High-Pressure Adsorption of CO2 and CH4 on Biochar—A Cost-Effective Sorbent for In Situ Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritchie, H.; Roser, M.; Rosado, P. CO₂ and Greenhouse Gas Emissions; Our World in Data: Oxford, UK, 2020. [Google Scholar]
- Pörtner, H.-O.; Roberts, D.C.; Adams, H.; Adler, C.; Aldunce, P.; Ali, E.; Begum, R.A.; Betts, R.; Kerr, R.B.; Biesbroek, R. Climate Change 2022: Impacts, Adaptation and Vulnerability; IPCC Sixth Assessment Report; IPCC: Geneva, Switzerland, 2022. [Google Scholar]
- Chen, S.; Liu, J.; Zhang, Q.; Teng, F.; McLellan, B.C. A Critical Review on Deployment Planning and Risk Analysis of Carbon Capture, Utilization, and Storage (CCUS) toward Carbon Neutrality. Renew. Sustain. Energy Rev. 2022, 167, 112537. [Google Scholar] [CrossRef]
- de Meyer, F.; Jouenne, S. Industrial Carbon Capture by Absorption: Recent Advances and Path Forward. Curr. Opin. Chem. Eng. 2022, 38, 100868. [Google Scholar] [CrossRef]
- Luis, P. Use of Monoethanolamine (MEA) for CO2 Capture in a Global Scenario: Consequences and Alternatives. Desalination 2016, 380, 93–99. [Google Scholar] [CrossRef]
- Riboldi, L.; Bolland, O. Overview on Pressure Swing Adsorption (PSA) as CO2 Capture Technology: State-of-the-Art, Limits and Potentials. Energy Procedia 2017, 114, 2390–2400. [Google Scholar] [CrossRef]
- Song, C.; Kansha, Y.; Ishizuka, M.; Fu, Q.; Tsutsumi, A. Design of a Low-Cost CO2 Capture Process Based on Heat Integration Technology. Energy Procedia 2014, 61, 365–368. [Google Scholar] [CrossRef]
- Riboldi, L.; Bolland, O. Evaluating Pressure Swing Adsorption as a CO2 Separation Technique in Coal-Fired Power Plants. Int. J. Greenh. Gas Control. 2015, 39, 1–16. [Google Scholar] [CrossRef]
- Myshakin, E.; Romanov, V.; Cygan, R. Natural Materials for Carbon Capture; U.S. Department of Commerce: Springfield, VA, USA, 2010.
- Hornbostel, M.D.; Bao, J.; Krishnan, G.; Nagar, A.; Jayaweera, I.; Kobayashi, T.; Sanjurjo, A.; Sweeney, J.; Carruthers, D.; Petruska, M.A.; et al. Characteristics of an Advanced Carbon Sorbent for CO2 Capture. Carbon 2013, 56, 77–85. [Google Scholar] [CrossRef]
- Hedin, N.; Andersson, L.; Bergström, L.; Yan, J. Adsorbents for the Post-Combustion Capture of CO2 Using Rapid Temperature Swing or Vacuum Swing Adsorption. Appl. Energy 2013, 104, 418–433. [Google Scholar] [CrossRef]
- Koytsoumpa, E.I.; Bergins, C.; Kakaras, E. The CO2 Economy: Review of CO2 Capture and Reuse Technologies. J. Supercrit. Fluids 2018, 132, 3–16. [Google Scholar] [CrossRef]
- Ahmed, R.; Liu, G.; Yousaf, B.; Abbas, Q.; Ullah, H.; Ali, M.U. Recent Advances in Carbon-Based Renewable Adsorbent for Selective Carbon Dioxide Capture and Separation-A Review. J. Clean. Prod. 2020, 242, 118409. [Google Scholar] [CrossRef]
- Gunawardene, O.H.P.; Gunathilake, C.A.; Vikrant, K.; Amaraweera, S.M. Carbon Dioxide Capture through Physical and Chemical Adsorption Using Porous Carbon Materials: A Review. Atmosphere 2022, 13, 397. [Google Scholar] [CrossRef]
- Sethupathi, S.; Zhang, M.; Rajapaksha, A.U.; Lee, S.R.; Mohamad Nor, N.; Mohamed, A.R.; Al-Wabel, M.; Lee, S.S.; Ok, Y.S. Biochars as Potential Adsorbers of CH4, CO2 and H2S. Sustainability 2017, 9, 121. [Google Scholar] [CrossRef]
- Zgureva, D. Carbon Dioxide Adsorption Studies on Fly Ash Zeolites. Coal Combust. Gasif. Prod. 2016, 8, 54–59. [Google Scholar] [CrossRef]
- Czuma, N.; Casanova, I.; Baran, P.; Szczurowski, J.; Zarębska, K. CO2 Sorption and Regeneration Properties of Fly Ash Zeolites Synthesized with the Use of Differentiated Methods. Sci. Rep. 2020, 10, 1825. [Google Scholar] [CrossRef] [PubMed]
- Lira-Zúñiga, S.; Sáez-Navarrete, C.; Rodríguez-Córdova, L.; Herrera-Zeppelin, L.; Herrera-Urbina, R. CO2 Adsorption on Agricultural Biomass Combustion Ashes. Maderas Cienc. Tecnol. 2016, 18, 607–616. [Google Scholar] [CrossRef]
- Sakiewicz, P.; Lutyński, M.; Sobieraj, J.; Piotrowski, K.; Miccio, F.; Kalisz, S. Adsorption of CO2 on In Situ Functionalized Straw Burning Ashes—An Innovative, Circular Economy-Based Concept for Limitation of Industrial-Scale Greenhouse Gas Emission. Energies 2022, 15, 1352. [Google Scholar] [CrossRef]
- Adamovsky, P. DŘEVĚNÉ PELETY TOP A1-sáček 15kg. Available online: https://eshop.biomac.cz/drevene-pelety-top-a1-sacek-15kg-g8900.html (accessed on 12 December 2022).
- AlOthman, Z.A.; Habila, M.A.; Ali, R.; Abdel Ghafar, A.; El-din Hassouna, M.S. Valorization of Two Waste Streams into Activated Carbon and Studying Its Adsorption Kinetics, Equilibrium Isotherms and Thermodynamics for Methylene Blue Removal. Arab. J. Chem. 2014, 7, 1148–1158. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Habila, M.A.; Moshab, M.S.; Al-Qahtani, K.M.; AlMasoud, N.; Al-Senani, G.M.; Al-Kadhi, N.S. Fabrication of Renewable Palm-Pruning Leaves Based Nano-Composite for Remediation of Heavy Metals Pollution. Arab. J. Chem. 2020, 13, 4936–4944. [Google Scholar] [CrossRef]
- McCarty, R.; Arp, V. A New Wide Range Equation of State for Helium. Adv. Cryog. Eng. 1990, 35, 1465–1475. [Google Scholar]
- Sakurovs, R.; Day, S.; Weir, S.; Duffy, G. Application of a Modified Dubinin-Radushkevich Equation to Adsorption of Gases by Coals under Supercritical Conditions. Energy Fuels 2007, 21, 992–997. [Google Scholar] [CrossRef]
- Donohue, M.D.; Aranovich, G.L. A New Classification of Isotherms for Gibbs Adsorption of Gases on Solids. Fluid Phase Equilibria 1999, 158–160, 557–563. [Google Scholar] [CrossRef]
- Dantas, T.L.; Amorim, S.M.; Luna, F.M.T.; Silva, I.J., Jr.; de Azevedo, D.C.; Rodrigues, A.E.; Moreira, R.F. Adsorption of Carbon Dioxide onto Activated Carbon and Nitrogen-Enriched Activated Carbon: Surface Changes, Equilibrium, and Modeling of Fixed-Bed Adsorption. Sep. Sci. Technol. 2009, 45, 73–84. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Oliveira, R.S.; Vieira, R.S.; Cavalcante Jr, C.L.; Azevedo, D.C. Adsorption of CO2 on Nitrogen-Enriched Activated Carbon and Zeolite 13X. Adsorption 2011, 17, 235–246. [Google Scholar] [CrossRef]
- Gensterblum, Y.; van Hemert, P.; Billemont, P.; Battistutta, E.; Busch, A.; Krooss, B.M.; De Weireld, G.; Wolf, K.-H.A.A. European Inter-Laboratory Comparison of High Pressure CO2 Sorption Isotherms II: Natural Coals. Int. J. Coal Geol. 2010, 84, 115–124. [Google Scholar] [CrossRef]
- Sudibandriyo, M.; Pan, Z.; Fitzgerald, J.E.; Robinson, R.L.; Gasem, K.A. Adsorption of Methane, Nitrogen, Carbon Dioxide, and Their Binary Mixtures on Dry Activated Carbon at 318.2 K and Pressures up to 13.6 MPa. Langmuir 2003, 19, 5323–5331. [Google Scholar] [CrossRef]
- Bae, J.-S.; Bhatia, S.K. High-Pressure Adsorption of Methane and Carbon Dioxide on Coal. Available online: https://pubs.acs.org/doi/pdf/10.1021/ef060318y (accessed on 2 January 2023).
- Baran, P.; Cygankiewicz, J.; Zarȩbska, K. Carbon Dioxide Sorption on Polish Ortholignite Coal in Low and Elevated Pressure. J. CO2 Util. 2013, 3–4, 44–48. [Google Scholar] [CrossRef]
- Siemons, N.; Busch, A. Measurement and Interpretation of Supercritical CO2 Sorption on Various Coals. Int. J. Coal Geol. 2007, 69, 229–242. [Google Scholar] [CrossRef]
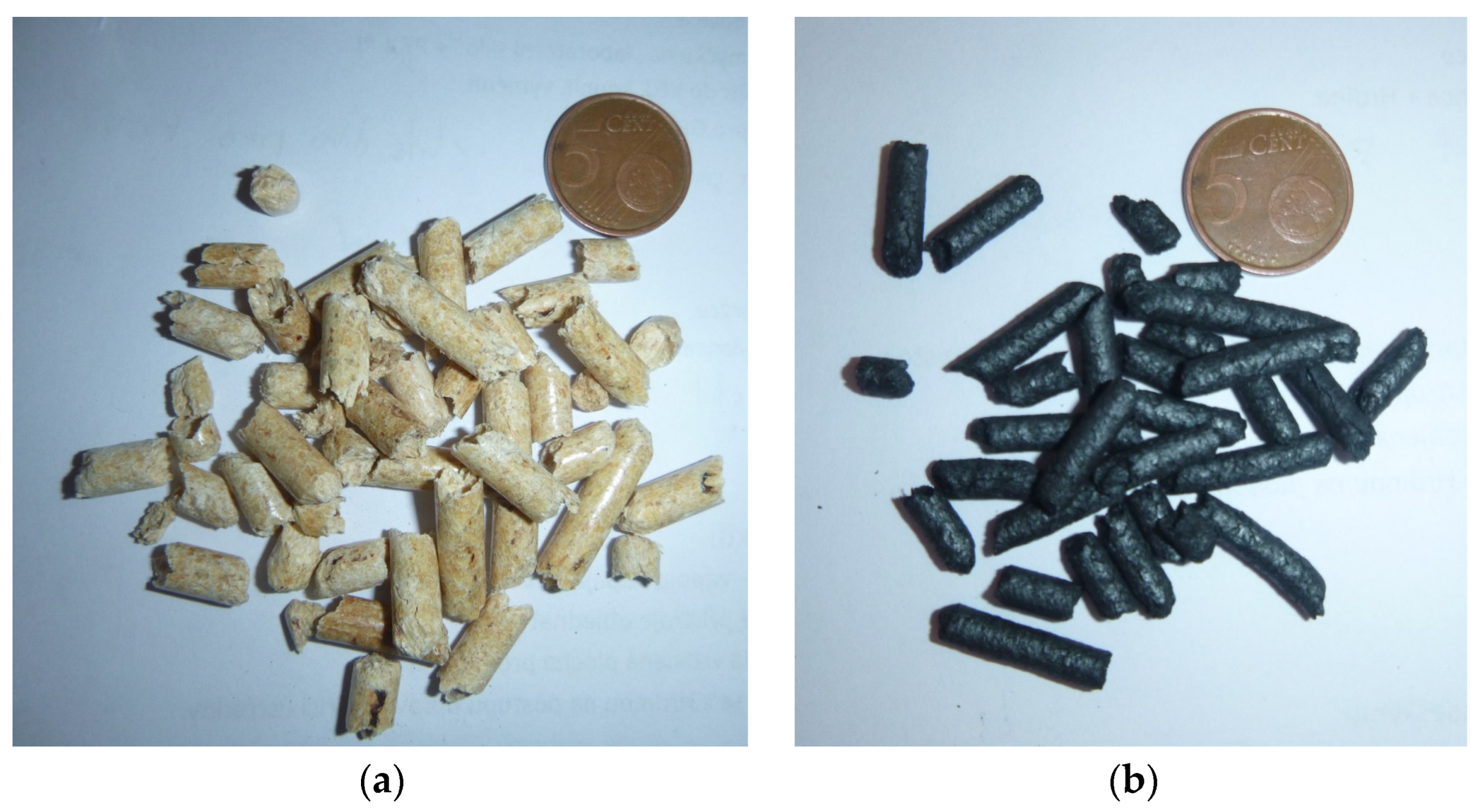
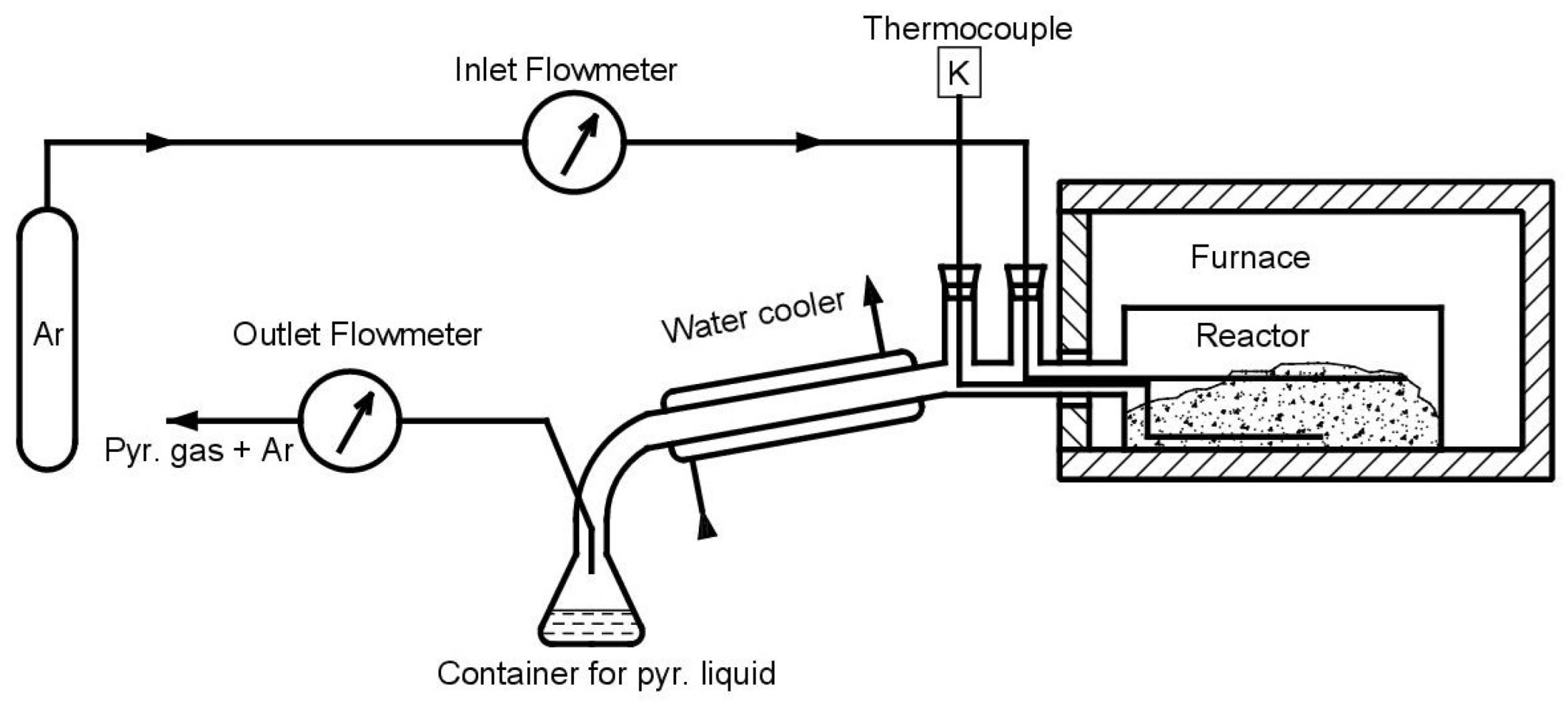

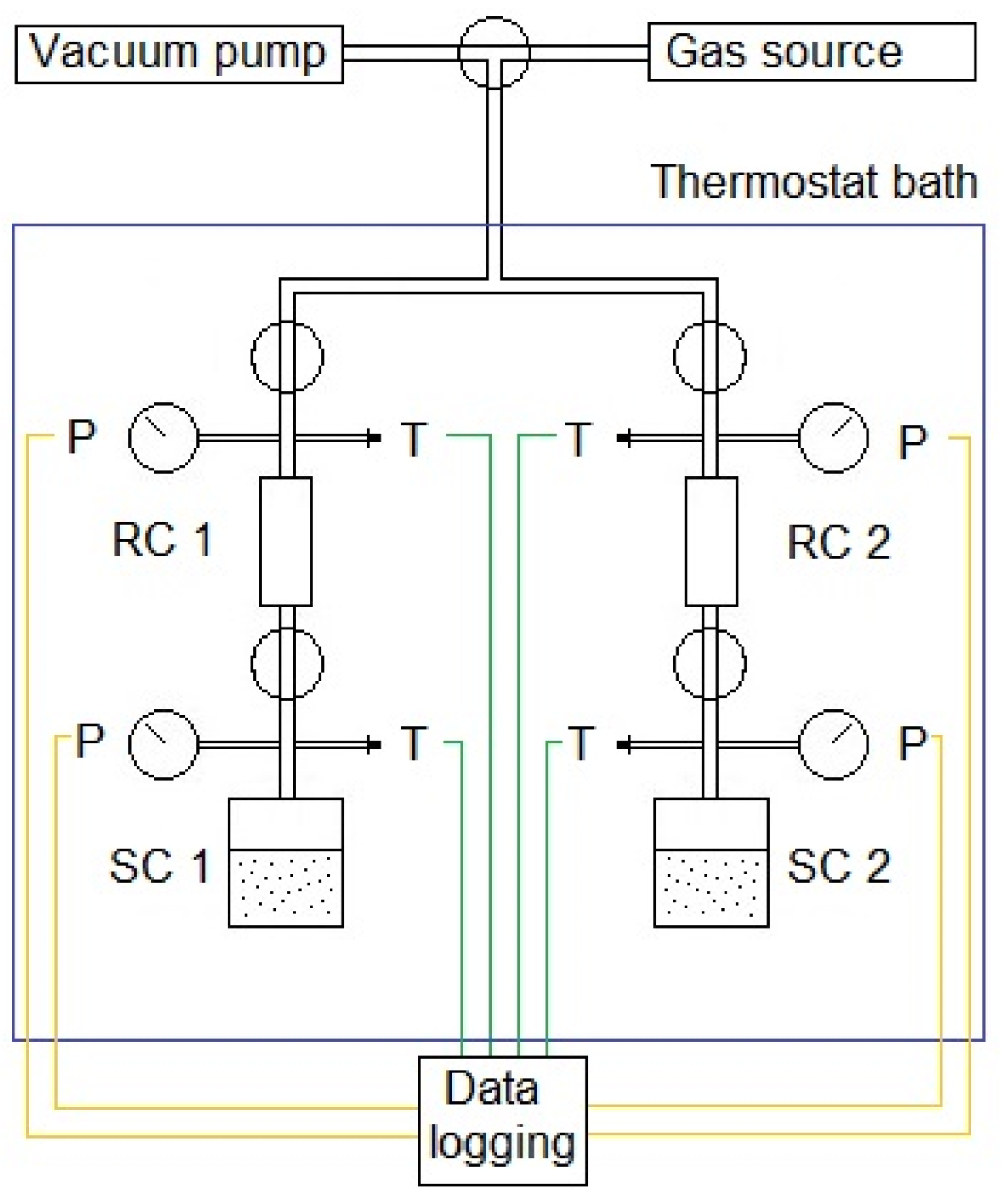
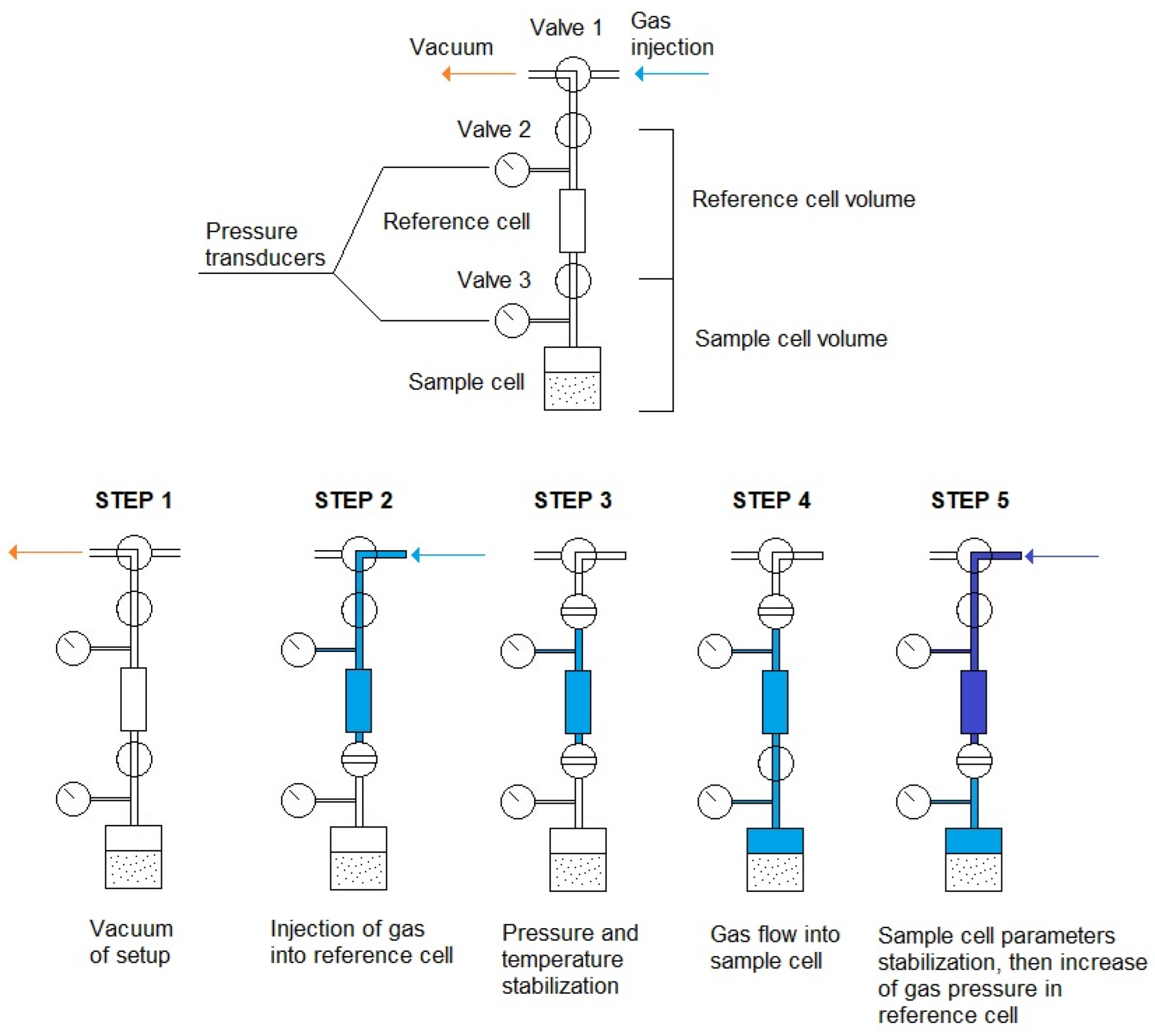
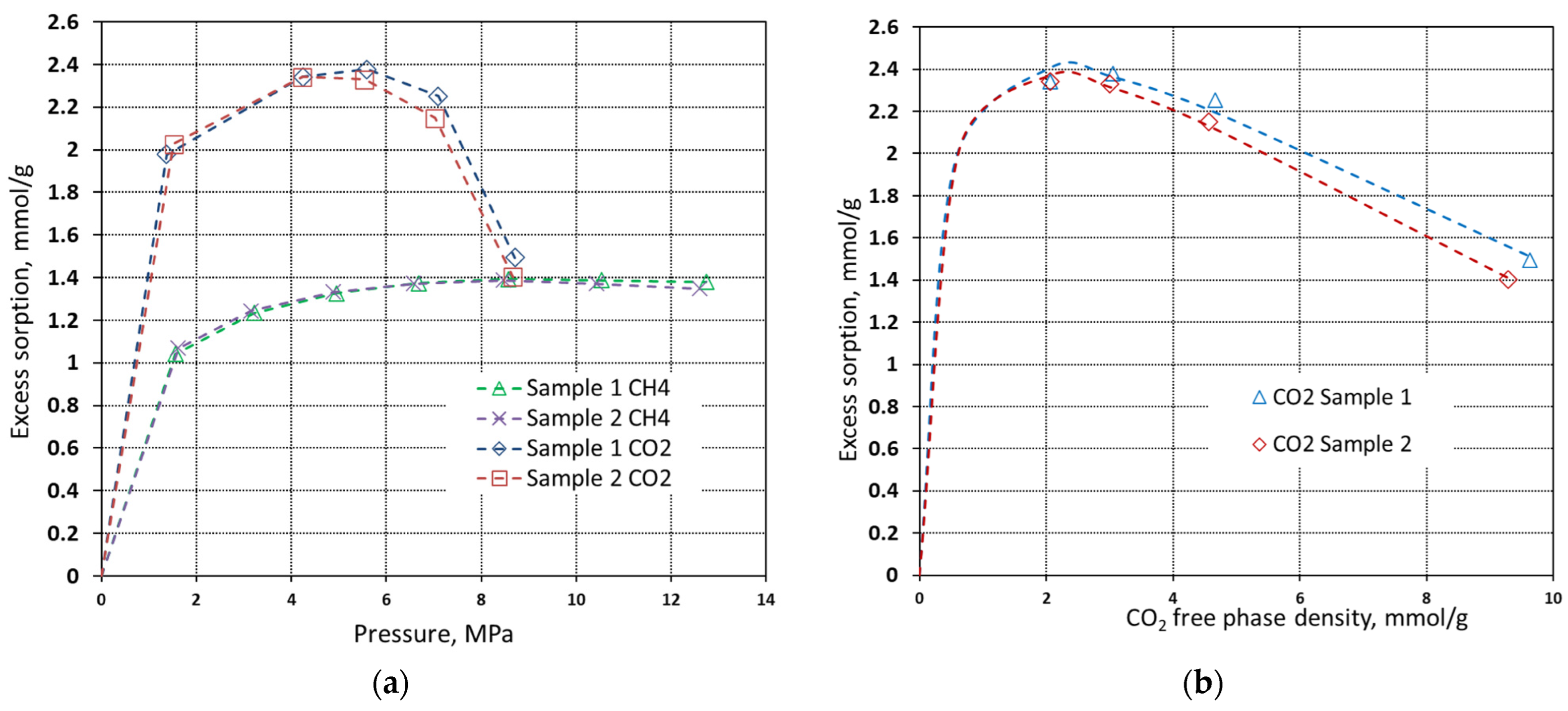
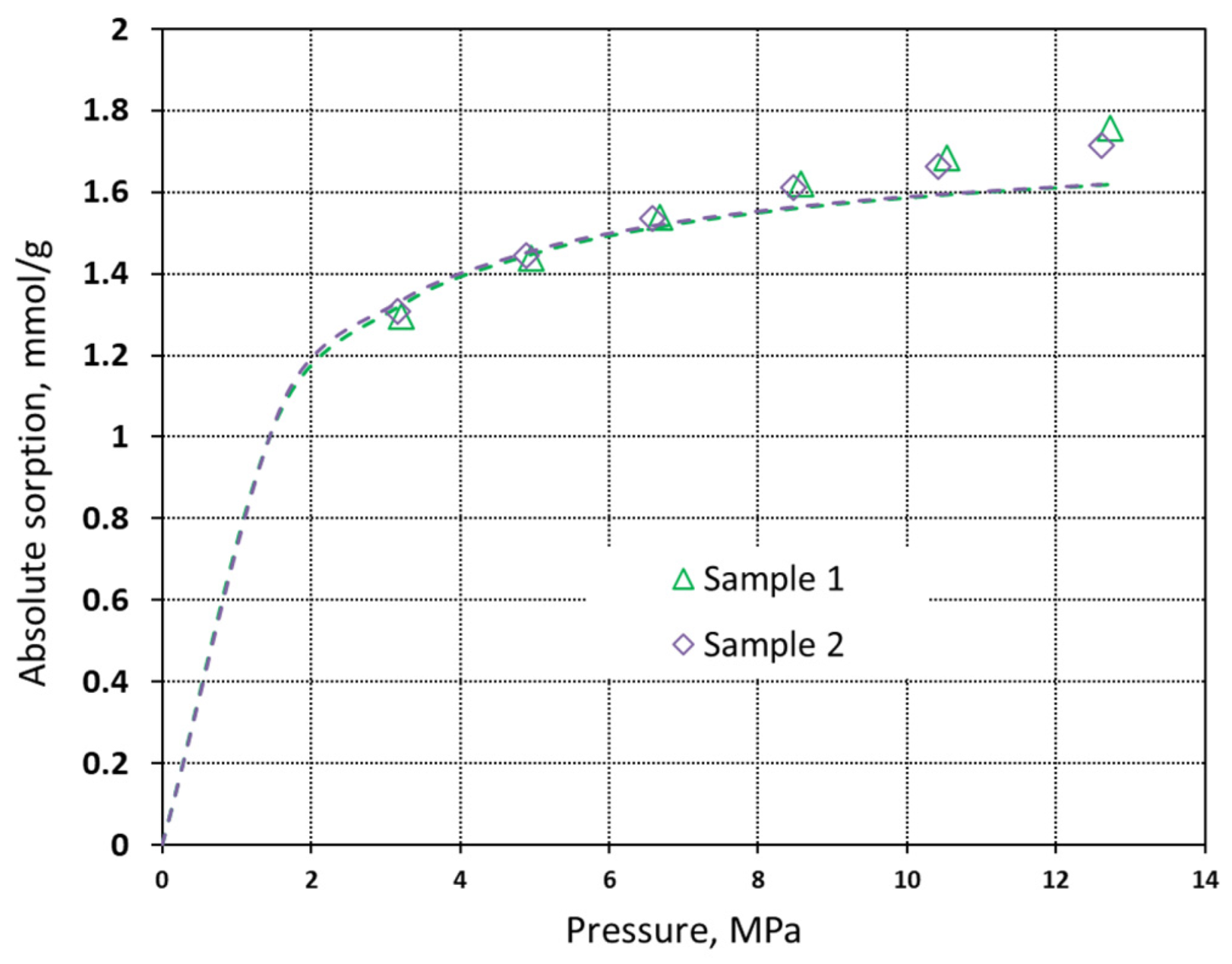
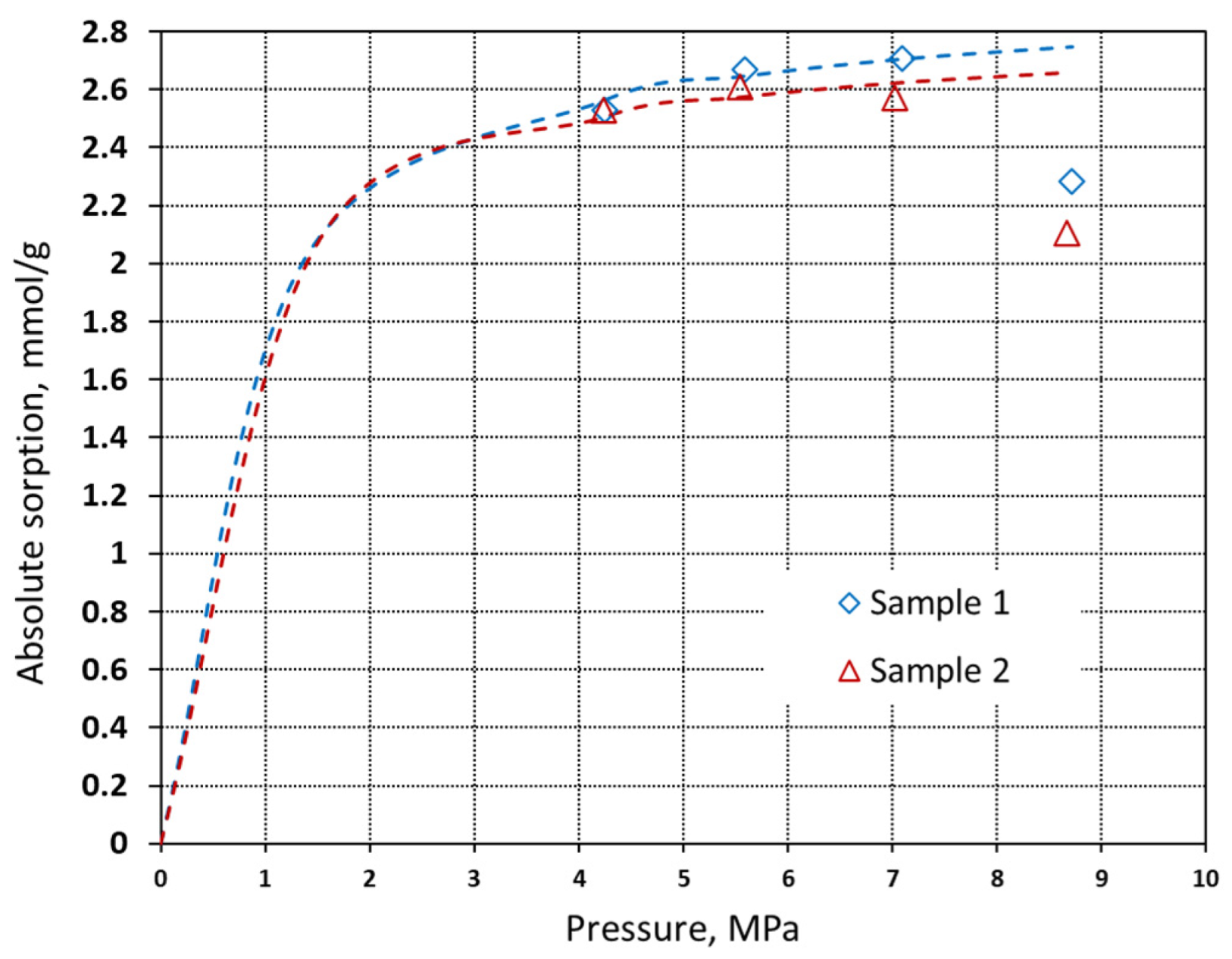
| Property | Symbol, Unit | Value |
|---|---|---|
| Ash content 1 | Ad, %mass | 0.53 |
| Volatile matter 2 | Vdaf, %mass | 83.7 |
| Carbon | Cdaf, %mass | 50.3 |
| Hydrogen | Hdaf, %mass | 6.16 |
| Oxygen | Odaf, %mass | 43.4 |
| Nitrogen | Ndaf, %mass | 0.12 |
| Chlorine | Cldaf, %mass | <0.01 |
| Sulphur | Sd, %mass | 0.01 |
| Heating value | Qp, net, d, MJ·kg−1 | 18.8 |
| Tannins | %mass | 8.75 ± 0.12 |
| Lignin | %mass | 23.29 ± 0.02 |
| Resinous substances | %mass | 3.49 ± 0.14 |
| Holocellulose | %mass | 54.92 |
| Deformation temperature | °C | 1044 |
| Softening temperature | °C | 1052 |
| Melting point | °C | 1257 |
| Creep temperature | °C | 1264 |
| Oxides/Element | Value |
|---|---|
| P2O5 | 3.53 |
| Al2O3 | 2.66 |
| Na2O | 0.59 |
| SO3 | 2.15 |
| SiO2 | 8.50 |
| CaO | 43.50 |
| K2O | 9.24 |
| Fe2O3 | 1.53 |
| MgO | 6.68 |
| TiO | 0.18 |
| MnO | 3.780 |
| Cl | 0.490 |
| Pb | 0.023 |
| Cd | 0.001 |
| Cu | 0.038 |
| Hg | <0.001 |
| Cr | 0.120 |
| Ni | 0.780 |
| V | BDL |
| Zn | 0.140 |
| Sample 1 | Sample 2 | Sample 1 | Sample 2 | |
|---|---|---|---|---|
| Adsorbate | CH4 | CO2 | ||
| ma, mmol·g−1 | 1.75 | 1.74 | 2.95 | 2.82 |
| bv, mol·dm−3 | 0.96 | 1.01 | 1.57 | 1.85 |
| R2 | 0.996 | 0.997 | 0.995 | 0.983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutyński, M.; Kielar, J.; Gajda, D.; Mikeska, M.; Najser, J. High-Pressure Adsorption of CO2 and CH4 on Biochar—A Cost-Effective Sorbent for In Situ Applications. Materials 2023, 16, 1266. https://doi.org/10.3390/ma16031266
Lutyński M, Kielar J, Gajda D, Mikeska M, Najser J. High-Pressure Adsorption of CO2 and CH4 on Biochar—A Cost-Effective Sorbent for In Situ Applications. Materials. 2023; 16(3):1266. https://doi.org/10.3390/ma16031266
Chicago/Turabian StyleLutyński, Marcin, Jan Kielar, Dawid Gajda, Marcel Mikeska, and Jan Najser. 2023. "High-Pressure Adsorption of CO2 and CH4 on Biochar—A Cost-Effective Sorbent for In Situ Applications" Materials 16, no. 3: 1266. https://doi.org/10.3390/ma16031266





