Phase Transition of High-Surface-Area Glycol–Thermal Synthesized Lanthanum Manganite
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis and Structural Calculations
2.2. Density Functional Theory Calculations
2.3. Material Characterization
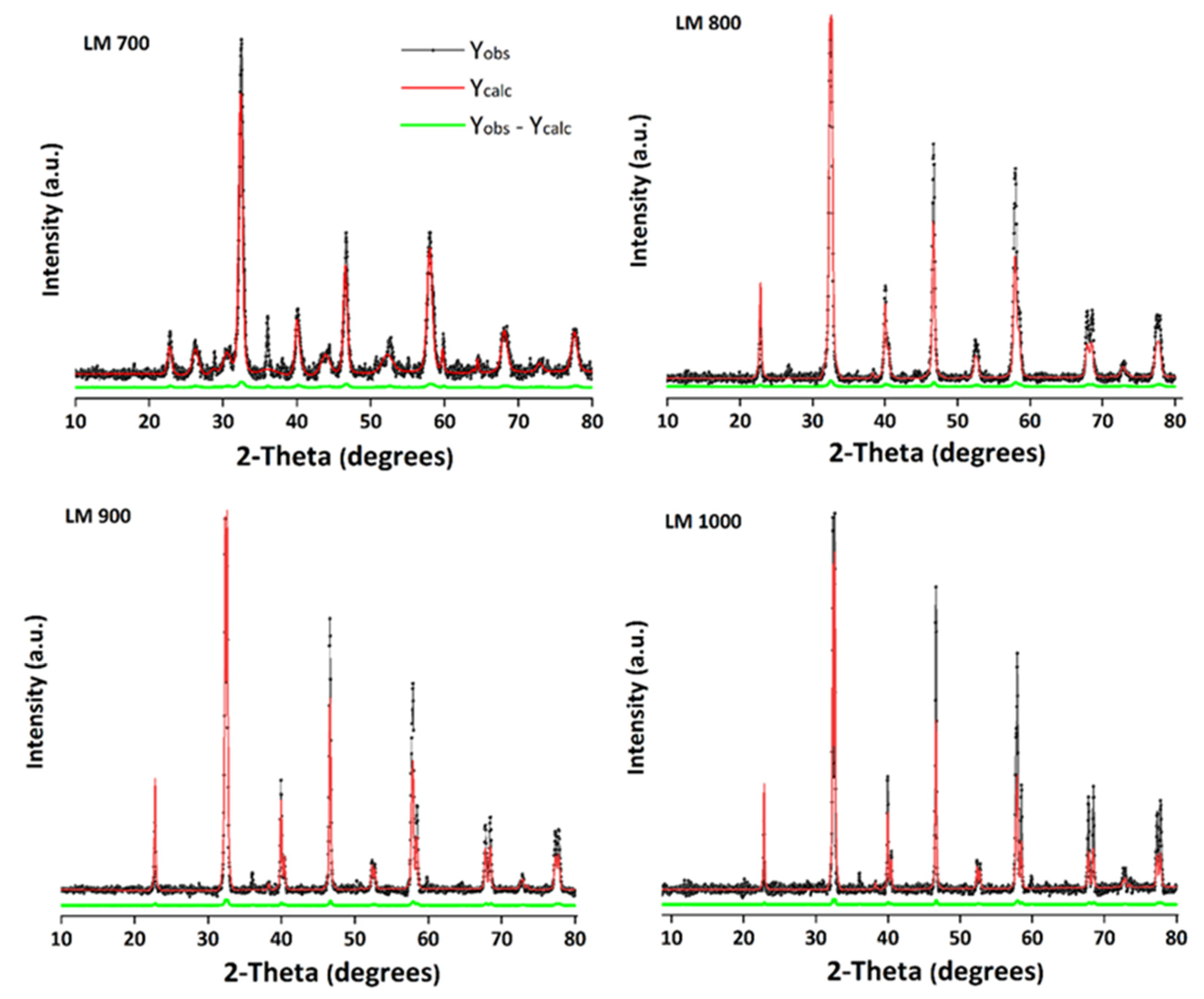
2.3.1. Phase Identification
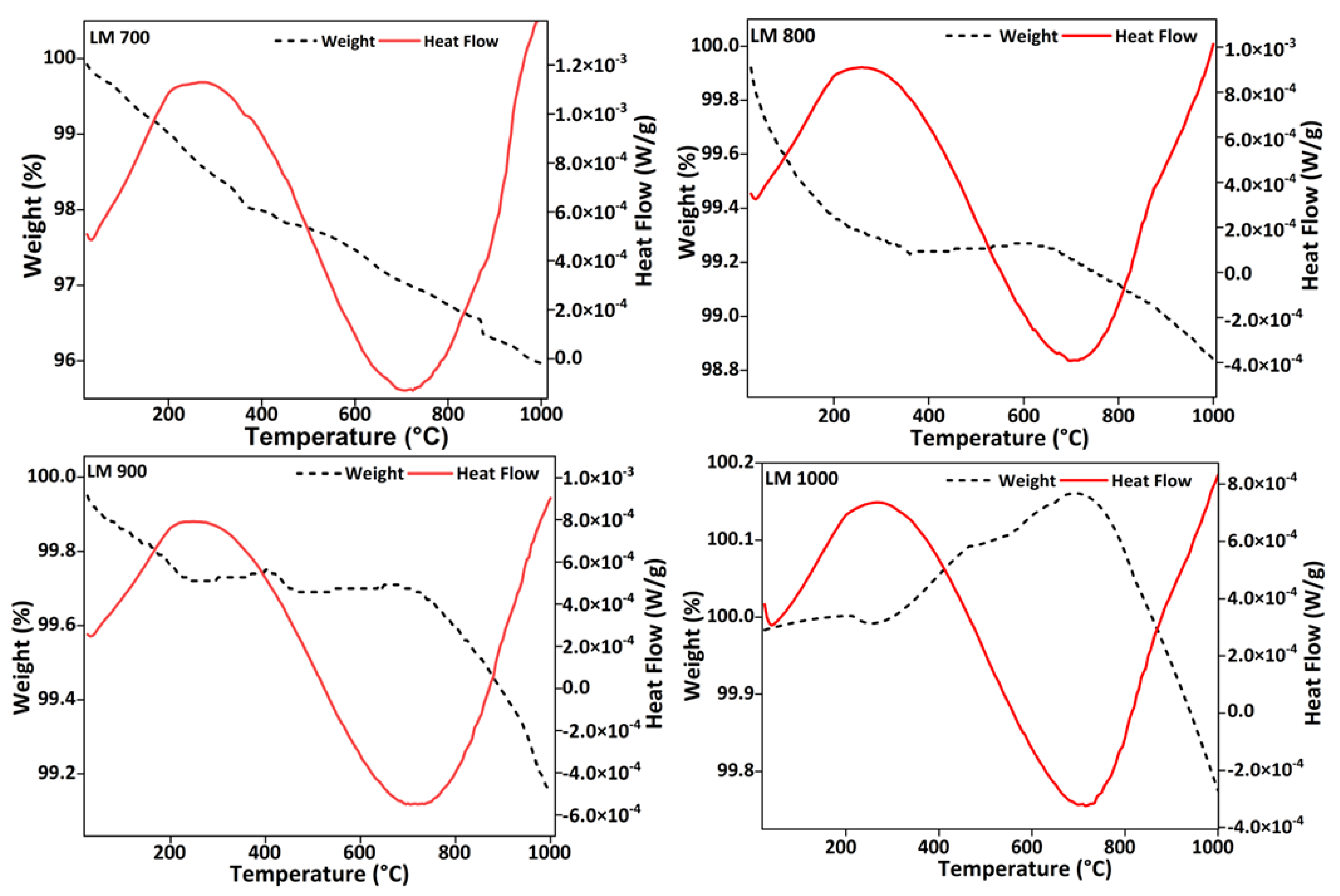
2.3.2. Thermogravimetric Analysis
2.3.3. Infrared Spectroscopy
2.3.4. Raman Spectroscopy
2.3.5. BET Surface Area
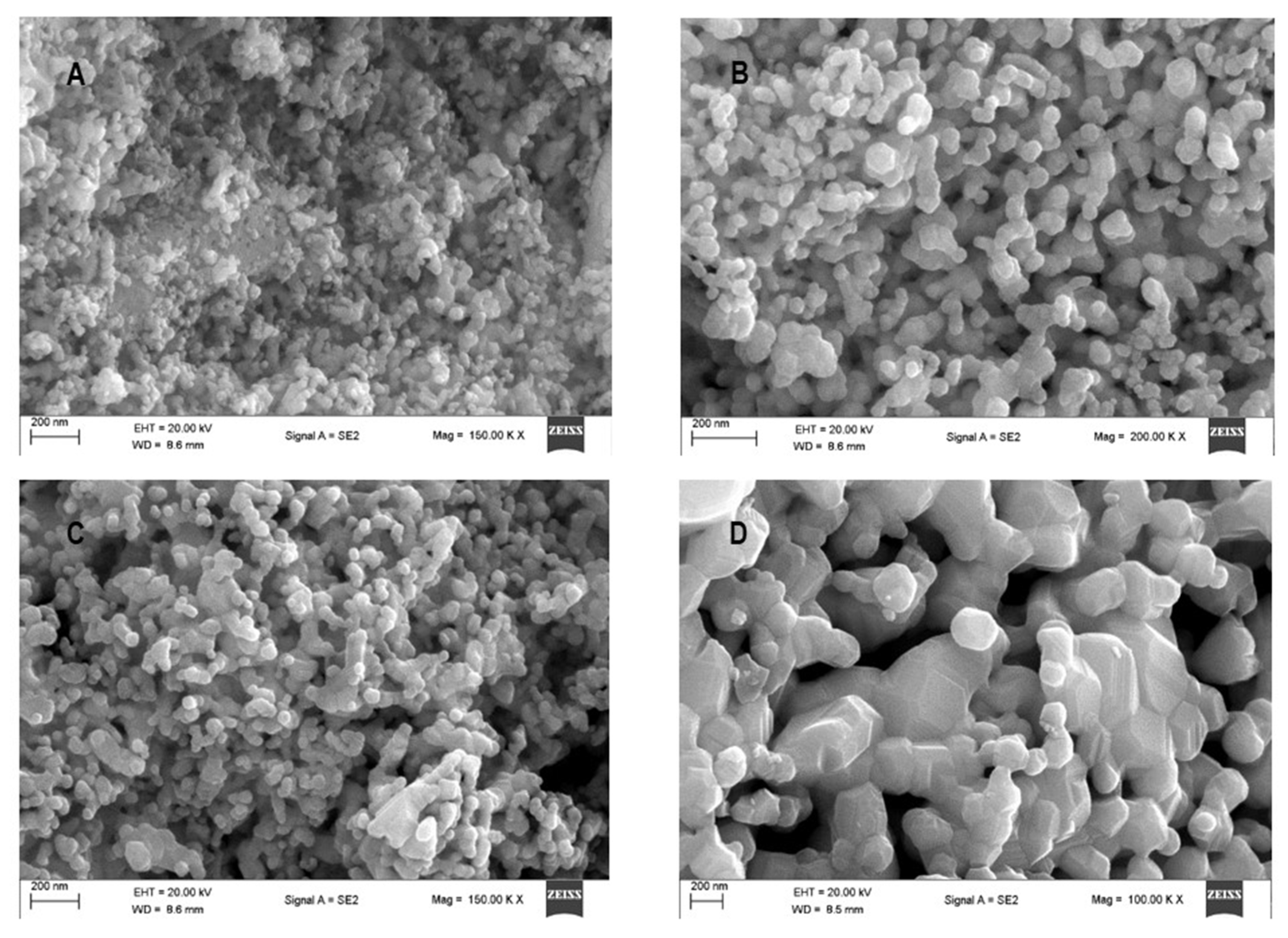
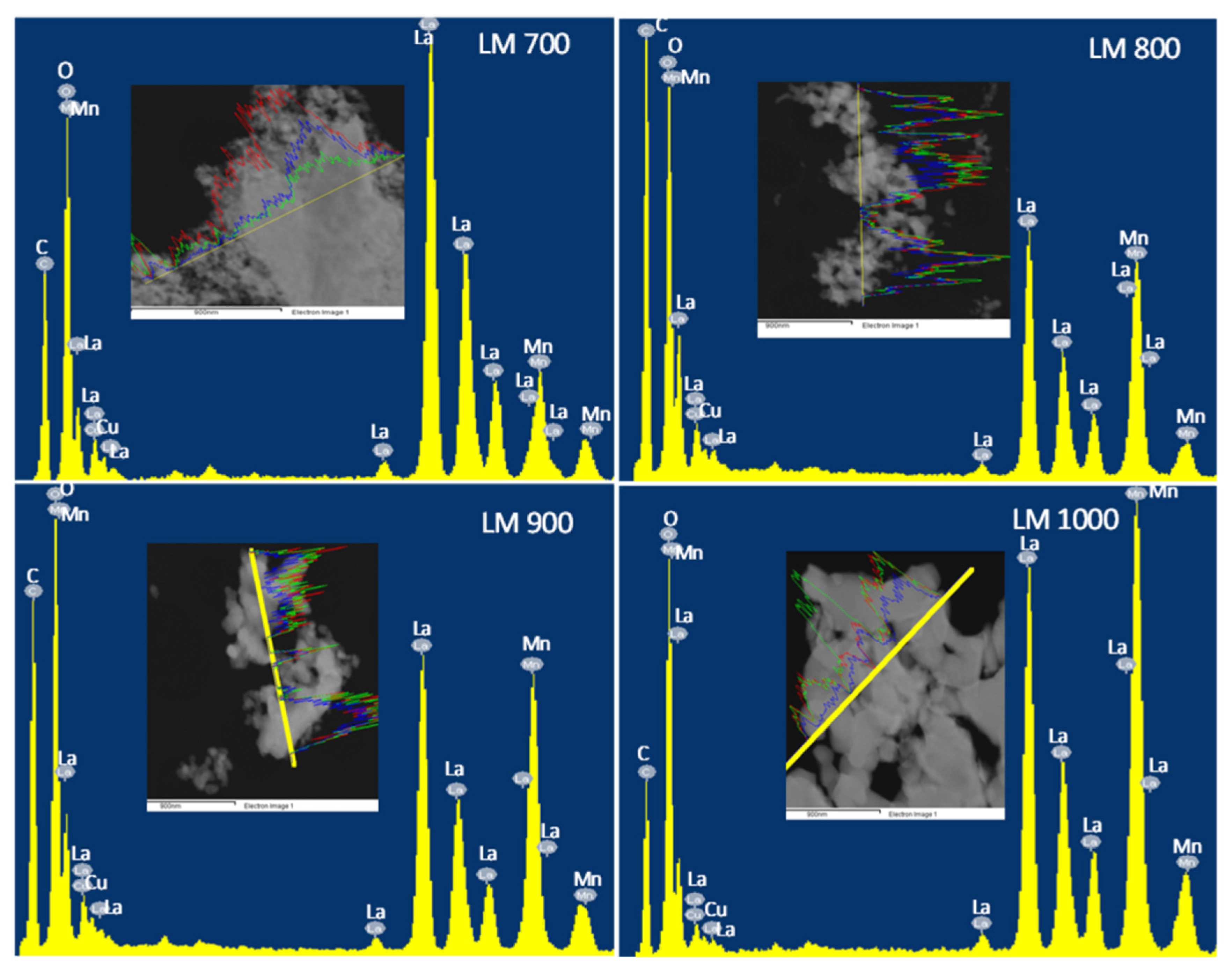
2.3.6. Electron Microscopy
2.3.7. Electrical Measurement
3. Results and Discussion
3.1. Thermal and Structural Analysis
3.2. Textural Properties
3.3. Electron Microscopy
3.4. Electrical Resistivity
3.5. Computational Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef] [PubMed]
- Parravano, G. Catalytic Activity of Lanthanum and Strontium Manganite. J. Am. Chem. Soc. 1953, 75, 1497–1498. [Google Scholar] [CrossRef]
- Ciambelli, P.; Cimino, S.; De Rossi, S.; Lisi, L.; Minelli, G.; Porta, P.; Russo, G. AFeO3 (A = La, Nd, Sm) and LaFe1−xMgxO3 perovskites as methane combustion and CO oxidation catalysts: Structural, redox and catalytic properties. Appl. Catal. B Environ. 2001, 29, 239–250. [Google Scholar] [CrossRef]
- Arandiyan, H.; Mofarah, S.S.; Sorrell, C.C.; Doustkhah, E.; Sajjadi, B.; Hao, D.; Wang, Y.; Sun, H.; Ni, B.J.; Rezaei, M.; et al. Defect engineering of oxide perovskites for catalysis and energy storage: Synthesis of chemistry and materials science. Chem. Soc. Rev. 2021, 50, 10116–10211. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.A.; Martínez-Lope, M.J.; Casais, M.T.; Fernández-Díaz, M.T. Evolution of the Jahn−Teller Distortion of MnO6 Octahedra in RMnO3 Perovskites (R = Pr, Nd, Dy, Tb, Ho, Er, Y): A Neutron Diffraction Study. Inorg. Chem. 2000, 39, 917–923. [Google Scholar] [CrossRef]
- Wang, X.; Huang, K.; Yuan, L.; Xi, S.; Yan, W.; Geng, Z.; Cong, Y.; Sun, Y.; Tan, H.; Wu, X.; et al. Activation of Surface Oxygen Sites in a Cobalt-Based Perovskite Model Catalyst for CO Oxidation. J. Phys. Chem. Lett. 2018, 9, 4146–4154. [Google Scholar] [CrossRef] [PubMed]
- Najjar, H.; Batis, H. Development of Mn-based perovskite materials: Chemical structure and applications. Catal. Rev. 2016, 58, 371–438. [Google Scholar] [CrossRef]
- Tofield, B.C.; Scott, W.R. Oxidative nonstoichiometry in perovskites, an experimental survey; the defect structure of an oxidized lanthanum manganite by powder neutron diffraction. J. Solid State Chem. 1974, 10, 183–194. [Google Scholar] [CrossRef]
- Angel, S.; Tapia, J.D.; Gallego, J.; Hagemann, U.; Wiggers, H. Spray-Flame Synthesis of LaMnO3+δ Nanoparticles for Selective CO Oxidation (SELOX). Energy Fuels 2021, 35, 4367–4376. [Google Scholar] [CrossRef]
- Valderrama, G.; Kiennemann, A.; de Navarro, C.U.; Goldwasser, M.R. LaNi1−xMnxO3 perovskite-type oxides as catalysts precursors for dry reforming of methane. Appl. Catal. A Gen. 2018, 565, 26–33. [Google Scholar] [CrossRef]
- Yamazoe, N.; Teraoka, Y. Oxidation catalysis of perovskites---relationships to bulk structure and composition (valency, defect, etc.). Catal. Today 1990, 8, 175–199. [Google Scholar] [CrossRef]
- Kulandaivelu, P.; Sakthipandi, K.; Senthil Kumar, P.; Rajendran, V. Mechanical properties of bulk and nanostructured La0.61Sr0.39MnO3 perovskite manganite materials. J. Phys. Chem. Solids 2013, 74, 205–214. [Google Scholar] [CrossRef]
- Zhong, W.; Chen, W.; Ding, W.P.; Zhang, N.; Hu, A.; Du, Y.W.; Yan, Q.J. Synthesis, structure and magnetic entropy change of polycrystalline La1−xKxMnO3+δ. J. Magn. Magn. Mater. 1999, 195, 112–118. [Google Scholar] [CrossRef]
- Iliev, M.N.; Abrashev, M.V.; Lee, H.G.; Popov, V.N.; Sun, Y.Y.; Thomsen, C.; Meng, R.L.; Chu, C.W. Raman spectroscopy of orthorhombic perovskite-like YMnO3 and LaMnO3. Phys. Rev. B 1998, 57, 2872–2877. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J.; Hennion, M.; Moussa, F.; Moudden, A.H.; Pinsard, L.; Revcolevschi, A. Neutron-diffraction study of the Jahn-Teller transition in stoichiometric LaMnO3. Phys. Rev. B 1998, 57, R3189–R3192. [Google Scholar] [CrossRef]
- Taboada-Moreno, C.A.; Sánchez-De Jesús, F.; Pedro-García, F.; Cortés-Escobedo, C.A.; Betancourt-Cantera, J.A.; Ramírez-Cardona, M.; Bolarín-Miró, A.M. Large magnetocaloric effect near to room temperature in Sr doped La0.7Ca0.3MnO3. J. Magn. Magn. Mater. 2020, 496, 165887. [Google Scholar] [CrossRef]
- Megaw, H.D.; Darlington, C.N.W. Geometrical and structural relations in the rhombohedral perovskites. Acta Crystallogr. Sect. A 1975, 31, 161–173. [Google Scholar] [CrossRef]
- Wang, G.; Xu, T.; Wen, S.; Pan, M. Structure-dependent electrocatalytic activity of La1−xSrxMnO3 for oxygen reduction reaction. Sci. China Chem. 2015, 58, 871–878. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Ponraj, J.; Mansour, S.A.; Tarlochan, F. Enhancing the electrocatalytic properties of LaMnO3 by tuning surface oxygen deficiency through salt assisted combustion synthesis. Catal. Today 2021, 375, 484–493. [Google Scholar] [CrossRef]
- Norby, P.; Andersen, I.G.K.; Andersen, E.K.; Andersen, N.H. The crystal structure of lanthanum manganate (III), LaMnO3, at room temperature and at 1273 K under N2. J. Solid State Chem. 1995, 119, 191–196. [Google Scholar] [CrossRef]
- Qiu, X.; Proffen, T.; Mitchell, J.F.; Billinge, S.J.L. Orbital Correlations in the Pseudocubic O and Rhombohedral R Phases of LaMnO3. Phys. Rev. Lett. 2005, 94, 177203. [Google Scholar] [CrossRef] [PubMed]
- Megarajan, S.K.; Rayalu, S.; Nishibori, M.; Teraoka, Y.; Labhsetwar, N. Effects of Surface and Bulk Silver on PrMnO3+δ Perovskite for CO and Soot Oxidation: Experimental Evidence for the Chemical State of Silver. ACS Catal. 2015, 5, 301–309. [Google Scholar] [CrossRef]
- Sardar, K.; Lees, M.R.; Kashtiban, R.J.; Sloan, J.; Walton, R.I. Direct Hydrothermal Synthesis and Physical Properties of Rare-Earth and Yttrium Orthochromite Perovskites. Chem. Mater. 2011, 23, 48–56. [Google Scholar] [CrossRef]
- Yuan, X.; Meng, L.; Zheng, C.; Zhao, H. Deep Insight into the Mechanism of Catalytic Combustion of CO and CH4 over SrTi1−xBxO3 (B = Co, Fe, Mn, Ni, and Cu) Perovskite via Flame Spray Pyrolysis. ACS Appl. Mater. Interfaces 2021, 13, 52571–52587. [Google Scholar] [CrossRef] [PubMed]
- Kaliaguine, S.; Van Neste, A.; Szabo, V.; Gallot, J.E.; Bassir, M.; Muzychuk, R. Perovskite-type oxides synthesized by reactive grinding: Part I. Preparation and characterization. Appl. Catal. A Gen. 2001, 209, 345–358. [Google Scholar] [CrossRef]
- Acharya, S.; Mondal, J.; Ghosh, S.; Roy, S.K.; Chakrabarti, P.K. Multiferroic behavior of lanthanum orthoferrite (LaFeO3). Mater. Lett. 2010, 64, 415–418. [Google Scholar] [CrossRef]
- Li, F.; Yu, X.; Chen, L.; Pan, H.; Xin, X. Solid-State Synthesis of LaCoO3 Perovskite Nanocrystals. J. Am. Ceram. Soc. 2002, 85, 2177–2180. [Google Scholar] [CrossRef]
- Ansari, A.A.; Ahmad, N.; Alam, M.; Adil, S.F.; Ramay, S.M.; Albadri, A.; Ahmad, A.; Al-Enizi, A.M.; Alrayes, B.F.; Assal, M.E.; et al. Physico-chemical properties and catalytic activity of the sol-gel prepared Ce-ion doped LaMnO3 perovskites. Sci. Rep. 2019, 9, 7747. [Google Scholar] [CrossRef]
- Li, Y.; Xue, L.; Fan, L.; Yan, Y. The effect of citric acid to metal nitrates molar ratio on sol–gel combustion synthesis of nanocrystalline LaMnO3 powders. J. Alloys Compd. 2009, 478, 493–497. [Google Scholar] [CrossRef]
- Töpfer, J.; Goodenough, J.B. LaMnO3+δ Revisited. J. Solid State Chem. 1997, 130, 117–128. [Google Scholar] [CrossRef]
- Nilsen, O.; Rauwel, E.; Fjellvåg, H.; Kjekshus, A. Growth of La1−xCaxMnO3 thin films by atomic layer deposition. J. Mater. Chem. 2007, 17, 1466–1475. [Google Scholar] [CrossRef]
- Berger, D.; Matei, C.; Papa, F.; Macovei, D.; Fruth, V.; Deloume, J.P. Pure and doped lanthanum manganites obtained by combustion method. J. Eur. Ceram. Soc. 2007, 27, 4395–4398. [Google Scholar] [CrossRef]
- Abdallah, H.M.I.; Moyo, T. Superparamagnetic behavior of MnxNi1−xFe2O4 spinel nanoferrites. J. Magn. Magn. Mater. 2014, 361, 170–174. [Google Scholar] [CrossRef]
- Masina, P.; Moyo, T.; Abdallah, H.M.I. Synthesis, structural and magnetic properties of ZnxMg1−xFe2O4 nanoferrites. J. Magn. Magn. Mater. 2015, 381, 41–49. [Google Scholar] [CrossRef]
- Tomaszewski, P.E.; Miniajluk, N.; Zawadzki, M.; Trawczyński, J. X-ray study of structural phase transitions in nanocrystalline LaMnO3+δ perovskite. Phase Transit. 2019, 92, 525–536. [Google Scholar] [CrossRef]
- Dlamini, W.B.; Msomi, J.Z.; Moyo, T. XRD, Mössbauer and magnetic properties of MgxCo1−xFe2O4 nanoferrites. J. Magn. Magn. Mater. 2015, 373, 78–82. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Q.; He, Q.; He, T. Double-perovskites A2FeMoO6−δ (A = Ca, Sr, Ba) as anodes for solid oxide fuel cells. J. Power Sources 2010, 195, 6356–6366. [Google Scholar] [CrossRef]
- Mefford, J.T.; Hardin, W.G.; Dai, S.; Johnston, K.P.; Stevenson, K.J. Anion charge storage through oxygen intercalation in LaMnO3 perovskite pseudocapacitor electrodes. Nat. Mater. 2014, 13, 726–732. [Google Scholar] [CrossRef]
- Hammami, R.; Harrouch Batis, N.; Batis, H.; Minot, C. Cation-deficient lanthanum manganite oxides: Experimental and theoretical studies. Solid State Sci. 2009, 11, 885–893. [Google Scholar] [CrossRef]
- Huang, Q.; Santoro, A.; Lynn, J.W.; Erwin, R.W.; Borchers, J.A.; Peng, J.L.; Greene, R.L. Structure and magnetic order in undoped lanthanum manganite. Phys. Rev. B 1997, 55, 14987–14999. [Google Scholar] [CrossRef]
- Wei, Z.; Xia, T.; Ma, J.; Feng, W.; Dai, J.; Wang, Q.; Yan, P. Investigation of the lattice expansion for Ni nanoparticles. Mater. Charact. 2007, 58, 1019–1024. [Google Scholar] [CrossRef]
- Ponce, S.; Peña, M.A.; Fierro, J.L.G. Surface properties and catalytic performance in methane combustion of Sr-substituted lanthanum manganites. Appl. Catal. B Environ. 2000, 24, 193–205. [Google Scholar] [CrossRef]
- Nagabhushana, B.M.; Chakradhar, R.P.S.; Ramesh, K.P.; Shivakumara, C.; Chandrappa, G.T. Combustion synthesis, characterization and metal–insulator transition studies of nanocrystalline La1−xCaxMnO3 (0.0 ≤ x ≤ 0.5). Mater. Chem. Phys. 2007, 102, 47–52. [Google Scholar] [CrossRef]
- Sui, Z.-J.; Vradman, L.; Reizner, I.; Landau, M.V.; Herskowitz, M. Effect of preparation method and particle size on LaMnO3 performance in butane oxidation. Catal. Commun. 2011, 12, 1437–1441. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.-L.; García-González, L.; Cancino-Gordillo, F.E.; Pal, U. Particle dispersion and lattice distortion induced magnetic behavior of La1−xSrxMnO3 perovskite nanoparticles grown by salt-assisted solid-state synthesis. Mater. Chem. Phys. 2020, 246, 122834. [Google Scholar] [CrossRef]
- Symianakis, E.; Malko, D.; Ahmad, E.; Mamede, A.-S.; Paul, J.-F.; Harrison, N.; Kucernak, A. Electrochemical Characterization and Quantified Surface Termination Obtained by Low Energy Ion Scattering and X-ray Photoelectron Spectroscopy of Orthorhombic and Rhombohedral LaMnO3 Powders. J. Phys. Chem. C 2015, 119, 12209–12217. [Google Scholar] [CrossRef]
- Tran, T.H.; Bach, T.C.; Pham, N.H.; Nguyen, Q.H.; Sai, C.D.; Nguyen, H.N.; Nguyen, V.T.; Nguyen, T.T.; Ho, K.H.; Doan, Q.K. Phase transition of LaMnO3 nanoparticles prepared by microwave assisted combustion method. Mater. Sci. Semicond. Process. 2019, 89, 121–125. [Google Scholar] [CrossRef]
- Mahmood, A.; Warsi, M.F.; Ashiq, M.N.; Sher, M. Improvements in electrical and dielectric properties of substituted multiferroic LaMnO3 based nanostructures synthesized by co-precipitation method. Mater. Res. Bull. 2012, 47, 4197–4202. [Google Scholar] [CrossRef]
- Coşkun, M.; Polat, Ö.; Coşkun, F.M.; Durmuş, Z.; Çağlar, M.; Turut, A. Effect of Os doping on electrical properties of YMnO3 multiferroic perovskite-oxide compounds. Mater. Sci. Semicond. Process. 2019, 91, 281–289. [Google Scholar] [CrossRef]
- Koriba, I.; Lagoun, B.; Guibadj, A.; Belhadj, S.; Ameur, A.; Cheriet, A. Structural, electronic, magnetic and mechanical properties of three LaMnO3 phases: Theoretical investigations. Comput. Condens. Matter 2021, 29, e00592. [Google Scholar] [CrossRef]
- Moreno, L.C.; Valencia, J.S.; Landínez Téllez, D.A.; Arbey Rodríguez, M.J.; Martínez, M.L.; Roa-Rojas, J.; Fajardo, F. Preparation and structural study of LaMnO3 magnetic material. J. Magn. Magn. Mater. 2008, 320, e19–e21. [Google Scholar] [CrossRef]
- Iniama, G.; de la Presa, P.; Alonso, J.M.; Multigner, M.; Ita, B.I.; Cortés-Gil, R.; Ruiz-González, M.L.; Hernando, A.; Gonzalez-Calbet, J.M. Unexpected ferromagnetic ordering enhancement with crystallite size growth observed in La0.5Ca0.5MnO3 nanoparticles. J. Appl. Phys. 2014, 116, 113901. [Google Scholar] [CrossRef]
- Sarkar, T.; Ghosh, B.; Raychaudhuri, A.K.; Chatterji, T. Crystal structure and physical properties of half-doped manganite nanocrystals of less than 100-nm size. Phys. Rev. B 2008, 77, 235112. [Google Scholar] [CrossRef] [Green Version]






| Samples | Refinement | ||||
|---|---|---|---|---|---|
| Cell Volume (Å3) | Lattice Parameters (Å) | ||||
| Initial | Final | A | b | c | |
| LM 700 | 58.41 | 58.46 | 3.881 | 3.881 | 3.881 |
| LM 800 | 58.41 | 58.53 | 3.883 | 3.883 | 3.883 |
| LM 900 | 353.8 | 352.2 | 5.519 | 5.519 | 13.353 |
| LM 1000 | 353.2 | 353.0 | 5.524 | 5.524 | 13.358 |
| Sample | Crystallite Size (nm) | W–H Plot | Rietveld Refinement | DBET (nm) | DTEM (nm) | ||
|---|---|---|---|---|---|---|---|
| Size (nm) | Strain (%) | Size (nm) | Strain (%) | ||||
| LM 700 | 8.1 | 12.8 | 0.10 | 18.9 | 0.525 | 28 | 19 |
| LM 800 | 10.5 | 23 | 0.10 | 27.4 | 0.476 | 61 | 39 |
| LM 900 | 12.0 | 26 | 0.10 | 28.4 | 0.234 | 141 | 45 |
| LM 1000 | 15.2 | 48 | 0.02 | 62.3 | 0.034 | 459 | 90 |
| Samples | Surface Area (m2/g) | Pore Volume (cm³/g) | Pore Size (Å) |
|---|---|---|---|
| LM 700 | 33.06 | 0.2141 | 115.4 |
| LM 800 | 14.60 | 0.0441 | 143.7 |
| LM 900 | 6.635 | 0.0077 | 146.4 |
| LM 1000 | 2.195 | 0.0058 | 164.0 |
| Sample | O | Mn | La | |||
|---|---|---|---|---|---|---|
| wt.% | at.% | wt.% | at.% | wt.% | at.% | |
| LM 700 | 18.1 | 55.8 | 25.2 | 19.1 | 50.8 | 20.5 |
| LM 800 | 17.9 | 56.3 | 12.9 | 11.5 | 61.5 | 22.6 |
| LM 900 | 18.4 | 57.3 | 17.3 | 15.3 | 55.4 | 20.2 |
| LM 1000 | 14.9 | 51.0 | 20.0 | 20.2 | 59.5 | 23.7 |
| Samples | Sheet Resistance | ρ (kΩ cm) |
|---|---|---|
| LM 700 | 18.64 MΩ | 437.7 |
| LM 800 | 18.47 MΩ | 435.3 |
| LM 900 | 20.91 kΩ | 4.890 |
| LM 1000 | 3.665 kΩ | 0.894 |
| Cut-Off (eV) | Total Energy (eV) | Cell Volume Å3 | Final Energy (eV) | BFGS Final Enthalpy (×103 eV) | BFGS Bulk Modulus (GPa) | Lattice Parameters (Å) | Final Pressure | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Mn | La | a | b | c | ||||||
| 450 | −428.03 | −639.99 | −855.83 | Initial: 58.41 Final: 61.69 | −2821.51 | −2.8215 | 500 | Initial: 3.88 Final: 3.95 | Initial: 3.88 Final: 3.95 | Initial: 3.88 Final: 3.95 | 0.0892 |
| 650 | −428.09 | −640.01 | −855.85 | Initial: 58.41 Final: 61.72 | −2821.69 | −2.8217 | 500 | Initial: 3.88 Final: 3.95 | Initial: 3.88 Final: 3.95 | Initial: 3.88 Final: 3.95 | 0.0730 |
| 800 | −428.10 | −640.02 | −855.85 | Initial: 58.41 Final: 61.84 | −2821.73 | −2.8217 | 500 | Initial: 3.95 Final: 3.95 | Initial: 3.95 Final: 3.95 | Initial: 3.95 Final: 3.95 | 0.0852 |
| Cut-Off (eV) | Total Energy (eV) | Cell Vol (Å3) | Final Energy (eV) | BFGS Final Enthalpy (×104 eV) | BFGS Bulk Modulus (GPa) | Lattice Parameters (Å) | Final Pressure | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Mn | La | a | b | c | ||||||
| 450 | −430.67 | −612.74 | −967.81 | Initial: 354.03 Final: 365.05 | −17504.76 | −1.7505 | 179.37 | Initial: 5.53 Final: 5.55 | Initial: 5.53 Final: 5.55 | Initial: 13.4 Final: 13.39 | −0.0160 |
| 650 | −431.67 | −612.76 | −967.82 | Initial: 354.03 Final: 353.72 | −17515.00 | −1.7515 | 257.74 | Initial: 5.53 Final: 5.55 | Initial: 5.53 Final: 5.55 | Initial: 13.4 Final: 13.28 | 0.0053 |
| 800 | −431.75 | −612.84 | −970.07 | Initial: 354.03 Final: 353.20 | −17514.99 | −1.7515 | 76.78 | Initial: 5.53 Final: 5.54 | Initial: 5.53 Final: 5.54 | Initial: 13.4 Final: 13.28 | 0.0614 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anyanwu, V.O.; Friedrich, H.B.; Mahomed, A.S.; Singh, S.; Moyo, T. Phase Transition of High-Surface-Area Glycol–Thermal Synthesized Lanthanum Manganite. Materials 2023, 16, 1274. https://doi.org/10.3390/ma16031274
Anyanwu VO, Friedrich HB, Mahomed AS, Singh S, Moyo T. Phase Transition of High-Surface-Area Glycol–Thermal Synthesized Lanthanum Manganite. Materials. 2023; 16(3):1274. https://doi.org/10.3390/ma16031274
Chicago/Turabian StyleAnyanwu, Victor O., Holger B. Friedrich, Abdul S. Mahomed, Sooboo Singh, and Thomas Moyo. 2023. "Phase Transition of High-Surface-Area Glycol–Thermal Synthesized Lanthanum Manganite" Materials 16, no. 3: 1274. https://doi.org/10.3390/ma16031274





