Enhanced Organic Contaminant Retardation by CTMAB-Modified Bentonite Backfill in Cut-Off Walls: Laboratory Test and Numerical Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Test Materials
2.2. Consolidation Tests
2.3. Chloride Ion Diffusion Tests
2.4. Migration Breakdown Test of Organic Pollutants
3. Results and Discussion
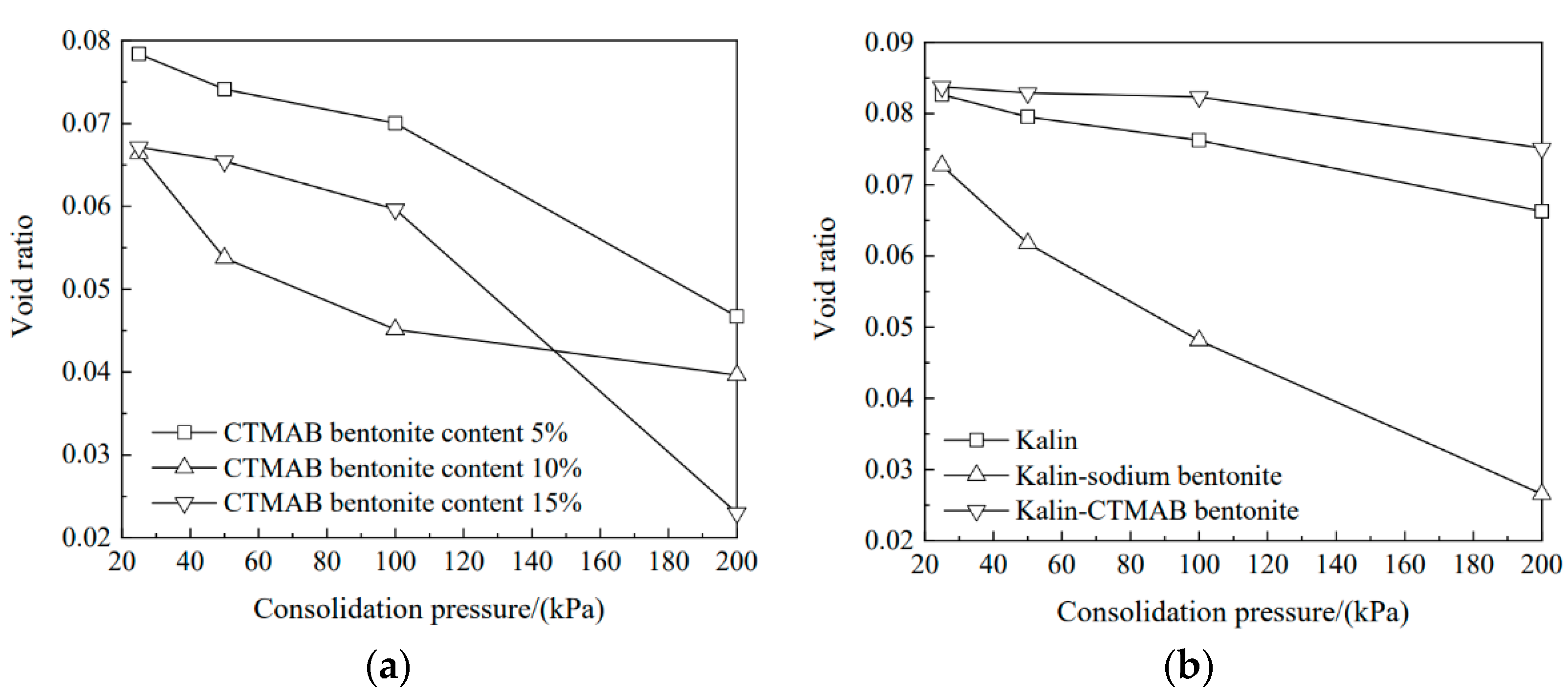
3.1. Void Ratio Variations of Soil Columns with Consolidation Pressure
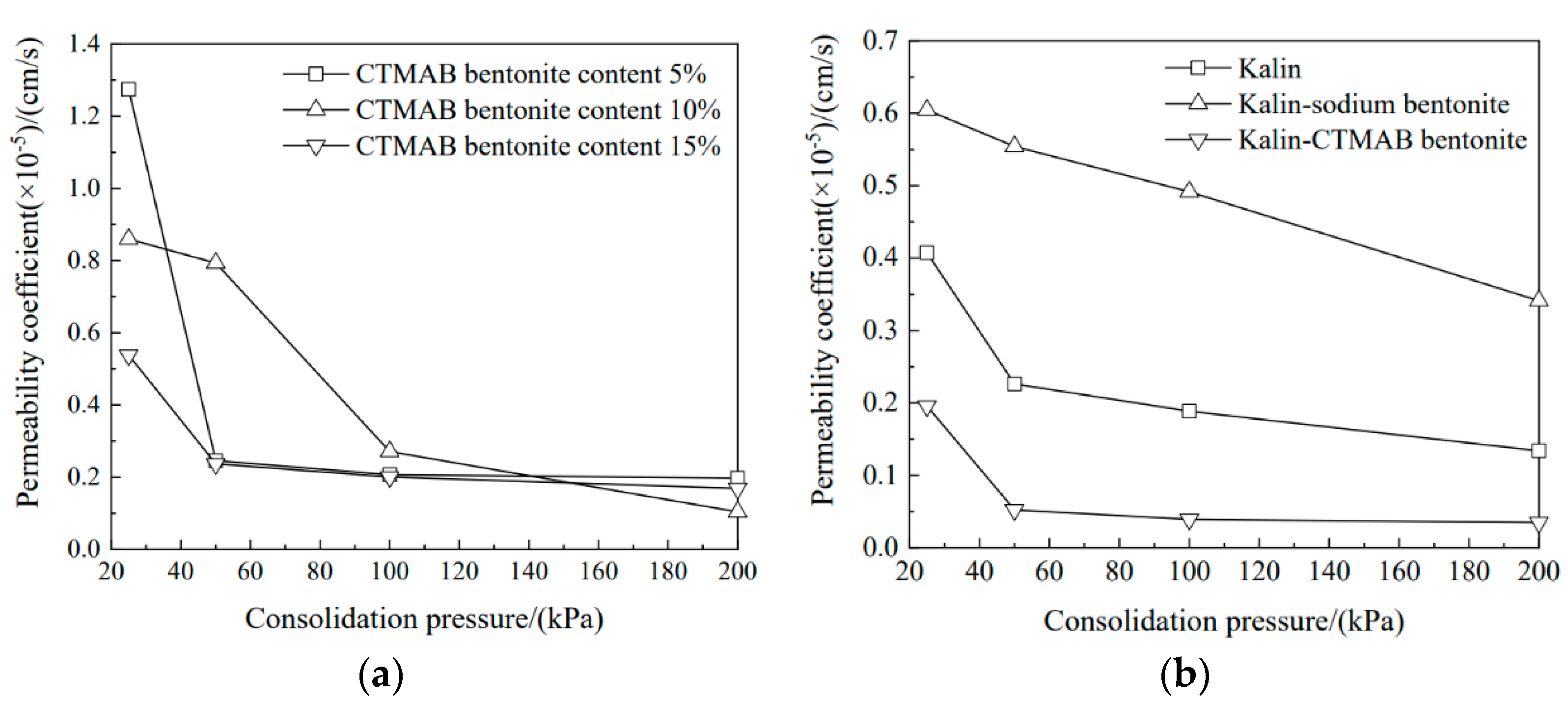
3.2. Permeability Coefficient Variations for Soil Columns with Consolidation Pressure
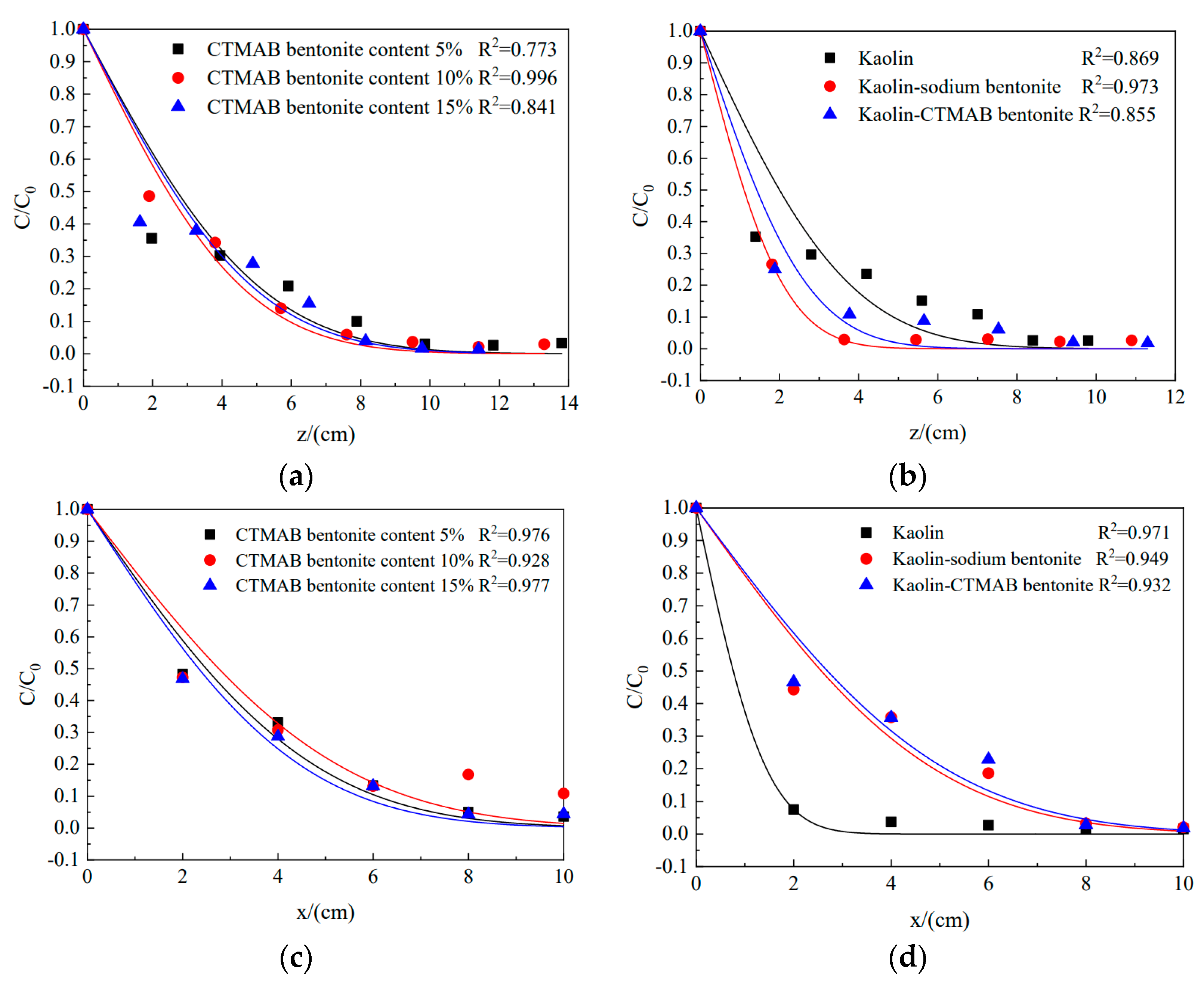
3.3. Diffusion of Chloride Ions in Different Soil Columns
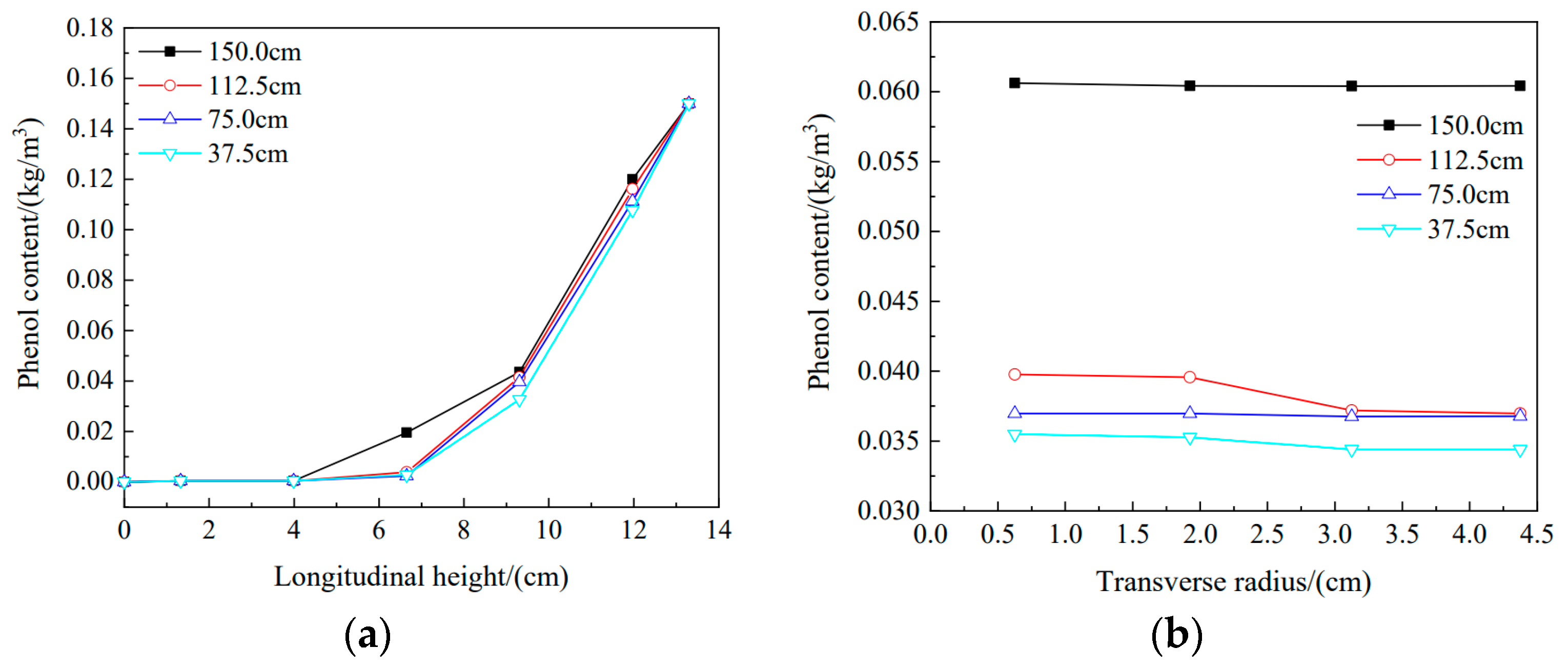
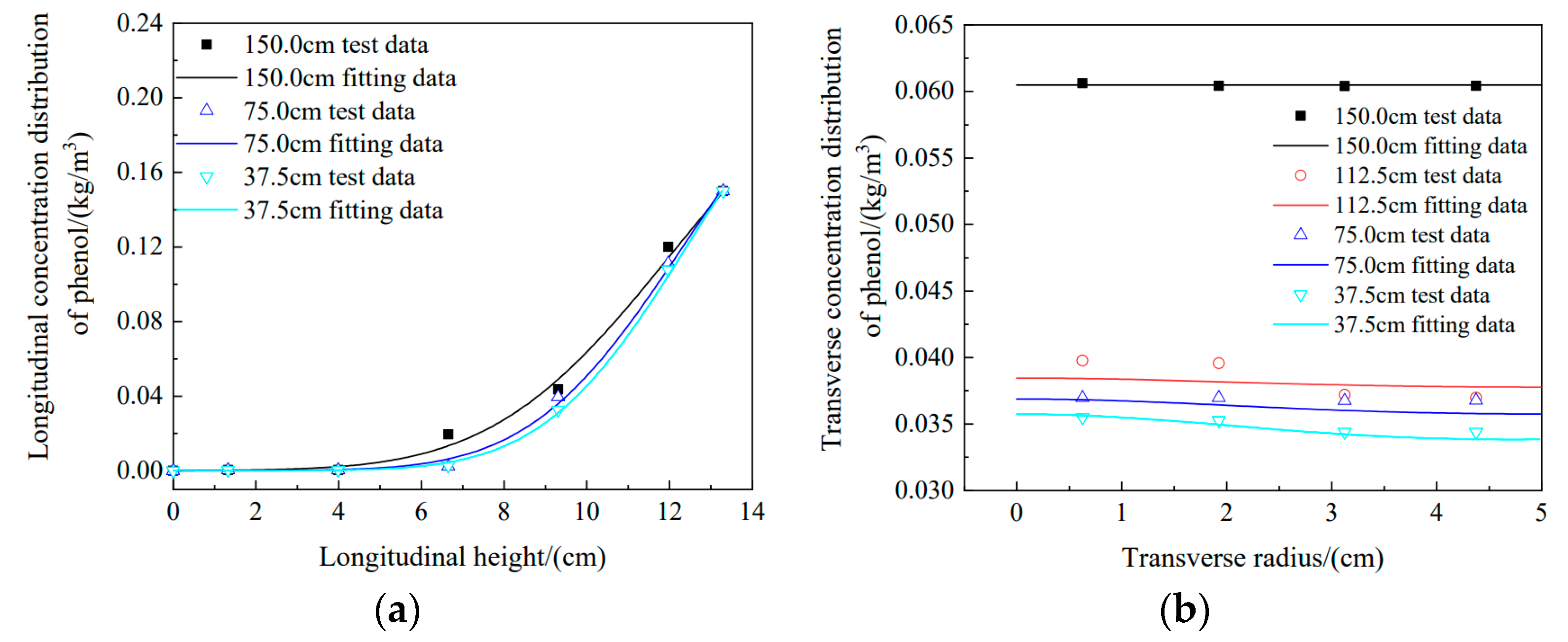
3.4. Effect of Different Water Head Heights on Phenol Migration in the Soil Column
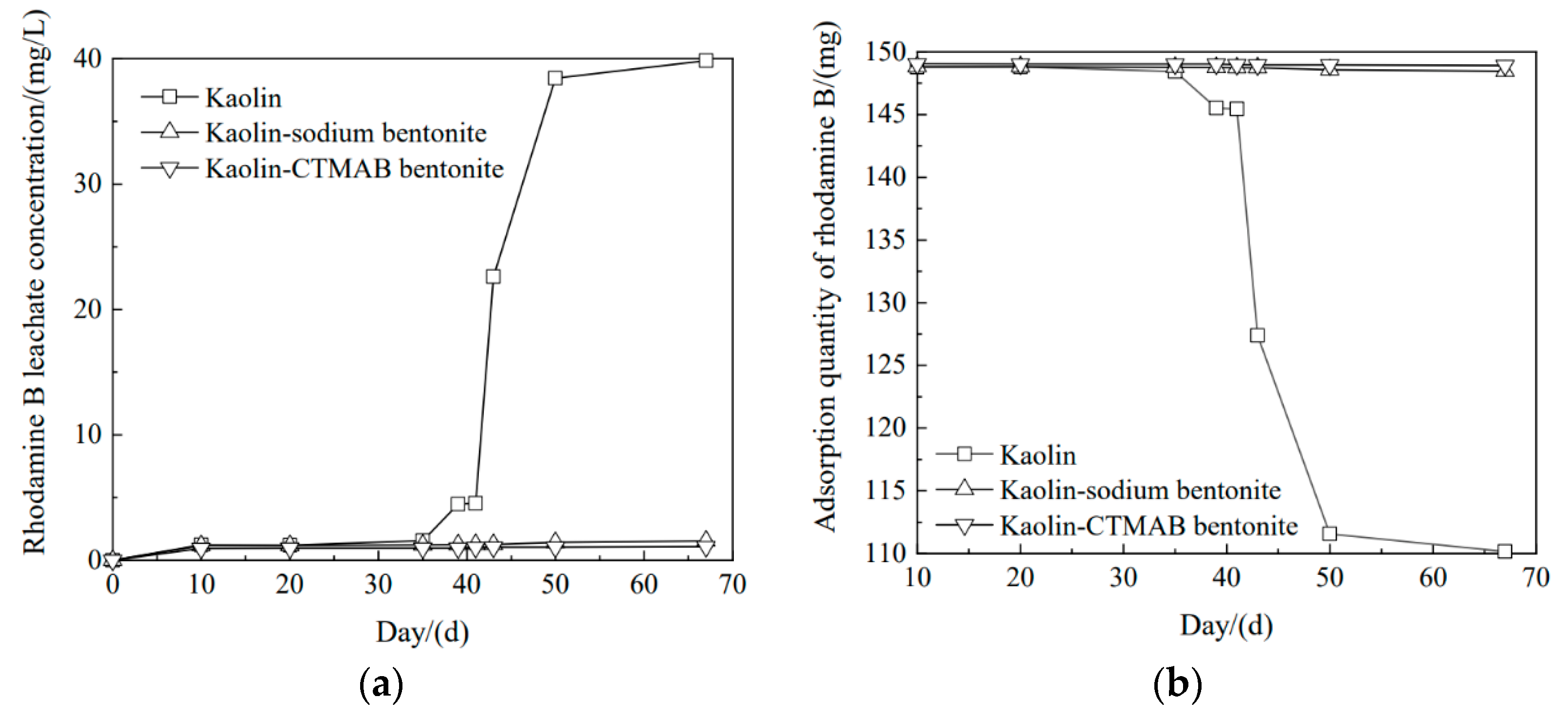
3.5. Effect of Time on the Removal of Rhodamine B from Different Soil Columns
4. Numerical Simulation of Organic Pollutant Migration Breakdown
4.1. Mathematical Model and Governing Equation
4.2. Numerical Results
4.2.1. Phenol Migration in the Soil Column
4.2.2. Migration of Rhodamine B in the Soil Column
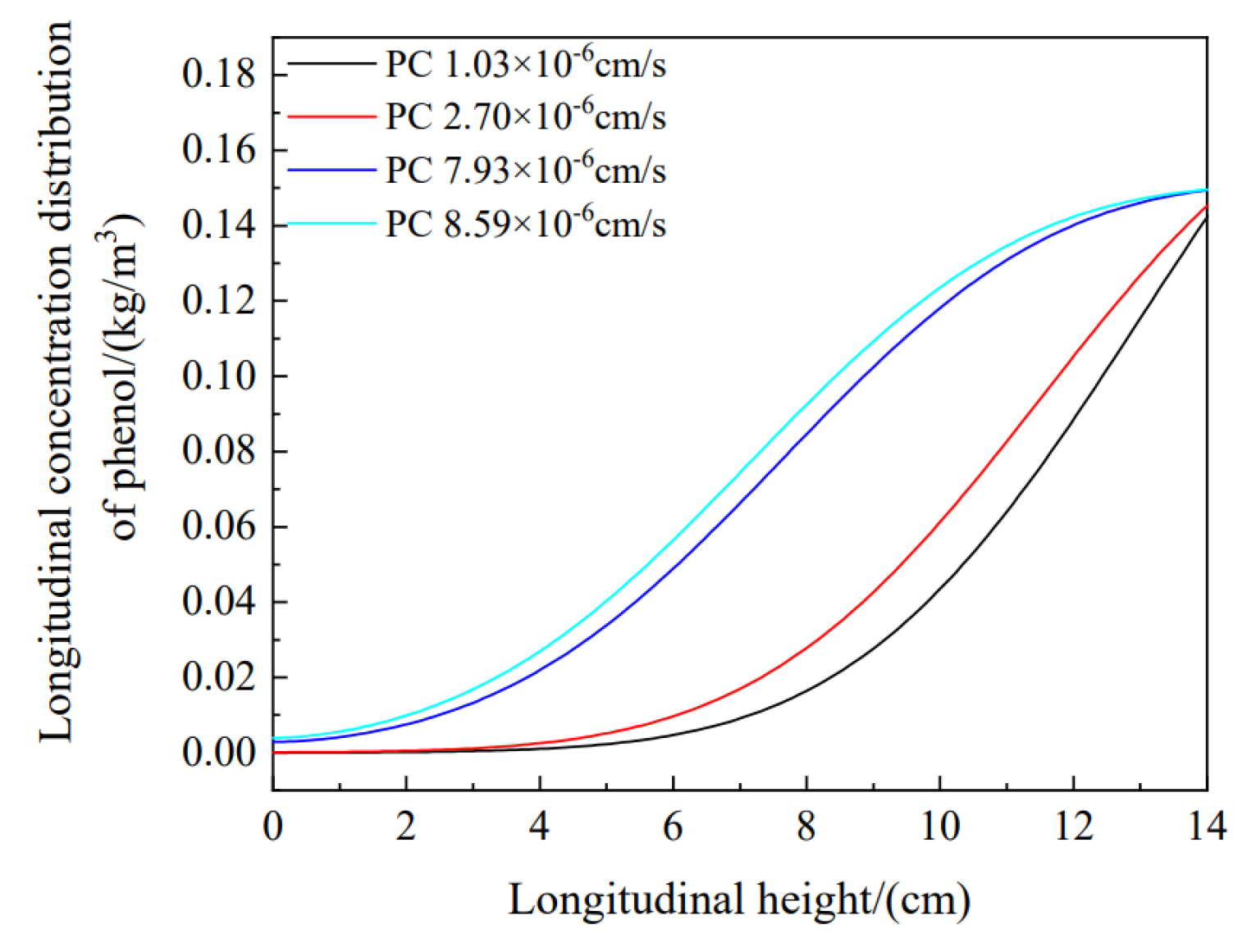
4.2.3. Parameter Sensitivity Analysis
5. Summary of the Migration of Phenol and Rhodamine B in the Soil Column
6. Conclusions
- With increasing consolidation pressure, the permeability coefficient decreased gradually. Sodium bentonite with 10% CTMAB showed the highest impermeability, and the permeability coefficient was 1.03 × 10−6 cm/s. With increasing CTMAB bentonite content, changes in the adsorbent pore ratio with higher CTMAB bentonite content exhibited a certain delay. Kaolin sodium bentonite showed good compressibility. The permeability coefficient for the soil sample with CTMAB bentonite was 3.49 × 10—7 cm/s, which was lower than those of kaolin and kaolin sodium bentonite and indicated good impermeability.
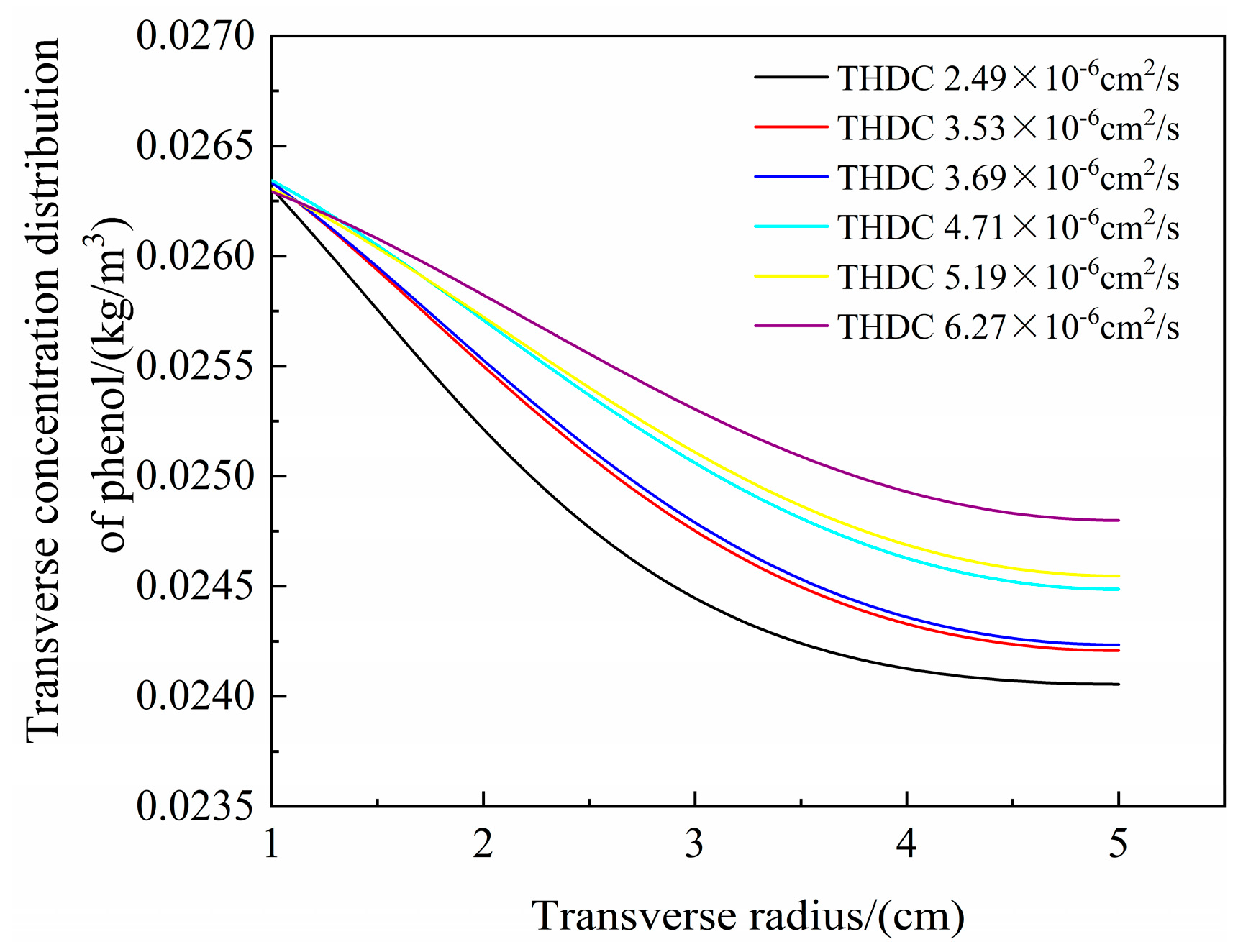
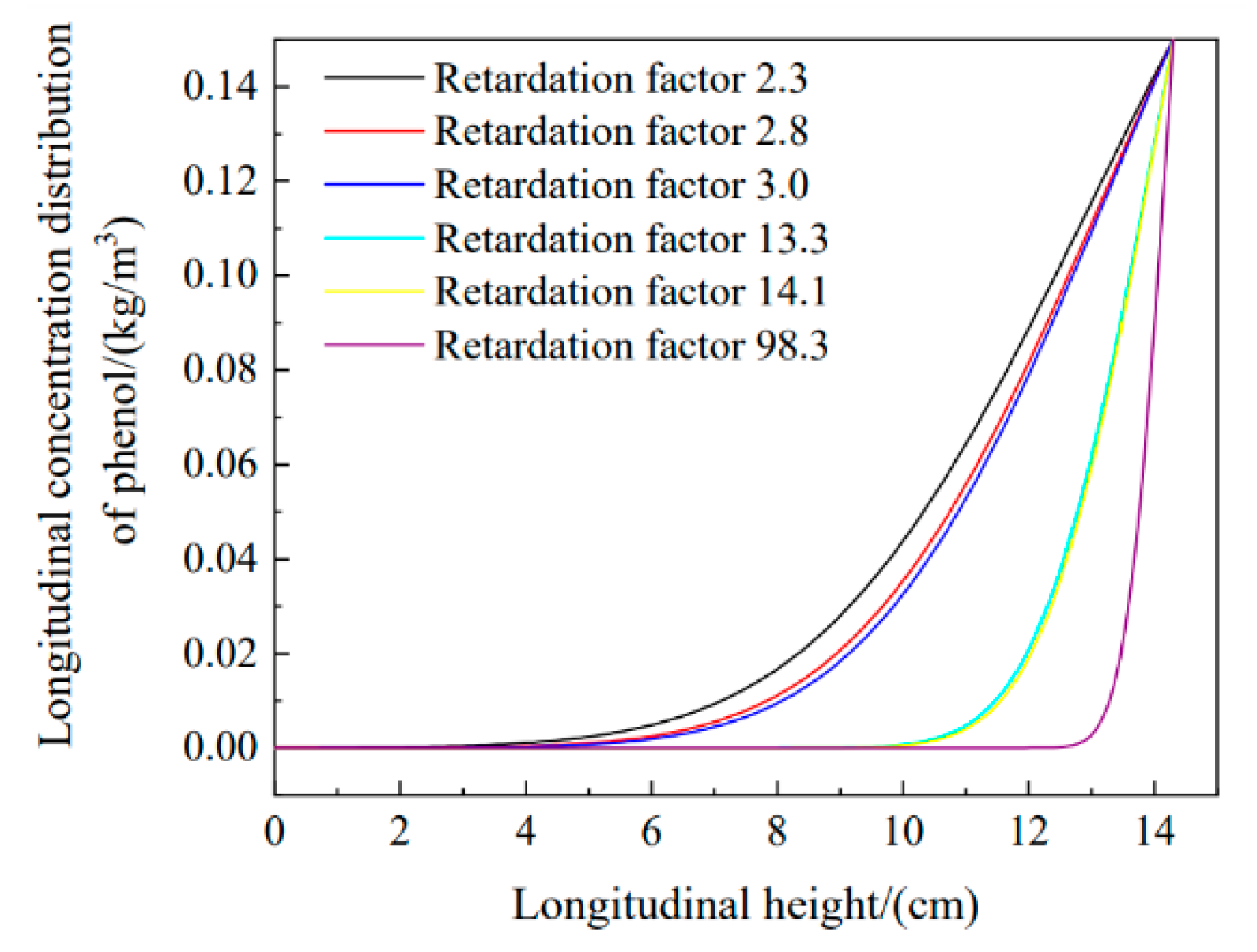
- The hydraulic transverse and longitudinal dispersion coefficients for phenol transported in sodium bentonite-10% CTMAB bentonite were the lowest at 4.71 × 10−6 cm2/s and 8.48 × 10−6 cm2/s, respectively. The retardation factor for the soil column with a CTMAB bentonite content of 15% was the largest at 3.0. The soil column with CTMAB bentonite showed good adsorption of rhodamine B; the retardation factor was 98.3, and the transverse and longitudinal hydraulic dispersion coefficients were the lowest at 2.49 × 10−6 cm2/s and 3.90 × 10−6 cm2/s, respectively. The test results for the indoor unit indicated that the kaolin CTMAB bentonite column adsorbed the most rhodamine B.
- Under longitudinal conditions, the simulated data for water head heights of 150 cm, 75 cm, and 37.5 cm were compared with experimental data. They all agreed well, which demonstrated the feasibility of simulating migration with Comsol software. Transverse diffusion became more evident from high to low water head heights, and the experimental data for the four water head heights were in good agreement with the simulation results. It was shown that the migration parameters measured experimentally basically described the actual migration states of pollutants in the soil column.
- The diffusion of pollutants in the soil column was tested by changing the migration parameters. The migration rule for pollutants in the soil column can be predicted with migration parameters. The higher the content of CTMAB bentonite is, the more obvious the retardation effect on the migration of organic pollutants.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukherjee, S.; Basak, B.; Bhunia, B.; Dey, A.; Mondal, B. Potential use of polyphenol oxidases (PPO) in the bioremediation of phenolic contaminants containing industrial wastewater. Rev. Environ. Sci. Bio/Technol. 2013, 12, 61–73. [Google Scholar] [CrossRef]
- Khan, T.A.; Dahiya, S.; Ali, I. Use of kaolinite as adsorbent: Equilibrium, dynamics and thermodynamic studies on the adsorption of Rhodamine B from aqueous solution. Appl. Clay Sci. 2012, 69, 58–66. [Google Scholar] [CrossRef]
- Li, Q.; Tang, X.; Sun, Y.; Wang, Y.; Long, Y.; Jiang, J.; Xu, H. Removal of Rhodamine B from wastewater by modified Volvariella volvacea: Batch and column study. RSC Adv. 2015, 5, 25337–25347. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, S.; Meng, K.; Wang, Y.; Ren, W.; Zhao, L.; Christie, P.; Teng, Y. Occurrence and risk assessment of potentially toxic elements and typical organic pollutants in contaminated rural soils. Sci. Total Environ. 2018, 630, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Xu, H.; Feng, Z.; Zhang, C.; Zhou, X.; Wang, Z.; Zheng, J.; Zou, X. Spatial distribution, deposition flux, and environmental impact of typical persistent organic pollutants in surficial sediments in the Eastern China Marginal Seas (ECMSs). J. Hazard. Mater. 2021, 407, 124343. [Google Scholar] [CrossRef]
- Hasan, M.; Kubra, K.T.; Hasan, N.; Awual, E.; Salman, S.; Sheikh, C.; Rehan, A.I.; Rasee, A.I.; Waliullah, R.; Islam, S.; et al. Sustainable ligand-modified based composite material for the selective and effective cadmium (II) capturing from wastewater. J. Mol. Liq. 2023, 371, 121125. [Google Scholar] [CrossRef]
- Awual, M.R. A novel facial composite adsorbent for enhanced copper (II) detection and removal from wastewater. Chem. Eng. J. 2015, 266, 368–375. [Google Scholar] [CrossRef]
- Hasan, M.N.; Salman, M.S.; Hasan, M.M.; Kubra, K.T.; Sheikh, M.C.; Rehan, A.I.; Khandaker, S.; Alsukaibi, A.K.D.; Alshammari, H.M.; Awual, M.R.; et al. Assessing sustainable Lutetium (III) ions adsorption and recovery using novel composite hybrid nanomaterials. J. Mol. Struct. 2023, 1276, 134795. [Google Scholar] [CrossRef]
- Joshi, K.; Kechavarzi, C.; Sutherland, K.; Ng MY, A.; Soga, K.; Tedd, P. Laboratory and insitu tests for long-term hydraulic conductivity of a cement-bentonite cutoff wall. J. Geotech. Geo-Environ. Eng. 2010, 136, 562–572. [Google Scholar] [CrossRef]
- Yeo, S.S.; Shackelford, C.D.; Evans, J.C. Con-solidation and hydraulic conductivity of nine model soil-bentonite backfills. J. Geotech. Geoenvironmental Eng. 2005, 131, 1189–1198. [Google Scholar] [CrossRef]
- Wang, F.; Xu, W.; Xu, Z.; Liu, H. CTMAB-Modified Bentonite–Based PRB in Remediating Cr (VI) Contaminated Groundwater. Water Air Soil Pollut. 2020, 231, 20. [Google Scholar] [CrossRef]
- Al Rubai, H.F.; Hassan, A.K.; Sultan, M.S.; Abood, W.M. Kinetics of Adsorption of Reactive Red 120 Using Bentonite Modified by CTAB and Study the Effect of Salts. Nat. Environ. Pollut. Technol. 2021, 20, 281–289. [Google Scholar] [CrossRef]
- Zahafa, F.; Maroufa, R.; Ouadjeniaa, F.; Schott, J. Kinetic and thermodynamic studies of the adsorption of Pb (II), Cr (III) and Cu (II) onto modified bentonite. Desalination Water Treat. 2018, 131, 282–290. [Google Scholar] [CrossRef]
- Guan, C.; Xie, H.J.; Wang, Y.Z.; Chen, Y.M.; Jiang, Y.S.; Tang, X.W. Analytical models for solute transport through a GCL based two-layered liner considering bio-degradation. Sci. Total Environ. 2014, 466–467, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Liu, S.Y.; Xu, L.; Bian, H.; Kou, B.; Cai, G. Effects of bentonite fraction on surface electrical conductivity of bentonite-kaolinite mixed soils. China Civ. Eng. J. 2019, 52, 23–29. (In Chinese) [Google Scholar]
- Feng, S.J.; Peng, M.Q.; Chen, Z.L.; Chen, H.X. The study of one-dimensional transport of organic contaminant transient diffusion-advection through composite liners. Chin. J. Geotech. Eng. 2021, 1–12. Available online: http://kns.cnki.net/kcms/detail/32.1124.TU.20211129.1315.002.html (accessed on 30 November 2021). (In Chinese).
- He, Y.; Hu, G.; Wu, D.Y.; Zhu, K.F.; Zhang, K.N. Contaminant migration and the retention behavior of a laterite–bentonite mixture engineered barrier in a landfill. J. Environ. Manag. 2022, 304, 114338. [Google Scholar] [CrossRef] [PubMed]
- Alkizwini, R.S. The Use of an Organo-Kaolinite Sorbent in a Permeable Reactive Barrier for Remediating Groundwater Contaminated with Methylene Blue Dye: Experimental and Theoretical Investigation. Environ. Process. 2021, 8, 889–910. [Google Scholar] [CrossRef]
- Dagher, E.E.; Nguyen, T.S.; Sedano, J.A.I. Development of a mathematical model for gas migration (two-phase flow) in natural and engineered barriers for radioactive waste disposal. Geol. Soc. Lond. Spec. Publ. 2019, 482, 115–148. [Google Scholar] [CrossRef]
- Lin, T.; Wang, J.; Yin, X.; Wei, X. Modification of Bentonite and Its Application in Antimicrobial Material. Bentonite Modif. 2020, 5, 54–61. [Google Scholar]
- Gelhar, L.; Welty, C.; Rehfeldt, K.A. Critical review of data on field-scale dispersion in aquifers. Water Resour. Res. 1992, 28, 1955–1974. [Google Scholar] [CrossRef]
- Hashemi, S.H.; Kaykhaii, M.; Keikha, A.J.; Parkaz, A. Application of Box–Behnken design in the optimization of a simple graphene oxide/zinc oxide nanocomposite-based pipette tip micro-solid phase extraction for the determination of Rhodamine B and Malachite green in seawater samples by spectrophotometry. Anal. Methods 2018, 10, 5707–5714. [Google Scholar] [CrossRef]
- Magdalena, A.; Tomasz, B. Modification of Bentonite with Cationic and Nonionic Surfactants: Structural and Textural Features. Materials 2019, 12, 3772–3794. [Google Scholar]
- Ren, X.; Zeng, G.; Tang, L.; Wang, J.; Wan, J.; Liu, Y.; Yu, J.; Yi, H.; Ye, S.; Deng, R. Sorption, transport and biodegradation–an insight into bioavailability of persistent organic pollutants in soil. Sci. Total Environ. 2018, 610, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
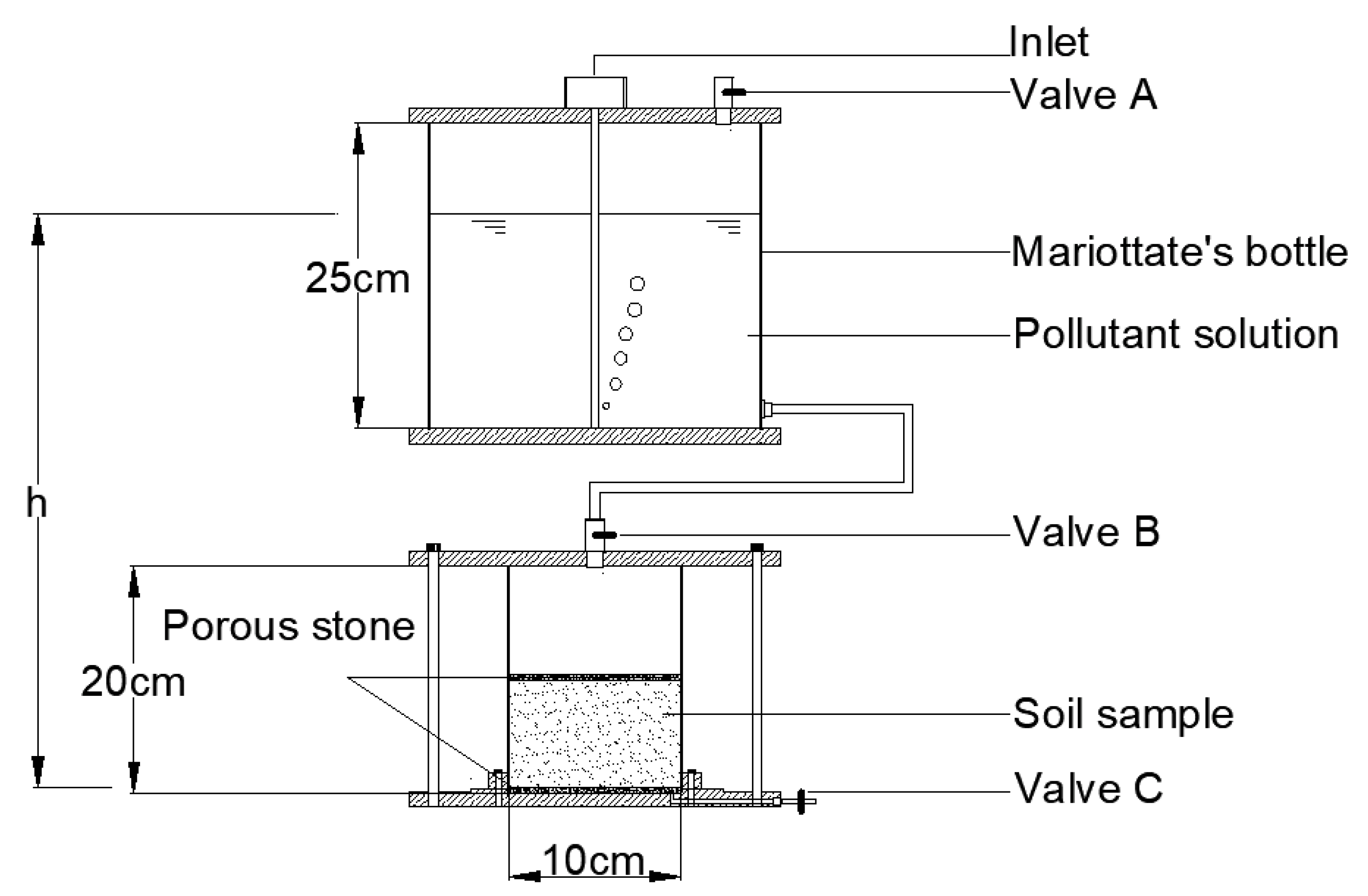


| Soil Proportion in the Column | Consolidation Pressure (kPa) | Water Head Height (cm) | Solute (mg/L) | Test Time (Days) |
|---|---|---|---|---|
| Sodium bentonite-5% CTMAB bentonite | 25, 50, 100, and 200 | 37.5, 75, 112.5, and 150 | Phenol (150) | 20 |
| Sodium bentonite-10% CTMAB bentonite | 25, 50, 100, and 200 | 37.5, 75, 112.5, and 150 | Phenol (150) | 20 |
| Sodium bentonite-15% CTMAB bentonite | 25, 50, 100, and 200 | 37.5, 75, 112.5, and 150 | Phenol (150) | 20 |
| Kaolin | 25, 50, 100, and 200 | 150 | Rhodamine B (150) | 10, 20, 35, 39, 41, 43, 50, and 67 |
| Kaolin-10% sodium bentonite | 25, 50, 100, and 200 | 150 | Rhodamine B (150) | 10, 20, 35, 39, 41, 43, 50, and 67 |
| Kaolin-10% CTMAB bentonite | 25, 50, 100, and 200 | 150 | Rhodamine B (150) | 10, 20, 35, 39, 41, 43, 50, and 67 |
| Sodium bentonite-5% CTMAB bentonite | 25, 50, 100, and 200 | 150 | Chloride ion solution (150) | 5 |
| Sodium bentonite-10% CTMAB bentonite | 25, 50, 100, and 200 | 150 | Chloride ion solution (150) | 5 |
| Sodium bentonite-15% CTMAB bentonite | 25, 50, 100, and 200 | 150 | Chloride ion solution (150) | 5 |
| Kaolin | 25, 50, 100, and 200 | 150 | Chloride ion solution (150) | 5 |
| Kaolin-10% sodium bentonite | 25, 50, 100, and 200 | 150 | Chloride ion solution (150) | 5 |
| Kaolin-10% CTMAB bentonite | 25, 50, 100, and 200 | 150 | Chloride ion solution (150) | 5 |
| Type | Consolidation Pressure/(kPa) | Natural Density/(g/cm3) | Permeability Coefficient × 10−6/(cm/s) | Soil Column Height/(cm) | Void Ratio | Bending Factor | Longitudinal Effective Diffusion Coefficient × 10−6/(cm2/s) | Transverse Effective Diffusion Coefficient × 10−6/(cm2/s) | Longitudinal Hydraulic Dispersion Coefficient × 10−6 /(cm2/s) | Transverse Hydraulic Dispersion Coefficient × 10−6 /(cm2/s) | Retardation Factor |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium bentonite-5% CTMAB bentonite | 25 | 2.056 | 12.700 | 13.8 | 0.0783 | — | — | — | — | — | 2.3 |
| 50 | 2.056 | 2.450 | 13.8 | 0.0741 | — | — | — | — | — | 2.3 | |
| 100 | 2.056 | 2.070 | 13.8 | 0.0700 | — | — | — | — | — | 2.3 | |
| 200 | 2.056 | 1.970 | 13.8 | 0.0466 | 0.7419 | 7.180 | 3.970 | 9.500 | 5.190 | 2.3 | |
| Sodium bentonite-10% CTMAB bentonite | 25 | 2.027 | 8.590 | 13.3 | 0.0663 | — | — | — | — | — | 2.8 |
| 50 | 2.027 | 7.930 | 13.3 | 0.0537 | — | — | — | — | — | 2.8 | |
| 100 | 2.027 | 2.700 | 13.3 | 0.0451 | — | — | — | — | — | 2.8 | |
| 200 | 2.027 | 1.030 | 13.3 | 0.0396 | 0.7440 | 7.200 | 3.990 | 8.480 | 4.710 | 2.8 | |
| Sodium bentonite-15% CTMAB bentonite | 25 | 2.053 | 5.380 | 11.4 | 0.0671 | — | — | — | — | — | 3.0 |
| 50 | 2.053 | 2.360 | 11.4 | 0.0654 | — | — | — | — | — | 3.0 | |
| 100 | 2.053 | 2.000 | 11.4 | 0.0596 | — | — | — | — | — | 3.0 | |
| 200 | 2.053 | 1.680 | 11.4 | 0.0229 | 0.8510 | 8.230 | 4.560 | 10.100 | 6.270 | 3.0 | |
| Kaolin | 25 | 2.005 | 4.070 | 9.8 | 0.0826 | — | — | — | — | — | 14.1 |
| 50 | 2.005 | 2.260 | 9.8 | 0.0795 | — | — | — | — | — | 14.1 | |
| 100 | 2.005 | 1.880 | 9.8 | 0.0762 | — | — | — | — | — | 14.1 | |
| 200 | 2.005 | 1.330 | 9.8 | 0.0662 | 0.5008 | 4.840 | 2.680 | 5.660 | 3.530 | 14.1 | |
| Kaolin-sodium bentonite | 25 | 1.983 | 6.040 | 10.9 | 0.0727 | — | — | — | — | — | 13.3 |
| 50 | 1.983 | 5.540 | 10.9 | 0.0617 | — | — | — | — | — | 13.3 | |
| 100 | 1.983 | 4.910 | 10.9 | 0.0481 | — | — | — | — | — | 13.3 | |
| 200 | 1.983 | 3.410 | 10.9 | 0.0265 | 0.1525 | 1.470 | 0.817 | 4.890 | 3.690 | 13.3 | |
| Kaolin-CTMAB bentonite | 25 | 1.980 | 1.950 | 11.3 | 0.0837 | — | — | — | — | — | 98.3 |
| 50 | 1.980 | 0.521 | 11.3 | 0.0829 | — | — | — | — | — | 98.3 | |
| 100 | 1.980 | 0.391 | 11.3 | 0.0823 | — | — | — | — | — | 98.3 | |
| 200 | 1.980 | 0.349 | 11.3 | 0.0751 | 0.2543 | 2.460 | 1.360 | 3.900 | 2.490 | 98.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Wu, T.; Shu, X.; Chai, K.; Qiu, Z.; Wang, S.; Yao, J. Enhanced Organic Contaminant Retardation by CTMAB-Modified Bentonite Backfill in Cut-Off Walls: Laboratory Test and Numerical Investigation. Materials 2023, 16, 1255. https://doi.org/10.3390/ma16031255
He H, Wu T, Shu X, Chai K, Qiu Z, Wang S, Yao J. Enhanced Organic Contaminant Retardation by CTMAB-Modified Bentonite Backfill in Cut-Off Walls: Laboratory Test and Numerical Investigation. Materials. 2023; 16(3):1255. https://doi.org/10.3390/ma16031255
Chicago/Turabian StyleHe, Haijie, Tao Wu, Xiaole Shu, Kuan Chai, Zhanhong Qiu, Shifang Wang, and Jun Yao. 2023. "Enhanced Organic Contaminant Retardation by CTMAB-Modified Bentonite Backfill in Cut-Off Walls: Laboratory Test and Numerical Investigation" Materials 16, no. 3: 1255. https://doi.org/10.3390/ma16031255




