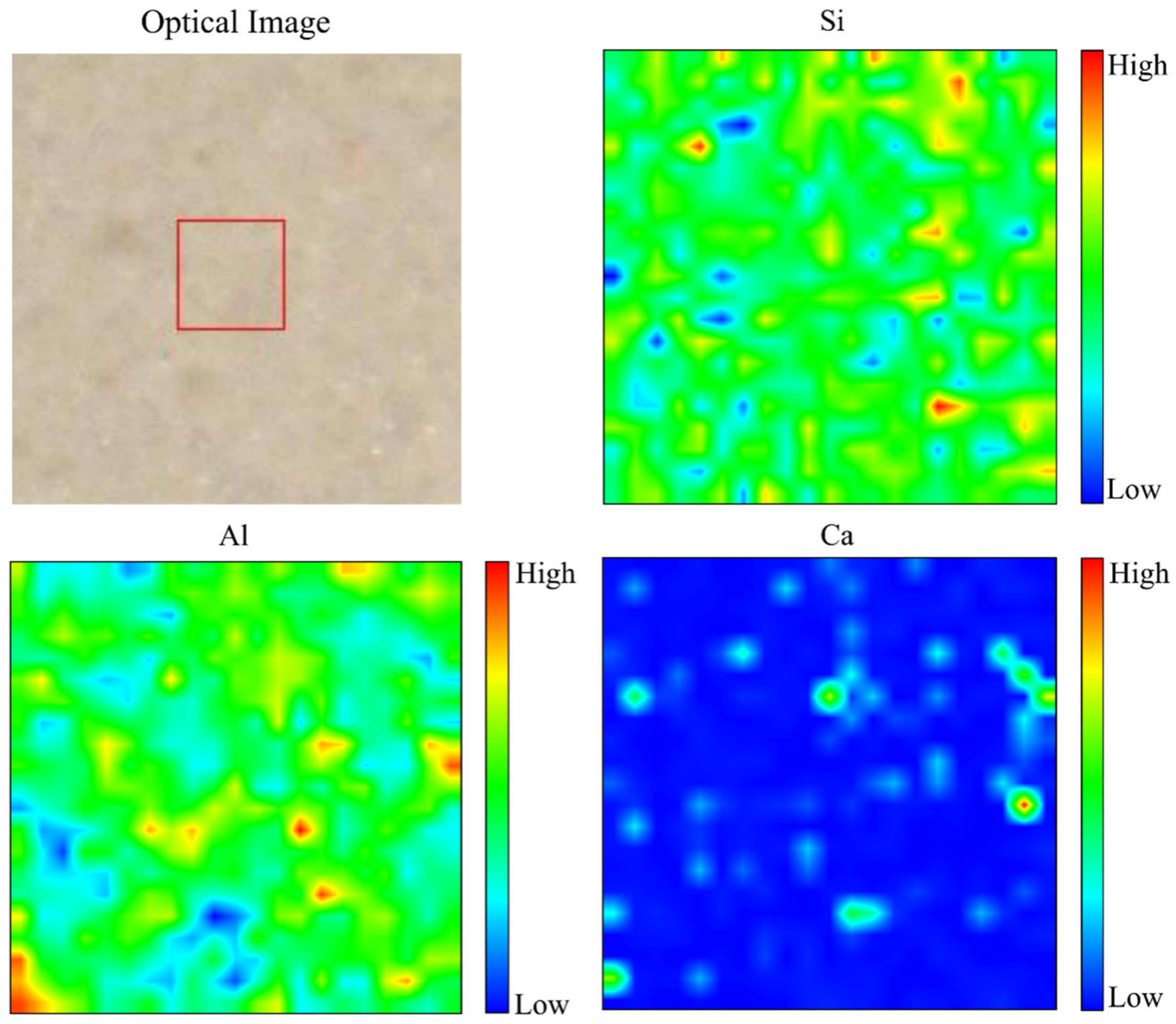
3.3.6. Elemental Distribution from Micro-XRF Analysis
3.4. Adsorption of Copper Ions Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, Y.; Yuan, Y.; Wang, K.; He, Y.; Cui, X. Preparation of geopolymer-based inorganic membrane for removing Ni2+ from wastewater. J. Hazard Mater. 2015, 299, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Awual, M.R. Novel ligand functionalized composite material for efficient copper(II) capturing from wastewater sample. Compos. B Eng. 2019, 172, 387–396. [Google Scholar] [CrossRef]
- Meseldzija, S.; Petrovic, J.; Onjia, A.; Volkov-Husovic, T.; Nesic, A.; Vukelic, N. Utilization of agro-industrial waste for removal of copper ions from aqueous solutions and mining-wastewater. J. Ind. Eng. Chem. 2019, 75, 246–252. [Google Scholar] [CrossRef]
- El-Sayed, W.N.; Elwakeel, K.Z.; Shahat, A.; Awual, M.R. Investigation of novel nanomaterial for the removal of toxic substances from contaminated water. RSC Adv. 2019, 9, 14167–14175. [Google Scholar] [CrossRef]
- Rocha, A.C.D.C.; Scaratti, G.; Moura-Nickel, C.D.; Da Silva, T.L.; Vieira, M.G.A.; Peralta, R.M.; Peralta, R.A.; De Noni, A.; Moreira, R.D.F.P.M. Economical and Technological Aspects of Copper Removal from Water Using a Geopolymer and Natural Zeolite. Water Air Soil Pollut. 2020, 231, 361. [Google Scholar] [CrossRef]
- Su, Q.; Ye, Q.; Deng, L.; He, Y.; Cui, X. Prepared self-growth supported copper catalyst by recovering Cu (II) from wastewater using geopolymer microspheres. J. Clean. Prod. 2020, 272, 122571. [Google Scholar] [CrossRef]
- Arokiasamy, P.; Abdullah, M.M.A.B.; Rahim, S.Z.A.; Zainol, M.R.R.M.A.; Salleh, M.A.A.M.; Kheimi, M.; Chaiprapa, J.; Sandu, A.V.; Vizureanu, P.; Razak, R.A.; et al. Metakaolin/sludge based geopolymer adsorbent on high removal efficiency of Cu2+. Case Stud. Constr. Mater. 2022, 17, e01428. [Google Scholar] [CrossRef]
- Chen, W.S.; Chen, Y.C.; Lee, C.H. Modified Activated Carbon for Copper Ion Removal from Aqueous Solution. Processes 2022, 10, 150. [Google Scholar] [CrossRef]
- Emenike, E.C.; Adeniyi, A.G.; Omuku, P.E.; Okwu, K.C.; Iwuozor, K.O. Recent advances in nano-adsorbents for the sequestration of copper from water. J. Water Process Eng. 2022, 47, 102715. [Google Scholar] [CrossRef]
- Nowicka, B. Heavy Metal–Induced Stress in Eukaryotic Algae—Mechanisms of Heavy Metal Toxicity and Tolerance with Particular Emphasis on Oxidative Stress in Exposed Cells and the Role of Antioxidant Response. Environ. Sci. Pollut. Res. 2022, 29, 16860–16911. [Google Scholar] [CrossRef]
- Malacas, M.; Balberan, M.C.; Bederi, N.A.J.; Ramos, C.J.; Rato, M.; Salazar, A.G.; Roque, E. The removal of copper (II) and lead (II) from aqueous solution using Fuller’s earth and Fuller’s earth-immobilized nanoscale zero valent iron (FE-NZVI) by adsorption. MATEC Web Conf. 2019, 268, 05006. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; Ibrahim, W.M.W.; Abdullah, M.M.A.B.; Sauffi, A.S. A Review of Geopolymer Based Metakaolin Membrane as an Effective Adsorbent for Waste Water Treatment. IOP Conf. Ser. Mater. Sci. Eng. 2020, 864, 012128. [Google Scholar] [CrossRef]
- Mužek, M.N.; Svilović, S.; Zelić, J. Fly ash-based geopolymeric adsorbent for copper ion removal from wastewater. Desalin. Water Treat 2014, 52, 2519–2526. [Google Scholar] [CrossRef]
- Kubra, K.T.; Salman, M.S.; Hasan, M.N.; Islam, A.; Hasan, M.M.; Awual, M.R. Utilizing an alternative composite material for effective copper(II) ion capturing from wastewater. J. Mol. Liq. 2021, 336, 116325. [Google Scholar] [CrossRef]
- Awual, M.R. New type mesoporous conjugate material for selective optical copper(II) ions monitoring & removal from polluted waters. Chem. Eng. J. 2017, 307, 85–94. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Rahman, M.M.; Asiri, A.M. Novel composite material for selective copper(II) detection and removal from aqueous media. J. Mol. Liq. 2019, 283, 772–780. [Google Scholar] [CrossRef]
- Purbasari, A.; Ariyanti, D.; Sumardiono, S. Preparation and application of fly ash-based geopolymer for heavy metal removal. AIP Conf. Proc. 2020, 2197, 050006. [Google Scholar] [CrossRef]
- Es-Sahbany, H.; Berradi, M.; Nkhili, S.; Hsissou, R.; Allaoui, M.; Loutfi, M.; Bassir, D.; Belfaquir, M.; El Youbi, M. Removal of heavy metals (nickel) contained in wastewater-models by the adsorption technique on natural clay. Mater. Today Proc. 2019, 13, 866–875. [Google Scholar] [CrossRef]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Rasaki, S.A.; Bingxue, Z.; Guarecuco, R.; Thomas, T.; Minghui, Y. Geopolymer for use in heavy metals adsorption, and advanced oxidative processes: A critical review. J. Clean. Prod. 2019, 213, 42–58. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.H.; Seabra, M.P.; Labrincha, J.A. Novel porous fly-ash containing geopolymer monoliths for lead adsorption from wastewaters. J. Hazard Mater. 2016, 318, 631–640. [Google Scholar] [CrossRef]
- Darmayanti, L.; Notodarmojo, S.; Damanhuri, E.; Kadja, G.T.M.; Mukti, R. Preparation of alkali-activated fly ash-based geopolymer and their application in the adsorption of copper (II) and zinc (II) ions. MATEC Web Conf. 2019, 276, 06012. [Google Scholar] [CrossRef] [Green Version]
- Mihaly-Cozmuta, L.; Peter, A.; Nicula, C.; Tutu, H.; Silipas, D.; Indrea, E. Adsorption of heavy metal cations by Na-clinoptilolite: Equilibrium and selectivity studies. J. Environ. Manag. 2014, 137, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Panda, L.; Jena, S.K.; Rath, S.S.; Misra, P.K. Heavy metal removal from water by adsorption using a low-cost geopolymer. Environ. Sci. Pollut. Res. 2020, 27, 24284–24298. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Song, W.; Li, J.; Li, Q. Improved simultaneous adsorption of Cu(II) and Cr(VI) of organic modified metakaolin-based geopolymer. Arab. J. Chem. 2020, 13, 4811–4823. [Google Scholar] [CrossRef]
- Kara, I.; Tunc, D.; Sayin, F.; Akar, S.T. Study on the performance of metakaolin based geopolymer for Mn(II) and Co(II) removal. Appl. Clay Sci. 2018, 161, 184–193. [Google Scholar] [CrossRef]
- Onutai, S.; Kobayashi, T.; Thavorniti, P.; Jiemsirilers, S. The adsorption of cadmium ions on fly ash based geopolymer particles. Key Eng. Mater. 2018, 766, 65–70. [Google Scholar] [CrossRef]
- Luukkonen, T.; Heponiemi, A.; Runtti, H.; Pesonen, J.; Yliniemi, J.; Lassi, U. Application of alkali-activated materials for water and wastewater treatment: A review. Rev. Environ. Sci. Biotechnol. 2019, 18, 271–297. [Google Scholar] [CrossRef] [Green Version]
- Mohd, M.A.B.A.; Jamaludin, L.; Hussin, K.; Binhussain, M.; Ghazali, C.M.R.; Izzat, A.M. Study on Fly Ash Based Geopolymer for Coating Applications. Adv. Mater. Res. 2013, 686, 227–233. [Google Scholar] [CrossRef]
- Sauffi, A.S.; Mastura, W.; Ibrahim, W.; Mustafa, M.; Bakri, A. Phase Analysis of Different Liquid Ratio on Metakaolin/Dolomite Geopolymer. Arch. Metall. Mater. 2022, 67, 247–250. [Google Scholar]
- Ibrahim, W.M.W.; Hussin, K.; Abdullah, M.M.A.B.; Kadir, A.A. Geopolymer lightweight bricks manufactured from fly ash and foaming agent. AIP Conf. Proc. 2017, 1835, 020048. [Google Scholar] [CrossRef]
- Bumanis, G.; Novais, R.M.; Carvalheiras, J.; Bajare, D.; Labrincha, J.A. Applied Clay Science Metals removal from aqueous solutions by tailored porous waste-based granulated alkali-activated materials. Appl. Clay Sci. 2019, 179, 105147. [Google Scholar] [CrossRef]
- Perumal, P.; Luukkonen, T.; Sreenivasan, H.; Kinnunen, P.; Illikainen, M. Porous alkali-activated materials. New Mater. Civ. Eng. 2020, 529–563. [Google Scholar] [CrossRef]
- Awual, M.R. A novel facial composite adsorbent for enhanced copper(II) detection and removal from wastewater. Chem. Eng. J. 2015, 266, 368–375. [Google Scholar] [CrossRef]
- Tang, Q.; Ge, Y.-Y.; Wang, K.-T.; He, Y.; Cui, X.-M. Preparation and characterization of porous metakaolin-based inorganic polymer spheres as an adsorbent. JMADE 2015, 88, 1244–1249. [Google Scholar] [CrossRef]
- Maleki, A.; Hajizadeh, Z.; Sharifi, V.; Emdadi, Z. A green, porous and eco-friendly magnetic geopolymer adsorbent for heavy metals removal from aqueous solutions. J. Clean. Prod. 2019, 215, 1233–1245. [Google Scholar] [CrossRef]
- Mondal, S.K.; Welz, A.; Rownaghi, A.; Wang, B.; Ma, H.; Rezaei, F.; Kumar, A.; Okoronkwo, M.U. Investigating the microstructure of high-calcium fly ash-based alkali-activated material for aqueous Zn sorption. Environ. Res. 2021, 198, 110484. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A Review on Geopolymers as Emerging Materials for the Adsorption of Heavy Metals and Dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef]
- Ibrahim, W.M.W.; Ahmad, R.; Coman, B.T.; Abdullah, M.M.A.B.; Puskas, A.; Jaganathan, V.S. The Effects of Solid to Liquid Ratio on Fly Ash Based Lightweight Geopolymer. IOP Conf. Ser. Mater. Sci. Eng. 2020, 877, 012013. [Google Scholar] [CrossRef]
- Ibrahim, W.M.W.; Abdullah, M.M.A.B.; Ahmad, R.; Sandu, A.V.; Vizureanu, P.; Benjeddou, O.; Rahim, A.; Ibrahim, M.; Sauffi, A.S. Chemical Distributions of Different Sodium Hydroxide Molarities on Fly Ash/Dolomite-Based Geopolymer. Materials 2022, 15, 6163. [Google Scholar] [CrossRef]
- Ma, C.K.; Awang, A.Z.; Omar, W. Structural and material performance of geopolymer concrete: A review. Constr. Build. Mater. 2018, 186, 90–102. [Google Scholar] [CrossRef]
- Farhana, Z.F.; Kamarudin, H.; Rahmat, A.; Al Bakri, A.M.M. A Study on Relationship between Porosity and Compressive Strength for A Study on Relationship between Porosity and Compressive Strength for Geopolymer Paste. Key Eng. Mater. 2013, 594–595, 1112–1116. [Google Scholar] [CrossRef]
- Thokchom, S.; Ghosh, S. Effect of water absorption, porosity and sorptivity on durability of geopolymer mortars. ARPN J. Eng. Appl. Sci. 2009, 4, 28–32. [Google Scholar]
- Bai, C.; Franchin, G.; Elsayed, H.; Zaggia, A.; Conte, L.; Li, H.; Colombo, P. High-porosity geopolymer foams with tailored porosity for thermal insulation and wastewater treatment. J. Mater. Res. 2017, 32, 3251–3259. [Google Scholar] [CrossRef]
- He, J.; Gao, Q.; Song, X.; Bu, X.; He, J. Effect of foaming agent on physical and mechanical properties of alkali-activated slag foamed concrete. Constr. Build. Mater. 2019, 226, 280–287. [Google Scholar] [CrossRef]
- Jaya, N.A.; Yun-Ming, L.; Cheng-Yong, H.; Abdullah, M.M.A.B.; Hussin, K. Correlation between pore structure, compressive strength and thermal conductivity of porous metakaolin geopolymer. Constr. Build. Mater. 2020, 247, 118641. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. ASTM C642 Standard Test Method for Density, Absorption and Voids in Hardened Concrete; American Society for Testing and Materials: West Conshohocken, PA, USA, 2001. [Google Scholar]
- ASTM Committee D-20 on Plastics. Standard Test Methods for Density and Specific Gravity (Relative Density) of Plastics by Displacement 1; American Society for Testing and Materials: West Conshohocken, PA, USA, 2008. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. ASTM C109 Standard Test Method for Compressive Strength of Hydraulic Cement Mortars; American Society for Testing and Materials: West Conshohocken, PA, USA, 2008. [Google Scholar]
- American Society for Testing and Materials. ASTM D3663-20 Standard Test Method for Surface Area of Catalysts and Catalyst Carriers; American Society for Testing and Materials: West Conshohocken, PA, USA, 2020. [Google Scholar]
- Cheng, T.W.; Lee, M.L.; Ko, M.S.; Ueng, T.H.; Yang, S.F. The heavy metal adsorption characteristics on metakaolin-based geopolymer. Appl. Clay Sci. 2012, 56, 90–96. [Google Scholar] [CrossRef]
- Ge, Y.; Cui, X.; Kong, Y.; Li, Z.; He, Y.; Zhou, Q. Porous geopolymeric spheres for removal of Cu(II) from aqueous solution: Synthesis and evaluation. J. Hazard Mater. 2015, 283, 244–251. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. ASTMC618—12 Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; American Society for Testing and Materials: West Conshohocken, PA, USA, 2014. [Google Scholar] [CrossRef]
- Dabbaghi, F.; Sadeghi-Nik, A.; Libre, N.A.; Nasrollahpour, S. Characterizing fiber reinforced concrete incorporating zeolite and metakaolin as natural pozzolans. Structures 2021, 34, 2617–2627. [Google Scholar] [CrossRef]
- Weise, K.; Ukrainczyk, N.; Duncan, A.; Koenders, E. Enhanced Metakaolin Reactivity in Blended Cement with Additional Calcium Hydroxide. Materials 2022, 15, 367. [Google Scholar] [CrossRef]
- Salimi, J.; Ramezanianpour, A.M.; Moradi, M.J. Studying the effect of low reactivity metakaolin on free and restrained shrinkage of high performance concrete. J. Build. Eng. 2019, 28, 101053. [Google Scholar] [CrossRef]
- Kadhim, A.; Sadique, M.; Al-Mufti, R.; Hashim, K. Developing one-part alkali-activated metakaolin/natural pozzolan binders using lime waste. Adv. Cem. Res. 2021, 33, 342–356. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer foam concrete: An emerging material for sustainable construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Jaya, N.A.; Liew, Y.M.; Heah, C.Y.; Abdullah, M.M.A.B. Effect of solid-to-liquid ratios on metakaolin geopolymers. AIP Conf. Proc. 2018, 2045, 020099. [Google Scholar] [CrossRef]
- Zahid, M.; Shafiq, N.; Nuruddin, M.F.; Nikbakht, E.; Jalal, A. Effect of partial replacement of fly ash by metakaolin on strength development of fly ash based geopolymer mortar. Key Eng. Mater. 2017, 744, 131–135. [Google Scholar] [CrossRef]
- Petlitckaia, S.; Poulesquen, A. Design of lightweight metakaolin based geopolymer foamed with hydrogen peroxide. Ceram. Int. 2019, 45, 1322–1330. [Google Scholar] [CrossRef]
- Jaya, N.U.R.A.; Yun-ming, L.; Mustafa, M.; Bakri, A. Thermophysical Properties of Metakaolin Geopolymers Based on Na 2 SiO 3/NaOH Ratio. Solid State Phenom. 2018, 280, 487–493. [Google Scholar] [CrossRef]
- Jaya, N.A.; Yun-Ming, L.; Abdullah, M.M.A.B.; Cheng-Yong, H.; Hussin, K. Effect of Sodium Hydroxide Molarity on Physical, Mechanical and Thermal Conductivity of Metakaolin Geopolymers. IOP Conf. Ser. Mater. Sci. Eng. 2018, 343, 012015. [Google Scholar] [CrossRef]
- El Alouani, M.; Alehyen, S.; El Achouri, M.; Taibi, M. Preparation, Characterization, and Application of Metakaolin-Based Geopolymer for Removal of Methylene Blue from Aqueous Solution. J. Chem. 2019, 2019, 4212901. [Google Scholar] [CrossRef]
- Hossain, M.M.; Karim, M.R.; Hossain, M.K.; Islam, M.N.; Zain, M.F.M. Durability of mortar and concrete containing alkali-activated binder with pozzolans: A review. Constr. Build. Mater. 2015, 93, 95–109. [Google Scholar] [CrossRef]
- Kwek, S.Y.; Awang, H.; Cheah, C.B. Influence of liquid-to-solid and alkaline activator (Sodium silicate to sodium hydroxide) ratios on fresh and hardened properties of alkali-activated palm oil fuel ash geopolymer. Materials 2021, 14, 4253. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.L.; Huynh, T.P. Effect of alkali-activator and rice husk ash content on strength development of fly ash and residual rice husk ash-based geopolymers. Constr. Build. Mater. 2015, 101, 1–9. [Google Scholar] [CrossRef]
- Böke, N.; Birch, G.D.; Nyale, S.M.; Petrik, L.F. New synthesis method for the production of coal fly ash-based foamed geopolymers. Constr. Build. Mater. 2015, 75, 189–199. [Google Scholar] [CrossRef]
- Do, N.; Oznuroz, H.; Bilgil, A.; Vural, T.; Süzgec, E. The effect of alkali activation solutions with different water glass/NaOH solution ratios on geopolymer composite materials. IOP Conf. Ser. Mater. Sci. Eng. 2019, 660, 012003. [Google Scholar] [CrossRef]
- Samal, S.; Thanh, N.P.; Marvalova, B.; Petrikova, I. Thermal Characterization of Metakaolin-Based Geopolymer. JOM 2017, 69, 2480–2484. [Google Scholar] [CrossRef]
- Skoczylas, K.; Rucińska, T. The effects of low curing temperature on the properties of cement mortars containing nanosilica. Nanotechnol. Constr. A Sci. Internet J. 2019, 11, 536–544. [Google Scholar] [CrossRef]
- Azevedo, A.G.S.; Strecker, K.; Barros, L.A.; Tonholo, L.F.; Lombardi, C.T. Effect of curing temperature, activator solution composition and particle size in Brazilian fly-ash based geopolymer production. Mater. Res. 2019, 22, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kong, H.; Cheu, S.-C.; Othman, N.S.; Song, S.-T.; Saman, N.; Johari, K.; Mat, H. Surfactant modification of banana trunk as low-cost adsorbents and their high benzene adsorptive removal performance from aqueous solution. RSC Adv. 2016, 6, 24738–24751. [Google Scholar] [CrossRef]
- Tamjidi, S.; Moghadas, B.K.; Esmaeili, H.; Khoo, F.S.; Gholami, G.; Ghasemi, M. Improving the surface properties of adsorbents by surfactants and their role in the removal of toxic metals from wastewater: A review study. Process Saf. Environ. Prot. 2021, 148, 775–795. [Google Scholar] [CrossRef]
- Salam, M.A.; Abukhadra, M.R.; Mostafa, M. Effective decontamination of As(V), Hg(II), and U(VI) toxic ions from water using novel muscovite/zeolite aluminosilicate composite: Adsorption behavior and mechanism. Environ. Sci. Pollut. Res. 2020, 27, 13247–13260. [Google Scholar] [CrossRef]
- Zhou, W.; Niu, J.; Xiao, W.; Ou, L. Adsorption of bulk nanobubbles on the chemically surface-modified muscovite minerals. Ultrason. Sonochem. 2019, 51, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Y.L.; He, D.D.; Liu, G.S. Adsorption of cationic collectors and water on muscovite (0 0 1) surface: A molecular dynamics simulation study. Miner. Eng. 2013, 53, 101–107. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Li, L.-Y.; Jaya, N.A.; Abdullah, M.M.A.B.; Tan, S.J.; Hussin, K. Formation of one-part-mixing geopolymers and geopolymer ceramics from geopolymer powder. Constr. Build. Mater. 2017, 156, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, X.; Lu, Y.; Bai, X. Effects of Na/Al ratio on mechanical properties and microstructure of red mud-coal metakaolin geopolymer. Constr. Build. Mater. 2020, 263, 120653. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Sawan, S.E.A.; Khattab, R.M.; Abdel-Shafi, A.A. Effect of nano sand on the properties of metakaolin-based geopolymer: Study on its low rate sintering. Constr. Build. Mater. 2020, 246, 118486. [Google Scholar] [CrossRef]
- Lahoti, M.; Wong, K.K.; Yang, E.H.; Tan, K.H. Effects of Si/Al molar ratio on strength endurance and volume stability of metakaolin geopolymers subject to elevated temperature. Ceram. Int. 2018, 44, 5726–5734. [Google Scholar] [CrossRef]
- Awual, M.R.; Ismael, M.; Khaleque, M.A.; Yaita, T. Ultra-trace copper(II) detection and removal from wastewater using novel meso-adsorbent. J. Ind. Eng. Chem. 2014, 20, 2332–2340. [Google Scholar] [CrossRef]
- Awual, R.; Eldesoky, G.E.; Yaita, T.; Naushad, M.; Shiwaku, H.; AlOthman, Z.A.; Suzuki, S. Schiff based ligand containing nano-composite adsorbent for optical copper(II) ions removal from aqueous solutions. Chem. Eng. J. 2015, 279, 639–647. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Khaleque, M.A.; Sheikh, M.C. Treatment of copper(II) containing wastewater by a newly developed ligand based facial conjugate materials. Chem. Eng. J. 2016, 288, 368–376. [Google Scholar] [CrossRef]
- Wang, F.; Kong, L.; Zhou, Z. Study on Pore Structure and Mechanical Property of Expansive Soil under Different Dehydration Conditions. Appl. Sci. 2022, 12, 5981. [Google Scholar] [CrossRef]
- Chen, S.; Ruan, S.; Zeng, Q.; Liu, Y.; Zhang, M.; Tian, Y.; Yan, D. Pore structure of geopolymer materials and its correlations to engineering properties: A review. Constr. Build. Mater. 2022, 328, 127064. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Liu, L.; Xiao, J.; Zhang, G.; Guo, F.; Li, L. Influence of MgO on the Hydration and Shrinkage Behavior of Low Heat Portland Cement-Based Materials via Pore Structural and Fractal Analysis. Fractal Fraction. 2022, 6, 40. [Google Scholar] [CrossRef]



| Number | Component | Wt% |
|---|---|---|
| 1 | Al2O3 | 34.7 |
| 2 | SiO2 | 56.7 |
| 3 | P2O5 | 1.68 |
| 4 | K2O | 0.607 |
| 5 | CaO | 0.700 |
| 6 | TiO2 | 3.13 |
| 7 | Fe2O3 | 2.09 |
| 8 | CuO | 0.0383 |
| 9 | Ga2O3 | 0.0398 |
| 10 | SrO | 0.0530 |
| 11 | ZrO2 | 0.199 |
| Sample | Specific Surface Area, SBET (m2/g) | Total Pore Volume, VT (cm³/g) |
|---|---|---|
| MK | 4.475 | 0.012366 |
| 60 °C | 24.608 | 0.171588 |
| 900 °C | 9.669 | 0.028246 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.; Wan Ibrahim, W.M.; Abdullah, M.M.A.B.; Nabialek, M.; Putra Jaya, R.; Setkit, M.; Ahmad, R.; Jeż, B. Synthesis of Metakaolin Based Alkali Activated Materials as an Adsorbent at Different Na2SiO3/NaOH Ratios and Exposing Temperatures for Cu2+ Removal. Materials 2023, 16, 1221. https://doi.org/10.3390/ma16031221
Ibrahim M, Wan Ibrahim WM, Abdullah MMAB, Nabialek M, Putra Jaya R, Setkit M, Ahmad R, Jeż B. Synthesis of Metakaolin Based Alkali Activated Materials as an Adsorbent at Different Na2SiO3/NaOH Ratios and Exposing Temperatures for Cu2+ Removal. Materials. 2023; 16(3):1221. https://doi.org/10.3390/ma16031221
Chicago/Turabian StyleIbrahim, Masdiyana, Wan Mastura Wan Ibrahim, Mohd Mustafa Al Bakri Abdullah, Marcin Nabialek, Ramadhansyah Putra Jaya, Monthian Setkit, Romisuhani Ahmad, and Bartłomiej Jeż. 2023. "Synthesis of Metakaolin Based Alkali Activated Materials as an Adsorbent at Different Na2SiO3/NaOH Ratios and Exposing Temperatures for Cu2+ Removal" Materials 16, no. 3: 1221. https://doi.org/10.3390/ma16031221








