The Safety of Fluoride Compounds and Their Effect on the Human Body—A Narrative Review
Abstract
1. Introduction
2. Cytotoxicity of Fluoride
2.1. Acute Poisoning with Fluoride Compounds
2.2. Chronic Poisoning with Fluoride Compounds
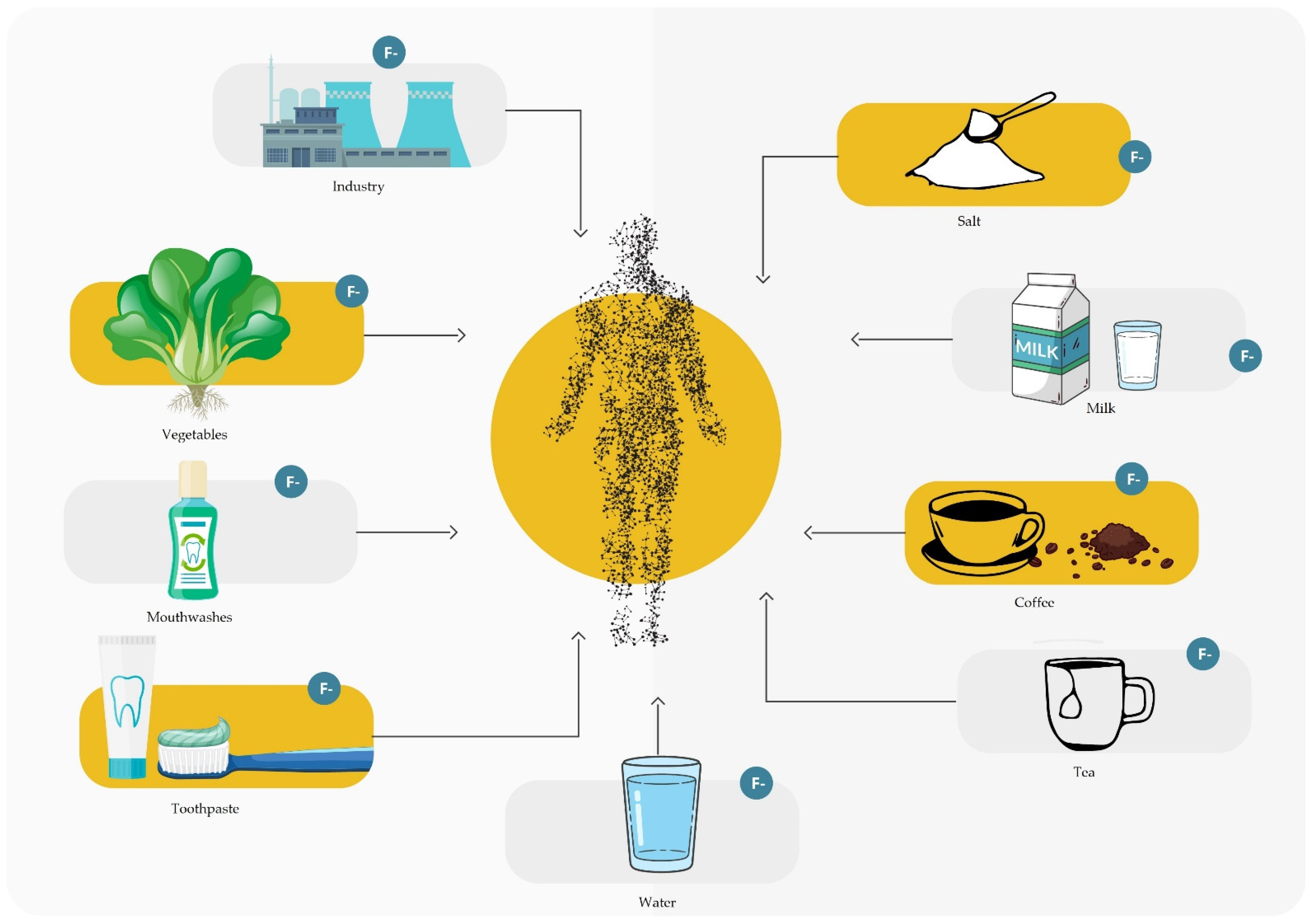
3. Sources of Fluoride
3.1. Groceries
3.2. Water and Drinks
3.3. Drugs and Agents Used in Dentistry
3.4. Endogenous Fluoridation
3.5. Exogenous Fluoridation
3.6. Fluoride Mouth Rinse
3.7. Fluoride Varnish
3.8. Fluoride Foam and Gel
3.9. Filling Materials
3.10. Fissure Sealants
3.11. Medicines and Agents Used in Medicine
3.12. Industry and Econoamy Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanduti, D.; Sterbenk, P. Artnik, and fluoride: A review of use and effects on health. Mater. Socio Med. 2016, 28, 133. [Google Scholar] [CrossRef]
- Sikorska-Jaroszyńska, M.H.; Czelej, G. Fluor w Stomatologii i Medycynie; Wydawnictwo Czelej: Lublin, Poland, 2000. [Google Scholar]
- Dhar, V.; Bhatnagar, M. Physiology and toxicity of fluoride. Indian J. Dent. Res. 2009, 20, 350. [Google Scholar] [CrossRef]
- Ozsvath, D.L. Fluoride and environmental health: A review. Rev. Environ. Sci. Biotechnol. 2009, 8, 59–79. [Google Scholar] [CrossRef]
- Jha, S.K.; Mishra, V.K.; Sharma, D.K.; Damodaran, T. Fluoride in the environment and its metabolism in humans. Rev. Environ. Contam. Toxicol. 2011, 211, 121–142. [Google Scholar]
- Naseem, S.; Rafique, T.; Bashir, E.; Bhanger, M.I.; Laghari, A.; Usmani, T.H. Lithological Influences on occurrence of high-fluoride groundwater in Nagar Parkar Area, Thar Desert, Pakistan. Chemosphere 2010, 78, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Whitford, G.M. The physiological and toxicological characteristics of fluoride. J. Dent. Res. 1990, 69, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Jańczuk, Z.; Kaczmarek, U.; Lipski, M. (Eds.) Stomatologia Zachowawcza z Endodoncją; PZWL Wydawncitwo Lekarskie: Warszawa, Poland, 2014. [Google Scholar]
- Nouri, M.-R.; Titley, K.C. Paediatrics: A review of the antibacterial effect of fluoride. Oral Health 2003, 93, 8–12. [Google Scholar]
- Kaczmarek, U.; Jackowska, T.; Mielnik-Błaszczak, M.; Jurczak, A.; Olczak-Kowalczyk, D. Indywidualna profilaktyka fluorkowa u dzieci i młodzieży-rekomendacje polskich ekspertów streszczenie. Nowa Stomatol. 2019, 24, 70–85. [Google Scholar] [CrossRef]
- Jańczuk, Z.K.U.; Mariusz, L. Stomatologia Zachowawcza z Endodoncją. Zarys Kliniczny; Wyd. Lekarskie PZWL: Warszawa, Poland, 2014. [Google Scholar]
- Karunanidhi, D.; Aravinthasamy, P.; Subramani, T.; Balakumar, K.G.; Chandran, N.S. Health threats for the inhabitants of a textile hub (tiruppur region) in southern india due to multipath entry of fluoride ions from groundwater. Ecotoxicol. Environ. Saf. 2020, 204, 111071. [Google Scholar] [CrossRef]
- Ibiyemi, O.; Zohoori, F.V.; Valentine, R.A.; Maguire, A. Fluoride intake and urinary fluoride excretion in 4- and 8-year-old children living in urban and rural areas of Southwest Nigeria. Community Dent. Oral Epidemiology 2018, 46, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Toumba, J.; Lygidakis, N.; Oulis, C.; Parnell, C.; Espelid, I.; Poulsen, S.; Twetman, S. Guidelines on the use of fluoride in children: An EAPD policy document. Eur. Arch. Paediatr. Dent. 2009, 10, 129–135. [Google Scholar] [CrossRef]
- Olczak-Kowalczyk, D.; Borysewicz-Lewicka, M.; Adamowicz-Klepalska, B.; Jackowska, T.; Kaczmarek, U. Consensus statement of polish experts on individual caries prevention with fluoride in children and adolescents. Nowa Stomatol. 2016, 21, 47–73. [Google Scholar] [CrossRef]
- Lussi, A.; Hellwig, E.; Klimek, J. Fluorides—Mode of action and recommendations for use. Schweiz Mon. Zahnmed. 2012, 122, 1030–1042. [Google Scholar]
- Olczak-Kowalczyk, D.; Szczepańska, J.; Kaczmarek, U. Modern Dentistry of Developmental Age; Med Press International: Otwock, Poland, 2017. [Google Scholar]
- Whitford, G.M. Fluoride in dental products: Safety considerations. J. Dent. Res. 1987, 66, 1056–1060. [Google Scholar] [CrossRef]
- Battaleb-Looie, S.; Moore, F.; Jacks, G.; Ketabdari, M.R. Geological sources of fluoride and acceptable intake of fluoride in an endemic fluorosis area, Southern Iran. Env. Geochem. Health 2012, 34, 641–650. [Google Scholar] [CrossRef]
- Susheela, A.K.; Mudgal, A.; Keast, G. Fluoride in water: An overview. Water front 1999, 1, 11–13. [Google Scholar]
- Fishwick, T. Hazards of hydrogen fluoride. Loss Prev. Bull. 2012, 224, 25–28. [Google Scholar]
- Lima-Arsati, Y.B.D.O.; Gomes, A.R.L.F.; Santos, H.K.A.; Arsati, F.; Oliveira, M.C.; Freitas, V.S. Exposição a fluoreto por crianças na faixa etária crítica para fluorose dentária, residentes no semiárido brasileiro. Cien. Saude Colet. 2018, 23, 1045–1054. [Google Scholar] [CrossRef]
- Gupta, S.K.; Deshpande, R.D.; Agarwal, M.; Raval, B.R. Origin of high fluoride in groundwater in the North Gujarat-Cambay Region, India. Hydrogeol. J. 2005, 13, 596–605. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Ma, T.; Ma, R. Geochemical processes controlling the elevated fluoride concentrations in groundwaters of the Taiyuan Basin, Northern China. J. Geochem. Explor. 2007, 93, 1–12. [Google Scholar] [CrossRef]
- Dean, H.T.; Arnold, J.F.A.; Jay, P.; Knutson, J.W. Studies on mass control of dental caries through fluoridation of the public water supply. Public Heal. Rep. (1896–1970) 1950, 65, 1403. [Google Scholar] [CrossRef]
- Brahman, K.D.; Kazi, T.G.; Baig, J.A.; Afridi, H.I.; Khan, A.; Arain, S.S.; Arain, M.B. Fluoride and arsenic exposure through water and grain crops in Nagarparkar, Pakistan. Chemosphere 2014, 100, 182–189. [Google Scholar] [CrossRef]
- Chachra, D.; Vieira, A.P.G.F.; Grynpas, M.D. Fluoride and mineralized tissues. Crit. Rev. Biomed. Eng. 2008, 36, 183–233. [Google Scholar] [CrossRef]
- Dean, H.T. Classification of mottled enamel diagnosis. J. Am. Dent. Assoc. (1922) 1934, 21, 1421–1426. [Google Scholar] [CrossRef]
- Gabuda, S.P.; Gaidash, A.A.; Kozlova, S.G.; Allan, N.L. Structural forms of fluorides in bone tissue of animals under chronic fluoride intoxication. J. Struct. Chem. 2006, 47, 258–266. [Google Scholar] [CrossRef]
- Kebede, A.; Retta, N.; Abuye, C.; Whiting, S.; Kassaw, M.; Zeru, T.; Tessema, M.; Kjellevold, M. Dietary fluoride intake and associated skeletal and dental fluorosis in school age children in Rural Ethiopian Rift Valley. Int. J. Environ. Res. Public Health 2016, 13, 756. [Google Scholar] [CrossRef]
- Guney, M.; Oral, B.; Take, G.; Giray, S.; Mungan, T. Effect of fluoride intoxication on endometrial apoptosis and lipid peroxidation in rats: Role of vitamins E and C. Toxicology 2007, 231, 215–223. [Google Scholar] [CrossRef]
- Cicek, E.; Aydin, G.; Akdogan, M.; Okutan, H. Effects of chronic ingestion of sodium fluoride on myocardium in a second generation of rats. Hum. Exp. Toxicol. 2005, 24, 79–87. [Google Scholar] [CrossRef]
- Perera, T.; Ranasinghe, S.; Alles, N.; Waduge, R. Effect of fluoride on major organs with the different time of exposure in rats. Environ. Health Prev. Med. 2018, 23, 17. [Google Scholar] [CrossRef]
- Lubkowska, A.; Chlubek, D.; Machoy-MokrzyåSka, A.; Nowacki, P. Distribution of fluoride in selected structures of the central nervous system in rats exposed to NaF and AlCl3 in drinking water. Trace Elem. Electrolytes 2012, 29, 162–171. [Google Scholar] [CrossRef]
- Choi, A.L.; Sun, G.; Zhang, Y.; Grandjean, P. Developmental fluoride neurotoxicity: A systematic review and meta-analysis. Env. Health Perspect 2012, 120, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Nkpaa, K.W.; Onyeso, G.I. Rutin attenuates neurobehavioral deficits, oxidative stress, neuro-inflammation and apoptosis in fluoride treated rats. Neurosci. Lett. 2018, 682, 92–99. [Google Scholar] [CrossRef]
- Basha, M.P.; Saumya, S.M. Influence of fluoride on streptozotocin induced diabetic nephrotoxicity in mice: Protective Role of Asian Ginseng (Panax Ginseng) & Banaba (Lagerstroemia Speciosa) on mitochondrial oxidative stress. Indian J. Med. Res. 2013, 137, 370–379. [Google Scholar] [PubMed]
- Niu, R.; Chen, H.; Manthari, R.K.; Sun, Z.; Wang, J.; Zhang, J.; Wang, J. Effects of fluoride on synapse morphology and myelin damage in mouse hippocampus. Chemosphere 2018, 194, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Dec, K.; Łukomska, A.; Baranowska-Bosiacka, I.; Pilutin, A.; Maciejewska, D.; Skonieczna-Żydecka, K.; Derkacz, R.; Goschorska, M.; Wąsik, A.; Rębacz-Maron, E.; et al. Pre-and postnatal exposition to fluorides induce changes in rats liver morphology by impairment of antioxidant defense mechanisms and COX induction. Chemosphere 2018, 211, 112–119. [Google Scholar] [CrossRef]
- Inkielewicz, I.; Czarnowski, W.; Krechniak, J. Determination of fluoride in soft tissues. Fluoride 2003, 36, 16–20. [Google Scholar]
- Ruxton, C. Fluoride in the UK diet. Nurs. Stand. 2014, 28, 52–59. [Google Scholar] [CrossRef]
- Levy, F.M.; Olympio, K.P.K.; Philippi, S.T.; Buzalaf, M.A.R. Fluoride intake from food items in 2- to 6-year-old Brazilian children living in a non-fluoridated area using a semiquantitative food frequency questionnaire. Int. J. Paediatr. Dent. 2012, 23, 444–451. [Google Scholar] [CrossRef]
- Cantoral, A.; Luna-Villa, L.C.; Mantilla-Rodriguez, A.A.; Mercado, A.; Lippert, F.; Liu, Y.; Peterson, K.E.; Hu, H.; Téllez-Rojo, M.M.; Martinez-Mier, E.A. Fluoride content in foods and beverages from mexico city markets and supermarkets. Food Nutr. Bull. 2019, 40, 514–531. [Google Scholar] [CrossRef]
- World Health Organization (Ed.) Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st Addendum; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Ali, S.; Thakur, S.K.; Sarkar, A.; Shekhar, S. Worldwide contamination of water by fluoride. Environ. Chem. Lett. 2016, 14, 291–315. [Google Scholar] [CrossRef]
- Public Health Service (PHS). Recommendation|FAQs|Community Water Fluoridation|Division of Oral Health|CDC. Available online: https://www.cdc.gov/fluoridation/faqs/public-service-recommendations.html (accessed on 6 January 2023).
- Doull, J.; Boekelheide, K.; Farishian, B.G.; Isaacson, R.L.; Klotz, J.B.; Kumar, J.V.; Limeback, H.; Poole, C.; Puzas, J.E.; Ruby Reed, N.; et al. Fluoride in Drinking Water; National Academies Press: Washington, DC, USA, 2006; ISBN 978-0-309-10128-8. [Google Scholar]
- Iheozor-Ejiofor, Z.; Worthington, H.V.; Walsh, T.; O’Malley, L.; Clarkson, J.E.; Macey, R.; Alam, R.; Tugwell, P.; Welch, V.; Glenny, A.-M. Water fluoridation for the prevention of dental caries. Cochrane Database Syst. Rev. 2015, 2015, CD010856. [Google Scholar] [CrossRef] [PubMed]
- Gallego Reyes, S.M.; Martínez Beneyto, Y.; Ser-na-Muñoz, C.; Pérez-Silva, A.; Aparecido Cury, J.; Ortiz Ruiz, A.J. Concentración de flúor y metales pesados en aguas embotella-das: Medidas barrera frente a caries dental y fluorosis. Rev. Esp. Salud Pública 2019, 93, 17–18. [Google Scholar]
- Sawangjang, B.; Hashimoto, T.; Wongrueng, A.; Wattanachira, S.; Takizawa, S. Assessment of fluoride intake from groundwater and intake reduction from delivering bottled water in Chiang Mai Province, Thailand. Heliyon 2019, 5, e02391. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, R.; Zhu, G. Evaluation of physicochemical characteristics in drinking water sources emphasized on fluoride: A case study of Yancheng, China. Int. J. Environ. Res. Public Health 2019, 16, 1030. [Google Scholar] [CrossRef] [PubMed]
- Wolska, J.; Janda, K.; Jakubczyk, K.; Szymkowiak, M.; Chlubek, D.; Gutowska, I. Levels of antioxidant activity and fluoride content in coffee infusions of Arabica, robusta and green coffee beans in according to their brewing methods. Biol. Trace Elem. Res. 2017, 179, 327–333. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Q.; Linforth, R.; Fisk, I.D.; Yang, N. Modifying robusta coffee aroma by green bean chemical pre-treatment. Food Chem. 2019, 272, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Cai, H.; Zhu, X.; Li, D.; Yang, Y.; Hou, R.; Wan, X. Analysis of naturally occurring fluoride in commercial teas and estimation of its daily intake through tea consumption. J. Food Sci. 2016, 81, H235–H239. [Google Scholar] [CrossRef]
- Validandi, V.; Viswanathan, G.; Khandare, A.L. Comparison of fluoride levels (total and extracted) in young, old tea leaves and market tea samples along with impact of tea infusion on dental fluorosis in fluoride endemic villages of Nalgonda District, India. Adv. Dent. Oral Health 2019, 10. [Google Scholar] [CrossRef]
- Lung, S.-C.C.; Hsiao, P.-K.; Chiang, K.-M. Fluoride concentrations in three types of commercially packed tea drinks in Taiwan. J. Expo Sci. Environ. Epidemiol. 2003, 13, 66–73. [Google Scholar] [CrossRef]
- Whyte, M.P. Fluoride levels in bottled teas. Am. J. Med. 2006, 119, 189–190. [Google Scholar] [CrossRef]
- Rirattanapong, P.; Rirattanapong, O. Fluoride content of commercially available ready-to-drink tea in Bangkok, Thailand. SouthEast Asian J. Trop. Med. Public Health 2017, 48, 690–693. [Google Scholar]
- Malinowska, E.; Inkielewicz, I.; Czarnowski, W.; Szefer, P. Assessment of fluoride concentration and daily intake by human from tea and herbal infusions. Food Chem. Toxicol. 2008, 46, 1055–1061. [Google Scholar] [CrossRef]
- Kłódka, D.; Telesiński, A.; Bońkowski, M. Określenie zależności pomiędzy zawartością fluoru oraz wybranych witamin w naparach różnych rodzajów herbat. Bromatol. I Chem. Toksykol. 2008, 41, 957–963. [Google Scholar]
- Kaczmarek, U. PH Values and Fluoride Levels in Some Tea Brands. Ann. Acad. Med. Stetin. 2004, 50 (Suppl. 1), 58–61. [Google Scholar]
- Kassahun, A.; Chandravanshi, S.B. Levels of fluoride in bottled soft drinks marketed in Addis Ababa, Ethiopia. Bull Chem. Soc. Ethiop. 2019, 33, 203. [Google Scholar] [CrossRef]
- American Dental Association Council on Scientific Affairs. Professionally applied topical fluoride: Evidence-based clinical recommendations. J. Am. Dent. Assoc. 2006, 137, 1151–1159. [Google Scholar] [CrossRef]
- Jasmin, K.; Matthias, H.; Pia, W.; Sabine, B.; Birgit, L.; Norbert, P.; Anna, K.; Christian, H. Influence of pure fluorides and stannous ions on the initial bacterial colonization in situ. Sci. Rep. 2019, 9, 18499. [Google Scholar] [CrossRef]
- American Academy of Pediatric Dentistry. Fluoride Therapy. The Reference Manual of Pediatric Dentistry; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2021. [Google Scholar]
- Carey, C.M. Focus on fluorides: Update on the use of fluoride for the prevention of dental caries. J. Evid. Based Dent. Pract. 2014, 14, 95–102. [Google Scholar] [CrossRef]
- American Academy of Pediatric Dentistry. Guideline on Management of Dental Patients with Special Health Care Needs; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2016. [Google Scholar]
- Lobo, P.L.D.; de Carvalho, C.B.M.; Fonseca, S.G.C.; de Castro, R.S.L.; Monteiro, A.J.; Fonteles, M.C.; Fonteles, C.S.R. Sodium fluoride and chlorhexidine effect in the inhibition of mutans streptococci in children with dental caries: A randomized, double-blind clinical trial. Oral Microbiol. Immunol. 2008, 23, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Aldrees, A.M.; AlBeshri, S.S.; AlSanie, I.S.; Alsarra, I.A. Assessment of fluoride concentrations in commercially available mouthrinses in central Saudi Arabia. Saudi Med. J. 2014, 35, 1278–1282. [Google Scholar] [PubMed]
- Sun, H.; Luo, F.; Wan, Q. The application of fluoride in dental caries. In Dental Caries; IntechOpen: London, UK, 2021. [Google Scholar]
- Carvalho, T.S.; Lussi, A. Combined effect of a fluoride-, stannous- and chitosan-containing toothpaste and stannous-containing rinse on the prevention of initial enamel erosion–abrasion. J. Dent. 2014, 42, 450–459. [Google Scholar] [CrossRef]
- Ijaimi, Z.A.; Abu-Bakr, N.H.; Ibrahim, Y.E. Assessment of the quality of composite resin restorations. Open J. Stomatol. 2015, 5, 19–25. [Google Scholar] [CrossRef]
- Asl Aminabadi, N.; Balaei, E.; Pouralibaba, F. The effect of 0.2% sodium fluoride mouthwash in prevention of dental caries according to the DMFT index. J. Dent. Res. Dent. Clin. Dent. Prospect. 2007, 1, 71–76. [Google Scholar] [CrossRef]
- Herman, K.; Czajczyńska-Waszkiewicz, A.; Kowalczyk-Zając, M.; Dobrzyński, M. Assessment of the influence of vegetarian diet on the occurrence of erosive and abrasive cavities in hard tooth tissues. Postep. Hig. Med. Dosw. 2011, 65, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Twetman, S.; Keller, M.K. Fluoride rinses, gels and foams: An update of controlled clinical trials. Caries Res. 2016, 50, 38–44. [Google Scholar] [CrossRef]
- Marinho, V.C.; Chong, L.-Y.; Worthington, H.V.; Walsh, T. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2016, 2021, CD002284. [Google Scholar] [CrossRef]
- Miller, E.; Vann, J.W. The use of fluoride Varnish in children: A critical review with treatment recommendations. J. Clin. Pediatr. Dent. 2008, 32, 259–264. [Google Scholar] [CrossRef]
- Chu, C.H.; Lo, E. Uses of sodium fluoride varnish in dental practice. Ann. R Australas. Coll. Dent. Surg. 2008, 19, 58–61. [Google Scholar]
- Mascarenhas, A.K. Is fluoride varnish safe? J. Am. Dent. Assoc. 2021, 152, 364–368. [Google Scholar] [CrossRef]
- Vaikuntam, J. Fluoride varnishes: Should we be using them? Pediatr. Dent. 2000, 22, 513–516. [Google Scholar]
- Seppä, L. Fluoride varnishes in caries prevention. Med. Princ. Pract. 2004, 13, 307–311. [Google Scholar] [CrossRef]
- Strohmenger, L.; Brambilla, E. The use of fluoride varnishes in the prevention of dental caries: A short review. Oral Dis. 2001, 7, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Lilia Adriana, J.-L.M.; Miriam, M.M.; Nelly, M.F. Comparative clinical trial of fluoridated varnishes with calcium compounds to prevent and reverse incipient carious lesions in school children. Eur. Sci. J. ESJ 2020, 16, 93. [Google Scholar] [CrossRef]
- Bijle, M.N.A.; Yiu, C.K.Y.; Ekambaram, M. Calcium-based caries preventive agents: A meta-evaluation of systematic reviews and meta-analysis. J. Evid.-Based Dent. Pract. 2018, 18, 203.e4–217.e4. [Google Scholar] [CrossRef] [PubMed]
- Noble, J. Fluoride varnish for the teeth. Vital 2008, 5, 39. [Google Scholar] [CrossRef]
- Wei, S.H.; Hattab, F.N. Fluoride retention following topical application of a new APF foam. Pediatr. Dent. 1989, 11, 121–124. [Google Scholar] [PubMed]
- Marinho, V.C.; Worthington, H.V.; Walsh, T.; Clarkson, J.E. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2013, 7, CD002279. [Google Scholar] [CrossRef]
- Jiang, H.; Tai, B.; Du, M.; Peng, B. Effect of professional application of APF foam on caries reduction in permanent first molars in 6–7-year-old children: 24-month clinical trial. J. Dent. 2005, 33, 469–473. [Google Scholar] [CrossRef]
- O’Mullane, D.M.; Baez, R.J.; Jones, S.; Lennon, M.A.; Petersen, P.E.; Rugg-Gunn, A.J.; Whelton, H.; Whitford, G.M. Fluoride and oral health. Community Dent. Health 2016, 33, 69–99. [Google Scholar]
- Jiang, H.; Bian, Z.; Tai, B.J.; Du, M.Q.; Peng, B. The effect of a bi-annual professional application of APF foam on dental caries increment in primary teeth: 24-month clinical trial. J. Dent. Res. 2005, 84, 265–268. [Google Scholar] [CrossRef]
- Huang, B.; Lin, D. Staining potential of acidulated phosphate fluoride (APF) foam on dental restorations in vitro. J. Conserv. Dent. 2015, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Seyedakhavan, P.; Sayahpour, S.; Momeni, H.; Kharazi, M. Effect of fluoride gel and foam on salivary fluoride concentration. J. Res. Dent. Maxillofac. Sci. 2017, 2, 16–22. [Google Scholar] [CrossRef]
- Opydo-Szymaczek, J.; Opydo, J. Salivary fluoride concentrations and fluoride ingestion following application of preparations containing high concentration of fluoride. Biol. Trace Elem. Res. 2010, 137, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Bahsi, E.; Sagmak, S.; Dayi, B.; Cellik, O.; Akkus, Z. The evaluation of microleakage and fluoride release of different types of glass ionomer cements. Niger. J. Clin. Pract. 2019, 22, 961. [Google Scholar] [CrossRef]
- Garoushi, S.; Vallittu, P.K.; Lassila, L. Characterization of fluoride releasing restorative dental materials. Dent. Mater. J. 2018, 37, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Zietek, M.; Dobrzynski, M.; Fita, K.; Diakowska, D.; Watras, A.; Wiglusz, R.J. In vitro studies concerning selected properties of a composite material blended with nanofluoroapatite crystals. Materials 2021, 14, 7295. [Google Scholar] [CrossRef]
- Nascimento, P.L.D.M.M.; Fernandes, M.T.G.; De Figueiredo, F.E.D.; Faria-E-Silva, A. Fluoride-releasing materials to prevent white spot lesions around orthodontic brackets: A systematic review. Braz. Dent. J. 2016, 27, 101–107. [Google Scholar] [CrossRef]
- Kaczmarek, U. Fluoride release from dental restorative materials and secondary caries. Dent. Med. Probl. 2005, 42, 333–340. [Google Scholar]
- Moheet, I.A.; Luddin, N.; Ab Rahman, I.; Masudi, S.M.; Kannan, T.P.; Nik Abd Ghani, N.R. Analysis of ionic-exchange of selected elements between novel nano-hydroxyapatite-silica added glass ionomer cement and natural teeth. Polymers 2021, 13, 3504. [Google Scholar] [CrossRef] [PubMed]
- Łukomska-Szymańska, M.; Zarzycka, B.; Grzegorczyk, J.; Sokołowski, K.; Półtorak, K.; Sokołowski, J.; Łapińska, B. Antibacterial properties of calcium fluoride-based composite materials: In vitro study. Biomed. Res. Int. 2016, 2016, 1048320. [Google Scholar] [CrossRef]
- Sreedevi, A.; Brizuela, M.; Mohamed, S. Pit and Fissure Sealants; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kosior, P.; Dobrzyński, M.; Korczyński, M.; Herman, K.; Czajczyńska-Waszkiewicz, A.; Kowalczyk-Zając, M.; Piesiak-Pańczyszyn, D.; Fita, K.; Janeczek, M. Long-term release of fluoride from fissure sealants—In vitro study. J. Trace Elem. Med. Biol. 2017, 41, 107–110. [Google Scholar] [CrossRef]
- Fita, K.; Dobrzyński, M.; Ziętek, M.; Diakowska, D.; Watras, A.; Wiglusz, R.J. Assessment of microstructure and release of fluoride ions from selected fissure sealants: An in vitro study. Materials 2021, 14, 4936. [Google Scholar] [CrossRef]
- Kosior, P.; Dobrzynski, M.; Zakrzewska, A.; Grosman, L.; Korczynski, M.; Blicharski, T.; Gutbier, M.; Watras, A.; Wiglusz, R.J. Preliminary in vitro study of fluoride release from selected ormocer materials. Materials 2021, 14, 2244. [Google Scholar] [CrossRef] [PubMed]
- Herman, K.; Wujczyk, M.; Dobrzynski, M.; Diakowska, D.; Wiglusz, K.; Wiglusz, R.J. In vitro assessment of long-term fluoride ion release from nanofluorapatite. Materials 2021, 14, 3747. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J. The Epidemiology and Pathogenesis of Osteoporosis. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Sato, H.; Tanno, K.; Muro-oka, G.; Itai, K. Serum ionic fluoride concentrations are significantly decreased after treatment with alendronate in patients with osteoporosis. Clin. Chim. Acta 2011, 412, 2146–2149. [Google Scholar] [CrossRef]
- Choubisa, S.L. Toxic effects of fluoride on human bones. Adv. Pharmacol. Toxicol. 2012, 13, 9–13. [Google Scholar]
- Martin, K.J.; Olgaard, K.; Coburn, J.W.; Coen, G.M.; Fukagawa, M.; Langman, C.; Malluche, H.H.; McCarthy, J.T.; Massry, S.G.; Mehls, O.; et al. Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. Am. J. Kidney Dis. 2004, 43, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.L.; Blake, G.M.; Fogelman, I. 18F-fluoride PET in osteoporosis. PET Clin. 2010, 5, 259–274. [Google Scholar] [CrossRef]
- Bongo, G.; Mercier, G.; Chartier, M.; Dhenain, A.; Blais, J.-F. Treatment of aluminum plant hazardous wastes containing fluorides and PAH. J. Environ. Eng. 2009, 135, 159–166. [Google Scholar] [CrossRef]
- Peicher, K.; Maalouf, N.M. Skeletal fluorosis due to fluorocarbon inhalation from an air dust cleaner. Calcif. Tissue Int. 2017, 101, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Bartram, J.; Cotruvo, J.A.; Dufour, A.; Rees, G.; Pedley, S. (Eds.) Pathogenic Mycobacteria in Water: A Guide to Public Health Consequences, Monitoring and Management; IWA Publishing: London, UK, 2004. [Google Scholar]
- Armfield, J.M. When public action undermines public health: A critical examination of antifluoridationist literature. Aust N. Z. Health Policy 2007, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Curiel, J.A.; Sanders, A.E.; Christian, T.-M.L.; Lafferty-Hess, S.; Carsey, T.M.; Lampiris, L.N.; Slade, G.D. Fluoridation advocacy in referenda where media coverage is balanced yet biased. J. Am. Dent. Assoc. 2018, 149, 273.e3–280.e3. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.M.; Lakhkar, B.B.; Patil, S.S. Curse of fluorosis. Indian J. Pediatr. 2018, 85, 375–383. [Google Scholar] [CrossRef]
- Kosior, P.; Dobrzynski, M.; Zakrzewska, A.; Diakowska, D.; Nienartowicz, J.; Blicharski, T.; Nagel, S.; Sikora, M.; Wiglusz, K.; Watras, A.; et al. Comparison of the Fluoride Ion Release from Composite and Compomer Materials under Varying PH Conditions—Preliminary In Vitro Study. Appl. Sci. 2022, 12, 12540. [Google Scholar] [CrossRef]
- World Health Assembly Resolution Paves the Way for Better Oral Health Care. Available online: https://www.who.int/news/item/27-05-2021-world-health-assembly-resolution-paves-the-way-for-better-oral-health-care (accessed on 1 December 2022).
- Khan, S.A. Relationship between dental fluorosis and intelligence quotient of school going children in and around lucknow district: A Cross-Sectional Study. J. Clin. Diagn. Res. 2015, 9, ZC10–ZC15. [Google Scholar] [CrossRef]
- Grandjean, P. Developmental fluoride neurotoxicity: An updated review. Environ. Health 2019, 18, 110. [Google Scholar] [CrossRef]
- Till, C.; Green, R.; Flora, D.; Hornung, R.; Martinez-Mier, E.A.; Blazer, M.; Farmus, L.; Ayotte, P.; Muckle, G.; Lanphear, B. Fluoride exposure from infant formula and child IQ in a canadian birth cohort. Environ. Int. 2020, 134, 105315. [Google Scholar] [CrossRef]
- Green, R.; Lanphear, B.; Hornung, R.; Flora, D.; Martinez-Mier, E.A.; Neufeld, R.; Ayotte, P.; Muckle, G.; Till, C. Association between maternal fluoride exposure during pregnancy and IQ scores in offspring in Canada. JAMA Pediatr. 2019, 173, 940. [Google Scholar] [CrossRef]
- Sebastian, S.; Sunitha, S. A cross-sectional study to assess the intelligence quotient (IQ) of school going children aged 10–12 years in villages of Mysore district, India with different fluoride levels. J. Indian Soc. Pedod. Prev. Dent. 2015, 33, 307. [Google Scholar] [CrossRef]
- Maguire, A.; Omid, N.; Abuhaloob, L.; Moynihan, P.J.; Zohoori, F.V. Fluoride content of ready-to-feed (RTF) infant food and drinks in the UK. Community Dent. Oral Epidemiol. 2012, 40, 26–36. [Google Scholar] [CrossRef]
- Takahashi, R.; Ota, E.; Hoshi, K.; Naito, T.; Toyoshima, Y.; Yuasa, H.; Mori, R.; Nango, E. Fluoride supplementation (with tablets, drops, lozenges or chewing gum) in pregnant women for preventing dental caries in the primary teeth of their children. Cochrane Database Syst. Rev. 2017, 2017, CD011850. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.I.; Cury, J.A. Fluoride concentrations in salt marketed in Managua, Nicaragua. Braz. Oral Res. 2018, 32, e45. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Worthington, H.V.; Glenny, A.-M.; Marinho, V.C.; Jeroncic, A. fluoride toothpastes of different concentrations for preventing dental caries. Cochrane Database Syst. Rev. 2019, 3, CD007868. [Google Scholar] [CrossRef] [PubMed]

| Toxicity of Fluoride | ||
|---|---|---|
| 1 | Early symptoms of poisoning | 1 mgF/kg body weight |
| 2 | The probably toxic dose | 5 mgF/kg body weight |
| 3 | The lethal dose (LD) | 14–28 mgF/kg body weight |
| 4 | The certain lethal dose (CLD) | 32–64 mgF/kg body weight |
| The Degree of Fluorosis on a Numerical Scale | The Degree of Fluorosis | Clinical Features |
|---|---|---|
| 1 | Normal | Flawless, glittering surface with various shades of white, ecru, cream |
| 2 | Questionable | It differs from the normal degree, with the presence of single white spots or fleck changes |
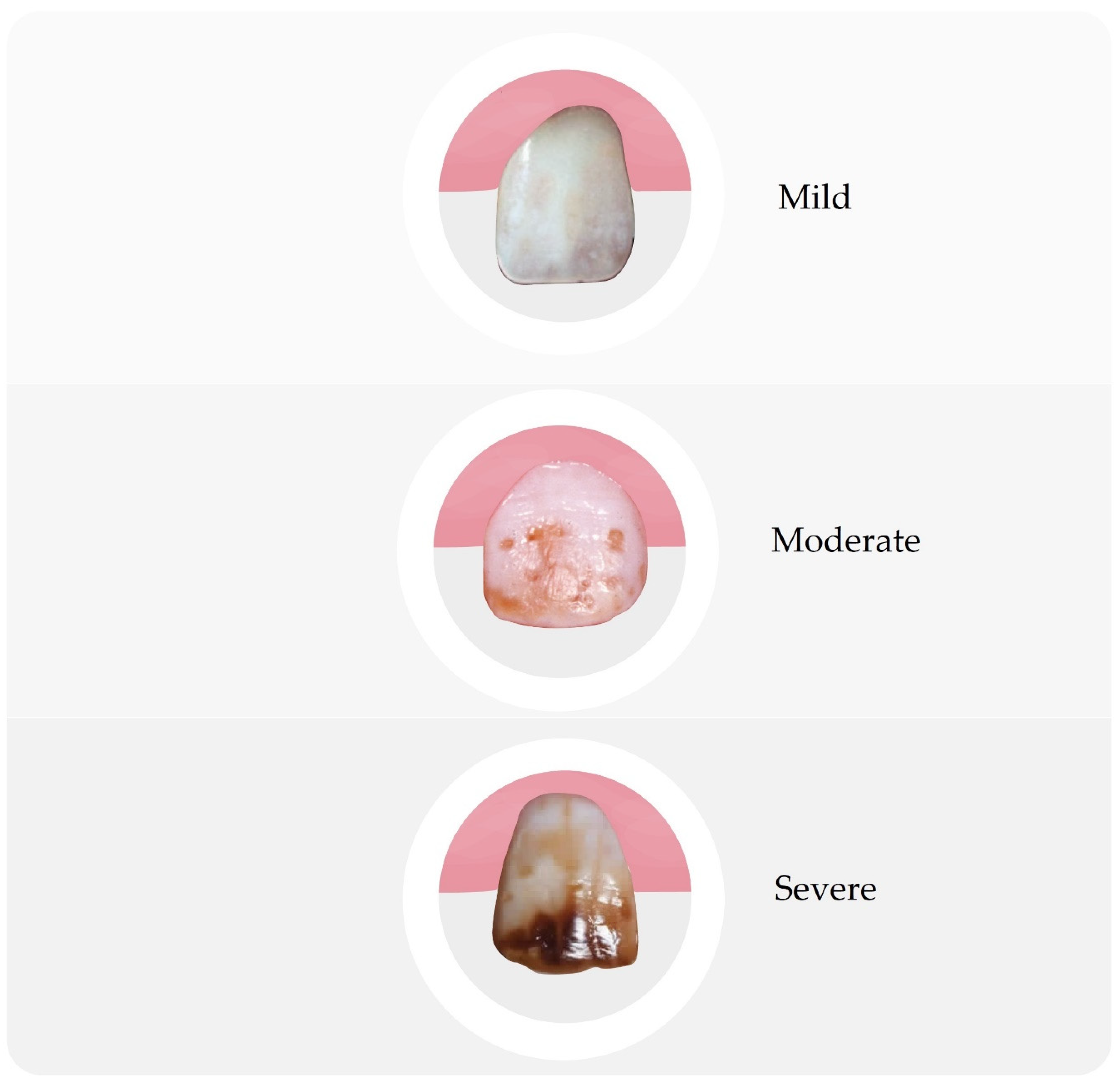
| 3 | Very Mild | Minor, intensely white regions with a lack of transparency on up to 25% of the surface, with no brown spots |
| 4 | Mild | The changes are similar to a lower degree, but cover up to 50% of the area |
| 5 | Moderate | There are already brown spots and the lesions cover most of the tooth surface, but the shape of the tooth usually does not change |
| 6 | Moderately Severe | Cavities are more frequent, and they are also deeper and more extensive |
| 7 | Severe | Visible hypoplasia in the form of comprehensive lesions and a color from brown to even black |
| Recommended Intake of Fluoride mg/day | 0.01–0.7 | 0.5–0.9 | 0.7–1.3 | 1.0–2.2 | 2.0–2.8 | 3.0–3.6 |
| Age | 0–6 months | 6–12 months | 1–3 years | 4–8 years | 9–13 years | 14–18 years |
| Recommendations Developed by | Age | Concentration of Fluoride in Drinking Water | ||
|---|---|---|---|---|
| <0.3 ppm | 0.3 ppm–0.6 ppm | >0.6 ppm | ||
| EAPD | 0–24 months | 0 mg | 0 mg | 0 mg |
| 2–6 years | 0.25 mg (0–3 years) | 0.25 mg (3–6 years) | 0 mg | |
| 7–18 years | 0.5 mg | 0.25 mg | 0 mg | |
| AAPD | 0–6 months | 0 mg | 0 mg | 0 mg |
| 6 months–3 years | 0.25 mg | 0 mg | 0 mg | |
| 3–6 years | 0.5 mg | 0.25 mg | 0 mg | |
| 6–16 years | 1.0 mg | 0.5 mg | 0 mg | |
| Selected Sources of Fluoride in the Human Body | |||
|---|---|---|---|
| Fluoride Source | Estimated Average Fluoride Content | Method of Product Administration | References |
| Fresh water | 0.5–40 mg F/L | Beverage | [24,25,48] |
| Salt | 100–400 mg F/kg | Food | [1,43,126] |
| Fluoridated water | 0.5–1 mg F/L | Beverage | [1,2] |
| Sea food | 1.9 mg F/kg | Food | [1,14,41] |
| Meat | 820 µg F/ kg | Food | [1,14,41] |
| Coffee | 0.013–0.502 mg F/L | Beverage | [53,54] |
| Tea, bottled tea | 1.79–803.94 mg/L | Beverage | [55,58] |
| Carbonated drinks | 0.03–0.27 mg F/L | Beverage | [63] |
| Fluoridated milk | 5 mg F/L | Beverage | [1,14,16,17] |
| Toothpaste for adults | 1450–5000 mg F/kg | Dental agent | [127] |
| Fluoride varnish | 22,600 mg F/L | Dental agent | [80] |
| Fluoride foam and gels | 5000–12,500 mg F/kg | Dental agent | [11,17,75] |
| Fluoride rinses | 225–900 mg F/L | Dental agent | [11,17,73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubojanski, A.; Piesiak-Panczyszyn, D.; Zakrzewski, W.; Dobrzynski, W.; Szymonowicz, M.; Rybak, Z.; Mielan, B.; Wiglusz, R.J.; Watras, A.; Dobrzynski, M. The Safety of Fluoride Compounds and Their Effect on the Human Body—A Narrative Review. Materials 2023, 16, 1242. https://doi.org/10.3390/ma16031242
Lubojanski A, Piesiak-Panczyszyn D, Zakrzewski W, Dobrzynski W, Szymonowicz M, Rybak Z, Mielan B, Wiglusz RJ, Watras A, Dobrzynski M. The Safety of Fluoride Compounds and Their Effect on the Human Body—A Narrative Review. Materials. 2023; 16(3):1242. https://doi.org/10.3390/ma16031242
Chicago/Turabian StyleLubojanski, Adam, Dagmara Piesiak-Panczyszyn, Wojciech Zakrzewski, Wojciech Dobrzynski, Maria Szymonowicz, Zbigniew Rybak, Bartosz Mielan, Rafal J. Wiglusz, Adam Watras, and Maciej Dobrzynski. 2023. "The Safety of Fluoride Compounds and Their Effect on the Human Body—A Narrative Review" Materials 16, no. 3: 1242. https://doi.org/10.3390/ma16031242
APA StyleLubojanski, A., Piesiak-Panczyszyn, D., Zakrzewski, W., Dobrzynski, W., Szymonowicz, M., Rybak, Z., Mielan, B., Wiglusz, R. J., Watras, A., & Dobrzynski, M. (2023). The Safety of Fluoride Compounds and Their Effect on the Human Body—A Narrative Review. Materials, 16(3), 1242. https://doi.org/10.3390/ma16031242










