Adding Value to Secondary Aluminum Casting Alloys: A Review on Trends and Achievements
Abstract
:1. Introduction
2. Materials and Methods
- Section 3—Aluminum Recycling Process: The primary focus of this section is to both comprehend the recycling cycle that generates the SAA casting alloys and highlight the key factors in each phase that may cause an alloy to deteriorate in quality and properties.
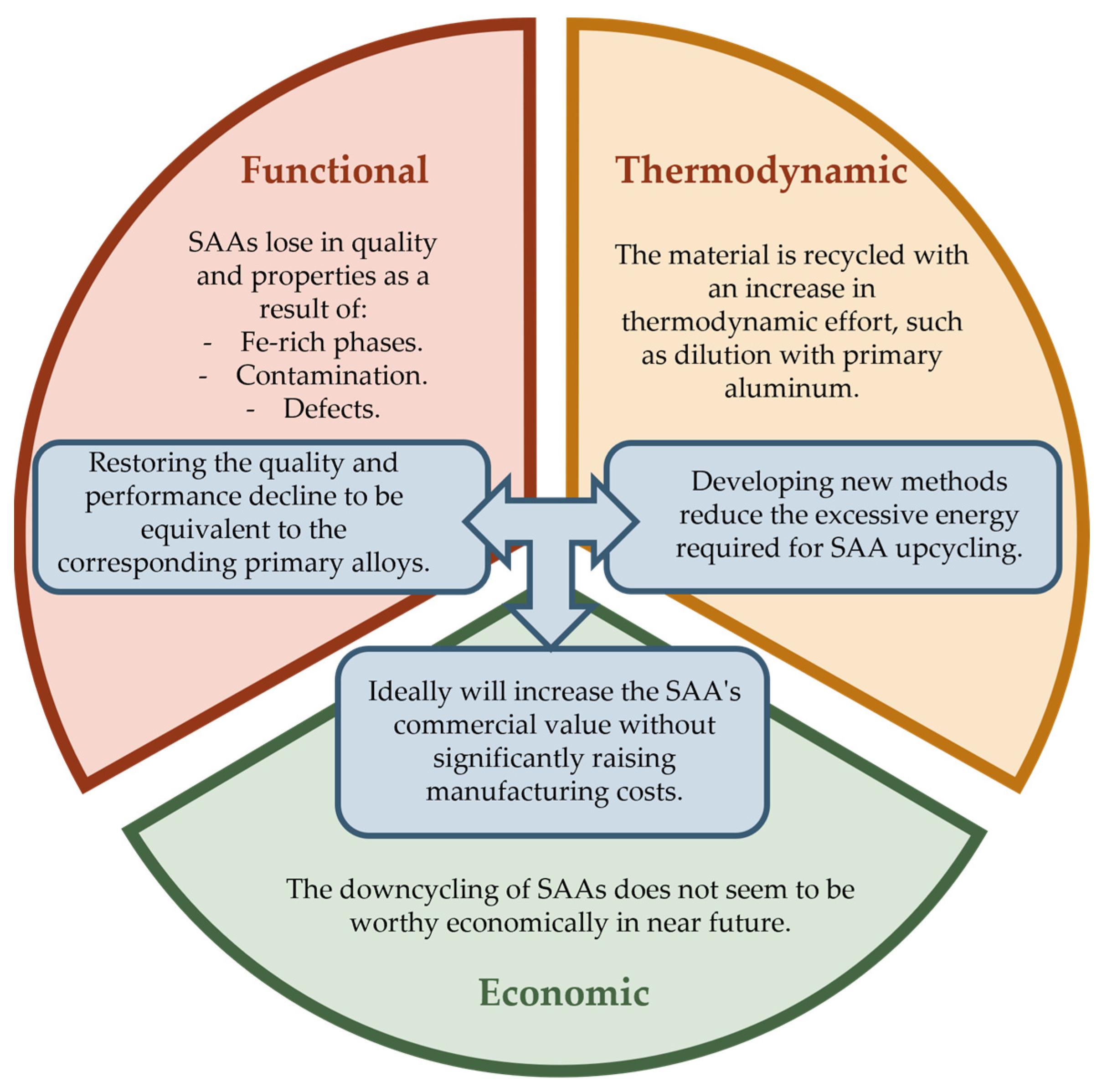
- Section 4—Aluminum Downcycling: This section emphasizes how the downgrade of aluminum fits into the three major downcycling aspects: functional, thermodynamic, and economic.
- Section 5—The Microstructure and Mechanical Performance of SAAs: Since the microstructure and mechanical properties of commercially used alloys have a close relationship to the alloy quality loss, this section describes the typical microstructure, chemical composition, and mechanical properties of some of the most used SAA alloys.
- Section 6—Valorization of SAAs: This section mainly focuses on strategies that attempt to lessen the effects of Fe-rich phases by modifying their phase and/or morphologies out of all the reported breakthroughs in techniques to add value to the SAA alloys. Emphasis is also placed on techniques that are simple to include into the often-utilized component manufacture cycles, such melt treatment or heat treatment.
3. Aluminum Recycling Process
3.1. Collection and Sorting
3.2. Remelting
3.3. Refining
4. Aluminum Downcycling
4.1. Functional Downcycling
Fe Contamination
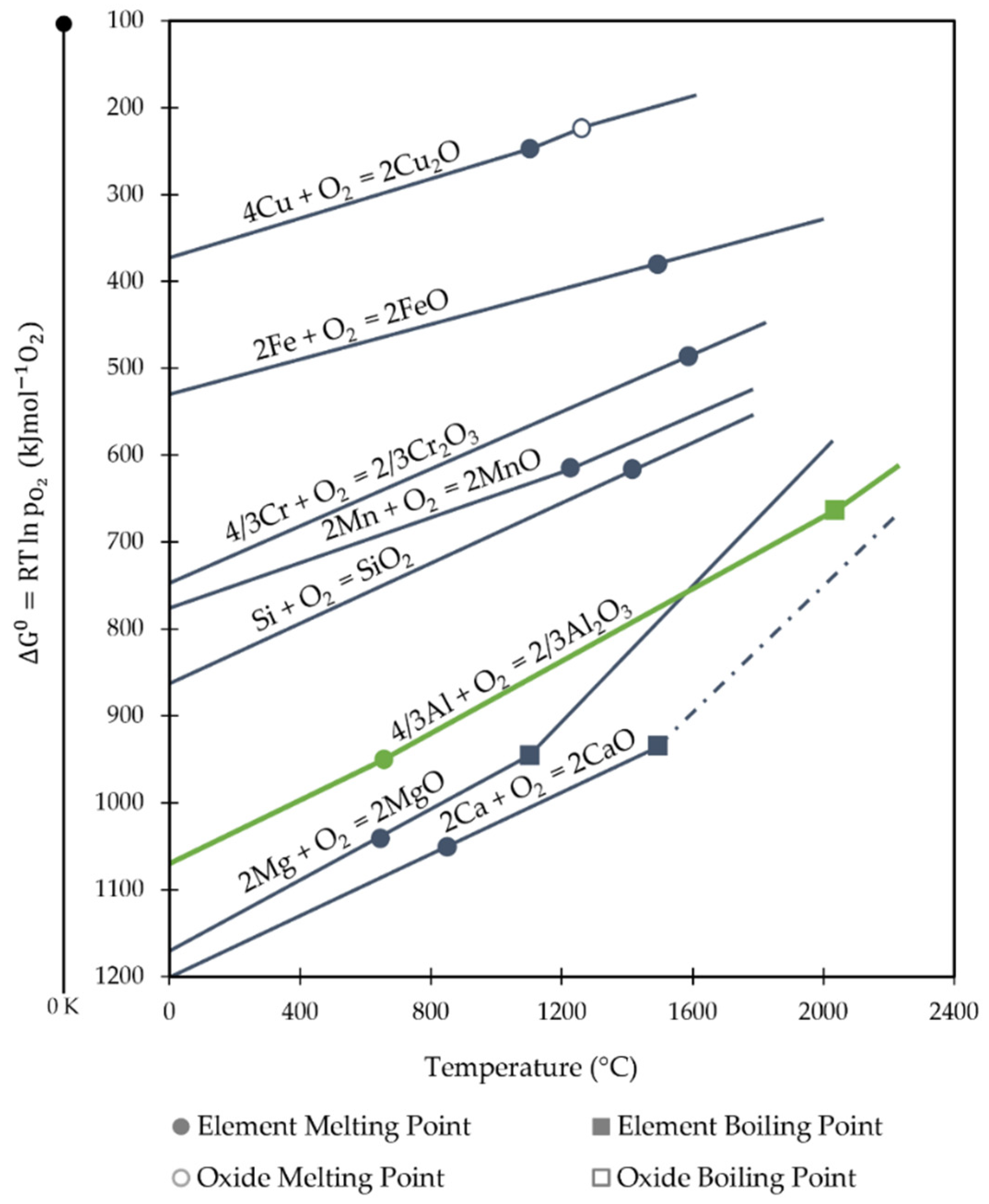
4.2. Thermodynamic Downcycling
4.3. Economic Downcycling
4.4. Prospects for Al Downcycling
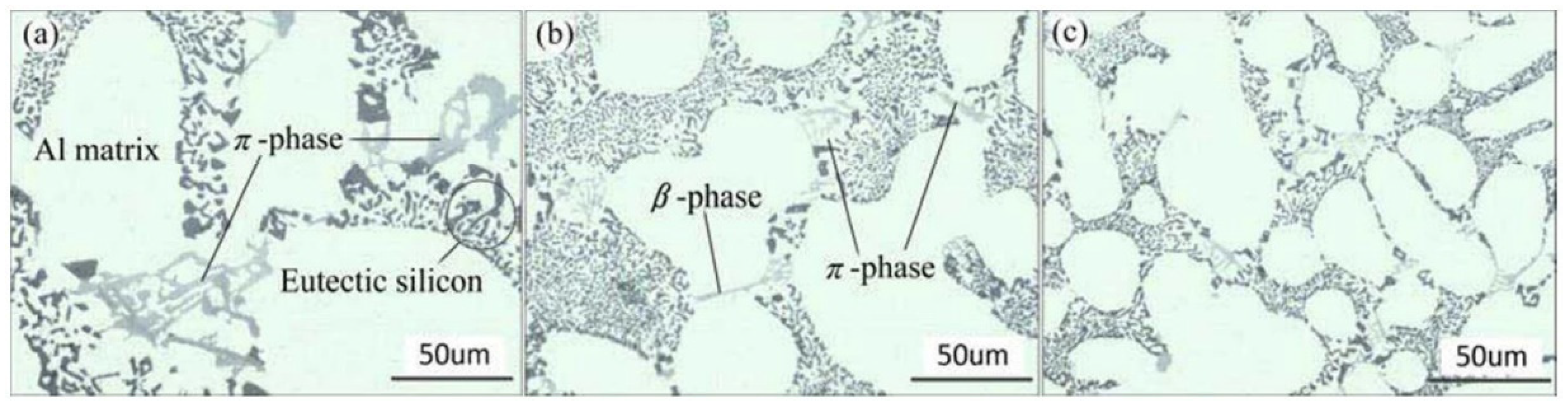
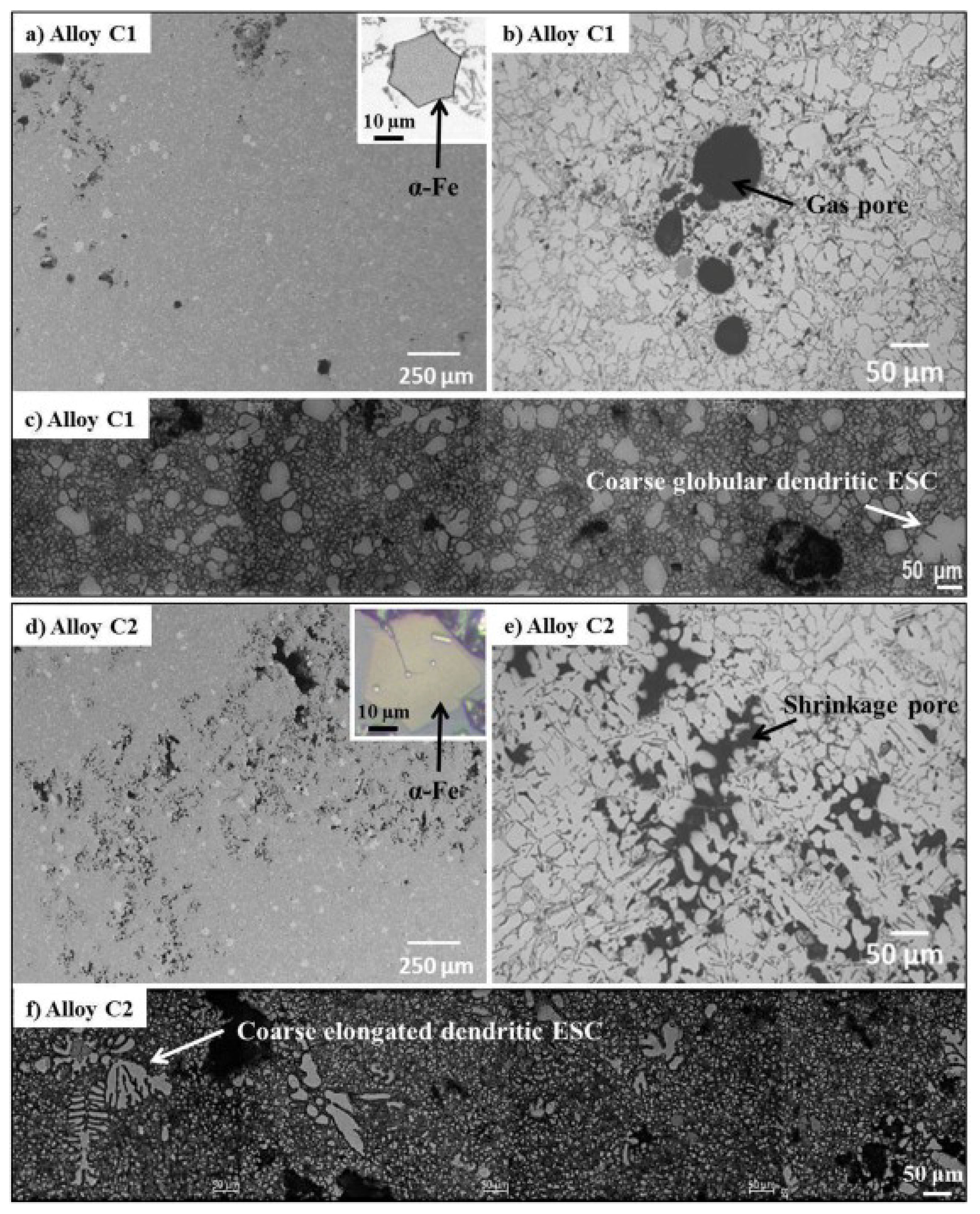
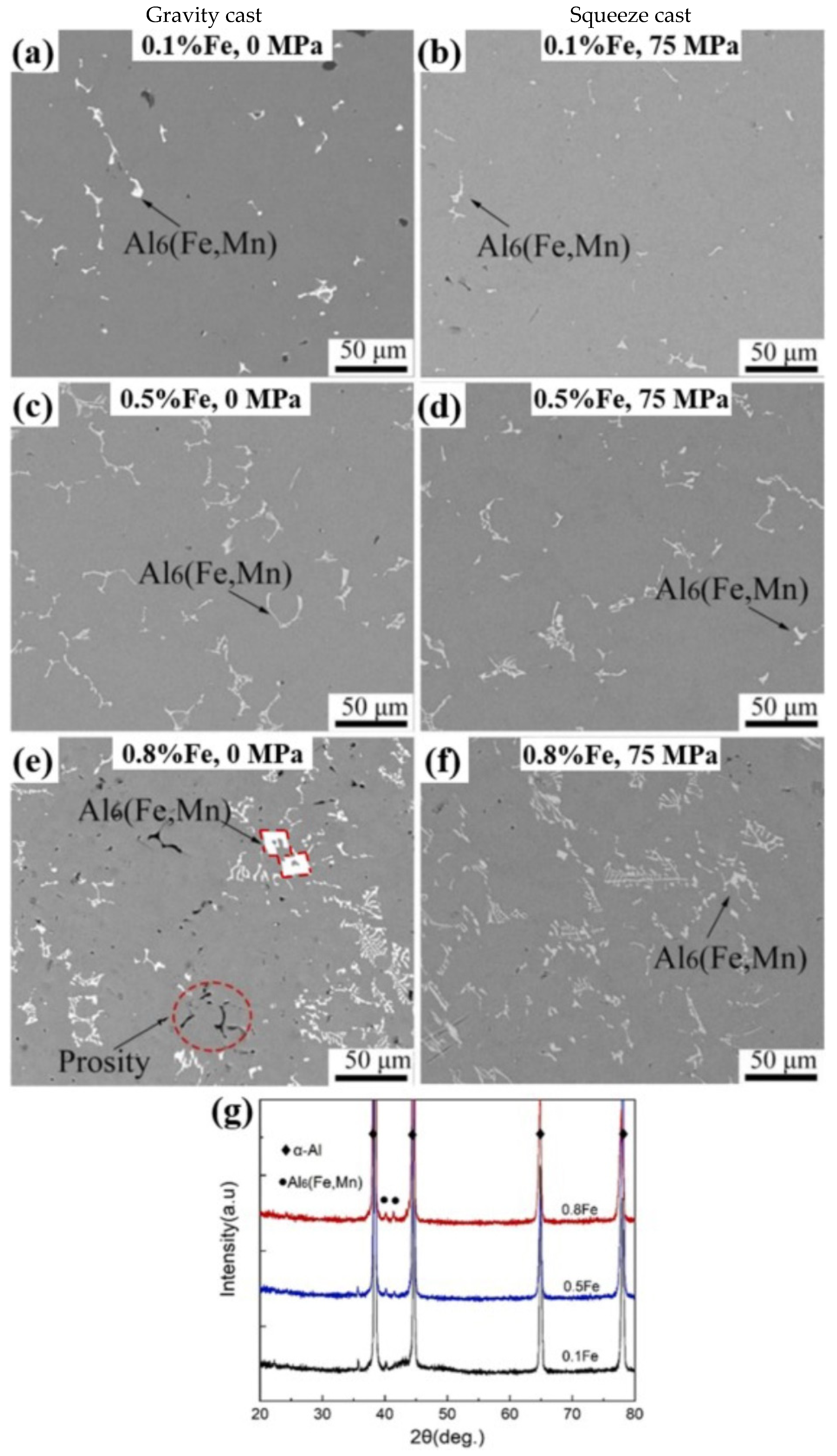
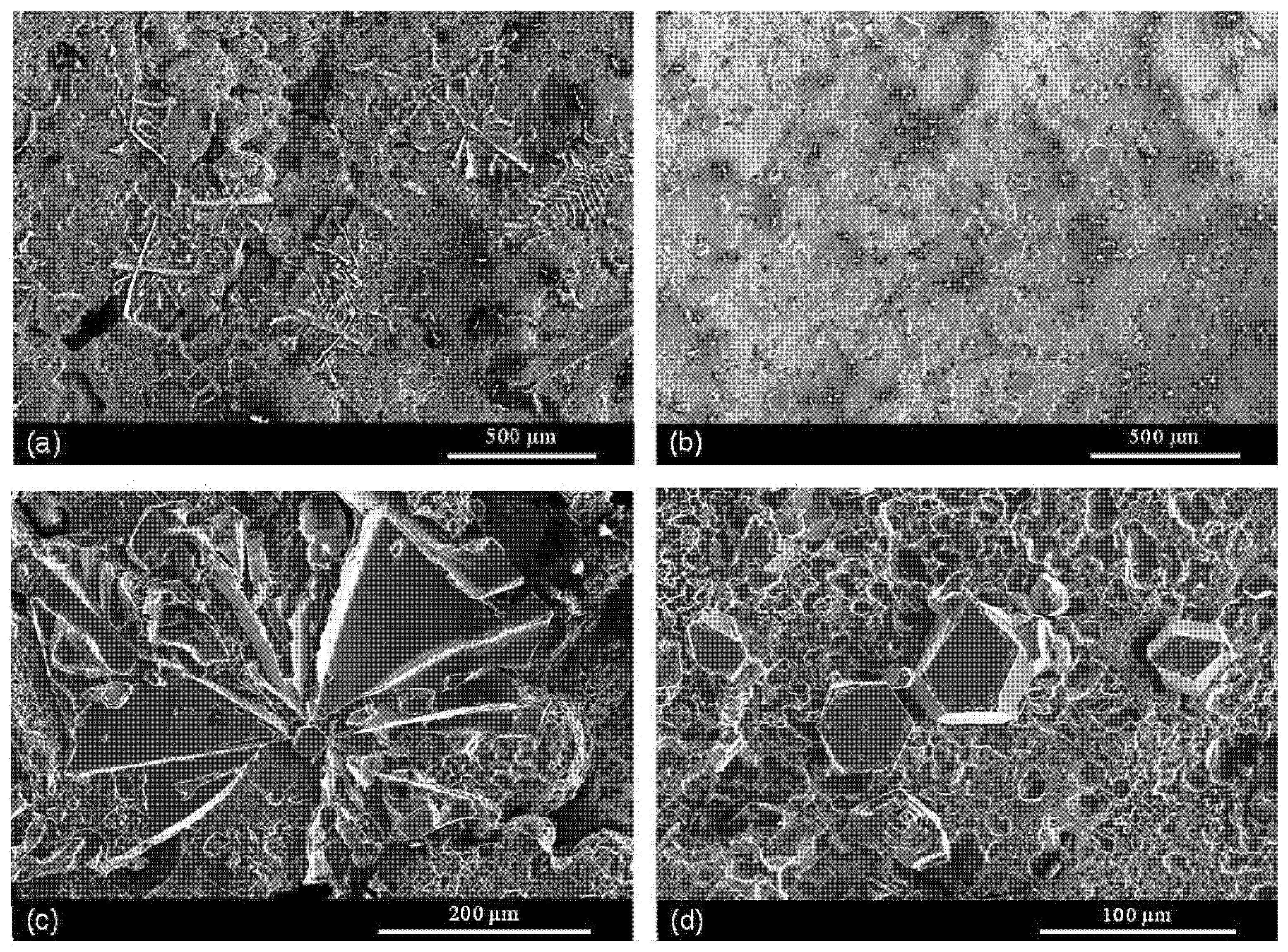
5. The Microstructure and Mechanical Performance of SAAs
6. Valorization of SAAs
6.1. New Aluminum Alloy Systems
| Element | Alloy | Fe (wt.%) | Mn/Fe | Observations | Reference |
|---|---|---|---|---|---|
| Be | AlSi7Mg0.3 | Up to 0.7 | ~0.02 | A small addition (500 ppm) of Be provoked the transformation of π into the β phase. Even though Be could avoid the effects of Fe phases on Al alloys, the researchers did not promote the use of these additions due to the toxic nature of Be. | [68,69] |
| Co | AlSi7Mg0.3 | Up to 1.0 | - | It was shown that Co/Fe ratios between 1 and 2 are ideal for intermetallic compounds with a Chinese-script shape. | [70] |
| Cr | AlSi3Mg0.6 | 0.1 | ~1.0 | The addition of Cr and Mn provoked the intermetallic phase shape to modify the needle-like morphology of the β and π phase to a more rounded α phase. | [71] |
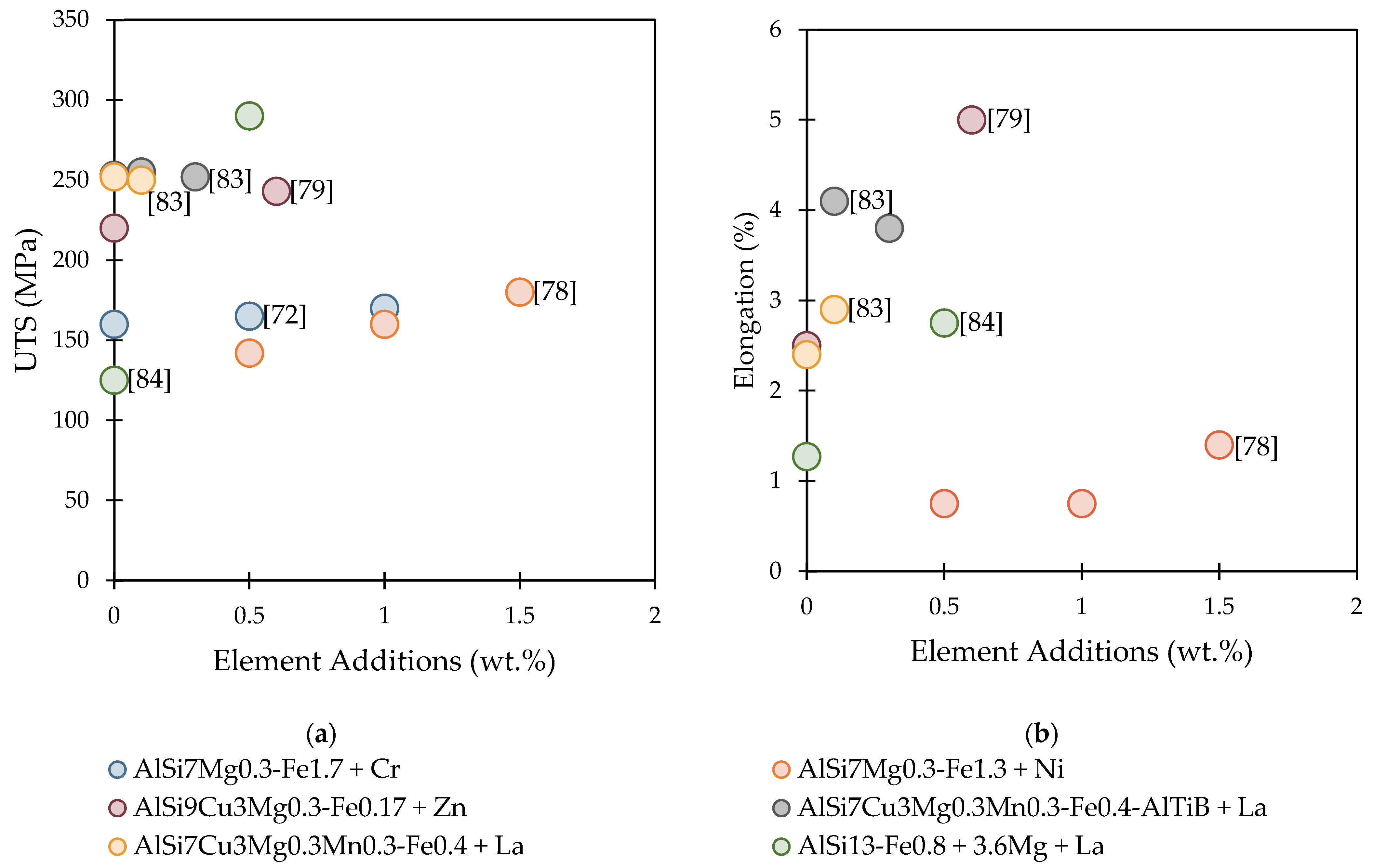
| AlSi7Mg0.3 | 1.7 | 0.3 | High Cr additions caused the β phase to become very long and thick, without significantly improving the mechanical properties of the alloy. | [72] | |
| AlSi9Cu3 | 0.8 | 0.6 | Cr addition up to 0.12 wt.% alongside a high cooling rate from HPDC caused the formation of α-Alx(Fe,Mn,Cr)ySiz. The Fe-rich intermetallic compounds’ volume fraction and size increased as the Cr content increased. | [73] | |
| AlSi9Cu3 | 4.0 | ~0.1 | A greater quantity of cubic α phase made up of several small particles results with the addition of Cr. Cr concentration gradients occurred in the cubic α phase with the Al13Cr4Si4 phase at the center of the particles. | [74] | |
| AlSi20 | 5.0 | - | High quantities of Cr additions (5%) achieved hardness above 200 HVN. This is due to Al3FeSi2 transforming to a more complex dendritic-shaped σ-CrFe compound. | [75] | |
| Li | AlSi7Mg0.3 | 0.1 | 0.5 | Li additions improved the hardness of the alloy due to the precipitation of AlLiSi phases in the Al matrix (HV increased between 10 and 25 depending on the cooling rate). However, other phases, such as β, occurred as the base alloy. | [76] |
| Mo | AlSi6Cu3 | 0.3 and 0.7 | - | In the alloys with low Fe content, it was possible to avoid β-phase precipitation with only 0.24% Mo addition. Moreover, in the high-Fe alloy, adding 0.41% was needed suppress the β-phase formation. | [77] |
| AlSi6Cu3 | 0.7 | 0.4 | Due to the lower solubility of Mo in Al, it was easier to form Al-Mo intermetallic than when adding Mn. Thus, Mn and Mo additions combined could achieve a better modification of the β phase. | [77] | |
| Ni | AlSi7Mg0.3 | 1.3 | 0.07 | Ni additions did not modify the β-phase morphology, without having any significant effect on tensile properties. | [78] |
| AlSi6Cu4 | 0.8 | 0.7 | Ni addition thickened not only the needed-like β phase but also the α phase. | [67] | |
| Ti | AlSi20 | 5.0 | - | With the addition of Ti, the significant Fe phase was the Al3FeSi2 with a plaque morphology. Additions of around 1 wt.% Ti formed Ti5Si3 during the acicular phase. While additions above 5 wt.% caused the segregation of eutectic Si around this binary intermetallic, the hardness increased from 106 to 144 HV. | [75] |
| Zn | AlSi9Cu3Mg0.3 | 0.2 | - | The Zn addition with the Sr eutectic modification helped to modify the morphology of the Fe-rich phases. The particle size was also reduced by almost half. With 0.58% Zn additions, thin and long β phases were still detected. The tensile properties of UTS and elongation were improved, but not YS. | [79] |
Rare Earth Element Microalloying
6.2. Ultrasonic Melt Treatment
6.3. Heat Treatment
6.4. Achivements and Future Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aluminium, E. A Strategy for Achieving Aluminium’s Full Potential for Circular Economy by 2030. In Circular Aluminium Action Plan; European Aluminium: Brussels, Belgium, 2020. [Google Scholar]
- Aluminium, E. European Aluminium’s Contribution to the Eu’s Mid-Century Low-Carbon Roadmap; European Aluminium: Brussels, Belgium, 2019. [Google Scholar]
- Tsakiridis, P.E.; Oustadakis, P.; Agatzini-Leonardou, S. Aluminium recovery during black dross hydrothermal treatment. J. Environ. Chem. Eng. 2013, 1, 23–32. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Allahverdi, A. Hazardous aluminum dross characterization and recycling strategies: A critical review. J. Environ. Manag. 2018, 223, 452–468. [Google Scholar] [CrossRef]
- Abdulkadir, A.; Ajayi, A.; Hassan, M. Evaluating the Chemical Composition and the Molar Heat Capacities of a white Aluminum Dross. Energy Procedia 2015, 75, 2099–2105. [Google Scholar] [CrossRef] [Green Version]
- Matejka, M.; Bolibruchová, D. Effect of Remelting on Microstructure of the AlSi9Cu3 Alloy with Higher Iron Content. Arch. Foundry Eng. 2018, 18, 25–30. [Google Scholar] [CrossRef]
- Singh, K.; Kashyap, B.P. Effects of Remelting on Variations in Composition, Microstructure, and Hardness Property of Binary Al-Si Alloys. J. Mater. Eng. Perform. 2022, 1–14. [Google Scholar] [CrossRef]
- Green, J.A. Aluminum Recycling and Processing for Energy Conservation and Sustainability; ASM International: Materials Park, OH, USA, 2007. [Google Scholar]
- Schmitz, C. Handbook of Aluminium Recycling—Mechanical Preparation—Metallurgical Processing—Heat Treatment, 2nd ed.; Vulkan Verlag: Essen, Germany, 2014. [Google Scholar]
- Gil, A. Management of the Salt Cake from Secondary Aluminum Fusion Processes. Ind. Eng. Chem. Res. 2005, 44, 8852–8857. [Google Scholar] [CrossRef]
- Tabereaux, A. The discovery, commercialization, and development of the aluminum industry in France. Light Met. Age 2012, 70, 28–33. [Google Scholar]
- Capuzzi, S.; Timelli, G. Preparation and Melting of Scrap in Aluminum Recycling: A Review. Metals 2018, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Kelly, S.; Apelian, D. Grave-to-Gate: Automotive Aluminum Recycling at End-of-Life. Light Met. Age 2017, 75, 40–43. [Google Scholar]
- Vicario, I.; Anza, I.; Tejada, F.; Alonso, J.; Galarraga, H.; Merchan, M. Development of New Al-Si9Cu3 Alloys for HPDC Components with Tailored Properties. In Proceedings of the 71st World Foundry Congress: Advanced Sustainable Foundry, Bilbao, Spain, 19–21 May 2014. [Google Scholar]
- Bösch, D.; Pogatscher, S.; Hummel, M.; Fragner, W.; Uggowitzer, P.J.; Göken, M.; Höppel, H.W. Secondary Al-Si-Mg High-pressure Die Casting Alloys with Enhanced Ductility. Metall. Mater. Trans. A 2015, 46, 1035–1045. [Google Scholar] [CrossRef]
- Curtolo, D.C.; Rodriguez-Rojas, M.J.; Friedrich, S.; Friedrich, B. Alternative fractional crystallization-based methods to produce high-purity aluminum. J. Mater. Res. Technol. 2021, 12, 796–806. [Google Scholar] [CrossRef]
- Lumley, R. Fundamentals of Aluminium Metallurgy: Production, Processing and Applications; Elsevier Science: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Schmitz, C. Handbook of Aluminium Recycling; Vulkan-Verlag: Essen, Germany, 2006. [Google Scholar]
- Gesing, A. Assuring the continued recycling of light metals in end-of-life vehicles: A global perspective. JOM 2004, 56, 18–27. [Google Scholar] [CrossRef]
- Løvik, A.N.; Modaresi, R.; Müller, D.B. Long-Term Strategies for Increased Recycling of Automotive Aluminum and Its Alloying Elements. Environ. Sci. Technol. 2014, 48, 4257–4265. [Google Scholar] [CrossRef] [PubMed]
- Hatayama, H.; Daigo, I.; Matsuno, Y.; Adachi, Y. Evolution of aluminum recycling initiated by the introduction of next-generation vehicles and scrap sorting technology. Resour. Conserv. Recycl. 2012, 66, 8–14. [Google Scholar] [CrossRef]
- den Eynde, S.V.; Diaz-Romero, D.J.; Engelen, B.; Zaplana, I.; Peeters, J.R. Assessing the efficiency of Laser-Induced Breakdown Spectroscopy (LIBS) based sorting of post-consumer aluminium scrap. Procedia CIRP 2022, 105, 278–283. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Kwon, E.; Kim, D.; Shin, S.; Jeong, S.; Park, K. 3D Sensing System for Laser-Induced Breakdown Spectroscopy-Based Metal Scrap Identification. Int. J. Precis. Eng. Manuf.-Green Technol. 2022, 9, 695–707. [Google Scholar] [CrossRef]
- Velasco, E.; Nino, J. Recycling of aluminium scrap for secondary Al-Si alloys. Waste Manag. Res. 2011, 29, 686–693. [Google Scholar] [CrossRef]
- Cagala, M.; Břuska, M.; Lichý, P.; Beňo, J.; Špirutová, N. Influence of aluminium-alloy remelting on the structure and mechanical properties. Mater. Technol. 2013, 2, 239–243. [Google Scholar]
- Matejka, M.; Bolibruchová, D. Influence of Remelting AlSi9Cu3 Alloy with Higher Iron Content on Mechanical Properties. Arch. Foundry Eng. 2018, 18, 25–30. [Google Scholar] [CrossRef]
- Kasińska, J.; Bolibruchová, D.; Matejka, M. The Influence of Remelting on the Properties of AlSi9Cu3 Alloy with Higher Iron Content. Materials 2020, 13, 575. [Google Scholar] [CrossRef] [Green Version]
- Matejka, M.; Bolibruchová, D.; Podprocká, R. The Influence of Returnable Material on Internal Homogeneity of the High-Pressure Die-Cast AlSi9Cu3(Fe) Alloy. Metals 2021, 11, 1084. [Google Scholar] [CrossRef]
- Kasińska, J.; Matejka, M.; Bolibruchová, D.; Kuriš, M.; Širanec, L. Effect of Returnable Material in Batch on Hot Tearing Tendency of AlSi9Cu3 Alloy. Materials 2021, 14, 1583. [Google Scholar] [CrossRef]
- Yorulmaz, A.; Erzi, E.; Gursoy, O.; Dispinar, D. End product rejection rate and its correlation with melt treatment in direct-chill casted hot rolling slabs. Int. J. Cast Met. Res. 2019, 32, 164–170. [Google Scholar] [CrossRef]
- Shi, M.; Li, Y. Performance Improvement in Aluminum Alloy Treated by Salt Flux with Different Fluorides. J. Mater. Eng. Perform. 2022, 1–8. [Google Scholar] [CrossRef]
- Ibragimov, V.E.; Bazhin, V.Y. Remelting of highly polluted metallic aluminium scrap with ecological refining reagents. IOP Conf. Ser. Mater. Sci. Eng. 2019, 537, 062087. [Google Scholar] [CrossRef]
- Çolak, M.; Kayikci, R.; Dispinar, D. Melt Cleanliness Comparison of Chlorine Fluxing and Ar Degassing of Secondary Al-4Cu. Metall. Mater. Trans. B 2016, 47, 2705–2709. [Google Scholar] [CrossRef]
- Jang, H.S.; Kang, H.J.; Park, J.Y.; Choi, Y.S.; Shin, S. Effects of Casting Conditions for Reduced Pressure Test on Melt Quality of Al-Si Alloy. Metals 2020, 10, 1422. [Google Scholar] [CrossRef]
- Bakedano, A.; Niklas, A.; Fernández-Calvo, A.I.; Plata, G.; Lozares, J.; Berlanga-Labari, C. Comparative Study of the Metallurgical Quality of Primary and Secondary AlSi10MnMg Aluminium Alloys. Metals 2021, 11, 1147. [Google Scholar] [CrossRef]
- Helbig, C.; Huether, J.; Joachimsthaler, C.; Lehmann, C.; Raatz, S.; Thorenz, A.; Faulstich, M.; Tuma, A. A terminology for downcycling. J. Ind. Ecol. 2022, 26, 1164–1174. [Google Scholar] [CrossRef]
- Koffler, C.; Florin, J. Tackling the Downcycling Issue—A Revised Approach to Value-Corrected Substitution in Life Cycle Assessment of Aluminum (VCS 2.0). Sustainability 2013, 5, 4546–4560. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Zhang, Z.; Hiraki, T.; Takeda, O.; Zhu, H.; Matsubae, K.; Nagasaka, T. A solid-state electrolysis process for upcycling aluminium scrap. Nature 2022, 606, 511–515. [Google Scholar] [CrossRef]
- Van den Eynde, S.; Bracquené, E.; Diaz-Romero, D.; Zaplana, I.; Engelen, B.; Duflou, J.R.; Peeters, J.R. Forecasting global aluminium flows to demonstrate the need for improved sorting and recycling methods. Waste Manag. 2022, 137, 231–240. [Google Scholar] [CrossRef]
- Gaustad, G.; Olivetti, E.; Kirchain, R. Improving aluminum recycling: A survey of sorting and impurity removal technologies. Resour. Conserv. Recycl. 2012, 58, 79–87. [Google Scholar] [CrossRef]
- Modaresi, R.; Løvik, A.N.; Müller, D.B. Component- and Alloy-Specific Modeling for Evaluating Aluminum Recycling Strategies for Vehicles. JOM 2014, 66, 2262–2271. [Google Scholar] [CrossRef] [Green Version]
- Stotz, P.M.; Niero, M.; Bey, N.; Paraskevas, D. Environmental screening of novel technologies to increase material circularity: A case study on aluminium cans. Resour. Conserv. Recycl. 2017, 127, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Reuter, M.A.; Schaik, A.v.; Gutzmer, J.; Bartie, N.; Abadías-Llamas, A. Challenges of the Circular Economy: A Material, Metallurgical, and Product Design Perspective. Annu. Rev. Mater. Res. 2019, 49, 253–274. [Google Scholar] [CrossRef]
- Nakajima, K.; Takeda, O.; Miki, T.; Matsubae, K.; Nakamura, S.; Nagasaka, T. Thermodynamic Analysis of Contamination by Alloying Elements in Aluminum Recycling. Environ. Sci. Technol. 2010, 44, 5594–5600. [Google Scholar] [CrossRef]
- DoITPoMS. The Ellingham Diagram. Available online: https://www.doitpoms.ac.uk/tlplib/ellingham_diagrams/ellingham.php (accessed on 1 November 2022).
- Stefániay, V.; Griger, Á.; Turmezey, T. Intermetallic phases in the aluminium-side corner of the AlFeSi-alloy system. J. Mater. Sci. 1987, 22, 539–546. [Google Scholar] [CrossRef]
- Moustafa, M.A. Effect of iron content on the formation of β-Al5FeSi and porosity in Al–Si eutectic alloys. J. Mater. Process. Technol. 2009, 209, 605–610. [Google Scholar] [CrossRef]
- Yan, F.; Kumar, S.; McKay, B.J.; O’Reilly, K.A.Q. Effect of Mn on Fe containing phase formation in high purity aluminium. Int. J. Cast Met. Res. 2014, 27, 202–206. [Google Scholar] [CrossRef]
- Ji, S.; Yang, W.; Gao, F.; Watson, D.; Fan, Z. Effect of iron on the microstructure and mechanical property of Al–Mg–Si–Mn and Al–Mg–Si diecast alloys. Mater. Sci. Eng. A 2013, 564, 130–139. [Google Scholar] [CrossRef]
- Ferraro, S.; Bjurenstedt, A.; Seifeddine, S. On the Formation of Sludge Intermetallic Particles in Secondary Aluminum Alloys. Metall. Mater. Trans. A 2015, 46, 3713–3722. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Doty, H.W.; Kaufman, M.J. The effects of Mn additions on the microstructure and mechanical properties of Al–Si–Cu casting alloys. Mater. Sci. Eng. A 2008, 488, 496–504. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, Y.; Xu, B.; Gu, C.; Wang, Y.; Tang, Q. Effect of Microstructure Evolution of Iron-Rich Intermetallic Compounds on Mechanical Property of Al–7Si–0.3Mg Casting Alloy with Low Iron Content. Metall. Mater. Trans. B 2022, 53, 548–560. [Google Scholar] [CrossRef]
- Modaresi, R.; Müller, D.B. The Role of Automobiles for the Future of Aluminum Recycling. Environ. Sci. Technol. 2012, 46, 8587–8594. [Google Scholar] [CrossRef]
- Dhinakar, A.; Lu, P.-Y.; Tang, N.-K.; Chen, J.-K. Iron Reduction in 356 Secondary Aluminum Alloy by Mn and Cr Addition for Sediment Separation. Int. J. Met. 2021, 15, 182–192. [Google Scholar] [CrossRef]
- Shaffer, B.; Auffhammer, M.; Samaras, C. Make electric vehicles lighter to maximize climate and safety benefits. Nature 2021, 598, 254–256. [Google Scholar] [CrossRef]
- ANSYS. GRANTA EduPack, Version 21.1.1; ANSYS Inc.: Canonsburg, PA, USA, 2021. [Google Scholar]
- Chen, R.; Shi, Y.-f.; Xu, Q.-y.; Liu, B.-c. Effect of cooling rate on solidification parameters and microstructure of Al-7Si-0.3Mg-0.15Fe alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 1645–1652. [Google Scholar] [CrossRef]
- Salas Reyes, A.E.; Altamirano Guerrero, G.; Rodríguez Ortiz, G.; Reyes Gasga, J.; García Robledo, J.F.; Lozada Flores, O.; Costa, P.S. Microstructural, microscratch and nanohardness mechanical characterization of secondary commercial HPDC AlSi9Cu3-type alloy. J. Mater. Res. Technol. 2020, 9, 8266–8282. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, D.; Wang, H.; Jia, Y.; Lin, B.; Tang, Y.; Tang, Y.; Shu, D.; Sun, Z.; Fu, Y.; et al. Revealing the influence of Fe on Fe-rich phases formation and mechanical properties of cast Al-Mg-Mn-Fe alloys. J. Alloys Compd. 2022, 901, 163666. [Google Scholar] [CrossRef]
- NP EN 1706 2000; Alumínio e ligas de alumínio: Produtos vazados: Composição química e características mecânicas. Instituto Português da Qualidade: Caparica, Portugal, 2000.
- Santamaría, J.A.; Sertucha, J.; Redondo, A.; Lizarralde, I.; Ochoa de Zabalegui, E.; Rodríguez, P. Towards the Prediction of Tensile Properties in Automotive Cast Parts Manufactured by LPDC with the A356.2 Alloy. Metals 2022, 12, 656. [Google Scholar] [CrossRef]
- Timelli, G.; Bonollo, F. The influence of Cr content on the microstructure and mechanical properties of AlSi9Cu3(Fe) die-casting alloys. Mater. Sci. Eng. A 2010, 528, 273–282. [Google Scholar] [CrossRef]
- Outmani, I.; FOUILLAND-PAILLE, L.; ISSELIN, J.; El Mansori, M. Effect of Si, Cu and processing parameters on Al-Si-Cu HPDC castings. J. Mater. Process. Technol. 2017, 249, 559–569. [Google Scholar] [CrossRef]
- Cecchel, S.; Panvini, A.; Cornacchia, G. Low Solution Temperature Heat Treatment of AlSi9Cu3(Fe) High-Pressure Die-Casting Actual Automotive Components. J. Mater. Eng. Perform. 2018, 27, 3791–3802. [Google Scholar] [CrossRef]
- Song, D.; Zhao, Y.; Jia, Y.; Li, R.; Zhou, N.; Zheng, K.; Fu, Y.; Zhang, W. Study of the evolution mechanisms of Fe-rich phases in Al-Si-Fe alloys with Mn modification using synchrotron X-ray imaging. J. Alloys Compd. 2022, 915, 165378. [Google Scholar] [CrossRef]
- Timelli, G.; Capuzzi, S.; Fabrizi, A. Precipitation of primary Fe-rich compounds in secondary AlSi9Cu3(Fe) alloys. J. Therm. Anal. Calorim. 2016, 123, 249–262. [Google Scholar] [CrossRef]
- Bolibruchová, D.; Brůna, M.; Macko, J. Elimination of Negative Effect of Fe in Secondary Alloys AlSi6Cu4 (En Ac 45 000, A 319) by Nickel. Arch. Metall. Mater. 2014, 59, 717–721. [Google Scholar] [CrossRef]
- Elsharkawi, E.A.; Samuel, E.; Samuel, A.M.; Samuel, F.H. Effects of Mg, Fe, Be additions and solution heat treatment on the π-AlMgFeSi iron intermetallic phase in Al–7Si–Mg alloys. J. Mater. Sci. 2010, 45, 1528–1539. [Google Scholar] [CrossRef]
- Elsharkawi, E.A.; Ibrahim, M.F.; Samuel, A.M.; Doty, H.W.; Samuel, F.H. Understanding the Effect of Be Addition on the Microstructure and Tensile Properties of Al–Si–Mg Cast Alloys. Int. J. Met. 2021, 16, 1777–1795. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Zhang, F.; Ma, Z.; Jia, L.; Yang, B.; Tao, T.; Zhang, H. Effect of cooling rate and Co content on the formation of Fe-rich intermetallics in hypoeutectic Al7Si0.3Mg alloy with 0.5%Fe. Mater. Charact. 2018, 139, 116–124. [Google Scholar] [CrossRef]
- Tocci, M.; Donnini, R.; Angella, G.; Pola, A. Effect of Cr and Mn addition and heat treatment on AlSi3Mg casting alloy. Mater. Charact. 2017, 123, 75–82. [Google Scholar] [CrossRef]
- Bolibruchová, D.; Richtárech, L. Elimination of Iron Based Particles in Al-Si Alloy. Arch. Foundry Eng. 2015, 15, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Timelli, G.; Fabrizi, A.; Capuzzi, S.; Bonollo, F.; Ferraro, S. The role of Cr additions and Fe-rich compounds on microstructural features and impact toughness of AlSi9Cu3(Fe) diecasting alloys. Mater. Sci. Eng. A 2014, 603, 58–68. [Google Scholar] [CrossRef]
- Dietrich, B.G.; Becker, H.; Smolka, M.; Keßler, A.; Leineweber, A.; Wolf, G. Intermetallic Sludge Formation in Fe Containing Secondary Al–Si Alloys Influenced by Cr and Mn as Preparative Tool for Metal Melt Filtration. Adv. Eng. Mater. 2017, 19, 1700161. [Google Scholar] [CrossRef] [Green Version]
- Aranda, V.A.; Figueroa, I.A.; González, G.; García-Hinojosa, J.A.; Lara-Rodríguez, G.A. Effect of Small Additions of Cr, Ti, and Mn on the Microstructure and Hardness of Al–Si–Fe–X Alloys. Metals 2019, 9, 136. [Google Scholar] [CrossRef] [Green Version]
- Petrič, M.; Zeka, B.; Mrvar, P.; Nagode, A.; Vončina, M.; Balaško, T. Solidification behaviour and microstructure of AlSi7Mg cast alloy with addition of Li. J. Mater. Res. Technol. 2022, 19, 2084–2092. [Google Scholar] [CrossRef]
- Jin, L.; Liu, K.; Chen, X.G. Evolution of Fe-Rich Intermetallics in Al-Si-Cu 319 Cast Alloy with Various Fe, Mo, and Mn Contents. Metall. Mater. Trans. B 2019, 50, 1896–1907. [Google Scholar] [CrossRef]
- Brůna, M.; Richtárech, L.; Bolibruchová, D.; Caiss, J. Influence of Nickel Addition on Properties of Secondary AlSi7Mg0.3 Alloy. Arch. Foundry Eng. 2015, 15, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.K.; Zhang, B.R. Effects of Zn on Microstructure Modification and Mechanical Properties Improvement of Al-Si-Cu-Mg Alloys. Metall. Mater. Trans. A 2020, 51, 4158–4167. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, X.; Wang, Z.; Chen, K.; Pan, S.; Zhu, Y.; Wang, Y. Synergistic influence of La and Zr on microstructure and mechanical performance of an Al-Si-Mg alloy at casting state. J. Alloys Compd. 2022, 902, 163829. [Google Scholar] [CrossRef]
- Jin, H.; Sui, Y.; Yang, Y.; Jiang, Y.; Wang, Q. Effect of Ce content on the microstructure and mechanical properties of squeeze-cast Al–5Mg-2.2Si-0.6Mn alloys. J. Mater. Res. Technol. 2022, 19, 1798–1804. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, J.; Cao, J.; Luo, L.; Guo, S.; Ou, L.; Liu, Z.; Bai, S. Effect of Minor Er Additions on the Microstructures and Mechanical Properties of Cast Al-Cu-Mg-Ag Alloys. Materials 2021, 14, 4212. [Google Scholar] [CrossRef]
- Liu, X.; Wang, B.; Li, Q.; Wang, J.; Zhang, C.; Xue, C.; Yang, X.; Tian, G.; Liu, X.; Tang, H. Quantifying the Effects of Grain Refiners Al-Ti-B and La on the Microstructure and Mechanical Properties of W319 Alloy. Metals 2022, 12, 627. [Google Scholar] [CrossRef]
- Li, D.; Cui, C.; Wang, X.; Wang, Q.; Chen, C.; Liu, S. Microstructure evolution and enhanced mechanical properties of eutectic Al–Si die cast alloy by combined alloying Mg and La. Mater. Des. 2016, 90, 820–828. [Google Scholar] [CrossRef]
- Uludağ, M.; Çetin, R.; Dispinar, D.; Tiryakioğlu, M. Effect of Degassing and Grain Refinement on Hot Tearing Tendency in Al8Si3Cu Alloy. Int. J. Met. 2018, 12, 589–595. [Google Scholar] [CrossRef]
- Tao, C.; Huang, H.; Yuan, X.; Yue, C.; Su, M.; Zuo, X. Effect of Y Element on Microstructure and Hot Tearing Sensitivity of As-Cast Al–4.4Cu–1.5Mg–0.15Zr Alloy. Int. J. Met. 2022, 16, 1010–1019. [Google Scholar] [CrossRef]
- Jang, H.S.; Lee, G.H.; Jeon, J.B.; Choi, Y.S.; Shin, S. Effect of ultrasonic melt treatment conditions on melt quality of Al–Mg alloy. J. Mater. Res. Technol. 2022, 19, 2645–2656. [Google Scholar] [CrossRef]
- Grilo, J.; Carneiro, V.H.; Teixeira, J.C.; Puga, H. Effect of Ultrasonic Melt Treatment on Solidification Behavior of Al7SiMg Alloy. Int. J. Met. 2022, 19, 2645–2656. [Google Scholar] [CrossRef]
- Kotadia, H.R.; Qian, M.; Das, A. Microstructural modification of recycled aluminium alloys by high-intensity ultrasonication: Observations from custom Al–2Si–2Mg–1.2Fe–(0.5,1.0)Mn alloys. J. Alloys Compd. 2020, 823, 153833. [Google Scholar] [CrossRef]
- Xuan, Y.; Liu, T.; Nastac, L.; Brewer, L.; Levin, I.; Arvikar, V. The Influence of Ultrasonic Cavitation on the Formation of Fe-Rich Intermetallics in A383 Alloy. Metall. Mater. Trans. A 2018, 49, 3346–3357. [Google Scholar] [CrossRef]
- So, T.I.; Jung, H.C.; Lee, C.D.; Shin, K.S. Effects of T6-treatment on the defect susceptibility of tensile strength to microporosity variation in low pressure die-cast A356 alloy. Met. Mater. Int. 2015, 21, 842–849. [Google Scholar] [CrossRef]
- Tillová, E.; Chalupová, M.; Kuchariková, L. Evolution of Phases in a Recycled Al-Si Cast Alloy During Solution Treatment. In Scanning Electron Microscopy; Kazmiruk, V., Ed.; InTech Open: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
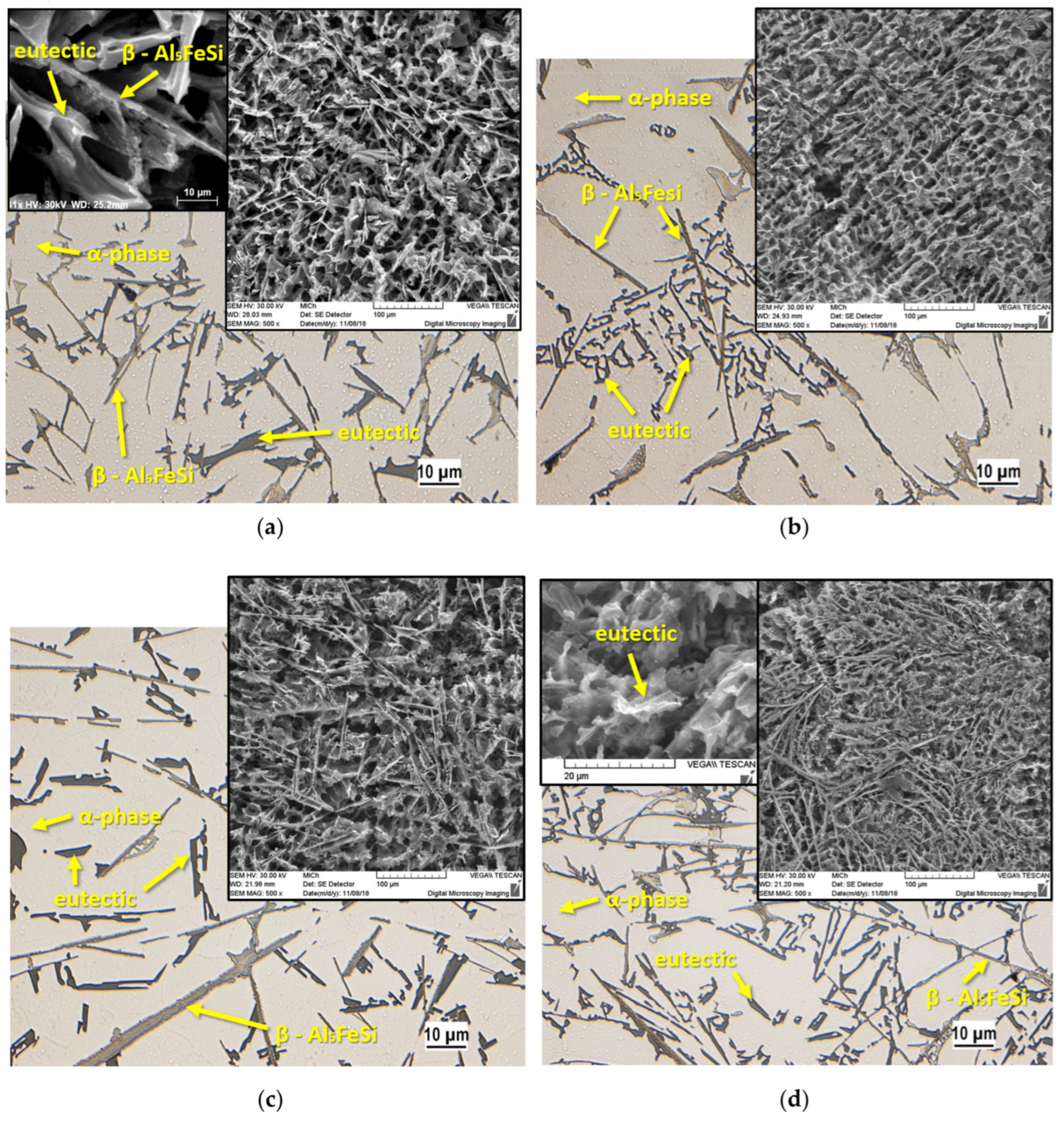

| θ-AlFe(Si) | α-AlFeSi | β-AlFeSi | ||||
|---|---|---|---|---|---|---|
| Si | Fe | Si | Fe | Si | Fe | |
| Non-equilibrium (as-cast state) | 1–6 | 35–42 | 6–13 | 31–35 | 13–16 | 25–29 |
| Equilibrium (heat-treated at 600 °C) | 1–5 | 36–41 | 6–9.5 | 32–36 | 14–16 | 27–28 |
| Example formula | Al3Fe or Al13Fe4 | Al8Fe2Si | Al5FeSi | |||
| Alloy | Al | Si | Mg | Cu | Fe | Mn | Cr | Ti | Zn | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| AlSi7Mg0.3(Fe) 356.0 | 90–93 | 6.5–7.5 | 0.2–0.45 | <0.25 | <0.6 | <0.35 | - | <0.25 | <0.35 | <0.15 |
| AlSi9Cu(Fe) A308 | 80–90 | 8–11 | 0.05–0.55 | 2–4 | <1.3 | <0.55 | <0.15 | <0.25 | <1.2 | Ni, Pb, Si, Sn |
| AlSi12 413.0 | 82–89 | 11–13 | <0.1 | <1 | <2 | <0.35 | - | - | <0.5 | <0.25 |
| AlZn3Mg1(Fe) 705.0 | 92–95 | <0.2 | 1.4–1.8 | <0.2 | <0.8 | 0.4–0.6 | 0.2–0.4 | <0.25 | 2.7–3.3 | <0.15 |
| Alloy | Class | Process | Fe (wt.%) | UTS (MPa) | YS (MPa) | A (%) | Reference |
|---|---|---|---|---|---|---|---|
| AlSi7Mg0.3 | NP EN 1706 | Gravity | <0.15 | min. 170 | min. 90 | min. 2.5 | [60] |
| Primary alloy | LPDC | ~0.1 | 304 ± 8 | 229 ± 8 | 11 ± 3 | [61] | |
| Gravity | 0.09 | 186 | 80 | 8 | [52] | ||
| Secondary alloy | Gravity | 0.2 | 151 | 69 | 5 | ||
| 0.33 | 167 | 75 | 6 | ||||
| AlSi9Cu3(Fe) | NP EN 1706 | HPDC | 0.6–1.1 | min. 240 | min. 140 | min. 1 | [60] |
| Secondary alloy | HPDC | 0.8 | 323 | 252 | 3.8 | [62] | |
| 0.8 | ~200 | 152 | 1 | [63] | |||
| 1.1 | 262 ± 3 | 158 ± 4 | 2 ± 0.1 | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, H.; Emadinia, O.; Soares, R.; Vieira, M.F.; Reis, A. Adding Value to Secondary Aluminum Casting Alloys: A Review on Trends and Achievements. Materials 2023, 16, 895. https://doi.org/10.3390/ma16030895
Nunes H, Emadinia O, Soares R, Vieira MF, Reis A. Adding Value to Secondary Aluminum Casting Alloys: A Review on Trends and Achievements. Materials. 2023; 16(3):895. https://doi.org/10.3390/ma16030895
Chicago/Turabian StyleNunes, Helder, Omid Emadinia, Rui Soares, Manuel F. Vieira, and Ana Reis. 2023. "Adding Value to Secondary Aluminum Casting Alloys: A Review on Trends and Achievements" Materials 16, no. 3: 895. https://doi.org/10.3390/ma16030895
APA StyleNunes, H., Emadinia, O., Soares, R., Vieira, M. F., & Reis, A. (2023). Adding Value to Secondary Aluminum Casting Alloys: A Review on Trends and Achievements. Materials, 16(3), 895. https://doi.org/10.3390/ma16030895









