Active Biohybrid Nanocomposite Films Made from Chitosan, ZnO Nanoparticles, and Stearic Acid: Optimization Study to Develop Antibacterial Films for Food Packaging Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
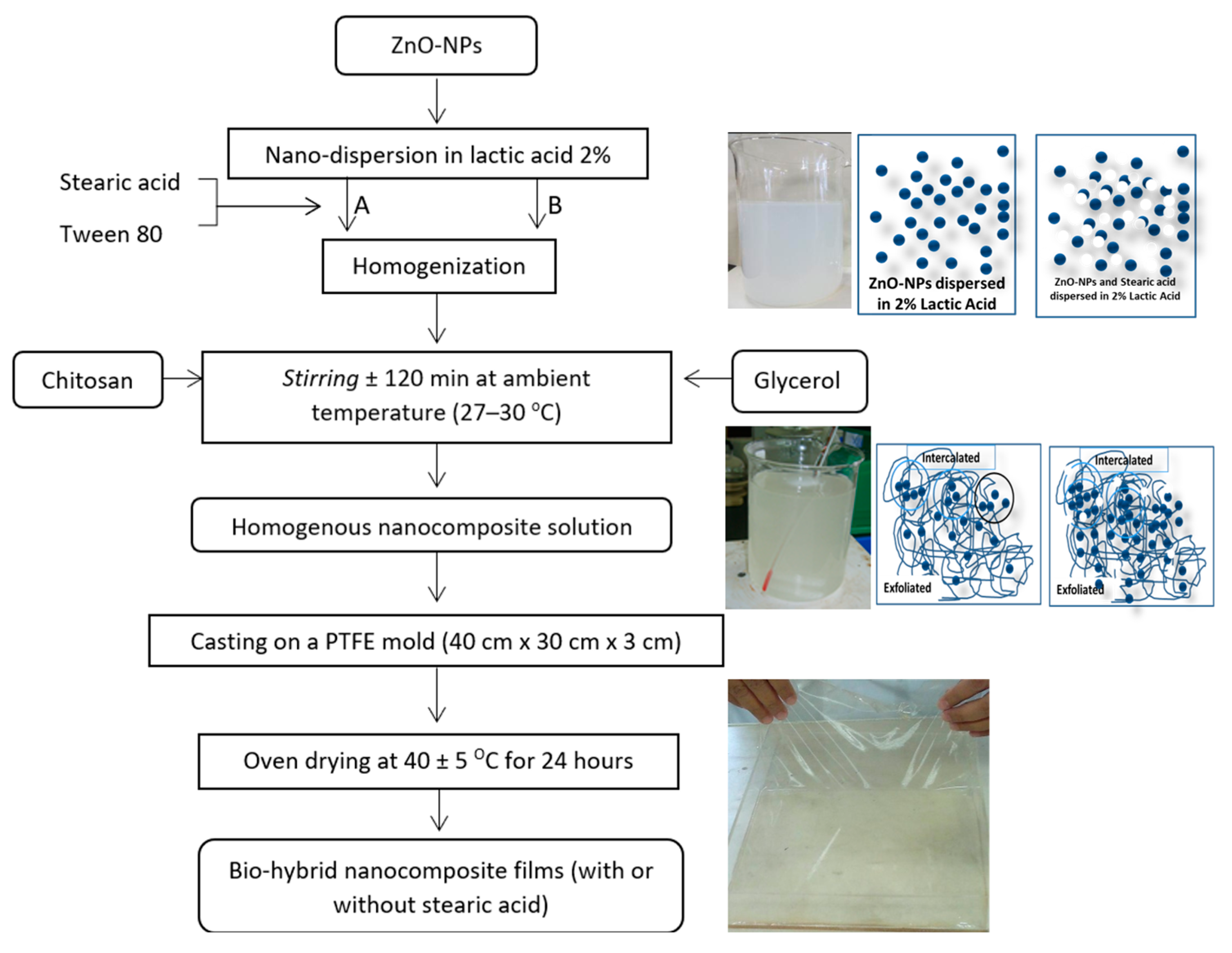
2.2. Nanocomposite Film/Coating Solution Preparation
2.3. Optimization Using a Full Factorial Design and Statistical Analysis
2.4. Analysis of Biohybrid Nanocomposite Films
2.4.1. Water Vapor Transmission Test Using Gravimetric Method
2.4.2. Mechanical Properties
2.4.3. Antibacterial Activity with Agar Well Diffusion Assay
2.4.4. Scanning Electron Microscopy
2.4.5. Thermal Analysis by DSC
2.4.6. FTIR Spectroscopy Analysis
2.4.7. Water Activity
3. Results and Discussion
3.1. Optimization Process with Full Factorial Design
3.1.1. Number of Runs and Multiple Responses for the Optimization
3.1.2. Analysis of Variance (ANOVA) and Independent Variables’ Effect on Multiple Responses
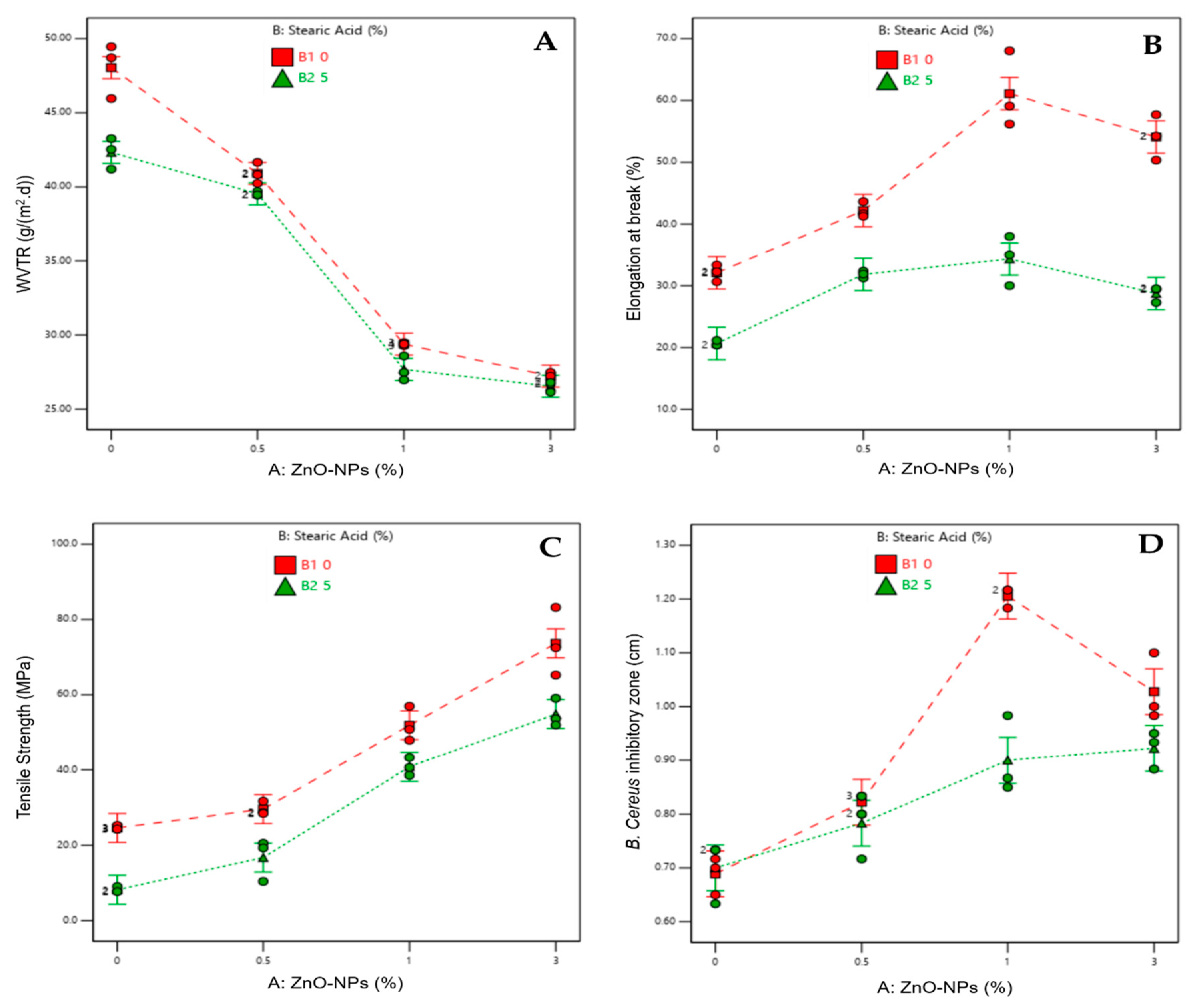
Main Factor and the Interaction Effects on WVTR
Main Factor and Interaction Effects on Mechanical Properties
Main Factor and Interaction Effects on Antibacterial Activity
3.1.3. Optimization of the Multiple Responses
3.1.4. Confirmation of the Optimum Formula
3.2. Films Morphology Observed by SEM
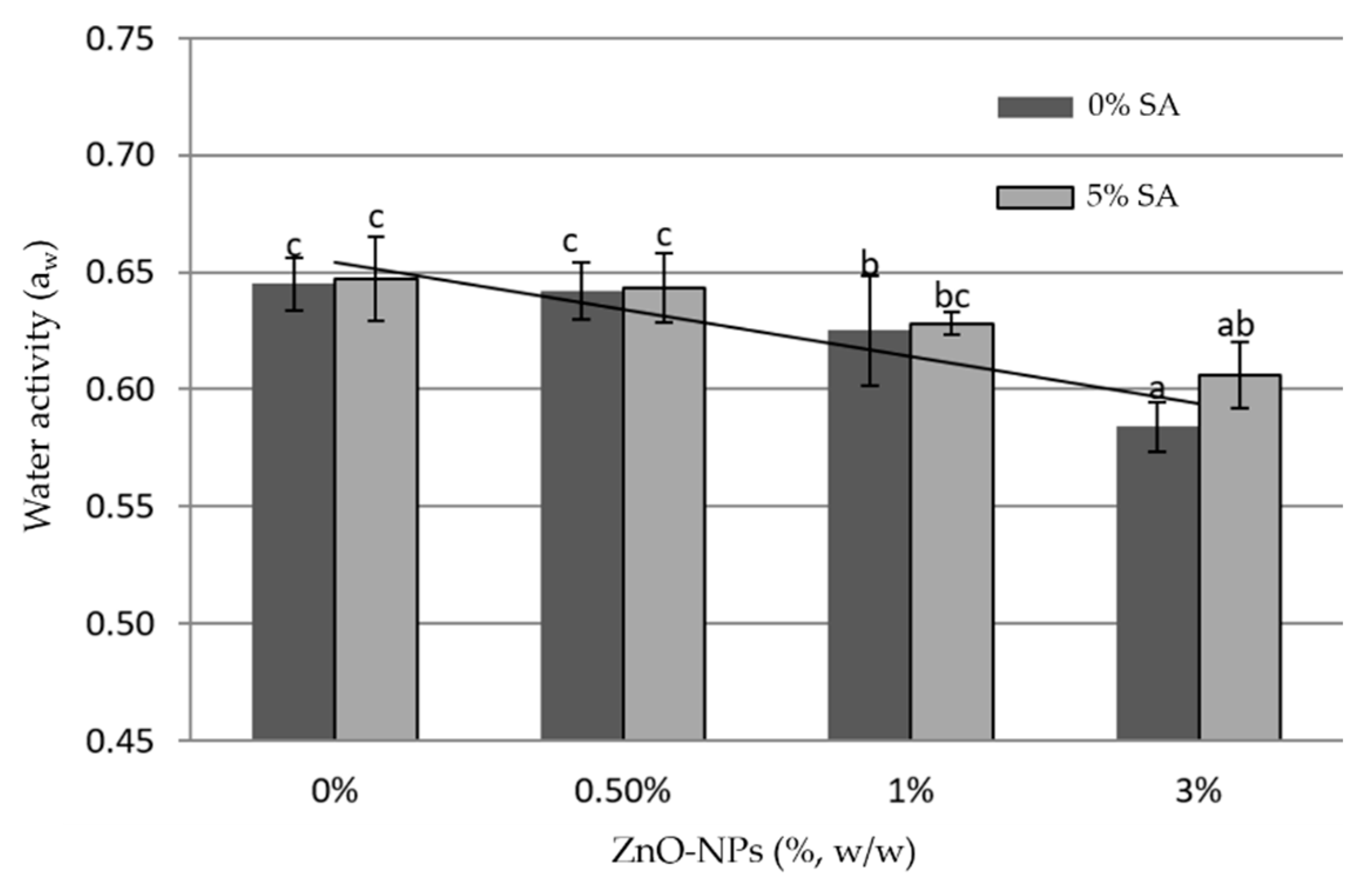
3.3. Water Activity
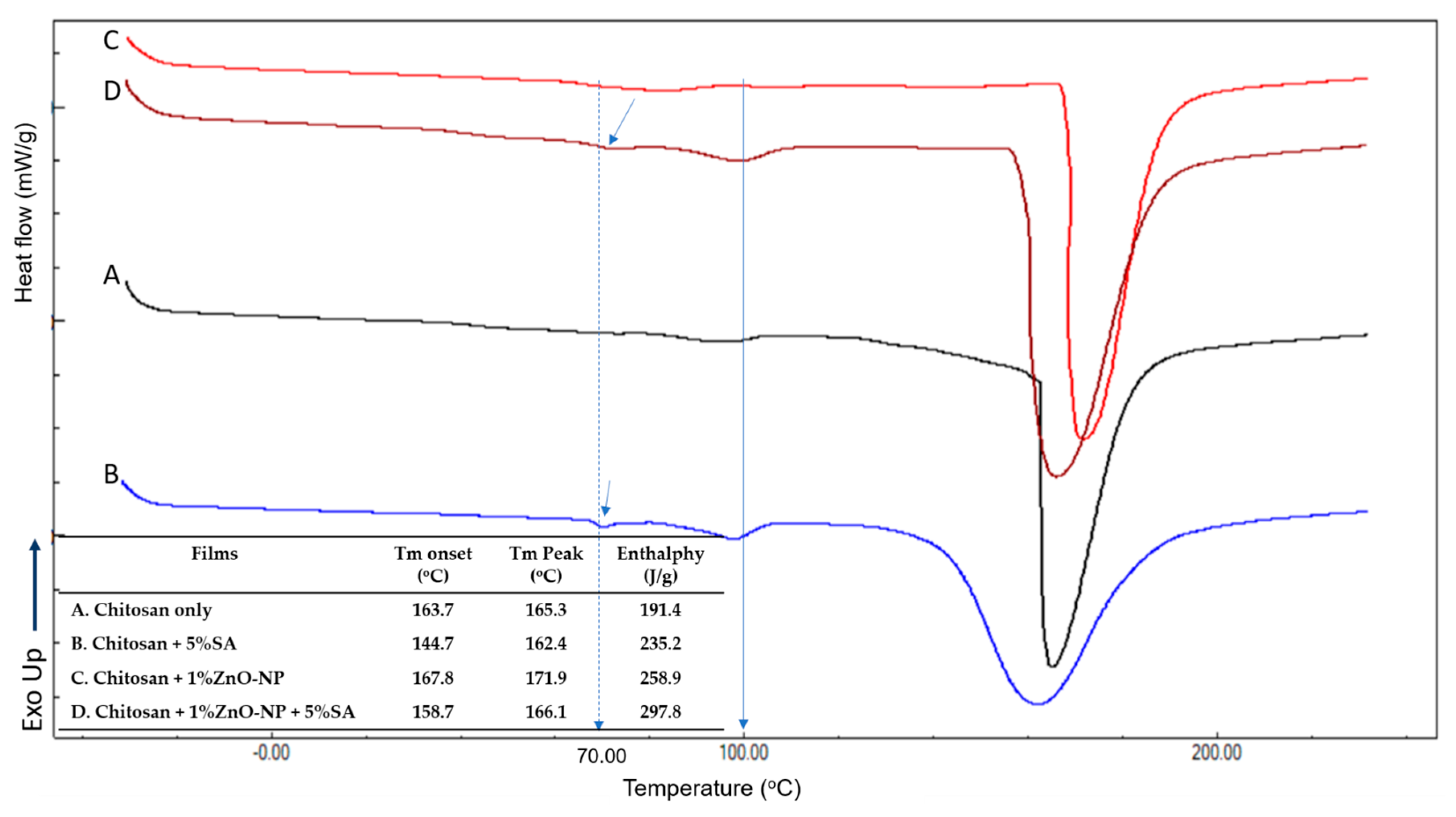
3.4. Thermal Properties
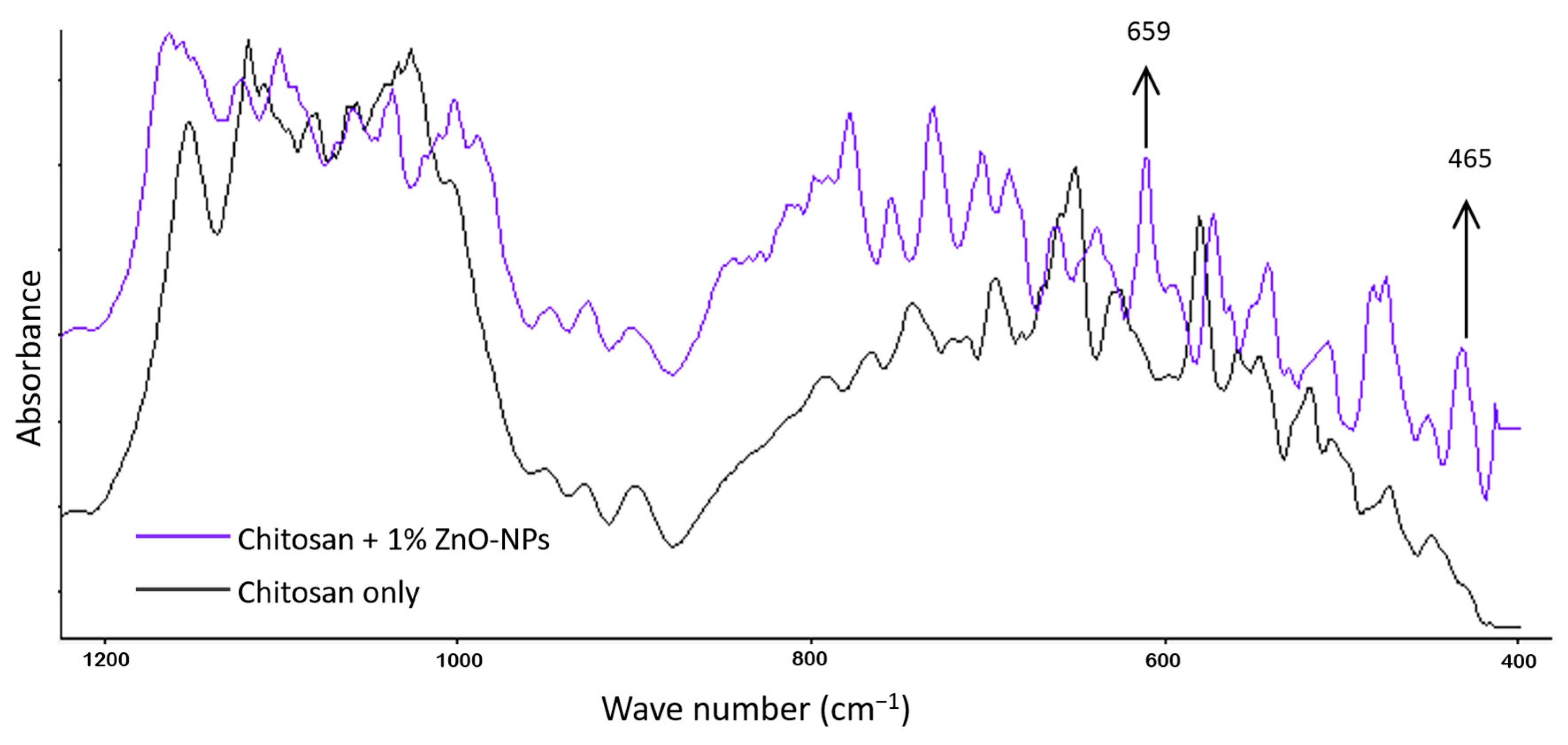
3.5. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA. Food Contact Materials. Available online: https://www.efsa.europa.eu/en/topics/topic/food-contact-materials (accessed on 6 December 2022).
- The Union of International Associations (UIA). Using Plastic Packaging for Food in The Encyclopedia of World Problems & Human Potential. Available online: http://encyclopedia.uia.org/en/strategy/211421 (accessed on 2 December 2022).
- Plastics Europe. Plastics–The Facts 2020. Available online: https://plasticseurope.org/fr/knowledge-hub/plastics-the-facts-2020/ (accessed on 5 December 2022).
- Macena, M.W.; Carvalho, R.; Cruz-Lopes, L.P.; Guiné, R.P.F. Plastic food packaging: Perceptions and attitudes of portuguese consumers about environmental impact and recycling. Sustainability 2021, 13, 9953. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Fattahi, E.; Cerruti, P.; Santagata, G. Edible polymers and secondary bioactive compounds for food packaging applications: Antimicrobial, mechanical, and gas barrier properties. Polymers 2022, 14, 2395. [Google Scholar] [CrossRef] [PubMed]
- Janik, W.; Nowotarski, M.; Shyntum, D.Y.; Banaś, A.; Krukiewicz, K.; Kudła, S.; Dudek, G. Antibacterial and biodegradable polysaccharide-based films for food packaging applications: Comparative study. Materials 2022, 15, 3236. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Alves, M.M.; Santos, C.F.; Ribeiro, I.A.C.; Rodrigues, C.; Coelhoso, I.; Fernando, A.L. Biodegradable chitosan films with ZnO nanoparticles synthesized using food industry byproducts—Production and characterization. Coatings 2021, 11, 646. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Wang, Z.; Cai, R.; Yue, T.; Cui, L. Preparation and characterization of chitosan-nano-ZnO composite films for preservation of cherry tomatoes. Foods 2021, 10, 3135. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Sorrentino, A. Nanohybrid active fillers in food contact bio-based materials. In Bionanocomposites for Packaging Applications; Springer: Berlin/Heidelberg, Germany, 2017; pp. 71–94. [Google Scholar] [CrossRef]
- Aji, A.I.; Suyatma, N.E.; Arpah, M.; Jayanegara, A. Evaluation of different parameters of ZnO nanoparticles on bionanocomposite film properties: A meta-analysis. Int. J. Food Sci. Technol. 2022. Accepted. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.d.F.F.; Coimbra, J.S.d.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Select Committee on GRAS Substances Database. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=SCOGS (accessed on 29 November 2022).
- Siddiqi, K.S.; ur Rahman, A.; Tajuddin; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Jayasuriya, A.C.; Aryaei, A.; Jayatissa, A.H. ZnO nanoparticles induced effects on nanomechanical behavior and cell viability of chitosan films. Mater. Sci. Eng. C 2013, 33, 3688–3696. [Google Scholar] [CrossRef]
- Rahman, P.M.; Mujeeb, V.M.A.; Muraleedharan, K. Flexible chitosan-nano ZnO antimicrobial pouches as a new material for extending the shelf life of raw meat. Int. J. Biol. Macromol. 2017, 97, 382–391. [Google Scholar] [CrossRef]
- Wardana, A.A.; Suyatma, N.E.; Muchtadi, T.R.; Yuliani, S. Influence of zno nanoparticles and stearic acid on physical, mechanical and structural properties of cassava starch-based bionanocomposite edible films. Int. Food Res. J. 2018, 25, 837–1844. [Google Scholar]
- Reddy, C.K.; Choi, S.M.; Lee, D.-J.; Lim, S.-T. Complex formation between starch and stearic acid: Effect of enzymatic debranching for starch. Food Chem. 2018, 244, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.C.R.; Porto, L.M.; Laurindo, J.B.; Menegalli, F.C. Water vapor barrier and mechanical properties of starch films containing stearic acid. Ind. Crops Prod. 2013, 41, 227–234. [Google Scholar] [CrossRef]
- Shi, S.C.; Peng, Y.Q. Preparation and tribological studies of stearic acid-modified biopolymer coating. Prog. Org. Coat. 2020, 138, 105304. [Google Scholar] [CrossRef]
- Karnnet, S.; Potiyaraj, P.; Pimpan, V. Preparation and properties of biodegradable stearic acid-modified gelatin films. Polym. Degrad. Stab. 2005, 90, 106–110. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial Fatty Acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef]
- StatEase. Multilevel Categoric Factorial. Available online: https://www.statease.com/docs/v13/tutorials/general-multilevel-categoric-factorial/ (accessed on 29 November 2022).
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.L.; Hu, C.; Shao, L.Q. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.; Deng, J.; Yang, F.; Gong, Y.; Zhao, N.; Zhang, X. Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials 2003, 24, 2871–2880. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Shahvalizadeh, R.; Ahmadi, R.; Davandeh, I.; Pezeshki, A.; Moslemi, S.A.S.; Karimi, S.; Rahimi, M.; Hamishehkar, H.; Mohammadi, M. Antimicrobial bio-nanocomposite films based on gelatin, tragacanth, and zinc oxide nanoparticles–microstructural, mechanical, thermo-physical, and barrier properties. Food Chem. 2021, 354, 129492. [Google Scholar] [CrossRef] [PubMed]
- Erol, I.; Hazman, O.; Aksu, M.; Bulut, E. Synergistic effect of ZnO nanoparticles and hesperidin on the antibacterial properties of chitosan. J. Biomater. Sci. Polym. Ed. 2022, 33, 1973–1997. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Ma, X.; Wang, X.; Yao, J. Effect of water on the thermal transition in chitosan films. Polym. Cryst. 2019, 2, e10092. [Google Scholar] [CrossRef]
- Carvalho, J.D.S.; Rabelo, R.S.; Hubinger, M.D. Thermo-rheological properties of chitosan hydrogels with hydroxypropyl methylcellulose and methylcellulose. Int. J. Biol. Macromol. 2022, 209, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Singleton, W.S.; Ward, T.L.; Dollear, F.G. Physical properties of fatty acids. I. Some dilatometric and thermal properties of stearic acid in two polymorphic forms. J. Am. Oil Chem. Soc. 1950, 27, 143–146. [Google Scholar] [CrossRef]
- INCHEM. Stearic acid: Physical & Chemical Information. Available online: https://inchem.org/documents/icsc/icsc/eics0568.htm (accessed on 4 December 2022).
- Dong, Y.; Ruan, Y.; Wang, H.; Zhao, Y.; Bi, D. Studies on glass transition temperature of chitosan with four techniques. J. Appl. Polym. Sci. 2004, 93, 1553–1558. [Google Scholar] [CrossRef]
- Akalin, G.O.; Oztuna Taner, O.; Taner, T. The preparation, characterization and antibacterial properties of chitosan/pectin silver nanoparticle films. Polym. Bull. 2022, 79, 3495–3512. [Google Scholar] [CrossRef]
- Cervera, M.F.; Heinämäki, J.; Räsänen, M.; Maunu, S.L.; Karjalainen, M.; Acosta, O.M.N.; Colarte, A.I.; Yliruusi, J. Solid-state characterization of chitosans derived from lobster chitin. Carbohyd. Polym. 2004, 58, 401–408. [Google Scholar] [CrossRef]
- Shuai, X.; He, Y.; Asakawa, N.; Inoue, Y. Miscibility and phase structure of binary blends of poly(L-lactide) and poly(vinyl alcohol). J. Appl. Polym. Sci. 2001, 81, 762–772. [Google Scholar] [CrossRef]
- Abdelhady, M. Preparation and characterization of chitosan/zinc oxide nanoparticles for imparting antimicrobial and UV protection to cotton fabric. Int. J. Carbohydr. Chem. 2012, 2012, 840591. [Google Scholar] [CrossRef]


| Run Number ** | Independent Variables | Dependent Variables (Responses for Optimization) | ||||||
|---|---|---|---|---|---|---|---|---|
| ZnO-NPs | SA | WVTR | %EB | TS | Inhibitory Zone (cm) | |||
| (%, w/w) | (%, w/w) | (g/m2/day) | (kPa) | B. cereus | S. aureus | E. coli | ||
| 1 | 1 | 5 | 28.59 | 38 | 40.6 | 0.98 | 0.75 | 1.08 |
| 2 | 3 | 0 | 26.98 | 54.2 | 72.5 | 1.10 | 1.03 | 1.13 |
| 3 | 0 | 5 | 43.26 | 20.4 | 7.8 | 0.73 | 0.77 | 0.75 |
| 4 | 3 | 5 | 26.8 | 29.5 | 52 | 0.93 | 0.88 | 0.85 |
| 5 | 1 | 0 | 29.48 | 68.0 | 50.8 | 1.22 | 1.15 | 1.15 |
| 6 | 0 | 0 | 48.7 | 32.3 | 24.3 | 0.72 | 0.82 | 0.68 |
| 7 | 3 | 0 | 27.23 | 50.3 | 83.2 | 1.00 | 0.97 | 1.12 |
| 8 | 1 | 0 | 29.29 | 59.1 | 56.9 | 1.22 | 1.23 | 1.18 |
| 9 | 0.5 | 0 | 40.24 | 41.3 | 28.5 | 0.80 | 0.97 | 1.00 |
| 10 | 1 | 5 | 27.48 | 35.0 | 43.3 | 0.87 | 0.85 | 1.07 |
| 11 | 1 | 0 | 29.39 | 56.2 | 47.9 | 1.18 | 1.10 | 1.20 |
| 12 | 3 | 0 | 27.48 | 57.7 | 65.2 | 0.98 | 1.17 | 1.02 |
| 13 | 0.5 | 5 | 39.44 | 31.3 | 20.5 | 0.83 | 0.82 | 0.82 |
| 14 | 0 | 0 | 45.96 | 33.3 | 24.3 | 0.65 | 0.72 | 0.63 |
| 15 | 0 | 5 | 41.2 | 20.4 | 9.1 | 0.63 | 0.72 | 0.73 |
| 16 | 0.5 | 5 | 39.7 | 32.4 | 10.4 | 0.72 | 0.87 | 0.88 |
| 17 | 0 | 0 | 49.45 | 30.6 | 25.3 | 0.70 | 0.83 | 0.68 |
| 18 | 0.5 | 0 | 40.83 | 41.7 | 31.7 | 0.83 | 1.22 | 0.9 |
| 19 | 1 | 5 | 26.98 | 30 | 38.6 | 0.85 | 0.68 | 0.92 |
| 20 | 0 | 5 | 42.53 | 21.2 | 7.7 | 0.73 | 0.70 | 0.70 |
| 21 | 0.5 | 0 | 41.66 | 43.6 | 28.7 | 0.83 | 1.08 | 1.02 |
| 22 | 3 | 5 | 26.15 | 29.4 | 59.1 | 0.88 | 0.98 | 0.83 |
| 23 | 0.5 | 5 | 39.47 | 31.9 | 19.3 | 0.80 | 0.95 | 0.83 |
| 24 | 3 | 5 | 26.69 | 27.3 | 53.6 | 0.95 | 0.78 | 0.85 |
| Response Parameter | p-Value FFD Model | p-Value Factor A | p-Value Factor B | p-Value Inter. AB | Adj-R2 Model | Pred-R2 Model | Adeq Precision |
|---|---|---|---|---|---|---|---|
| WVTR | <0.0001 **** | <0.0001 **** | <0.0001 **** | 0.0005 **** | 0.9887 | 0.9824 | 43.4378 |
| %EB | <0.0001 **** | <0.0001 **** | <0.0001 **** | 0.0002 **** | 0.9472 | 0.9173 | 23.1187 |
| TS | <0.0001 **** | <0.0001 **** | <0.0001 **** | 0.4524 | 0.9563 | 0.9316 | 25.5774 |
| B. Cereus | <0.0001 **** | <0.0001 **** | <0.0001 **** | 0.0002 **** | 0.9174 | 0.8707 | 18.1951 |
| S. aureus | <0.0001 **** | 0.0008 **** | <0.0001 **** | 0.0226 ** | 0.7565 | 0.6188 | 8.8458 |
| E. coli | <0.0001 **** | <0.0001 **** | <0.0001 **** | 0.0006 **** | 0.9188 | 0.8729 | 17.788 |
| Variable Name | Goal | Lower Limit | Upper Limit | Importance |
|---|---|---|---|---|
| A: ZnO-NP | in range | 0 | 3 | 3 |
| B: Stearic Acid | in range | 0 | 5 | 3 |
| WVTR | minimize | 26.15 | 49.45 | 5 |
| %EB | maximize | 20.41 | 68.02 | 5 |
| Tensile Strength | in range | 7.71 | 83.21 | 3 |
| B. cereus | maximize | 0.63 | 1.22 | 5 |
| S. aereus | maximize | 0.68 | 1.23 | 5 |
| E. coli | maximize | 0.63 | 1.20 | 5 |
| Solution No. | ZnO-NP | SA | WVTR | %EB | TS | B. cereus | S. aereus | E. coli | Desirability |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 29.386 | 61.095 | 51.875 | 1.206 | 1.161 | 1.178 | 0.904 |
| 2 | 3 | 0 | 27.228 | 54.093 | 73.651 | 1.028 | 1.056 | 1.089 | 0.757 |
| 3 | 0.5 | 0 | 40.909 | 42.191 | 29.637 | 0.822 | 1.089 | 0.972 | 0.474 |
| 4 | 1 | 5 | 27.681 | 34.333 | 40.838 | 0.9 | 0.761 | 1.022 | 0.414 |
| 5 | 3 | 5 | 26.55 | 28.731 | 54.873 | 0.922 | 0.883 | 0.844 | 0.41 |
| 6 | 0.5 | 5 | 39.538 | 31.829 | 16.749 | 0.783 | 0.878 | 0.844 | 0.322 |
| 7 | 0 | 0 | 48.037 | 32.079 | 24.614 | 0.689 | 0.789 | 0.667 | 0.11 |
| 8 | 0 | 5 | 42.33 | 20.657 | 8.199 | 0.7 | 0.729 | 0.728 | 0.076 |
| Run | WVTR | %EB | TS | B. cereus | S. aureus | E. coli |
|---|---|---|---|---|---|---|
| 1 | 28.52 | 63.4 | 51.9 | 1.21 | 1.32 | 1.30 |
| 2 | 31.17 | 62.6 | 52.3 | 1.38 | 1.32 | 1.09 |
| 3 | 30.75 | 61.4 | 52.4 | 1.28 | 1.31 | 1.10 |
| 4 | 29.84 | 66.2 | 49.9 | 1.17 | 1.30 | 0.99 |
| 5 | 31.79 | 62.7 | 48.0 | 1.26 | 1.28 | 1.12 |
| 6 | 30.28 | 65.2 | 50.6 | 1.34 | 1.39 | 1.23 |
| Response | Number of Replications | Prediction Value | 95% PI Low | Data Mean of 6 Replications | 95% PI High |
|---|---|---|---|---|---|
| WVTR | 6 | 29.39 | 28.1 | 30.39 | 30.67 |
| %EB | 6 | 61.1 | 56.6 | 63.6 | 65.6 |
| TS | 6 | 51.9 | 45.2 | 50.8 | 58.5 |
| B. cereus | 6 | 1.21 | 1.13 | 1.27 | 1.28 |
| S. aereus | 6 | 1.16 | 1.03 | 1.32 | 1.29 |
| E. coli | 6 | 1.18 | 1.1 | 1.14 | 1.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suyatma, N.E.; Gunawan, S.; Putri, R.Y.; Tara, A.; Abbès, F.; Hastati, D.Y.; Abbès, B. Active Biohybrid Nanocomposite Films Made from Chitosan, ZnO Nanoparticles, and Stearic Acid: Optimization Study to Develop Antibacterial Films for Food Packaging Application. Materials 2023, 16, 926. https://doi.org/10.3390/ma16030926
Suyatma NE, Gunawan S, Putri RY, Tara A, Abbès F, Hastati DY, Abbès B. Active Biohybrid Nanocomposite Films Made from Chitosan, ZnO Nanoparticles, and Stearic Acid: Optimization Study to Develop Antibacterial Films for Food Packaging Application. Materials. 2023; 16(3):926. https://doi.org/10.3390/ma16030926
Chicago/Turabian StyleSuyatma, Nugraha Edhi, Sanjaya Gunawan, Rani Yunia Putri, Ahmed Tara, Fazilay Abbès, Dwi Yuni Hastati, and Boussad Abbès. 2023. "Active Biohybrid Nanocomposite Films Made from Chitosan, ZnO Nanoparticles, and Stearic Acid: Optimization Study to Develop Antibacterial Films for Food Packaging Application" Materials 16, no. 3: 926. https://doi.org/10.3390/ma16030926





