Novel Ultrafine-Grain Mg-Gd/Nd-Y-Ca Alloys with an Increased Ignition Temperature
Abstract
:1. Introduction
- High flammability resistance,
- Solid mechanical properties,
- Acceptable price.
- The formation of AxOy oxide is favourable over MgO in terms of Gibbs free energy.
- The solubility limit and diffusivity of alloying element A are sufficiently high.
2. Materials and Methods
2.1. Material
2.2. Microstructure
2.3. Mechanical Properties
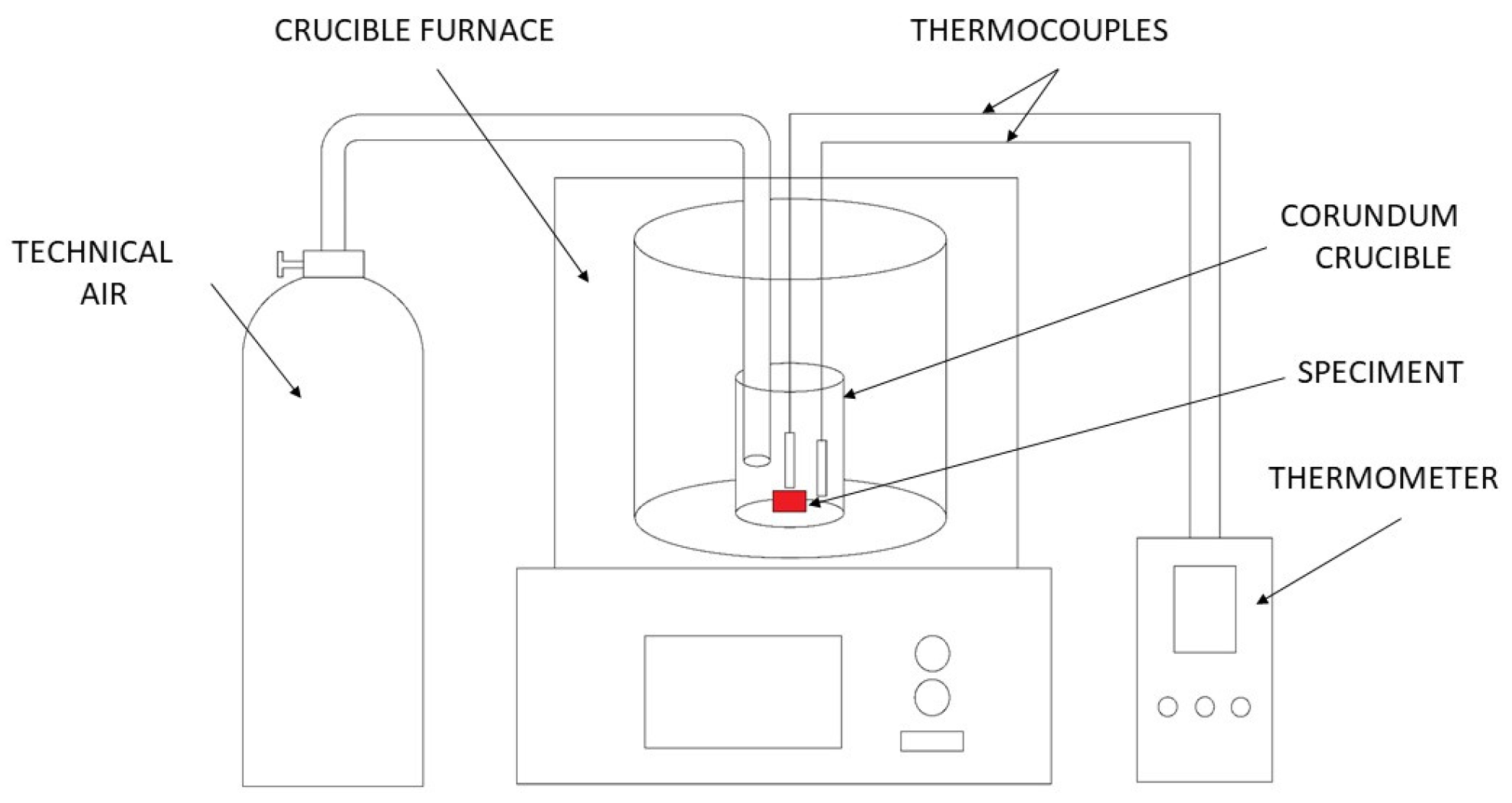
2.4. Ignition Temperature
3. Results and Discussion
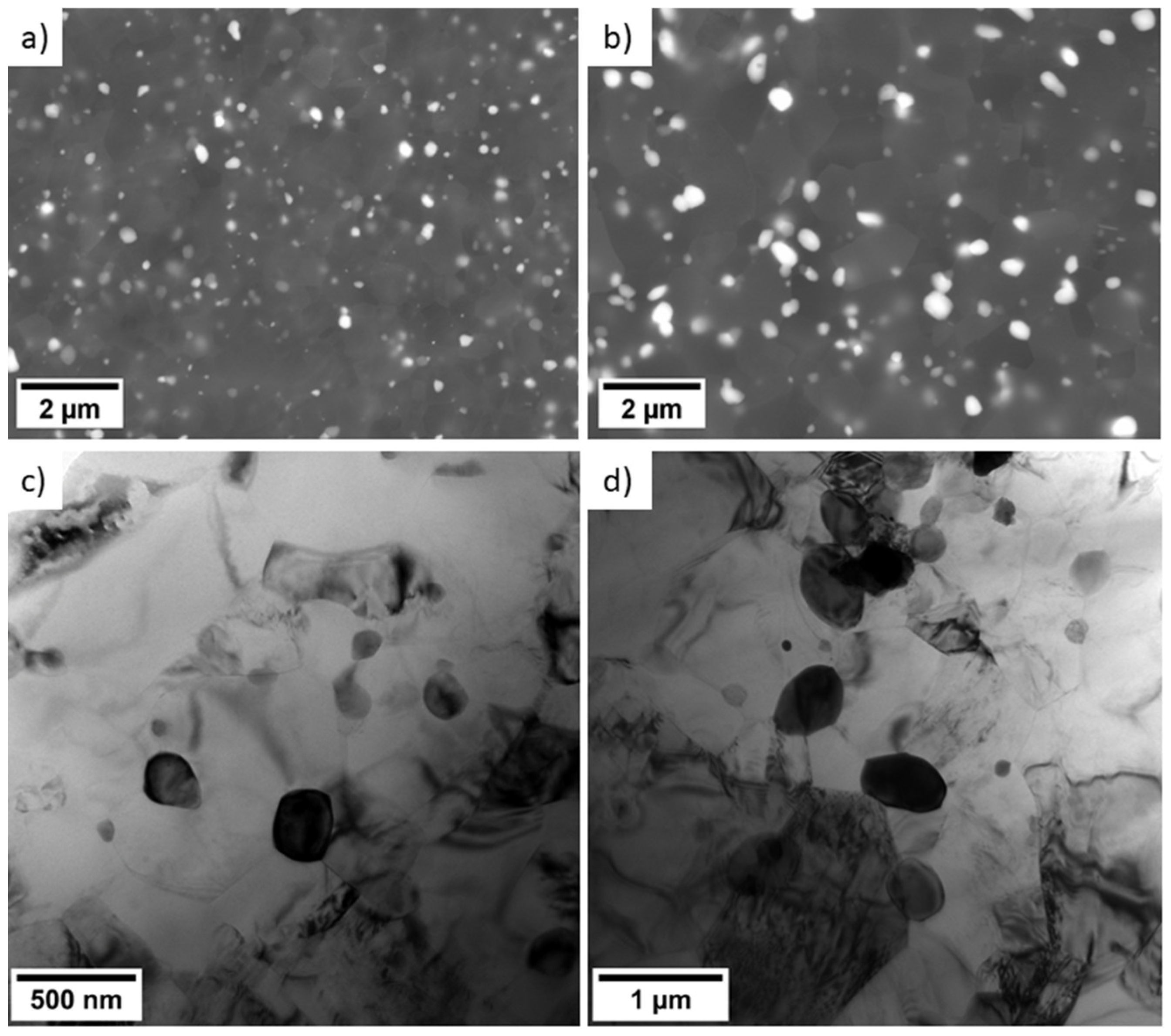
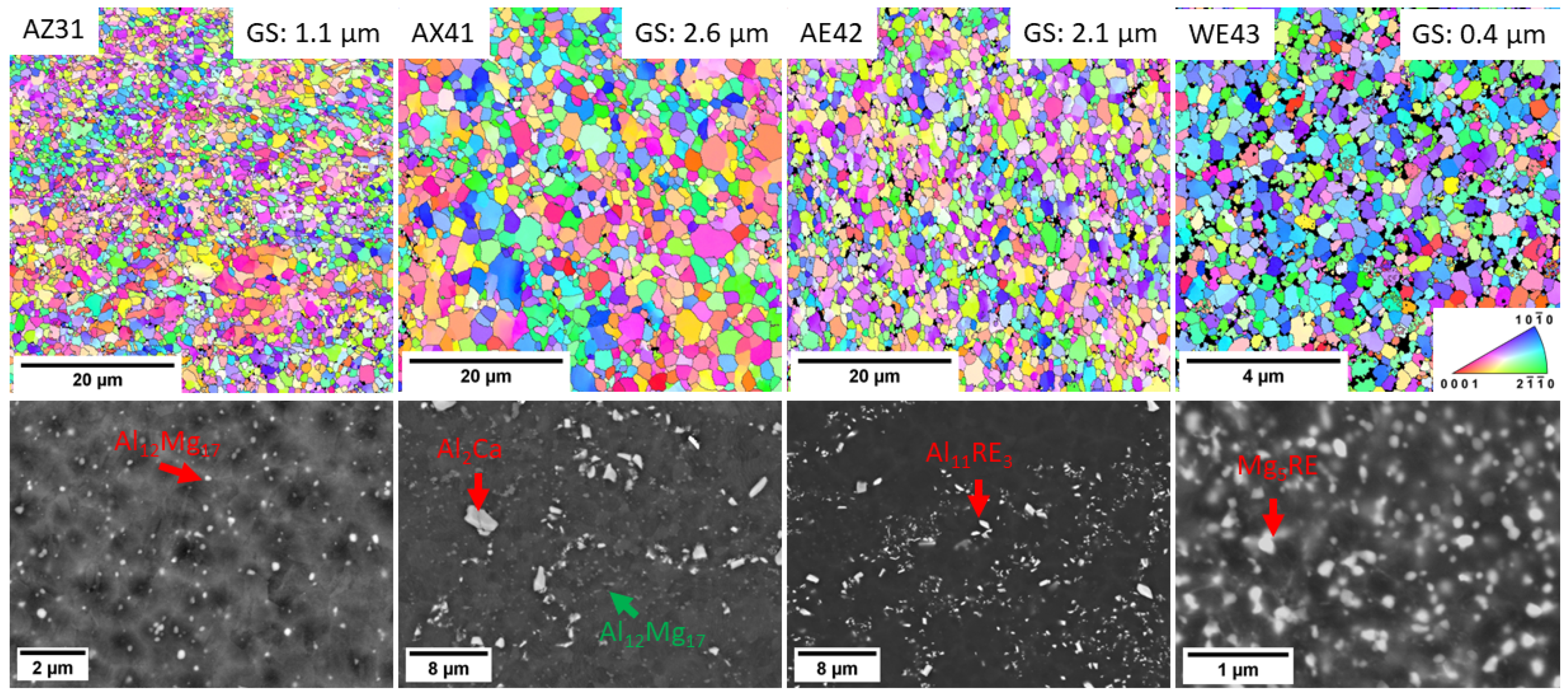
3.1. Microstructure
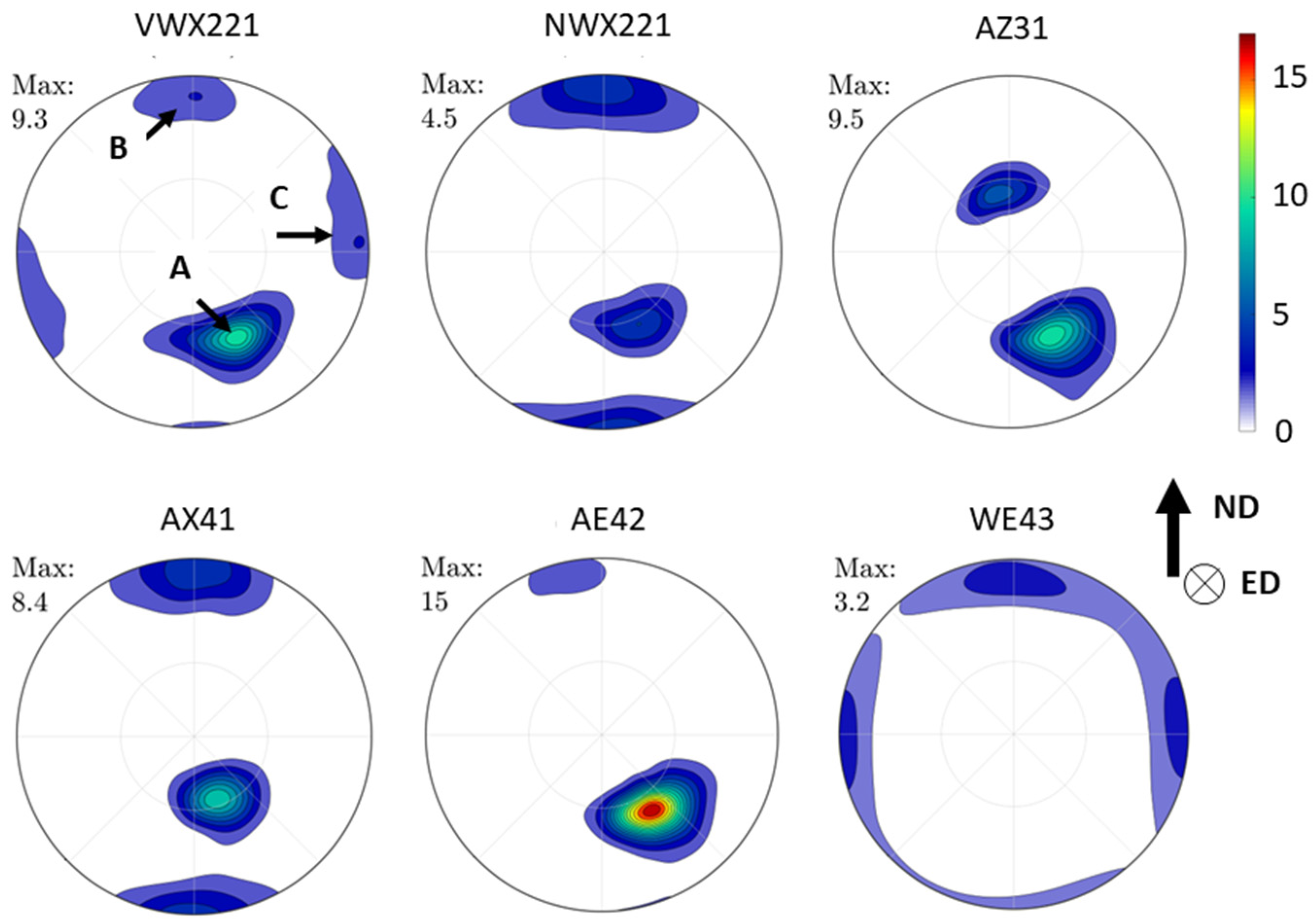
3.2. Texture
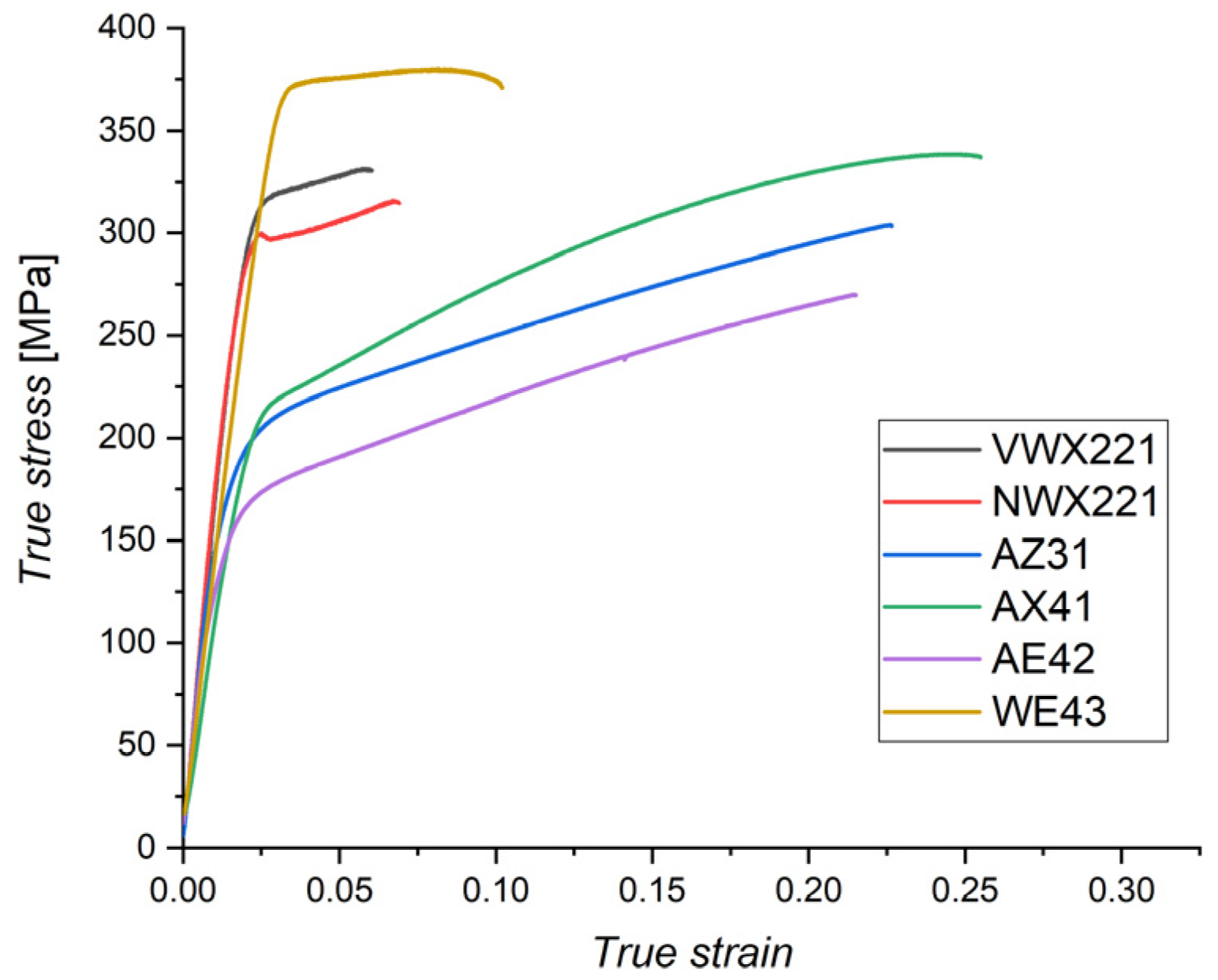
3.3. Mechanical Properties
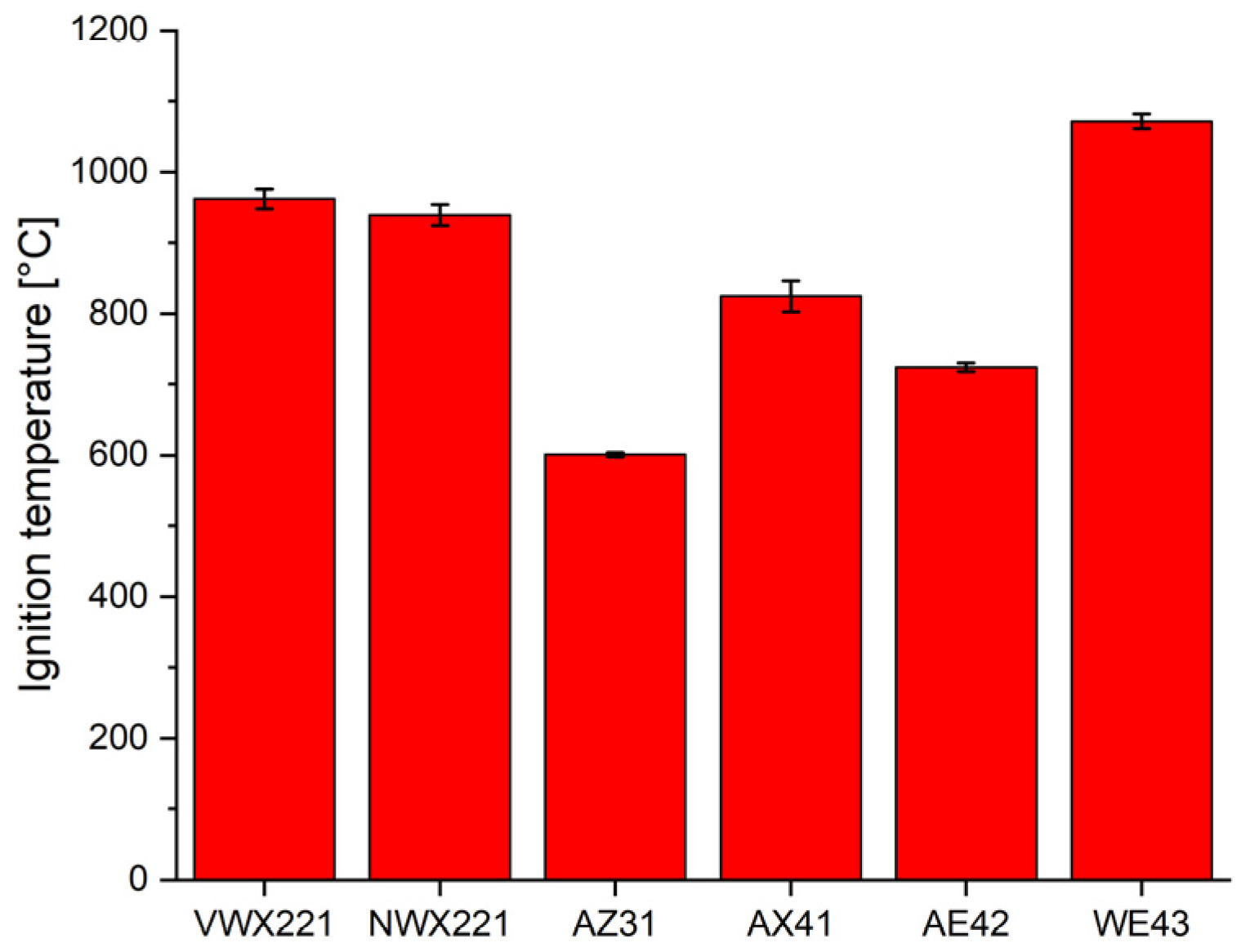
3.4. Ignition Temperature
4. Conclusions
- The grain structure of both alloys was refined by ECAP down to 1.1 µm and 1.5 µm for Mg-2Gd-2Y-1Ca and Mg-2Nd-1Y-1Ca, respectively. Both alloys contained dense distribution of small secondary phase particles. Higher refinement of Gd-containing alloy was attributed to its ability to form finer secondary phase particles.
- The mechanical strength of both novel alloys was very high: tensile-yield strength was ~290 MPa. However, the segregation of Ca into the grain boundaries caused a decrease in ductility below 6%. The strength of the novel alloys was much higher than the commercial ones, except for the WE43, but the ductility was lower.
- The ignition temperature of both novel alloys (~950 °C) was significantly higher than that of the commercial alloys, thanks to the combined effect of Gd/Nd, Y and Ca. The only exception was the WE43 alloy, which showed a higher ignition temperature because of the much higher amount of rare-earth elements and yttrium.
- This study showed that both alloys could be prepared in the condition exhibiting high strength and high ignition temperature. It was found that there is a small difference in the effect of Gd and Nd on the microstructure during the ECAP processing, but the strength and ignition temperature of both alloys was similar.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, M.; Sharon, N.M.L. Magnesium, Magnesium Alloys, and Magnesium Composites, 1st ed.; Wiley: New York, NY, USA, 2011. [Google Scholar]
- Tekumalla, S.; Gupta, M. An Insight into Ignition Factors and Mechanisms of Magnesium Based Materials: A Review. Mater. Des. 2017, 113, 84–98. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Langdon, T.G. Principles of Equal-Channel Angular Pressing as a Processing Tool for Grain Refinement. Prog. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Langdon, T.G. Twenty-Five Years of Ultrafine-Grained Materials: Achieving Exceptional Properties through Grain Refinement. Acta Mater. 2013, 61, 7035–7059. [Google Scholar] [CrossRef]
- Han, D.; Zhang, J.; Huang, J.; Lian, Y.; He, G. A Review on Ignition Mechanisms and Characteristics of Magnesium Alloys. J. Magnes. Alloy. 2020, 8, 329–344. [Google Scholar] [CrossRef]
- Czerwinski, F. Controlling the Ignition and Flammability of Magnesium for Aerospace Applications. Corros. Sci. 2014, 86, 1–16. [Google Scholar] [CrossRef]
- Aydin, D.S.; Bayindir, Z.; Hoseini, M.; Pekguleryuz, M.O. The High Temperature Oxidation and Ignition Behavior of Mg–Nd Alloys Part I: The Oxidation of Dilute Alloys. J. Alloys Compd. 2013, 569, 35–44. [Google Scholar] [CrossRef]
- Aydin, D.S.; Bayindir, Z.; Pekguleryuz, M.O. The High Temperature Oxidation Behavior of Mg–Nd Alloys. Part II: The Effect of the Two-Phase Microstructure on the on-Set of Oxidation and on Oxide Morphology. J. Alloys Compd. 2014, 584, 558–565. [Google Scholar] [CrossRef]
- Kubásek, J.; Minárik, P.; Hosová, K.; Šašek, S.; Knapek, M.; Veselý, J.; Stráská, J.; Dvorský, D.; Čavojský, M.; Vojtěch, D. Novel Magnesium Alloy Containing Y, Gd and Ca with Enhanced Ignition Temperature and Mechanical Properties for Aviation Applications. J. Alloys Compd. 2021, 877, 160089. [Google Scholar] [CrossRef]
- Kim, Y.M.; Yim, C.D.; Kim, H.S.; You, B.S. Key Factor Influencing the Ignition Resistance of Magnesium Alloys at Elevated Temperatures. Scr. Mater. 2011, 65, 958–961. [Google Scholar] [CrossRef]
- Minárik, P.; Veselý, J.; Král, R.; Bohlen, J.; Kubásek, J.; Janeček, M.; Stráská, J. Exceptional Mechanical Properties of Ultra-Fine Grain Mg-4Y-3RE Alloy Processed by ECAP. Mater. Sci. Eng. A 2017, 708, 193–198. [Google Scholar] [CrossRef]
- Li, F.; Peh, W.Y.; Nagarajan, V.; Ho, M.K.; Danno, A.; Chua, B.W.; Tan, M.J. Development of Non-Flammable High Strength AZ91+Ca Alloys via Liquid Forging and Extrusion. Mater. Des. 2016, 99, 37–43. [Google Scholar] [CrossRef]
- Kawamura, Y. Flame-Resistant Magnesium Alloys with High Strength. In Proceedings of the Seventh Triennial International Fire & Cabin Safety Research Conference, Philadelphia, PA, USA, 2–5 December 2013; Volume 2013. [Google Scholar]
- Ding, J.; Zhao, W.M.; Qin, L.; Li, Y.Y. Study of Ca and Ce Additions on Different Ignition Resistance Behavior of Magnesium Alloy. Mater. Sci. Forum 2014, 788, 7–11. [Google Scholar] [CrossRef]
- Zeng, Z.R.; Bian, M.Z.; Xu, S.W.; Davies, C.H.J.; Birbilis, N.; Nie, J.F. Effects of Dilute Additions of Zn and Ca on Ductility of Magnesium Alloy Sheet. Mater. Sci. Eng. A 2016, 674, 459–471. [Google Scholar] [CrossRef]
- Villegas-Armenta, L.A.; Drew, R.A.L.; Pekguleryuz, M.O. The Ignition Behavior of Mg–Ca Binary Alloys: The Role of Heating Rate. Oxid. Met. 2020, 93, 545–558. [Google Scholar] [CrossRef]
- Ravi Kumar, N.V.; Blandin, J.J.; Suéry, M.; Grosjean, E. Effect of Alloying Elements on the Ignition Resistance of Magnesium Alloys. Scr. Mater. 2003, 49, 225–230. [Google Scholar] [CrossRef]
- Janeček, M.; Yi, S.; Král, R.; Vrátná, J.; Kainer, K.U. Texture and Microstructure Evolution in Ultrafine-Grained AZ31 Processed by EX-ECAP. J. Mater. Sci. 2010, 45, 4665–4671. [Google Scholar] [CrossRef]
- Krajňák, T.; Minárik, P.; Gubicza, J.; Máthis, K.; Kužel, R.; Janeček, M. Influence of Equal Channel Angular Pressing Routes on Texture, Microstructure and Mechanical Properties of Extruded AX41 Magnesium Alloy. Mater. Charact. 2017, 123, 282–293. [Google Scholar] [CrossRef]
- Minárik, P.; Král, R.; Čížek, J.; Chmelík, F. Effect of Different c/a Ratio on the Microstructure and Mechanical Properties in Magnesium Alloys Processed by ECAP. Acta Mater. 2016, 107, 83–95. [Google Scholar] [CrossRef]
- Bachmann, F.; Hielscher, R.; Schaeben, H. Texture Analysis with MTEX—Free and Open Source Software Toolbox. Solid State Phenom. 2010, 160, 63–68. [Google Scholar] [CrossRef]
- Krajňák, T.; Minárik, P.; Stráský, J.; Máthis, K.; Janeček, M. Mechanical Properties of Ultrafine-Grained AX41 Magnesium Alloy at Room and Elevated Temperatures. Mater. Sci. Eng. A 2018, 731, 438–445. [Google Scholar] [CrossRef]
- Orlov, D.; Pelliccia, D.; Fang, X.; Bourgeois, L.; Kirby, N.; Nikulin, A.Y.; Ameyama, K.; Estrin, Y. Particle Evolution in Mg–Zn–Zr Alloy Processed by Integrated Extrusion and Equal Channel Angular Pressing: Evaluation by Electron Microscopy and Synchrotron Small-Angle X-Ray Scattering. Acta Mater. 2014, 72, 110–124. [Google Scholar] [CrossRef]
- Minárik, P.; Zemková, M.; Veselý, J.; Bohlen, J.; Knapek, M.; Král, R. The Effect of Zr on Dynamic Recrystallization during ECAP Processing of Mg-Y-RE Alloys. Mater. Charact. 2021, 174, 111033. [Google Scholar] [CrossRef]
- Massalski, T.B.; Okamoto, H. Binary Alloy Phase Diagrams, 2nd ed.; The Materials Information Society: Materials Park, OH, USA, 1990; ISBN 978-0-87170-403-0. [Google Scholar]
- Krajňák, T.; Minárik, P.; Stráská, J.; Gubicza, J.; Máthis, K.; Janeček, M. Influence of Equal Channel Angular Pressing Temperature on Texture, Microstructure and Mechanical Properties of Extruded AX41 Magnesium. J. Alloys Compd. 2017, 705, 273–282. [Google Scholar] [CrossRef]
- Hall, E.O. The Deformation and Ageing of Mild Steel: III Discussion of Results. Proc. Phys. Society. Sect. B 1951, 64, 747–753. [Google Scholar] [CrossRef]
- Petch, N.J. The Cleavage Strength of Polycrystals; The Iron and Steel Institute: London, UK, 1953; pp. 25–28. [Google Scholar]
- Zhu, G.; Wang, L.; Wang, J.; Wang, J.; Park, J.-S.; Zeng, X. Highly Deformable Mg–Al–Ca Alloy with Al2Ca Precipitates. Acta Mater. 2020, 200, 236–245. [Google Scholar] [CrossRef]
- Kashiwase, S.; Unekawa, M.; Hisazawa, H.; Terada, Y. Three-Dimensional Morphology of C15–Al2Ca Precipitates in a Mg–Al–Ca Alloy. Mater. Trans. 2019, 60, 2048–2052. [Google Scholar] [CrossRef]
- Nie, J.F.; Muddle, B.C. Precipitation Hardening of Mg-Ca(-Zn) Alloys. Scr. Mater. 1997, 37, 1475–1481. [Google Scholar] [CrossRef]
- Prasad, A.; Shi, Z.; Atrens, A. Influence of Al and Y on the Ignition and Flammability of Mg Alloys. Corros. Sci. 2012, 55, 153–163. [Google Scholar] [CrossRef]
- Prasad, A.; Shi, Z.; Atrens, A. Flammability of Mg–X Binary Alloys. Adv. Eng. Mater. 2012, 14, 772–784. [Google Scholar] [CrossRef]
- Zeng, Z.; Stanford, N.; Davies, C.H.J.; Nie, J.-F.; Birbilis, N. Magnesium Extrusion Alloys: A Review of Developments and Prospects. Int. Mater. Rev. 2019, 64, 27–62. [Google Scholar] [CrossRef]



| Y | Gd | Nd | Ce | Ca | Al | Zn | Zr | Mg | |
|---|---|---|---|---|---|---|---|---|---|
| VWX221 | 1.8 | 2.2 | - | - | 0.9 | - | - | - | Bal. |
| NWX221 | 1.5 | - | 2.0 | - | 1.0 | - | - | - | Bal. |
| AZ31 | - | - | - | - | - | 2.8 | 0.9 | - | Bal. |
| AX41 | - | - | - | - | 0.9 | 5.6 | - | - | Bal. |
| AE42 | - | - | 0.6 | 1.3 | - | 3.5 | - | - | Bal. |
| WE43 | 3.8 | 0.6 | 2.3 | - | - | - | - | 0.4 | Bal. |
| TYS [MPa] | UTS [MPa] | |
|---|---|---|
| VWX221 | 303 ± 10 | 308 ± 10 |
| NWX221 | 282 ± 11 | 284 ± 12 |
| AZ31 | 168 ± 5 | 245 ± 6 |
| AX41 | 178 ± 8 | 270 ± 26 |
| AE42 | 126 ± 9 | 220 ± 8 |
| WE43 | 356 ± 8 | 367 ± 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šašek, S.; Minárik, P.; Stráská, J.; Hosová, K.; Veselý, J.; Kubásek, J.; Král, R.; Krajňák, T.; Vojtěch, D. Novel Ultrafine-Grain Mg-Gd/Nd-Y-Ca Alloys with an Increased Ignition Temperature. Materials 2023, 16, 1299. https://doi.org/10.3390/ma16031299
Šašek S, Minárik P, Stráská J, Hosová K, Veselý J, Kubásek J, Král R, Krajňák T, Vojtěch D. Novel Ultrafine-Grain Mg-Gd/Nd-Y-Ca Alloys with an Increased Ignition Temperature. Materials. 2023; 16(3):1299. https://doi.org/10.3390/ma16031299
Chicago/Turabian StyleŠašek, Stanislav, Peter Minárik, Jitka Stráská, Klára Hosová, Jozef Veselý, Jiří Kubásek, Robert Král, Tomáš Krajňák, and Dalibor Vojtěch. 2023. "Novel Ultrafine-Grain Mg-Gd/Nd-Y-Ca Alloys with an Increased Ignition Temperature" Materials 16, no. 3: 1299. https://doi.org/10.3390/ma16031299
APA StyleŠašek, S., Minárik, P., Stráská, J., Hosová, K., Veselý, J., Kubásek, J., Král, R., Krajňák, T., & Vojtěch, D. (2023). Novel Ultrafine-Grain Mg-Gd/Nd-Y-Ca Alloys with an Increased Ignition Temperature. Materials, 16(3), 1299. https://doi.org/10.3390/ma16031299







