Hybrid Nano Flake-like Vanadium Diselenide Combined on Multi-Walled Carbon Nanotube as a Binder-Free Electrode for Sodium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of 1,3-Dimethyl-imidazoline-2-selenone
2.2. Synthesis of Nano Flake-like VSe2 and VSe2@MWCNT
2.3. Material Characterization
2.4. Electrochemical Properties Evaluation
3. Results and Discussion
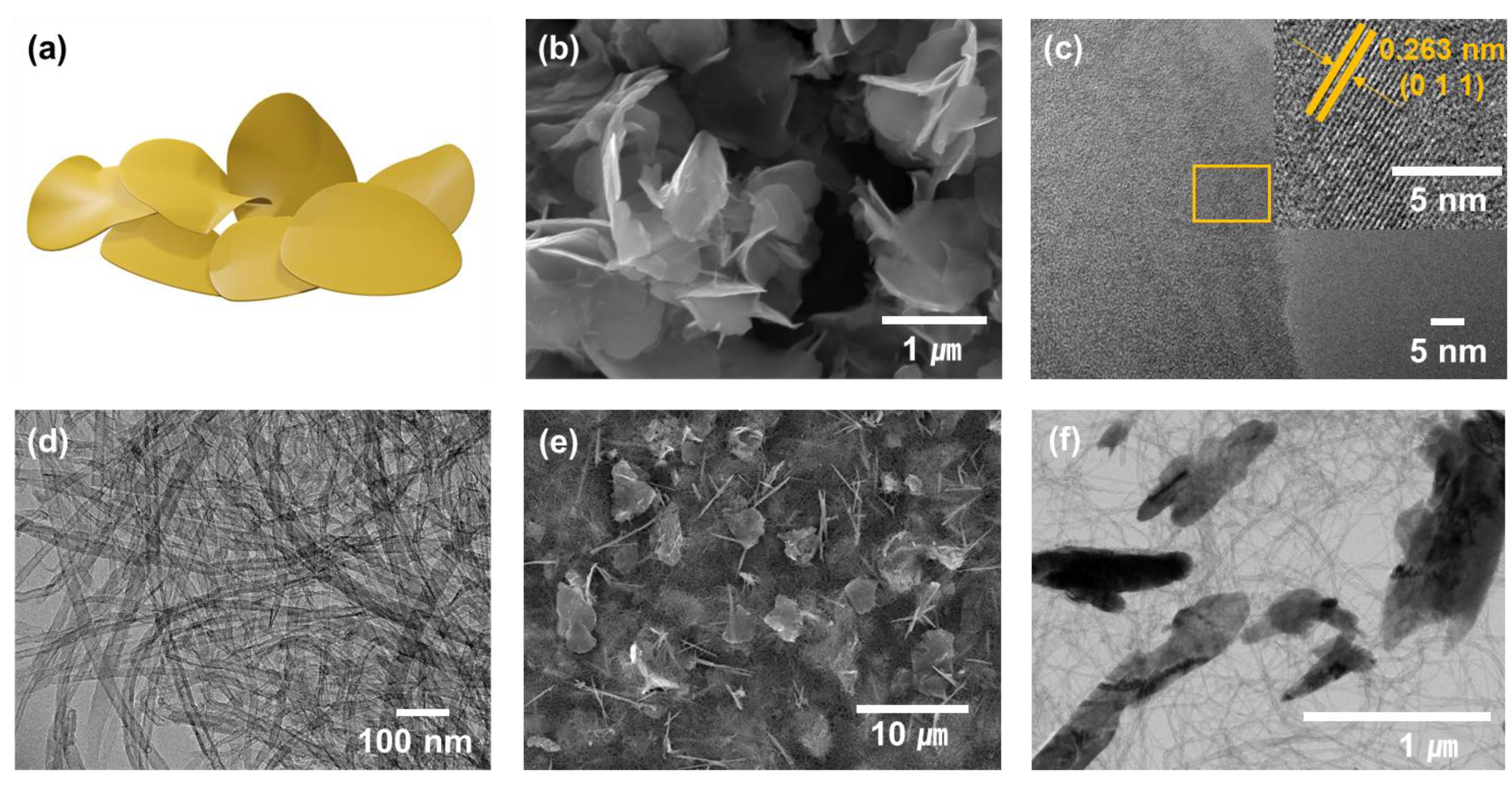
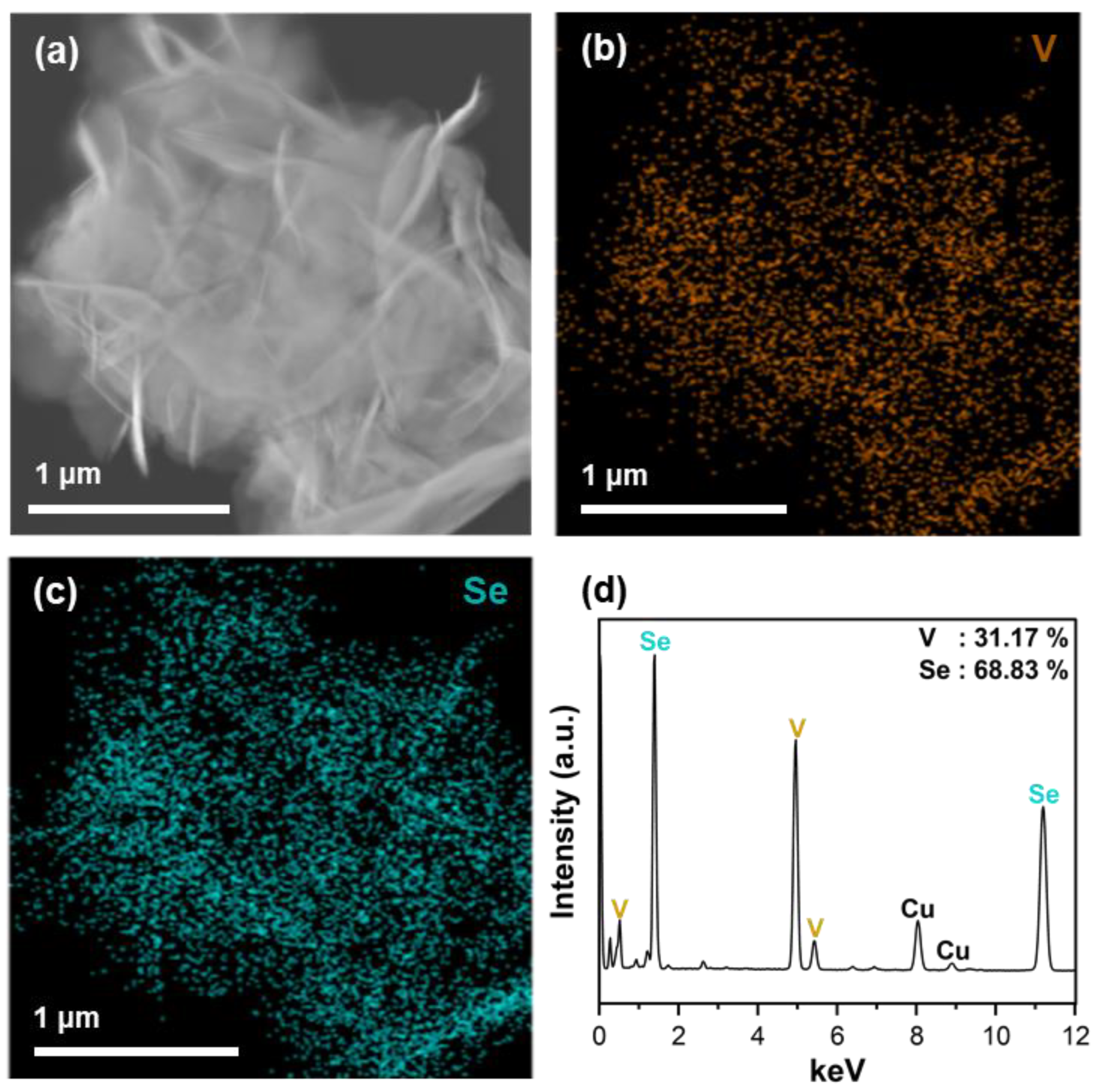
3.1. Morphology and Composition Analysis
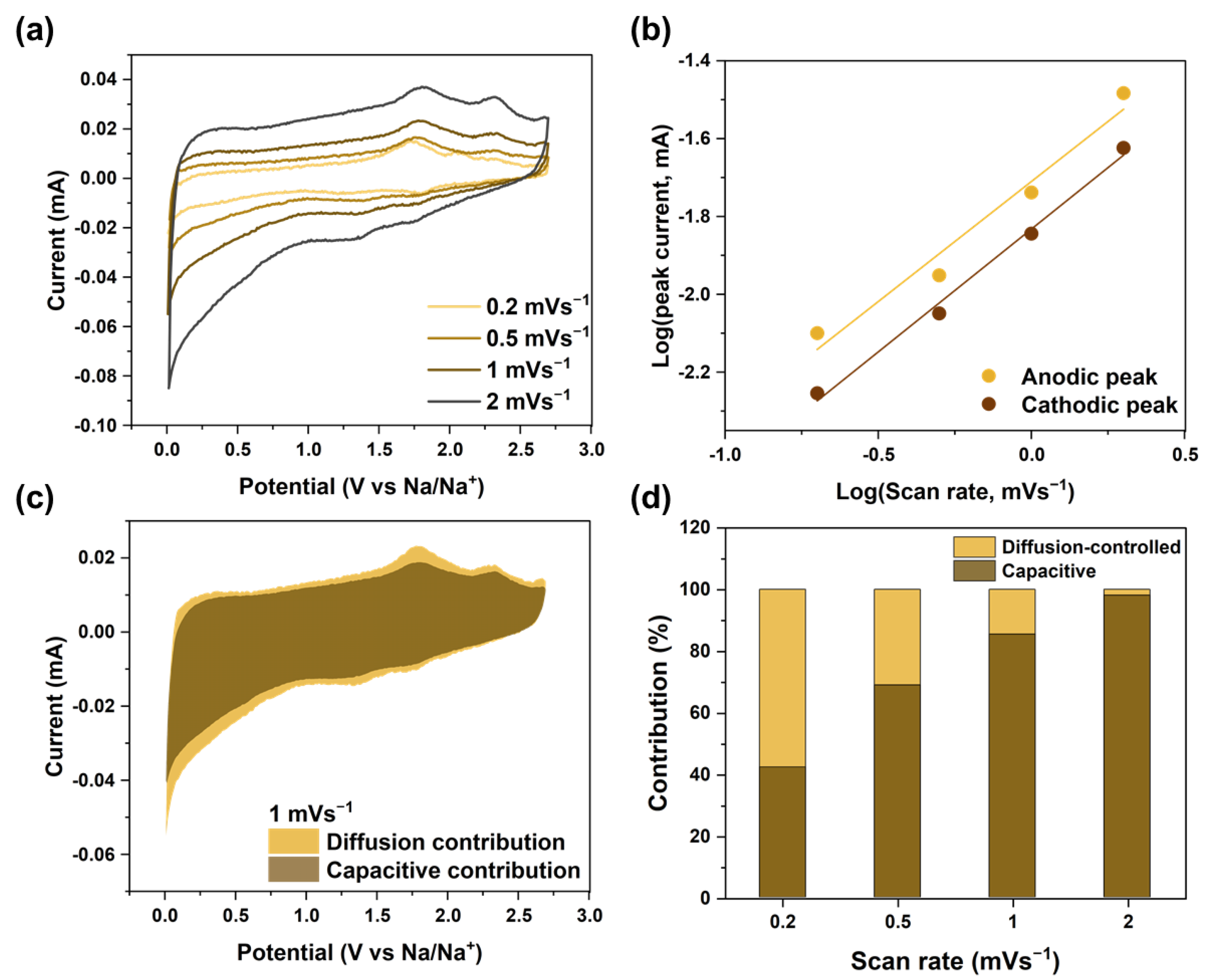
3.2. Electrochemical Properties and Sodium-Ion Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-Ion Batteries: Present and Future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Luo, W.B.; Chou, S.L.; Liu, H.K.; Dou, S.X. Sodium-Ion Batteries: From Academic Research to Practical Commercialization. Adv. Energy Mater. 2018, 8, 1701428. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Ding, Z.; Lee, M.H.; Lim, K.; Yoon, G.; Kang, K. Recent Progress in Electrode Materials for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600943. [Google Scholar] [CrossRef]
- Wang, S.; Xia, L.; Yu, L.; Zhang, L.; Wang, H.; Lou, X.W. Free-Standing Nitrogen-Doped Carbon Nanofiber Films: Integrated Electrodes for Sodium-Ion Batteries with Ultralong Cycle Life and Superior Rate Capability. Adv. Energy Mater. 2016, 6, 1502217. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Ma, L.; Li, H.; Xu, X.; Liu, X.; Lu, T.; Pan, L. Insights into the Storage Mechanism of 3D Nanoflower-like V3S4 Anode in Sodium-Ion Batteries. Chem. Eng. J. 2022, 427, 130936. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, X.; Bai, Y.; Yang, H.; Dong, R.; Wang, X.; Xu, H.; Wang, Q.; Li, H.; Gao, H.; et al. Probing the Energy Storage Mechanism of Quasi-Metallic Na in Hard Carbon for Sodium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2003854. [Google Scholar] [CrossRef]
- Luo, W.; Allen, M.; Raju, V.; Ji, X. An Organic Pigment as a High-Performance Cathode for Sodium-Ion Batteries. Adv. Energy Mater. 2014, 4, 1400554. [Google Scholar] [CrossRef]
- Meng, J.K.; Wang, W.W.; Wang, Q.C.; Cao, M.H.; Fu, Z.W.; Wu, X.J.; Zhou, Y.N. Graphene Supported Ultrafine Tin Oxide Nanoparticles Enable Conversion Reaction Dominated Mechanism for Sodium-Ion Batteries. Electrochim. Acta 2019, 303, 32–39. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.; Peng, J.; Yu, D.; Liang, Z.; Zhang, W.; Wu, J.; Wang, G.; Li, H.; Huang, S. Nanodot-in-Nanofiber Structured Carbon-Confined Sb2Se3 Crystallites for Fast and Durable Sodium Storage. Adv. Funct. Mater. 2022, 32, 2112776. [Google Scholar] [CrossRef]
- Ge, P.; Hou, H.; Li, S.; Yang, L.; Ji, X. Tailoring Rod-Like FeSe2 Coated with Nitrogen-Doped Carbon for High-Performance Sodium Storage. Adv. Funct. Mater. 2018, 28, 1801765. [Google Scholar] [CrossRef]
- Wei, X.; Li, W.; Shi, J.A.; Gu, L.; Yu, Y. FeS@C on Carbon Cloth as Flexible Electrode for Both Lithium and Sodium Storage. ACS Appl. Mater. Interfaces 2015, 7, 27804–27809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, T.; Fang, T.; Gao, Y.; Gao, S.; Wang, W.; Liao, L. A Novel MoS2@C Framework Architecture Composites with Three-Dimensional Cross-Linked Porous Carbon Supporting MoS2 Nanosheets for Sodium Storage. J. Alloys Compd. 2020, 818, 152821. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, W.; Tang, W.; Zhang, L.; Chen, H.; Li, Q.; Xu, M.; Bao, S. juan Flexible Electrode Constructed by Encapsulating Ultrafine VSe2 in Carbon Fiber for Quasi-Solid-State Sodium Ion Batteries. J. Power Sources 2020, 470, 228438. [Google Scholar] [CrossRef]
- Jin, T.; Han, Q.; Jiao, L. Binder-Free Electrodes for Advanced Sodium-Ion Batteries. Adv. Mater. 2020, 32, 1806304. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.L.; Knauth, P.; Djenizian, T. Three-Dimensional Self-Supported Metal Oxides for Advanced Energy Storage. Adv. Mater. 2014, 26, 3368–3397. [Google Scholar] [CrossRef]
- Landi, B.J.; Ganter, M.J.; Cress, C.D.; DiLeo, R.A.; Raffaelle, R.P. Carbon Nanotubes for Lithium Ion Batteries. Energy Environ. Sci. 2009, 2, 638–654. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, H.; Lu, X.F.; Tong, Y.X.; Li, G.R. Recent Progress on MOF-Derived Heteroatom-Doped Carbon-Based Electrocatalysts for Oxygen Reduction Reaction. Adv. Sci. 2018, 5, 1700515. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, M.; Lei, Y. Nanoarchitectured Array Electrodes for Rechargeable Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1502514. [Google Scholar] [CrossRef]
- Choi, J.; Myung, Y.; Kim, S.K. Flexible Sodium-Ion Battery Anodes Using Indium Sulfide-Based Nanohybrid Paper Electrodes. Appl. Surf. Sci. 2019, 467–468, 1040–1045. [Google Scholar] [CrossRef]
- Oliveira, T.M.B.F.; Morais, S. New Generation of Electrochemical Sensors Based on Multi-Walled Carbon Nanotubes. Appl. Sci. 2018, 8, 1925. [Google Scholar] [CrossRef]
- Abd El-Mageed, A.I.A.; Ogawa, T. Single-Walled Carbon Nanotube Absolute-Handedness Chirality Assignment Confirmation Using Metalized Porphyrin’s Supramolecular Structures via STM Imaging Technique. Chirality 2020, 32, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A. Functionalization of Single-Walled Carbon Nanotubes. Angew. Chem.-Int. Ed. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Hou, T.; Liu, B.; Sun, X.; Fan, A.; Xu, Z.; Cai, S.; Zheng, C.; Yu, G.; Tricoli, A. Covalent Coupling-Stabilized Transition-Metal Sulfide/Carbon Nanotube Composites for Lithium/Sodium-Ion Batteries. ACS Nano 2021, 15, 6735–6746. [Google Scholar] [CrossRef] [PubMed]
- El-Shafai, N.; El-Khouly, M.E.; El-Kemary, M.; Ramadan, M.S.; Masoud, M.S. Self-Assembly of Porphyrin on Graphene Oxide in Aqueous Medium: Fabrication, Characterization, and Photocatalytic Studies. Photochem. Photobiol. Sci. 2019, 18, 2071–2079. [Google Scholar] [CrossRef]
- Chen, M.; Chao, D.; Liu, J.; Yan, J.; Zhang, B.; Huang, Y.; Lin, J.; Shen, Z.X. Rapid Pseudocapacitive Sodium-Ion Response Induced by 2D Ultrathin Tin Monoxide Nanoarrays. Adv. Funct. Mater. 2017, 27, 1606232. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, D.; Shi, W.; Liu, B.; Sim, G.J.; Ge, Q.; Yang, H.Y. Ice Templated Free-Standing Hierarchically WS2/CNT-RGO Aerogel for High-Performance Rechargeable Lithium and Sodium Ion Batteries. Adv. Energy Mater. 2016, 6, 1601057. [Google Scholar] [CrossRef]
- Fan, H.N.; Zhang, Q.; Gu, Q.F.; Li, Y.; Luo, W.B.; Liu, H.K. Exploration of the Sodium Ion Ordered Transfer Mechanism in a MoSe2@Graphene Composite for Superior Rate and Lifespan Performance. J. Mater. Chem. A 2019, 7, 13736–13742. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.; Wang, G. WS2@graphene Nanocomposites as Anode Materials for Na-Ion Batteries with Enhanced Electrochemical Performances. Chem. Commun. 2014, 50, 4192–4195. [Google Scholar] [CrossRef]
- Tao, H.; Zhou, M.; Wang, R.; Wang, K.; Cheng, S.; Jiang, K. TiS2 as an Advanced Conversion Electrode for Sodium-Ion Batteries with Ultra-High Capacity and Long-Cycle Life. Adv. Sci. 2018, 5, 1801021. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Z.; Wang, Y.; Dong, Y.; Liu, Y.; Wang, X.; Qiu, J. Carbon-Stabilized Interlayer-Expanded Few-Layer MoSe2 Nanosheets for Sodium Ion Batteries with Enhanced Rate Capability and Cycling Performance. ACS Appl. Mater. Interfaces 2016, 8, 32324–32332. [Google Scholar] [CrossRef]
- Majumder, S.; Shao, M.; Deng, Y.; Chen, G. Two Dimensional WS2/C Nanosheets as a Polysulfides Immobilizer for High Performance Lithium-Sulfur Batteries. J. Electrochem. Soc. 2019, 166, A5386–A5395. [Google Scholar] [CrossRef]
- Salavati, M.; Rabczuk, T. Application of Highly Stretchable and Conductive Two-Dimensional 1T VS2 and VSe2 as Anode Materials for Li-, Na- and Ca-Ion Storage. Comput. Mater. Sci. 2019, 160, 360–367. [Google Scholar] [CrossRef]
- Yang, W.; Chen, D.; She, Y.; Zeng, M.; Lin, X.; Ang, E.H.; Yan, C.; Qin, Y.; Rui, X. Rational Design of Vanadium Chalcogenides for Sodium-Ion Batteries. J. Power Sources 2020, 478, 228769. [Google Scholar] [CrossRef]
- Yang, C.; Feng, J.; Lv, F.; Zhou, J.; Lin, C.; Wang, K.; Zhang, Y.; Yang, Y.; Wang, W.; Li, J.; et al. Metallic Graphene-Like VSe2 Ultrathin Nanosheets: Superior Potassium-Ion Storage and Their Working Mechanism. Adv. Mater. 2018, 30, 1800036. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, D.; Lu, T.; Yao, Y.; Pan, L. An Advanced CoSe Embedded within Porous Carbon Polyhedra Hybrid for High Performance Lithium-Ion and Sodium-Ion Batteries. Chem. Eng. J. 2017, 325, 14–24. [Google Scholar] [CrossRef]
- Choi, J.; Kang, N.; Yang, H.Y.; Kim, H.J.; Son, S.U. Colloidal Synthesis of Cubic-Phase Copper Selenide Nanodiscs and Their Optoelectronic Properties. Chem. Mater. 2010, 22, 3586–3588. [Google Scholar] [CrossRef]
- Choi, J.; Jin, J.; Jung, I.G.; Kim, J.M.; Kim, H.J.; Son, S.U. SnSe2 Nanoplate–Graphene Composites as Anode Materials for Lithium Ion Batteries. Chem. Commun. 2011, 47, 5241–5243. [Google Scholar] [CrossRef]
- Kuo, F.Y.; Lin, F.S.; Yeh, M.H.; Fan, M.S.; Hsiao, L.Y.; Lin, J.J.; Jeng, R.J.; Ho, K.C. Synthesis of Surfactant-Free and Morphology-Controllable Vanadium Diselenide for Efficient Counter Electrodes in Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces. 2019, 11, 25090–25099. [Google Scholar] [CrossRef]
- Nie, P.; Min, C.; Song, H.J.; Chen, X.; Zhang, Z.; Zhao, K. Preparation and Tribological Properties of Polyimide/Carboxyl-Functionalized Multi-Walled Carbon Nanotube Nanocomposite Films under Seawater Lubrication. Tribol. Lett. 2015, 58, 1–12. [Google Scholar] [CrossRef]
- Kwak, J.; Jung, S.; Lee, N.; Thiyagarajan, K.; Kim, J.K.; Giri, A.; Jeong, U. Microwave-Assisted Synthesis of Group 5 Transition Metal Dichalcogenide Thin Films. J. Mater. Chem. C 2018, 6, 11303–11311. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, K.; Xu, C.; Wu, S.; Guo, Z.; He, M.; Xu, X.; Xue, M. Cellulose/Lignin-Based Carbon Nanobelt Aerogels Coating on VSe2 Nanosheets as Anode for High Performance Potassium-Ion Batteries. J. Power Sources 2022, 548, 232033. [Google Scholar] [CrossRef]
- Yi, Y.; Du, X.; Zhao, Z.; Liu, Y.; Guan, H.; Liu, X.; Pei, X.; Zhang, S.; Li, D. Coupling of Metallic VSe2 and Conductive Polypyrrole for Boosted Sodium-Ion Storage by Reinforced Conductivity Within and Outside. ACS Nano 2022, 16, 7772–7782. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Wu, X.; Shao, Y.; Qi, J.; Cao, Y.; Huang, L.; Liu, C.; Wang, J.O.; Zheng, Q.; Zhu, Z.L.; et al. Epitaxially Grown Monolayer VSe2: An Air-Stable Magnetic Two-Dimensional Material with Low Work Function at Edges. Sci. Bull. 2018, 63, 419–425. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, Y.; Lu, S.; Li, K.; Li, Y.; Yang, B.; Chen, W.; Wang, L.; Li, D.; Deng, H.; et al. Accelerated Optimization of TiO2/Sb2Se3 Thin Film Solar Cells by High-Throughput Combinatorial Approach. Adv. Energy Mater. 2017, 7, 1700866. [Google Scholar] [CrossRef]
- Wang, J.; Guan, F. Solution-Synthesis of Sb2Se3 Nanorods Using KSeCN as a Molecular Selenium Source. CrystEngComm 2019, 22, 68–73. [Google Scholar] [CrossRef]
- Pandit, B.; Sankapal, B.R. Cerium Selenide Nanopebble/Multiwalled Carbon Nanotube Composite Electrodes for Solid-State Symmetric Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 3007–3017. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman Spectroscopy of Disordered, Amorphous, and Diamondlike Carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on Carbon Nanotubes and Graphene Raman Spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Puravankara, S. Nagmani Insights into the Plateau Capacity Dependence on the Rate Performance and Cycling Stability of a Superior Hard Carbon Microsphere Anode for Sodium-Ion Batteries. ACS. Appl. Energy Mater. 2020, 3, 10045–10052. [Google Scholar] [CrossRef]
- Ming, F.; Liang, H.; Lei, Y.; Zhang, W.; Alshareef, H.N. Solution Synthesis of VSe2 Nanosheets and Their Alkali Metal Ion Storage Performance. Nano Energy 2018, 53, 11–16. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z. Carbon-Coated Vanadium Selenide as Anode for Lithium-Ion Batteries and Sodium-Ion Batteries with Enhanced Electrochemical Performance. Mater. Lett. 2017, 189, 152–155. [Google Scholar] [CrossRef]
- Yan, B.; Li, X.; Fu, X.; Zhang, L.; Bai, Z.; Yang, X. An Elaborate Insight of Lithiation Behavior of V2O5 Anode. Nano Energy 2020, 78, 105233. [Google Scholar] [CrossRef]
- Lee, Y.S.; Ryu, K.S. Study of the Lithium Diffusion Properties and High Rate Performance of TiNb6O17 as an Anode in Lithium Secondary Battery. Sci. Rep. 2017, 7, 16617. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Jiang, Q.; Wu, T.; Wu, T.; Ding, Z.; Wu, J.; Yu, H.; Huang, K. Binary Iron Sulfide as a Low-Cost and High-Performance Anode for Lithium-/Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 52888–52898. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Calas, A.; Tang, C.; Li, F.; Zhou, L.; Mai, L. Ultralong Sb2Se3 Nanowire-Based Free-Standing Membrane Anode for Lithium/Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 35219–35226. [Google Scholar] [CrossRef]
- David, L.; Bhandavat, R.; Singh, G. MoS2/Graphene Composite Paper for Sodium-Ion Battery Electrodes. ACS Nano 2014, 8, 1759–1770. [Google Scholar] [CrossRef]
- Kim, T.; Choi, W.; Shin, H.C.; Choi, J.Y.; Kim, J.M.; Park, M.S.; Yoon, W.S. Applications of Voltammetry in Lithium Ion Battery Research. J. Electrochem. Sci. Technol. 2020, 11, 14–25. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J. Definitions of Pseudocapacitive Materials: A Brief Review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef]
- Yousaf, M.; Chen, Y.; Tabassum, H.; Wang, Z.; Wang, Y.; Abid, A.Y.; Mahmood, A.; Mahmood, N.; Guo, S.; Han, R.P.S.; et al. A Dual Protection System for Heterostructured 3D CNT/CoSe2/C as High Areal Capacity Anode for Sodium Storage. Adv. Sci. 2020, 7, 1902907. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Y.; Xu, Z.; Li, H.; Xu, M.; Li, J.; Dai, Y.; Zong, W.; Chen, R.; He, L.; et al. Rationally Designed Sodium Chromium Vanadium Phosphate Cathodes with Multi-Electron Reaction for Fast-Charging Sodium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2201065. [Google Scholar] [CrossRef]
- Puravankara, S.; Verma, P. Jute-Fiber Precursor-Derived Low-Cost Sustainable Hard Carbon with Varying Micro/Mesoporosity and Distinct Storage Mechanisms for Sodium-Ion and Potassium-Ion Batteries. Langmuir 2022, 38, 15703–15713. [Google Scholar] [CrossRef]
- Chao, D.; Zhu, C.; Yang, P.; Xia, X.; Liu, J.; Wang, J.; Fan, X.; Savilov, S.V.; Lin, J.; Fan, H.J.; et al. Array of Nanosheets Render Ultrafast and High-Capacity Na-Ion Storage by Tunable Pseudocapacitance. Nat. Commun. 2016, 7, 12122. [Google Scholar] [CrossRef] [PubMed]


| Material | Year | Potential | Templates | Synthetic Methods | Electrochemical Performance | Reference |
|---|---|---|---|---|---|---|
| VSe2@MWCNT | 2023 | 0.01–2.7 V | Free | Vacuum filtrate | 469.1 mAg−1 at 10 mAg−1 after 200 cycles | This work |
| FeS@C | 2022 | 0.5–3.0 V | Carbon cloth | Hydrothermal method and carbonization | 150 mAhg−1 at 12 C after 200 cycles | [11] |
| VSe2/NCNFs | 2020 | 0.01–3.0 V | Carbon fibers | Electrospinning | 420.8 mAhg−1 at 50 mAg−1 | [13] |
| Ultralong Sb2Se3 | 2016 | 0.01–3.0 V | Free | Vacuum filtrate | 289 mAhg−1 at 100 mAg−1 after 50 cycles | [56] |
| MoS2/graphene composite paper | 2014 | 0.0–2.25 V | Free | Vacuum filtrate | 218 mAhg−1 at 25 mAg−1 after 20 cycles | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Lee, M.E.; Kim, G.; Seong, H.; Nam, W.; Kim, S.K.; Moon, J.H.; Choi, J. Hybrid Nano Flake-like Vanadium Diselenide Combined on Multi-Walled Carbon Nanotube as a Binder-Free Electrode for Sodium-Ion Batteries. Materials 2023, 16, 1253. https://doi.org/10.3390/ma16031253
Jin Y, Lee ME, Kim G, Seong H, Nam W, Kim SK, Moon JH, Choi J. Hybrid Nano Flake-like Vanadium Diselenide Combined on Multi-Walled Carbon Nanotube as a Binder-Free Electrode for Sodium-Ion Batteries. Materials. 2023; 16(3):1253. https://doi.org/10.3390/ma16031253
Chicago/Turabian StyleJin, Youngho, Min Eui Lee, Geongil Kim, Honggyu Seong, Wonbin Nam, Sung Kuk Kim, Joon Ha Moon, and Jaewon Choi. 2023. "Hybrid Nano Flake-like Vanadium Diselenide Combined on Multi-Walled Carbon Nanotube as a Binder-Free Electrode for Sodium-Ion Batteries" Materials 16, no. 3: 1253. https://doi.org/10.3390/ma16031253
APA StyleJin, Y., Lee, M. E., Kim, G., Seong, H., Nam, W., Kim, S. K., Moon, J. H., & Choi, J. (2023). Hybrid Nano Flake-like Vanadium Diselenide Combined on Multi-Walled Carbon Nanotube as a Binder-Free Electrode for Sodium-Ion Batteries. Materials, 16(3), 1253. https://doi.org/10.3390/ma16031253






