Functionalized Fullerenes and Their Applications in Electrochemistry, Solar Cells, and Nanoelectronics
Abstract
:1. Introduction
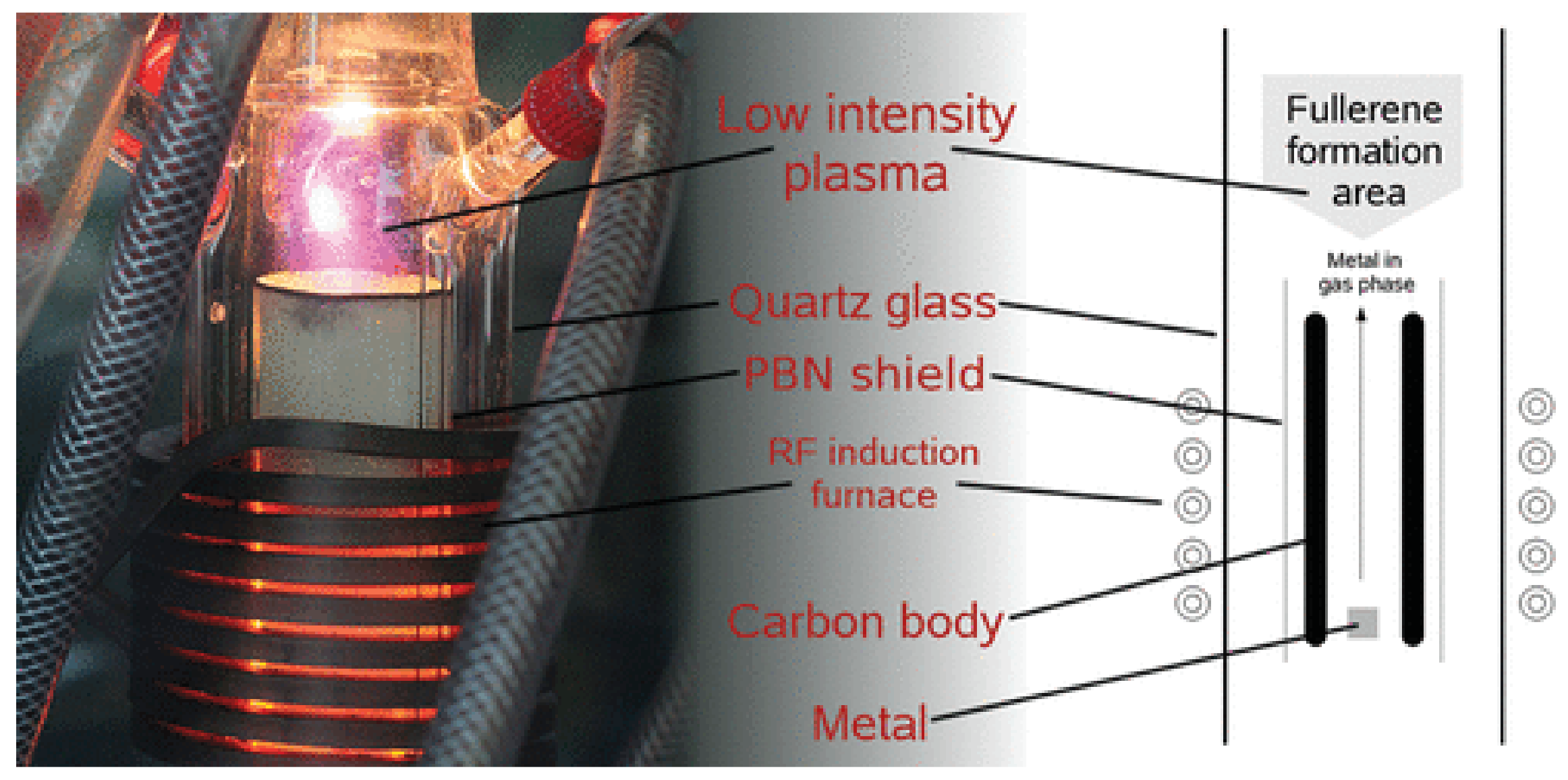
2. The Methods of Synthesis of Fullerenes
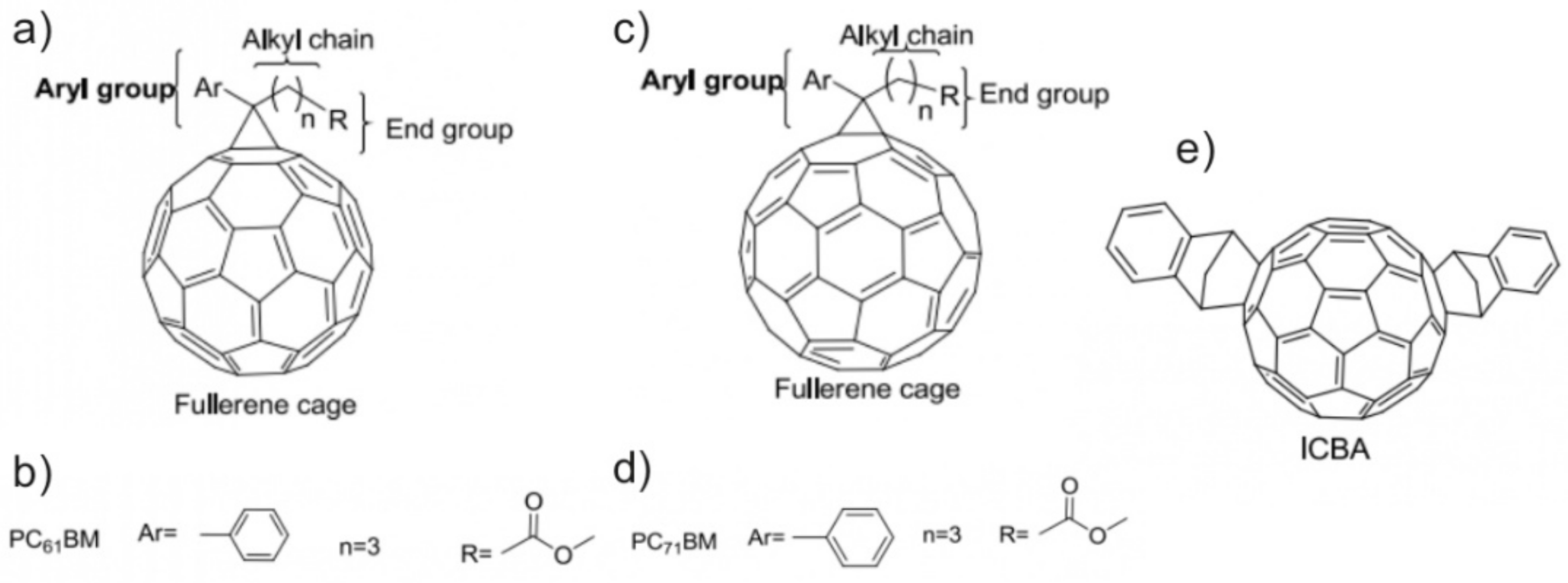
3. The Methods of Functionalization of Fullerenes

4. Applications in Electrochemistry
5. Application of Fullerenes in Solar Cells
6. Applications in Nanoelectronics
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, D.E.H. New Scientist; Freeman: Oxford, UK, 1966; p. 245. [Google Scholar]
- Osawa, E. Superaromaticity. Kagaku 1970, 25, 854–863. [Google Scholar]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Lange, H.; Huczko, A.; Byszewski, P.; Mizera, E.; Shinohara, H. Influence of Boron on Carbon Arc Plasma and Formation of Fullerenes and Nanotubes. Chem. Phys. Lett. 1998, 289, 174–180. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A New Form of Carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Sugai, T.; Omote, H.; Bandow, S.; Tanaka, N.; Shinohara, H. Production of Fullerenes and Single-Wall Carbon Nanotubes by High-Temperature Pulsed Arc Discharge. J. Chem. Phys 2000, 112, 6000–6005. [Google Scholar] [CrossRef]
- Johnson, R.D.; de Vries, M.S.; Salem, J.; Bethune, D.S.; Yannoni, C.S. Electron Paramagnetic Resonance Studies of Lanthanum-Containing C82. Nature 1992, 355, 239–240. [Google Scholar] [CrossRef]
- Alvarez, M.M.; Gillan, E.G.; Holczer, K.; Kaner, R.B.; Min, K.S.; Whetten, R.L. La2C80: A Soluble Dimetallofullerene. J. Phys. Chem. 1991, 95, 10561–10563. [Google Scholar] [CrossRef]
- Stevenson, S.; Mackey, M.A.; Thompson, M.C.; Coumbe, H.L.; Madasu, P.K.; Coumbe, C.E.; Phillips, J.P. Effect of Copper Metal on the Yield of Sc3N@C80 Metallofullerenes. Chem. Commun. 2007, 4263–4265. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, M.M.; de Bettencourt-Dias, A.; Duchamp, J.C.; Stevenson, S.; Dorn, H.C.; Balch, A.L. Isolation and Crystallographic Characterization of ErSc2N@C80: An Endohedral Fullerene Which Crystallizes with Remarkable Internal Order. J. Am. Chem. Soc. 2000, 122, 12220–12226. [Google Scholar] [CrossRef]
- Beavers, C.M.; Zuo, T.; Duchamp, J.C.; Harich, K.; Dorn, H.C.; Olmstead, M.M.; Balch, A.L. Tb3N@C84: An Improbable, Egg-Shaped Endohedral Fullerene That Violates the Isolated Pentagon Rule. J. Am. Chem. Soc. 2006, 128, 11352–11353. [Google Scholar] [CrossRef]
- A Scandium Carbide Endohedral Metallofullerene: (Sc2C2)@C84. Available online: https://www.researchgate.net/publication/229979823_A_Scandium_Carbide_Endohedral_Metallofullerene_Sc2C2C84 (accessed on 15 September 2022).
- Stevenson, S.; Rice, G.; Glass, T.; Harlch, K.; Cromer, F.; Jordan, M.R.; Craft, J.; Hadju, E.; Bible, R.; Olmstead, M.M.; et al. Small-Bandgap Endohedral Metallofullerenes in High Yield and Purity. Nature 1999, 401, 55–57. [Google Scholar] [CrossRef]
- Yang, S.; Popov, A.A.; Dunsch, L. The Role of an Asymmetric Nitride Cluster on a Fullerene Cage: The Non-IPR Endohedral DySc2N@C76. J. Phys. Chem. B 2007, 111, 13659–13663. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Dunsch, L. Gadolinium Nitride Gd3N in Carbon Cages: The Influence of Cluster Size and Bond Strength. Angew. Chem. Int. Ed. 2005, 44, 1557–1560. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Müller, K.H.; Skourski, Y.; Eckert, D.; Georgi, P.; Krause, M.; Dunsch, L. Magnetic Moments of the Endohedral Cluster Fullerenes Ho3N@C80 and Tb3N@C80: The Role of Ligand Fields. Angew. Chem. Int. Ed. 2005, 44, 3306–3309. [Google Scholar] [CrossRef]
- Chen, C.; Liu, F.; Li, S.; Wang, N.; Popov, A.A.; Jiao, M.; Wei, T.; Li, Q.; Dunsch, L.; Yang, S. Titanium/Yttrium Mixed Metal Nitride Clusterfullerene TiY2N@C80: Synthesis, Isolation, and Effect of the Group-III Metal. Inorg. Chem. 2012, 51, 3039–3045. [Google Scholar] [CrossRef]
- MacFarlane, R.M.; Bethune, D.; Stevenson, S.; Dorn, H.C. Fluorescence Spectroscopy and Emission Lifetimes of Er3+ in ErxSc3-XN@C80 (X = 1–3). Chem. Phys. Lett. 2001, 343, 229–234. [Google Scholar] [CrossRef]
- Mordkovich, V.Z.; Shiratori, Y.; Hiraoka, H.; Takeuchi, Y. Synthesis of Multishell Fullerenes by Laser Vaporization of Composite Carbon Targets. Phys. Solid State 2002, 44, 603–606. [Google Scholar] [CrossRef]
- Peters, G.; Jansen, M. A New Fullerene Synthesis. Angew. Chem. Int. Ed. Engl. 1992, 31, 223–224. [Google Scholar] [CrossRef]
- Ata, M.; Huang, H.; Akasaka, T. Nitrogen Radio Frequency Plasma Processing of Fullerenes. J. Phys. Chem. B 2004, 108, 4640–4646. [Google Scholar] [CrossRef]
- Krokos, E. Plasma Coupled Radio Frequency Furnace: The Synthesis, Separation, and Elucidation of the Elusive Sc4C82 Fullerene. J. Phys. Chem. C 2010, 114, 7626–7630. [Google Scholar] [CrossRef]
- Kaneko, T.; Abe, S.; Ishida, H.; Hatakeyama, R. An Electron Cyclotron Resonance Plasma Configuration for Increasing the Efficiency in the Yield of Nitrogen Endohedral Fullerenes. Phys. Plasmas 2007, 14, 110705. [Google Scholar] [CrossRef]
- Jansen, M.; Peters, G.; Wagner, N. Zur Bildung von Fullerenen Und Endohedralen Metallofullerenen: Darstellung Im Hochfrequenzofen. Z. Anorg. Allg. Chem. 1995, 621, 689–693. [Google Scholar] [CrossRef]
- Duan, S.; Liu, X.; Wang, Y.; Shao, D.; Meng, Y.; Hayat, T.; Alsaedi, A.; Li, J. Formation of C 60 Fullerene-Bonded-CNTs Using Radio Frequency Plasma. RSC Adv. 2017, 7, 21124–21127. [Google Scholar] [CrossRef]
- Barth, W.E.; Lawton, R.G. Dibenzo[Ghi,Mno]Fluoranthene. J. Am. Chem. Soc. 1966, 88, 380–381. [Google Scholar] [CrossRef]
- Sygula, A.; Rabideau, P.W. A Practical, Large Scale Synthesis of the Corannulene System. J. Am. Chem. Soc. 2000, 122, 6323–6324. [Google Scholar] [CrossRef]
- Scott, L.T. Methods for the Chemical Synthesis of Fullerenes. Angew. Chem. Int. Ed. 2004, 43, 4994–5007. [Google Scholar] [CrossRef]
- Plater, M.J. Fullerene Tectonics. Part 1. A Programmed Precursor to C60. J. Chem. Soc. Perkin 1 1997, 2897–2901. [Google Scholar] [CrossRef]
- Chibante, L.P.F.; Thess, A.; Alford, J.M.; Diener, M.D.; Smalley, R.E. Solar Generation of the Fullerenes. J. Phys. Chem. 1993, 97, 8696–8700. [Google Scholar] [CrossRef]
- Takehara, H.; Fujiwara, M.; Arikawa, M.; Diener, M.D.; Alford, J.M. Experimental Study of Industrial Scale Fullerene Production by Combustion Synthesis. Carbon 2005, 43, 311–319. [Google Scholar] [CrossRef]
- Howard, J.B.; McKinnon, J.T.; Makarovsky, Y.; Lafleur, A.L.; Johnson, M.E. Fullerenes C60 and C70 in Flames. Nature 1991, 352, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; O’Brien, S.C.; Heath, J.R.; Liu, Y.; Curl, R.F.; Kroto, H.W.; Smalley, R.E. Reactivity of Large Carbon Clusters: Spheroidal Carbon Shells and Their Possible Relevance to the Formation and Morphology of Soot. J. Phys. Chem. 1986, 90, 525–528. [Google Scholar] [CrossRef]
- McElvany, S.W.; Ross, M.M.; Goroff, N.S.; Diederich, F. Cyclocarbon Coalescence: Mechanisms for Tailor-Made Fullerene Formation. Science 1993, 259, 1594–1596. [Google Scholar] [CrossRef]
- Lozovik, Y.E.; Popov, A.M.; Belikov, A.V. Classification of Two-Shell Nanotubes with Commensurate Structures of Shells. Phys. Solid State 2003, 45, 1396–1402. [Google Scholar] [CrossRef]
- Askhabov, A.M. The Kvataron Model of Fullerene Formation. Phys. Solid State 2005, 47, 1186–1190. [Google Scholar] [CrossRef]
- Irle, S.; Zheng, G.; Wang, Z.; Morokuma, K. The C60 Formation Puzzle “Solved”: QM/MD Simulations Reveal the Shrinking Hot Giant Road of the Dynamic Fullerene Self-Assembly Mechanism. J. Phys. Chem. B 2006, 110, 14531–14545. [Google Scholar] [CrossRef] [PubMed]
- Chuvilin, A.; Kaiser, U.; Bichoutskaia, E.; Besley, N.A.; Khlobystov, A.N. Direct Transformation of Graphene to Fullerene. Nat. Chem. 2010, 2, 450–453. [Google Scholar] [CrossRef]
- Lungerich, D.; Hoelzel, H.; Harano, K.; Jux, N.; Amsharov, K.Y.; Nakamura, E. A Singular Molecule-to-Molecule Transformation on Video: The Bottom-Up Synthesis of Fullerene C60 from Truxene Derivative C60H30. ACS Nano. 2021, 15, 12804–12814. [Google Scholar] [CrossRef]
- Bartolomeo, D.; Kharlamova, M.V.; Burdanova, M.G.; Paukov, M.I.; Kramberger, C. Synthesis, Sorting, and Applications of Single-Chirality Single-Walled Carbon Nanotubes. Materials 2022, 15, 5898. [Google Scholar] [CrossRef]
- Ajie, H.; Alvarez, M.M.; Anz, S.J.; Beck, R.D.; Diederich, F.; Fostiropoulos, K.; Huffman, D.R.; Krätschmer, W.; Rubin, Y.; Schriver, K.E.; et al. Characterization of the Soluble All-Carbon Molecules C60 and C70. J. Phys. Chem. 1990, 94, 8630–8633. [Google Scholar] [CrossRef]
- Saito, Y.; Ohta, H.; Jinno, K. Peer Reviewed: Chromatographic Separation of Fullerenes. Anal. Chem. 2004, 76, 266. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Paukov, M.; Burdanova, M.G. Nanotube Functionalization: Investigation, Methods and Demonstrated Applications. Materials 2022, 15, 5386. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, S.; Alargova, R.G.; Tsujii, K. Stable Dispersions of Fullerenes, C60 and C70, in Water. Preparation and Characterization. Langmuir 2001, 17, 6013–6017. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Jin, Z.; Gu, Z.; Wu, Y.; Sun, Y. Solubility of Fullerene C60 and C70 in Toluene, o-Xylene and Carbon Disulfide at Various Temperatures. Full Sci. Technol. 2007, 5, 285–290. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Malhotra, R.; Huestis, D.L.; Tse, D.S.; Lorents, D.C. Anomalous Solubility Behaviour of C60. Nature 1993, 362, 140–141. [Google Scholar] [CrossRef]
- Sawamura, S.; Fujita, N. High-Pressure Solubility of Fullerene C60 in Toluene. Carbon 2007, 45, 965–970. [Google Scholar] [CrossRef]
- Teh, S.L.; Linton, D.; Sumpter, B.; Dadmun, M.D. Controlling Non-Covalent Interactions to Modulate the Dispersion of Fullerenes in Polymer Nanocomposites. Macromolecules 2011, 44, 7737–7745. [Google Scholar] [CrossRef]
- Li, F.; Yager, K.G.; Dawson, N.M.; Jiang, Y.B.; Malloy, K.J.; Qin, Y. Stable and Controllable Polymer/Fullerene Composite Nanofibers through Cooperative Noncovalent Interactions for Organic Photovoltaics. Chem. Mater. 2014, 26, 3747–3756. [Google Scholar] [CrossRef]
- Reddy, B.K.; Gadekar, S.C.; Anand, V.G. Non-Covalent Composites of Antiaromatic Isophlorin–Fullerene. Chem. Commun. 2015, 51, 8276–8279. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.M.; Posa, M.; Srdjenovic, B.; Simplício, A.L. Solubilization of Fullerene C60 in Micellar Solutions of Different Solubilizers. Colloids Surf. B Biointerfaces 2011, 82, 46–53. [Google Scholar] [CrossRef]
- Deng, J.P.; Mou, C.Y.; Han, C.C. Oxidation of Fullerenes by Ozone. Full Sci. Technol. 2006, 5, 1325–1336. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Z.; Zhao, Y.; Gao, X. Oxidation-Induced Water-Solubilization and Chemical Functionalization of Fullerenes C60, Gd@C60 and Gd@C82: Atomistic Insights into the Formation Mechanisms and Structures of Fullerenols Synthesized by Different Methods. Nanoscale 2015, 7, 2914–2925. [Google Scholar] [CrossRef] [PubMed]
- Zygouri, P.; Spyrou, K.; Mitsari, E.; Barrio, M.; Macovez, R.; Patila, M.; Stamatis, H.; Verginadis, I.I.; Velalopoulou, A.P.; Evangelou, A.M.; et al. A Facile Approach to Hydrophilic Oxidized Fullerenes and Their Derivatives as Cytotoxic Agents and Supports for Nanobiocatalytic Systems. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Haufler, R.E.; Conceicao, J.; Chibante, L.P.F.; Chai, Y.; Byrne, N.E.; Flanagan, S.; Haley, M.M.; O’Brien, S.C.; Pan, C.; Xiao, Z.; et al. Efficient Production of C60 (Buckminsterfullerene), C60H36, and the Solvated Buckide Ion. J. Phys. Chem. 1990, 94, 8634–8636. [Google Scholar] [CrossRef]
- Shigematsu, K.; Abe, K.; Mitani, M.; Tanaka, K. Catalytic Hydrogenation of Fullerenes in the Presence of Metal Catalysts in Toluene Solution. Full Sci. Technol. 2006, 1, 309–318. [Google Scholar] [CrossRef]
- Darwish, A.D.; Abdul-Sada, A.K.; Langley, G.J.; Kroto, H.W.; Taylor, R.; Walton, D.R.M. Polyhydrogenation of [60]- and [70]-Fullerenes. J. Chem. Soc. Perkin Trans. 2 1995, 2359–2365. [Google Scholar] [CrossRef]
- Cataldo, F.; Iglesias-Groth, S.; Manchado, A. Isotope Effect in the UV Photolysis of Hydrogenated and Perdeuterated Fulleranes. Fulleranes: Hydrog. Fuller. 2010, 2, 149–170. [Google Scholar] [CrossRef]
- Tayfun, U.; Kanbur, Y.; Abaci, U.; Guney, H.Y.; Bayramli, E. Mechanical, Flow and Electrical Properties of Thermoplastic Polyurethane/Fullerene Composites: Effect of Surface Modification of Fullerene. Compos. B Eng. 2015, 80, 101–107. [Google Scholar] [CrossRef]
- Shan, C.; Yen, H.J.; Wu, K.; Lin, Q.; Zhou, M.; Guo, X.; Wu, D.; Zhang, H.; Wu, G.; Wang, H.L. Functionalized Fullerenes for Highly Efficient Lithium Ion Storage: Structure-Property-Performance Correlation with Energy Implications. Nano Energy 2017, 40, 327–335. [Google Scholar] [CrossRef]
- Lee, K.C.; Chen, Y.L.; Wang, C.C.; Huang, J.H.; Cho, E.C. Refluxed Esterification of Fullerene-Conjugated P25 TiO 2 Promotes Free Radical Scavenging Capacity and Facilitates Antiaging Potentials in Human Cells. ACS Appl. Mater. Interfaces 2019, 11, 311–319. [Google Scholar] [CrossRef]
- Zhang, W.B.; He, J.; Dong, X.; Wang, C.L.; Li, H.; Teng, F.; Li, X.; Wesdemiotis, C.; Quirk, R.P.; Cheng, S.Z.D. Improved Synthesis of Fullerynes by Fisher Esterification for Modular and Efficient Construction of Fullerene Polymers with High Fullerene Functionality. Polymers 2011, 52, 4221–4226. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, H.; Wang, Y.X.; Dong, B.W.; Sun, B.Y.; Jiang, S.; da Gao, S. Amination of the Gd@C 82 Endohedral Fullerene: Tunable Substitution Effect on Quantum Coherence Behaviors. Chem. Sci. 2020, 11, 10737–10743. [Google Scholar] [CrossRef]
- Ohno, M.; Kojima, S.; Shirakawa, Y.; Eguchi, S. Hetero-Diels-Alder Reaction of Fullerene: Synthesis of Thiochroman-Fused C60 with o-Thioquinone Methide and Oxidation to Its S-Oxides. Tetrahedron Lett. 1995, 36, 6899–6902. [Google Scholar] [CrossRef]
- Ohno, M.; Kojima, S.; Eguchi, S. Dihydrothiopyran-Fused [60]Fullerene from Hetero-Diels–Alder Reaction with Thioacrylamide and Acyl Chloride. J. Chem. Soc. Chem. Commun. 1995, 565–566. [Google Scholar] [CrossRef]
- Khakina, E.A.; Peregudov, A.S.; Yurkova, A.A.; Piven, N.P.; Shestakov, A.F.; Troshin, P.A. Unusual Multistep Reaction of C70Cl10 with Thiols Producing C70[SR]5H. Tetrahedron Lett. 2016, 57, 1215–1219. [Google Scholar] [CrossRef]
- Khakina, E.A.; Yurkova, A.A.; Peregudov, A.S.; Troyanov, S.I.; Trush, V.V.; Vovk, A.I.; Mumyatov, A.V.; Martynenko, V.M.; Balzarini, J.; Troshin, P.A. Highly Selective Reactions of C60Cl6 with Thiols for the Synthesis of Functionalized [60]Fullerene Derivatives. Chem. Commun. 2012, 48, 7158–7160. [Google Scholar] [CrossRef]
- Iskin, B.; Yilmaz, G.; Yagci, Y. Mono-Addition Synthesis of Polystyrene-Fullerene (C60) Conjugates by Thiol-Ene Chemistry. Chemistry 2012, 18, 10254–10257. [Google Scholar] [CrossRef] [PubMed]
- Kniaź, K.; Fischer, J.E.; Kniaź, K.; Fischer, J.E.; Vaughan, G.B.M.; Romanow, W.J.; McCauley, J.P.; Strongin, R.M.; Smith, A.B.; Selig, H.; et al. Fluorinated Fullerenes: Synthesis, Structure, and Properties. J. Am. Chem. Soc. 1993, 115, 6060–6064. [Google Scholar] [CrossRef]
- Troshin, P.A.; Avent, A.G.; Darwish, A.D.; Martsinovich, N.; Abdul-Sada, A.K.; Street, J.M.; Taylor, R. Isolation of Two Seven-Membered Ring C58 Fullerene Derivatives: C58F17CF3 and C58F18. Science 2005, 309, 278–281. [Google Scholar] [CrossRef]
- Troshin, P.A.; Popkov, O.; Lyubovskaya, R.N. Some New Aspects of Chlorination of Fullerenes. Full Nanotub. Carbon Nanostructures 2007, 11, 165–185. [Google Scholar] [CrossRef]
- Troyanov, S.I.; Shustova, N.B.; Popov, A.A.; Sidorov, L.N. Synthesis and Structures of C60 Fullerene Chlorides. Russ. Chem. Bull. 2005, 54, 1656–1666. [Google Scholar] [CrossRef]
- Shustova, N.B.; Popov, A.A.; Sidorov, L.N.; Turnbull, A.P.; Kemnitz, E.; Troyanov, S.I. Preparation and Crystallographic Characterization of C60Cl24. Chem. Commun. 2005, 1411–1413. [Google Scholar] [CrossRef]
- Troshina, O.A.; Troshin, P.A.; Peregudov, A.S.; Kozlovskiy, V.I.; Balzarini, J.; Lyubovskaya, R.N. Chlorofullerene C60Cl6: A Precursor for Straightforward Preparation of Highly Water-Soluble Polycarboxylic Fullerene Derivatives Active against HIV. Org. Biomol. Chem. 2007, 5, 2783–2791. [Google Scholar] [CrossRef] [PubMed]
- Birkett, P.R.; Avent, A.G.; Darwish, A.D.; Kroto, H.W.; Taylor, R.; Walton, D.R.M. Formation and Characterisation of C70Cl10. J. Chem. Soc. Chem. Commun 1995, 683–684. [Google Scholar] [CrossRef]
- Troshin, P.A.; Kolesnikov, D.; Burtsev, A.V.; Lubovskaya, R.N.; Denisenko, N.I.; Popov, A.A.; Troyanov, S.I.; Boltalina, O.V. Bromination of [60]Fullerene. I. High-Yield Synthesis of C60Br x (X = 6, 8, 24). Full Nanotub. Carbon Nanostruct. 2011, 11, 47–60. [Google Scholar] [CrossRef]
- Birkett, P.R.; Hitchcock, P.B.; Kroto, H.W.; Taylor, R.; Walton, D.R.M. Preparation and Characterization of C60Br6 and C60Br8. Nature 1992, 357, 479–481. [Google Scholar] [CrossRef]
- Marastonı, M.; Salvadorı, S.; Balbonı, G.; Spısanı, S.; Gavıolı, R.; Tranıello, S.; Tomatıs, R. Synthesis, Metabolic Stability and Chemotactic Activity of Peptide T and Its Analogues. Int. J. Pept. Protein Res. 1990, 35, 81–88. [Google Scholar] [CrossRef]
- Yang, X.; Ebrahimi, A.; Li, J.; Cui, Q. Fullerene-Biomolecule Conjugates and Their Biomedicinal Applications. Int. J. Nanomed. 2013, 9, 77–92. [Google Scholar] [CrossRef]
- Toniolo, C.; Bianco, A.; Maggini, M.; Scorrano, G.; Prato, M.; Marastoni, M.; Tomatis, R.; Spisani, S.; Palú, G.; Blair, E.D. A Bioactive Fullerene Peptide. J. Med. Chem. 1994, 37, 4558–4562. [Google Scholar] [CrossRef]
- Bianco, A.; Ros, T.; da Prato, M.; Toniolo, C. Fullerene-Based Amino Acids and Peptides. J. Pept. Sci. 2001, 7, 208–219. [Google Scholar] [CrossRef]
- Yang, J.; Alemany, L.B.; Driver, J.; Hartgerink, J.D.; Barron, A.R. Fullerene-Derivatized Amino Acids: Synthesis, Characterization, Antioxidant Properties, and Solid-Phase Peptide Synthesis. Chemistry 2007, 13, 2530–2545. [Google Scholar] [CrossRef]
- Loutfy, R.O.; Katagiri, S. Fullerene Materials for Lithium-Ion Battery Applications. Perspect. Fuller. Nanotechnol. 2002, 357–367. [Google Scholar] [CrossRef]
- Mulet-Gas, M.; Rodríguez-Fortea, A.; Echegoyen, L.; Poblet, J.M. Relevance of Thermal Effects in the Formation of Endohedral Metallofullerenes: The Case of Gd3N@ C s(39663)-C 82 and Other Related Systems. Inorg. Chem. 2013, 52, 1954–1959. [Google Scholar] [CrossRef] [PubMed]
- Beavers, C.M.; Chaur, M.N.; Olmstead, M.M.; Echegoyen, L.; Balch, A.L. Large Metal Ions in a Relatively Small Fullerene Cage: The Structure of Gd3N@C2(22010)-C78 Departs from the Isolated Pentagon Rule. J. Am. Chem. Soc. 2009, 131, 11519–11524. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Walker, K.; Olmstead, M.M.; Melin, F.; Holloway, B.C.; Echegoyen, L.; Dorn, H.C.; Chaur, M.N.; Chancellor, C.J.; Beavers, C.M.; et al. New Egg-Shaped Fullerenes: Non-Isolated Pentagon Structures of Tm3N@Cs(51 365)-C84 and Gd3N@Cs(51 365)-C84. Chem. Commun. 2008, 9, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Yumura, T.; Sato, Y.; Suenaga, K.; Iijima, S. Which Do Endohedral Ti2C80 Metallofullerenes Prefer Energetically: Ti2@C80 or Ti2C 2@C78? A Theoretical Study. J. Phys. Chem. B 2005, 109, 20251–20255. [Google Scholar] [CrossRef]
- Chen, C.H.; Abella, L.; Cerón, M.R.; Guerrero-Ayala, M.A.; Rodríguez-Fortea, A.; Olmstead, M.M.; Powers, X.B.; Balch, A.L.; Poblet, J.M.; Echegoyen, L. Zigzag Sc2C2 Carbide Cluster inside a [88]Fullerene Cage with One Heptagon, Sc2C2@Cs(Hept)-C88: A Kinetically Trapped Fullerene Formed by C2 Insertion? J. Am. Chem. Soc. 2016, 138, 13030–13037. [Google Scholar] [CrossRef]
- Melin, F.; Chaur, M.N.; Engmann, S.; Elliott, B.; Kumbhar, A.; Athans, A.J.; Echegoyen, L. The Large Nd3N@C2n (40≤n≤49) Cluster Fullerene Family: Preferential Templating of a C88 Cage by a Trimetallic Nitride Cluster. Angew. Chem. Int. Ed. 2007, 46, 9032–9035. [Google Scholar] [CrossRef]
- Yang, Y.; Arias, F.; Echegoyen, L.; Felipe Chibante, L.P.; Flanagan, S.; Robertson, A.; Wilson, L.J. Reversible Fullerene Electrochemistry: Correlation with the HOMO-LUMO Energy Difference for C60, C70, C76, C78, and C84. J. Am. Chem. Soc. 1995, 117, 7801–7804. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nagase, S. Structures of the Ca@C82 Isomers: A Theoretical Prediction. Chem. Phys. Lett. 1997, 274, 226–230. [Google Scholar] [CrossRef]
- Xu, W.; Hao, Y.; Uhlik, F.; Shi, Z.; Slanina, Z.; Feng, L. Structural and Electrochemical Studies of Sm@D3h-C74 Reveal a Weak Metal–Cage Interaction and a Small Band Gap Species. Nanoscale 2013, 5, 10409–10413. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Hao, C.; Shi, Z.; Gu, Z. Synthesis, Isolation, Spectroscopic and Electrochemical Characterization of Some Calcium-Containing Metallofullerenes. Carbon 2006, 44, 475–479. [Google Scholar] [CrossRef]
- Lu, X.; Slanina, Z.; Akasaka, T.; Tsuchiya, T.; Mizorogi, N.; Nagase, S. Yb@C2n (n = 40, 41, 42): New Fullerene Allotropes with Unexplored Electrochemical Properties. J. Am. Chem. Soc. 2010, 132, 5896–5905. [Google Scholar] [CrossRef] [PubMed]
- Cardona, C.M.; Kitaygorodskiy, A.; Echegoyen, L. Trimetallic Nitride Endohedral Metallofullerenes: Reactivity Dictated by the Encapsulated Metal Cluster. J. Am. Chem. Soc. 2005, 127, 10448–10453. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Wakahara, T.; Lian, Y.; Tsuchiya, T.; Akasaka, T.; Waelchli, M.; Mizorogi, N.; Nagase, S.; Kadish, K.M. Analysis of Lanthanide-Induced NMR Shifts of the Ce@C82 Anion. J. Am. Chem. Soc. 2006, 128, 1400–1401. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, M.M.; de Bettencourt-Dias, A.; Stevenson, S.; Dorn, H.C.; Balch, A.L. Crystallographic Characterization of the Structure of the Endohedral Fullerene {Er2@C82 Isomer I} with Cs Cage Symmetry and Multiple Sites for Erbium along a Band of Ten Contiguous Hexagons. J. Am. Chem. Soc. 2002, 124, 4172–4173. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Hu, J.; Cui, P.; Jiang, L.; Chen, Z.; Zhang, Q.; Wang, C.; Luo, Y. Visible-Light Photoexcited Electron Dynamics of Scandium Endohedral Metallofullerenes: The Cage Symmetry and Substituent Effects. J. Am. Chem. Soc. 2015, 137, 8769–8774. [Google Scholar] [CrossRef]
- Olmstead, M.M.; Balch, A.L.; Pinzón, J.R.; Echegoyen, L.; Gibson, H.W.; Dorn, H.C. New Endohedral Metallofullerenes: Trimetallic Nitride Endohedral Fullerenes. Chem. Nanocarbons 2010, 239–259. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, J.; Wei, T.; Chen, X.; Yang, Q.; Yang, S. Micron-Sized Hexagonal Single-Crystalline Rods of Metal Nitride Clusterfullerene: Preparation, Characterization, and Photoelectrochemical Application. Nanoscale 2013, 5, 1993–2001. [Google Scholar] [CrossRef]
- Popov, A.A.; Dunsch, L. Structure, Stability, and Cluster-Cage Interactions in Nitride Clusterfullerenes M3N@C2n (M = Sc, Y; 2n = 68–98): A Density Functional Theory Study. J. Am. Chem. Soc. 2007, 129, 11835–11849. [Google Scholar] [CrossRef]
- Chen, N.; Mulet-Gas, M.; Li, Y.Y.; Stene, R.E.; Atherton, C.W.; Rodríguez-Fortea, A.; Poblet, J.M.; Echegoyen, L. Sc2S@C2(7892)–C70: A Metallic Sulfide Cluster inside a Non-IPR C70 Cage. Chem. Sci. 2012, 4, 180–186. [Google Scholar] [CrossRef]
- Zhang, M.; Hao, Y.; Li, X.; Feng, L.; Yang, T.; Wan, Y.; Chen, N.; Slanina, Z.; Uhlík, F.; Cong, H. Facile Synthesis of an Extensive Family of Sc2O@C2 n (n = 35–47) and Chemical Insight into the Smallest Member of Sc2O@ C 2(7892)-C70. J. Phys. Chem. C 2014, 118, 28883–28889. [Google Scholar] [CrossRef]
- Aoyagi, S.; Nishibori, E.; Sawa, H.; Sugimoto, K.; Takata, M.; Miyata, Y.; Kitaura, R.; Shinohara, H.; Okada, H.; Sakai, T.; et al. A Layered Ionic Crystal of Polar Li@C60 Superatoms. Nat. Chem. 2010, 2, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Feng, L.; Xu, W.; Gu, Z.; Hu, Z.; Shi, Z.; Slanina, Z.; Uhlík, F. Sm at C2v(19138)-C76: A Non-IPR Cage Stabilized by A Divalent Metal Ion. Inorg. Chem. 2015, 54, 4243–4248. [Google Scholar] [CrossRef]
- Hu, Z.; Hao, Y.; Slanina, Z.; Gu, Z.; Shi, Z.; Uhlík, F.; Zhao, Y.; Feng, L. Popular C82 Fullerene Cage Encapsulating a Divalent Metal Ion Sm2+: Structure and Electrochemistry. Inorg. Chem. 2015, 54, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Kuran, P.; Krause, M.; Bartl, A.; Dunsch, L. Preparation, Isolation and Characterisation of Eu@C74: The First Isolated Europium Endohedral Fullerene. Chem. Phys. Lett. 1998, 292, 580–586. [Google Scholar] [CrossRef]
- Sun, B.; Li, M.; Luo, H.; Shi, Z.; Gu, Z. Electrochemical Properties of Metallofullerenes and Their Anions. Electrochim. Acta 2002, 47, 3545–3549. [Google Scholar] [CrossRef]
- Zuo, T.; Xu, L.; Beavers, C.M.; Olmstead, M.M.; Fu, W.; Crawford, T.D.; Balch, A.L.; Dorn, H.C. M2@C79N (M = Y, Tb): Isolation and Characterization of Stable Endohedral Metallofullerenes Exhibiting M-M Bonding Interactions inside Aza[80]Fullerene Cages. J. Am. Chem. Soc. 2008, 130, 12992–12997. [Google Scholar] [CrossRef] [PubMed]
- Jakes, P.; Dinse, K.P. Chemically Induced Spin Transfer to an Encased Molecular Cluster: An EPR Study of Sc3N@C80 Radical Anions [2]. J. Am. Chem. Soc. 2001, 123, 8854–8855. [Google Scholar] [CrossRef]
- Popov, A.A.; Dunsch, L. Hindered Cluster Rotation and 45Sc Hyperfine Splitting Constant in Distonoid Anion Radical Sc3N@C80-, and Spatial Spin–Charge Separation as a General Principle for Anions of Endohedral Fullerenes with Metal-Localized Lowest Unoccupied Molecular Orbitals. J. Am. Chem. Soc. 2008, 130, 17726–17742. [Google Scholar] [CrossRef]
- Popov, A.A.; Shustova, N.B.; Svitova, A.L.; Mackey, M.A.; Coumbe, C.E.; Phillips, J.P.; Stevenson, S.; Strauss, S.H.; Boltalina, O.V.; Dunsch, L. Redox-Tuning Endohedral Fullerene Spin States: From the Dication to the Trianion Radical of Sc3N@C80(CF3)2 in Five Reversible Single-Electron Steps. Chem. Eur. J. 2010, 16, 4721–4724. [Google Scholar] [CrossRef] [PubMed]
- Shustova, N.B.; Peryshkov, D.V.; Kuvychko, I.V.; Chen, Y.S.; MacKey, M.A.; Coumbe, C.E.; Heaps, D.T.; Confait, B.S.; Heine, T.; Phillips, J.P.; et al. Poly(Perfluoroalkylation) of Metallic Nitride Fullerenes Reveals Addition-Pattern Guidelines: Synthesis and Characterization of a Family of Sc3N@C80(CF3)n (n = 2–16) and Their Radical Anions. J. Am. Chem. Soc. 2011, 133, 2672–2690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Schiemenz, S.; Popov, A.A.; Dunsch, L. Strain-Driven Endohedral Redox Couple CeIV/CeIII in Nitride Clusterfullerenes CeM2N@C80 (M = Sc, Y, Lu). J. Phys. Chem. Lett. 2013, 4, 2404–2409. [Google Scholar] [CrossRef]
- Popov, A.A.; Chen, N.; Pinzón, J.R.; Stevenson, S.; Echegoyen, L.A.; Dunsch, L. Redox-Active Scandium Oxide Cluster inside a Fullerene Cage: Spectroscopic, Voltammetric, Electron Spin Resonance Spectroelectrochemical, and Extended Density Functional Theory Study of Sc4O2@C80 and Its Ion Radicals. J. Am. Chem. Soc. 2012, 134, 19607–19618. [Google Scholar] [CrossRef]
- Svitova, A.L.; Ghiassi, K.B.; Schlesier, C.; Junghans, K.; Zhang, Y.; Olmstead, M.M.; Balch, A.L.; Dunsch, L.; Popov, A.A. Endohedral Fullerene with Μ3-Carbido Ligand and Titanium-Carbon Double Bond Stabilized inside a Carbon Cage. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Junghans, K.; Ghiassi, K.B.; Samoylova, N.A.; Deng, Q.; Rosenkranz, M.; Olmstead, M.M.; Balch, A.L.; Popov, A.A. Synthesis and Isolation of the Titanium–Scandium Endohedral Fullerenes—Sc2TiC@Ih-C80, Sc2TiC@D5h-C80 and Sc2TiC2@Ih-C80: Metal Size Tuning of the TiIV/TiIII Redox Potentials. Chem. Eur. J. 2016, 22, 13098–13107. [Google Scholar] [CrossRef] [PubMed]
- Junghans, K.; Schlesier, C.; Kostanyan, A.; Samoylova, N.A.; Deng, Q.; Rosenkranz, M.; Schiemenz, S.; Westerström, R.; Greber, T.; Büchner, B.; et al. Methane as a Selectivity Booster in the Arc-Discharge Synthesis of Endohedral Fullerenes: Selective Synthesis of the Single-Molecule Magnet Dy2TiC@C80 and Its Congener Dy2TiC2@C80. Angew. Chem. Int. Ed. 2015, 54, 13411–13415. [Google Scholar] [CrossRef]
- Xu, W.; Feng, L.; Calvaresi, M.; Liu, J.; Liu, Y.; Niu, B.; Shi, Z.; Lian, Y.; Zerbetto, F. An Experimentally Observed Trimetallofullerene Sm3@I H-C80: Encapsulation of Three Metal Atoms in a Cage without a Nonmetallic Mediator. J. Am. Chem. Soc. 2013, 135, 4187–4190. [Google Scholar] [CrossRef]
- Sathiyan, G.; Sivakumar, E.K.T.; Ganesamoorthy, R.; Thangamuthu, R.; Sakthivel, P. Review of Carbazole Based Conjugated Molecules for Highly Efficient Organic Solar Cell Application. Tetrahedron Lett. 2016, 57, 243–252. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. A Review on Solar Energy Use in Industries. Renew. Sustain. Energy Rev. 2011, 15, 1777–1790. [Google Scholar] [CrossRef]
- Zhennan, G.; Jiuxin, Q.; Xihuang, Z.; Yongqing, W.; Xing, Z.; Sunqi, F.; Zizhao, G. Buckminsterfullerene C60: Synthesis, Spectroscopic Characterization, and Structure Analysis. J. Phys. Chem. 1991, 95, 9615–9618. [Google Scholar] [CrossRef]
- Sariciftci, N.S.; Smilowitz, L.; Heeger, A.J.; Wudl, F. Photoinduced Electron Transfer from a Conducting Polymer to Buckminsterfullerene. Science 1992, 258, 1474–1476. [Google Scholar] [CrossRef]
- Seyler, H.; Wong, W.W.H.; Jones, D.J.; Holmes, A.B. Continuous Flow Synthesis of Fullerene Derivatives. J. Org. Chem. 2011, 76, 3551–3556. [Google Scholar] [CrossRef] [PubMed]
- Ganesamoorthy, R.; Sathiyan, G.; Sakthivel, P. Review: Fullerene Based Acceptors for Efficient Bulk Heterojunction Organic Solar Cell Applications. Sol. Energy Mater. Sol. Cells 2017, 161, 102–148. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789. [Google Scholar] [CrossRef]
- Troshin, P.A.; Hoppe, H.; Renz, J.; Egginger, M.; Mayorova, J.Y.; Goryachev, A.E.; Peregudov, A.S.; Lyubovskaya, R.N.; Gobsch, G.; Sariciftci, N.S.; et al. Material Solubility-Photovoltaic Performance Relationship in the Design of Novel Fullerene Derivatives for Bulk Heterojunction Solar Cells. Adv. Funct. Mater. 2009, 19, 779–788. [Google Scholar] [CrossRef]
- Zhang, Y.; Yip, H.-L.; Acton, O.; Hau, S.K.; Huang, F.; Jen, A.K.-Y. A Simple and Effective Way of Achieving Highly Efficient and Thermally Stable Bulk-Heterojunction Polymer Solar Cells Using Amorphous Fullerene Derivatives as Electron Acceptor. Chem. Mater. 2009, 21, 2598–2600. [Google Scholar] [CrossRef]
- Kim, H.U.; Kim, J.H.; Kang, H.; Grimsdale, A.C.; Kim, B.J.; Yoon, S.C.; Hwang, D.H. Naphthalene-, Anthracene-, and Pyrene-Substituted Fullerene Derivatives as Electron Acceptors in Polymer-Based Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 20776–20785. [Google Scholar] [CrossRef]
- Yang, C.; Jin, Y.K.; Cho, S.; Jae, K.L.; Heeger, A.J.; Wudl, F. Functionalized Methanofullerenes Used as N-Type Materials in Bulk-Heterojunction Polymer Solar Cells and in Field-Effect Transistors. J. Am. Chem. Soc. 2008, 130, 6444–6450. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-Efficiency Solution Processable Polymer Photovoltaic Cells by Self-Organization of Polymer Blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Zheng, L.; Zhou, Q.; Deng, X.; Yuan, M.; Yu, G.; Cao, Y. Methanofullerenes Used as Electron Acceptors in Polymer Photovoltaic Devices. J. Phys. Chem. B 2004, 108, 11921–11926. [Google Scholar] [CrossRef]
- Ge, J.; Liu, J.; Guo, X.; Qin, Y.; Luo, H.; Guo, Z.X.; Li, Y. Photovoltaic Properties of Dimeric Methanofullerenes Containing Hydroxyl Groups. Chem. Phys. Lett. 2012, 535, 100–105. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, S.; Shu, X.; Li, Y.; Xu, L.; Liu, T.; Yu, Y.; Zhang, L.; Liu, H.; Li, Y. Synthesis and Photovoltaic Properties of Novel Monoadducts and Bisadducts Based on Amide Methanofullerene. ACS Appl. Mater. Interfaces 2012, 4, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Kumar, C.P.; Sharma, G.D.; Kurchania, R.; Roy, M.S. Synthesis of a Modified PC 70BM and Its Application as an Electron Acceptor with Poly(3-Hexylthiophene) as an Electron Donor for Efficient Bulk Heterojunction Solar Cells. Adv. Funct. Mater. 2012, 22, 4087–4095. [Google Scholar] [CrossRef]
- Shin, W.S.; Lee, J.C.; Kim, J.R.; Lee, H.Y.; Lee, S.K.; Yoon, S.C.; Moon, S.J. Effect of the Alkyl Chain Length of C70-PCBX Acceptors on the Device Performance of P3HT:C70-PCBX Polymer Solar Cells. J. Mater. Chem. 2011, 21, 960–967. [Google Scholar] [CrossRef]
- Susarova, D.K.; Goryachev, A.E.; Novikov, D.V.; Dremova, N.N.; Peregudova, S.M.; Razumov, V.F.; Troshin, P.A. Material Solubility Effects in Bulk Heterojunction Solar Cells Based on the Bis-Cyclopropane Fullerene Adducts and P3HT. Sol. Energy Mater. Sol. Cells 2014, 120, 30–36. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, S.; Qian, D.; Wang, Q.; Hou, J. Application of Bis-PCBM in Polymer Solar Cells with Improved Voltage. J. Phys. Chem. C 2013, 117, 25360–25366. [Google Scholar] [CrossRef]
- Lin, Y.; Lim, J.A.; Wei, Q.; Mannsfeld, S.C.B.; Briseno, A.L.; Watkins, J.J. Cooperative Assembly Of-Bonded Diblock Copolythiophene/Fullerene for Photovoltaic Devices with Well-Defined Morphologies and Enhanced Stability. Chem. Mater. 2012, 24, 622–632. [Google Scholar] [CrossRef]
- Lenes, M.; Shelton, S.W.; Sieval, A.B.; Kronholm, D.F.; Hummelen, J.C.; Blom, P.W.M. Electron Trapping in Higher Adduct Fullerene-Based Solar Cells. Adv. Funct. Mater. 2009, 19, 3002–3007. [Google Scholar] [CrossRef]
- Lenes, M.; Wetzelaer, G.J.A.H.; Kooistra, F.B.; Veenstra, S.C.; Hummelen, J.C.; Blom, P.W.M. Fullerene Bisadducts for Enhanced Open-Circuit Voltages and Efficiencies in Polymer Solar Cells. Adv. Mater. 2008, 20, 2116–2119. [Google Scholar] [CrossRef]
- He, Y.; Chen, H.Y.; Hou, J.; Li, Y. Indene−C60 Bisadduct: A New Acceptor for High-Performance Polymer Solar Cells. J. Am. Chem. Soc. 2010, 132, 5532. [Google Scholar] [CrossRef]
- Zhao, G.J.; He, Y.J.; Li, Y. 6.5% Efficiency of Polymer Solar Cells Based on Poly(3-hexylthiophene) and Indene-C60 Bisadduct by Device Optimization. Adv. Mater. 2010, 22, 4355–4358. [Google Scholar] [CrossRef]
- Sun, S.; Salim, T.; Mathews, N.; Duchamp, M.; Boothroyd, C.; Xing, G.; Sum, T.C.; Lam, Y.M. The Origin of High Efficiency in Low-Temperature Solution-Processable Bilayer Organometal Halide Hybrid Solar Cells. Energy Environ. Sci. 2013, 7, 399–407. [Google Scholar] [CrossRef]
- Kumar, M.H.; Yantara, N.; Dharani, S.; Graetzel, M.; Boix, P.P.; Mathews, N. Flexible, Low-Temperature, Solution Processed ZnO-Based Perovskite Solid State Solar Cells. Chem. Commun. 2013, 49, 11089–11091. [Google Scholar] [CrossRef]
- Kumari, M.A.; Swetha, T.; Singh, S.P. Fullerene Derivatives: A Review on Perovskite Solar Cells. Mater. Express 2018, 8, 389–406. [Google Scholar] [CrossRef]
- Anthopoulos, T.; Singh, B.; De Leeuw, D. High performance n-channel organic field-effect transistors and ring oscillators based on C60 fullerene films. Appl. Phys. Lett. 2006, 89, 213504. [Google Scholar] [CrossRef]
- Haddon, R.C.; Perel, A.S.; Morris, R.C.; Palstra, T.T.M.; Hebard, A.F.; Fleming, R.M. C60 Thin Film Transistors. Appl. Phys. Lett. 1998, 67, 121. [Google Scholar] [CrossRef]
- Kitamura, M.; Aomori, S.; Na, J.H.; Arakawa, Y. Bottom-Contact Fullerene C60 Thin-Film Transistors with High Field-Effect Mobilities. Appl. Phys. Lett. 2008, 93, 033313. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takenobu, T.; Mon, S.; Fujiwara, A.; Iwasa, Y. Fabrication and Characterization of C60 Thin-Film Transistors with High Field-Effect Mobility. Appl. Phys. Lett. 2003, 82, 4581. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paukov, M.; Kramberger, C.; Begichev, I.; Kharlamova, M.; Burdanova, M. Functionalized Fullerenes and Their Applications in Electrochemistry, Solar Cells, and Nanoelectronics. Materials 2023, 16, 1276. https://doi.org/10.3390/ma16031276
Paukov M, Kramberger C, Begichev I, Kharlamova M, Burdanova M. Functionalized Fullerenes and Their Applications in Electrochemistry, Solar Cells, and Nanoelectronics. Materials. 2023; 16(3):1276. https://doi.org/10.3390/ma16031276
Chicago/Turabian StylePaukov, Maksim, Christian Kramberger, Ilia Begichev, Marianna Kharlamova, and Maria Burdanova. 2023. "Functionalized Fullerenes and Their Applications in Electrochemistry, Solar Cells, and Nanoelectronics" Materials 16, no. 3: 1276. https://doi.org/10.3390/ma16031276
APA StylePaukov, M., Kramberger, C., Begichev, I., Kharlamova, M., & Burdanova, M. (2023). Functionalized Fullerenes and Their Applications in Electrochemistry, Solar Cells, and Nanoelectronics. Materials, 16(3), 1276. https://doi.org/10.3390/ma16031276







