A p–n Junction by Coupling Amine-Enriched Brookite–TiO2 Nanorods with CuxS Nanoparticles for Improved Photocatalytic CO2 Reduction
Abstract
1. Introduction
2. Experimental
2.1. Reagents
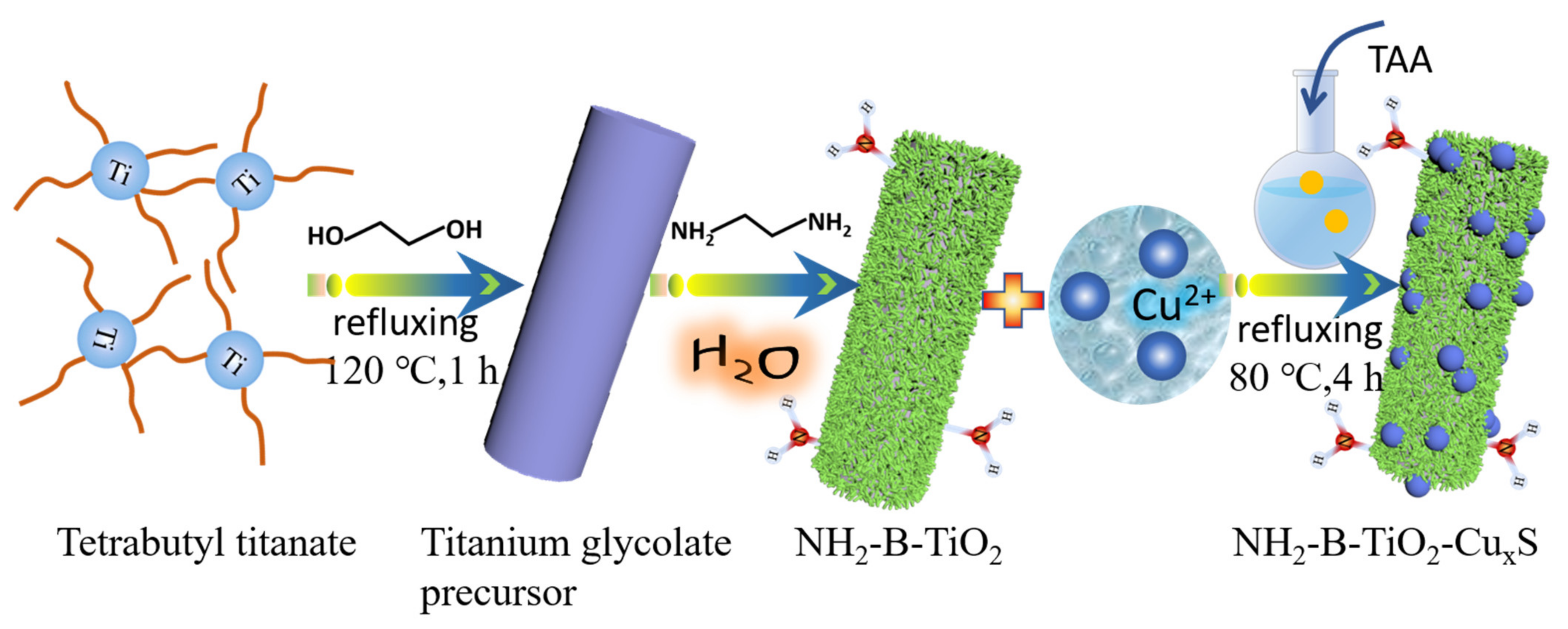
2.2. Materials Synthesis
2.3. Characterization of Photocatalysts
2.4. Evaluation of Photocatalytic CO2 Reduction
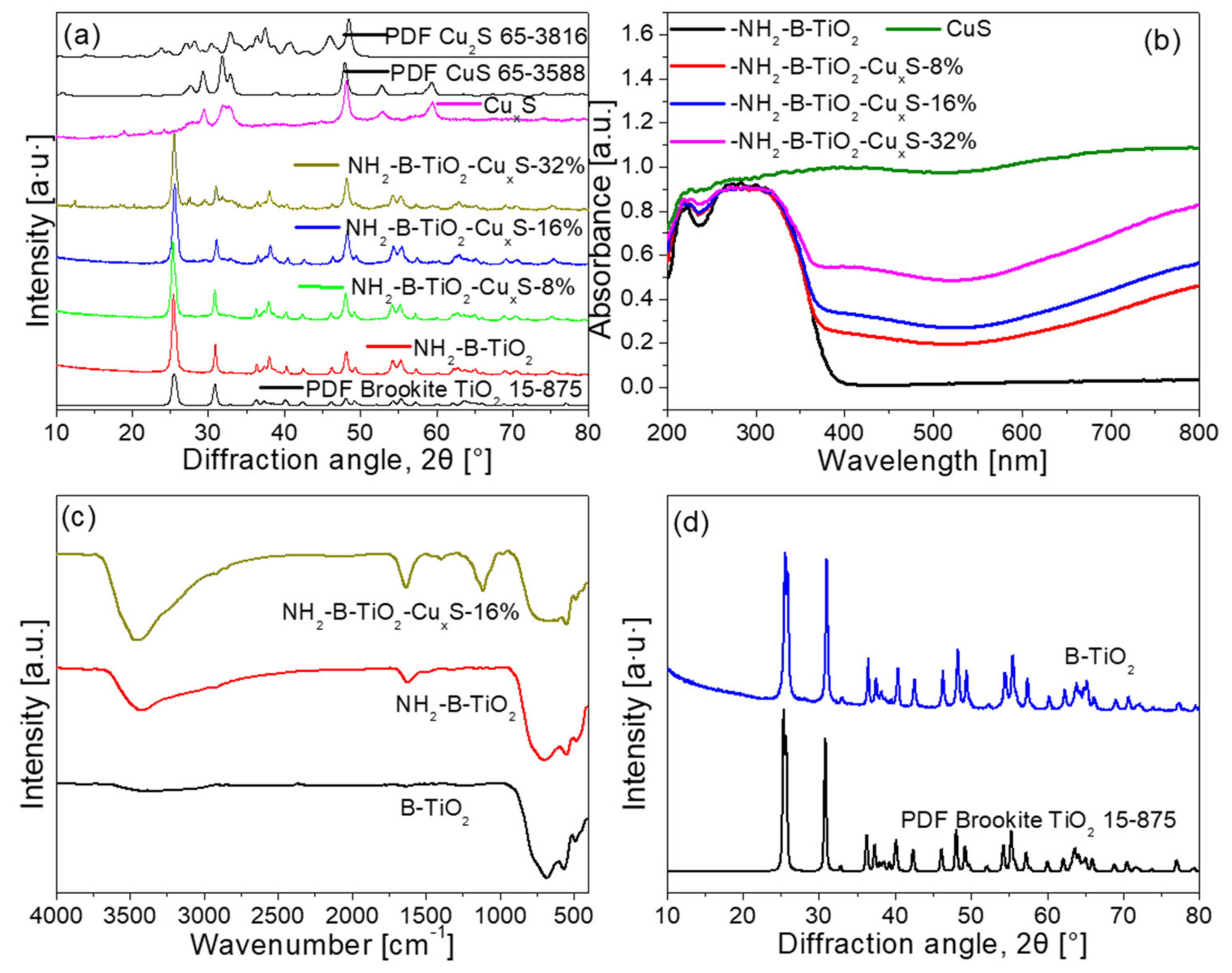
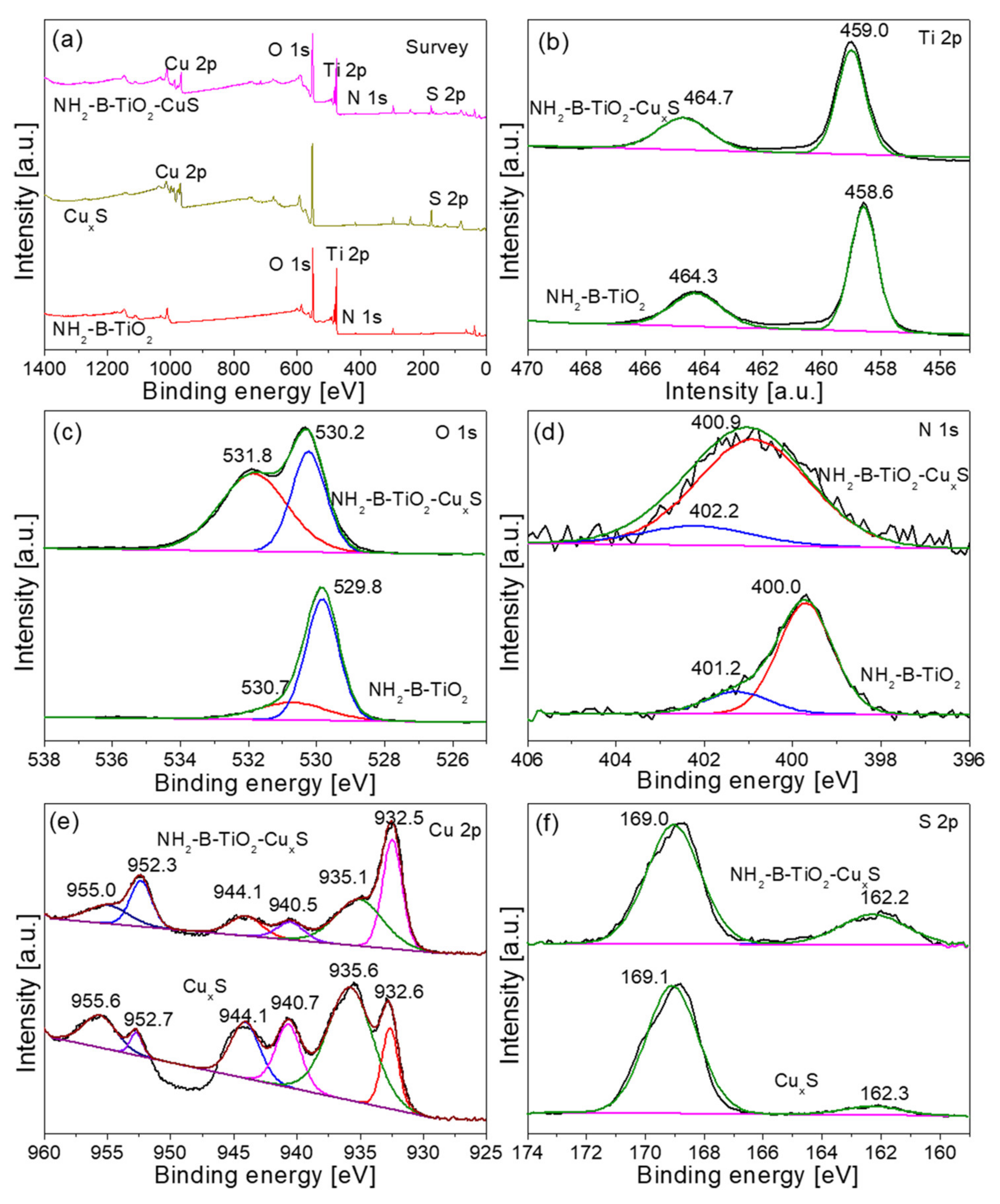
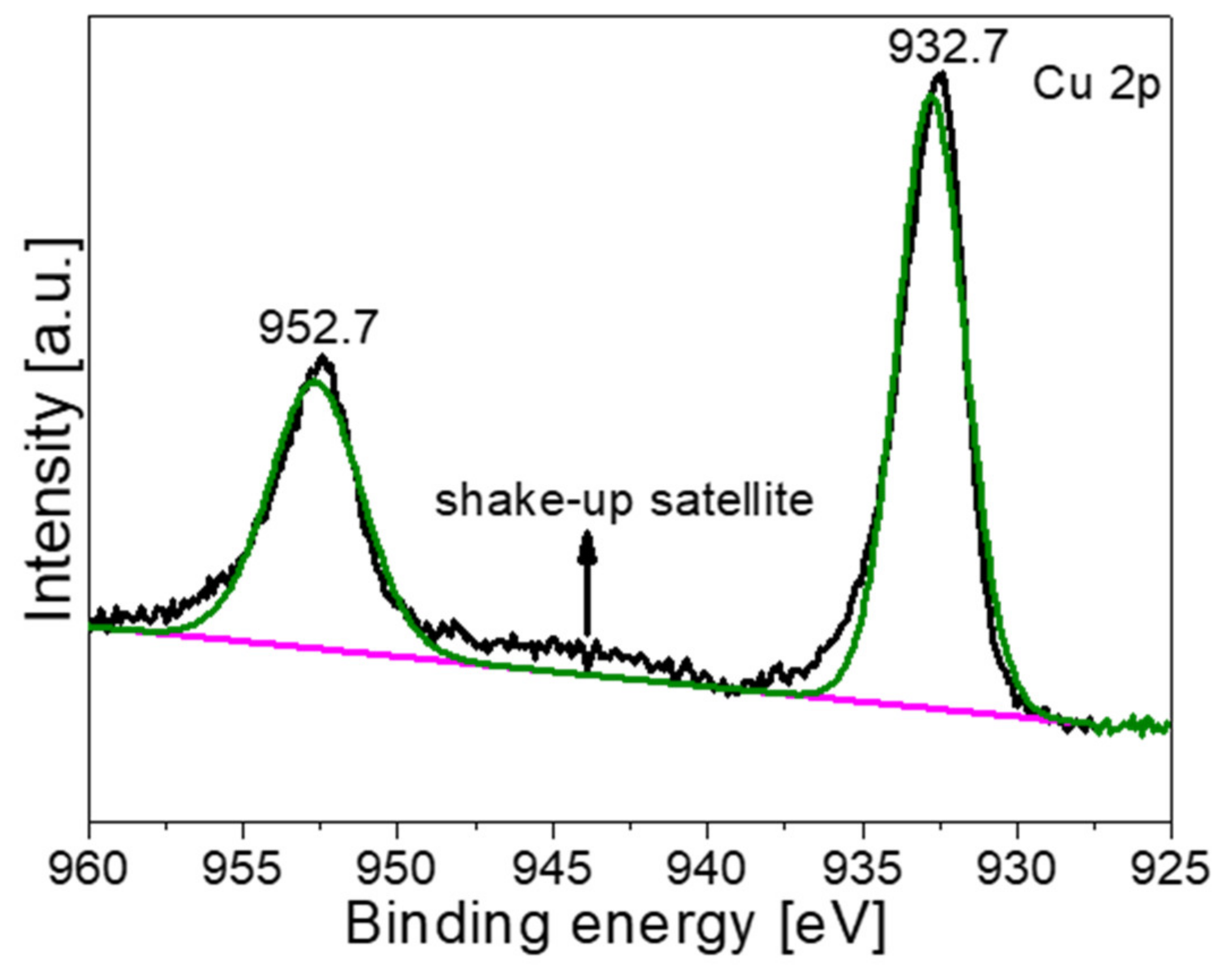
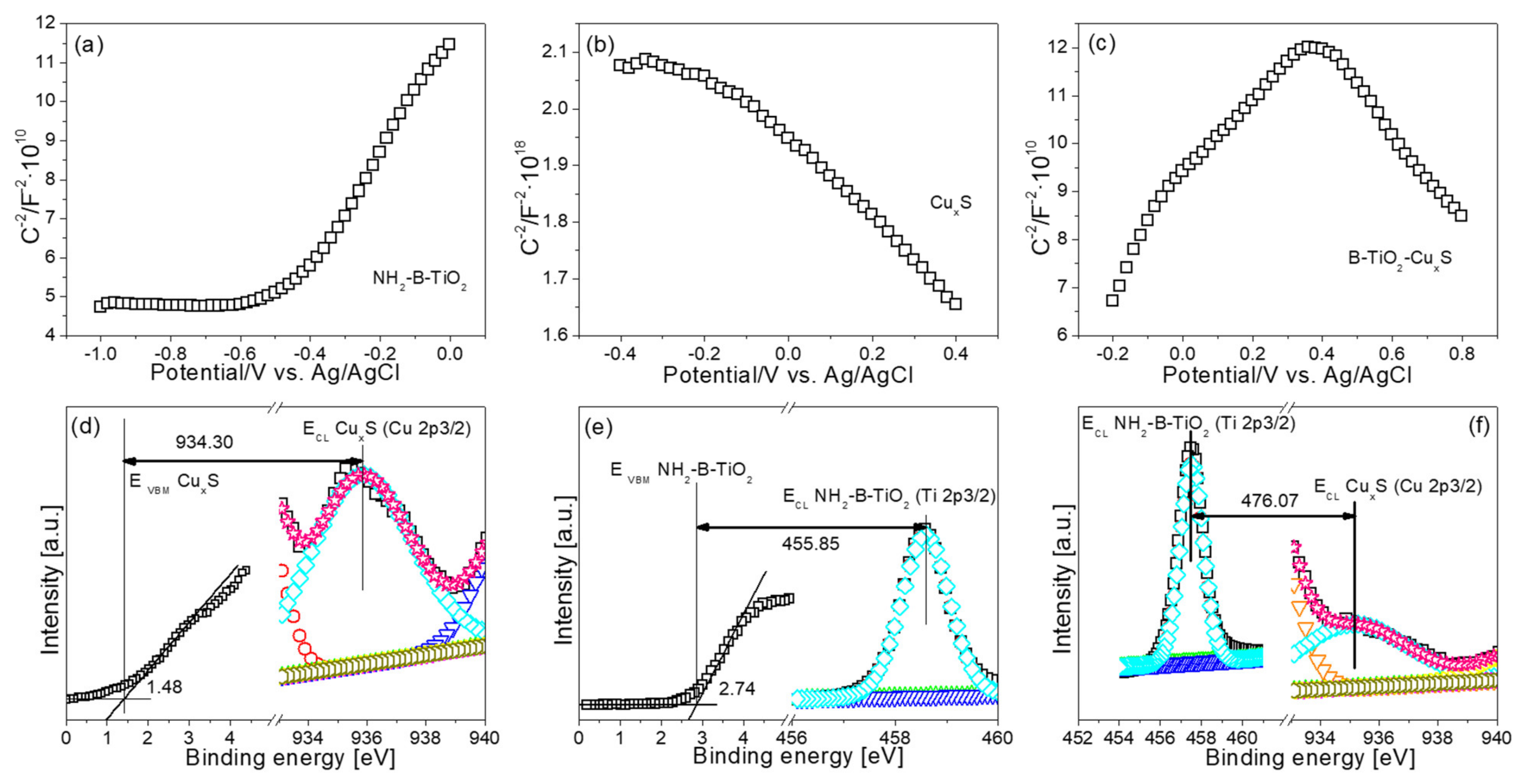
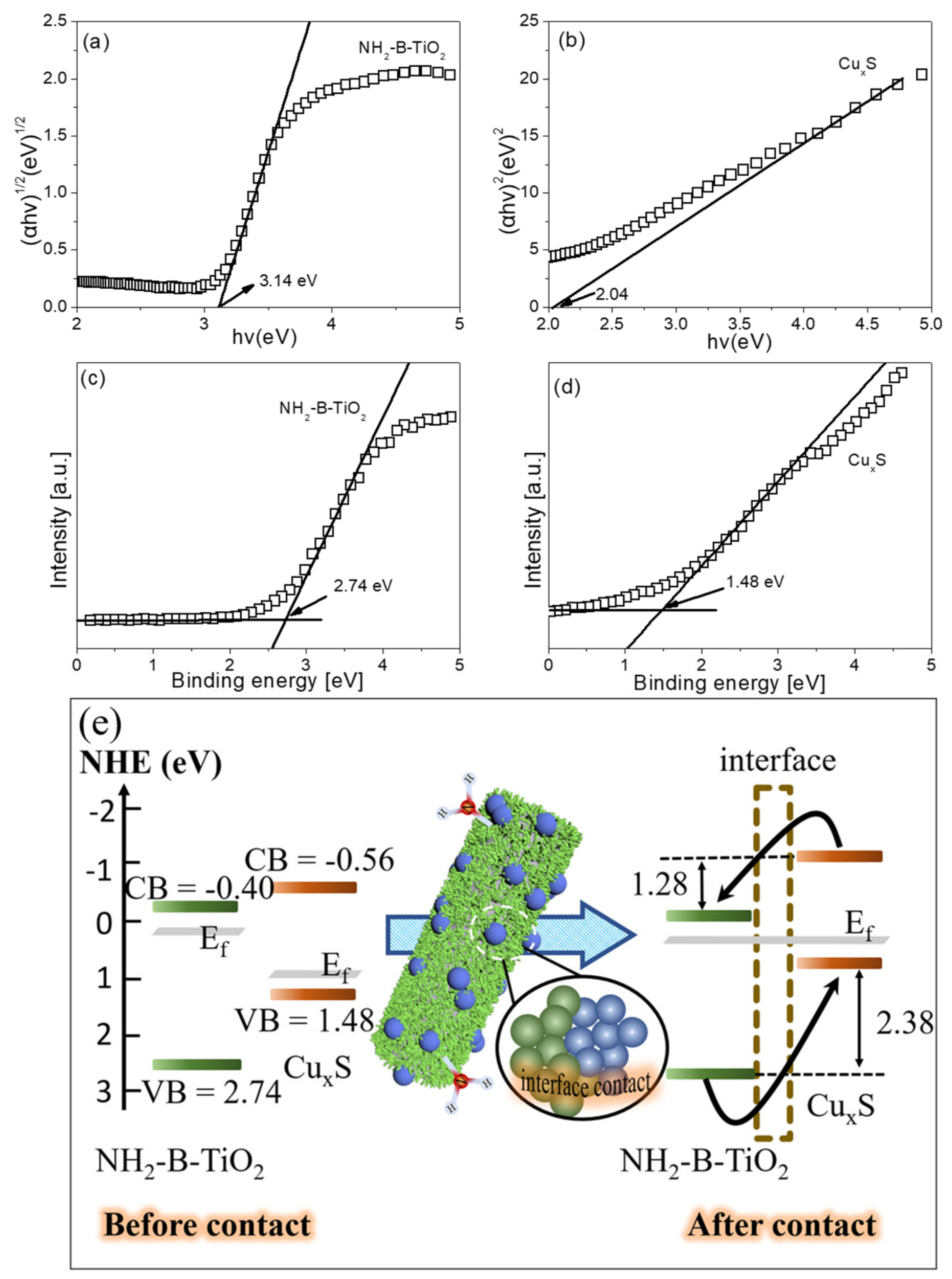
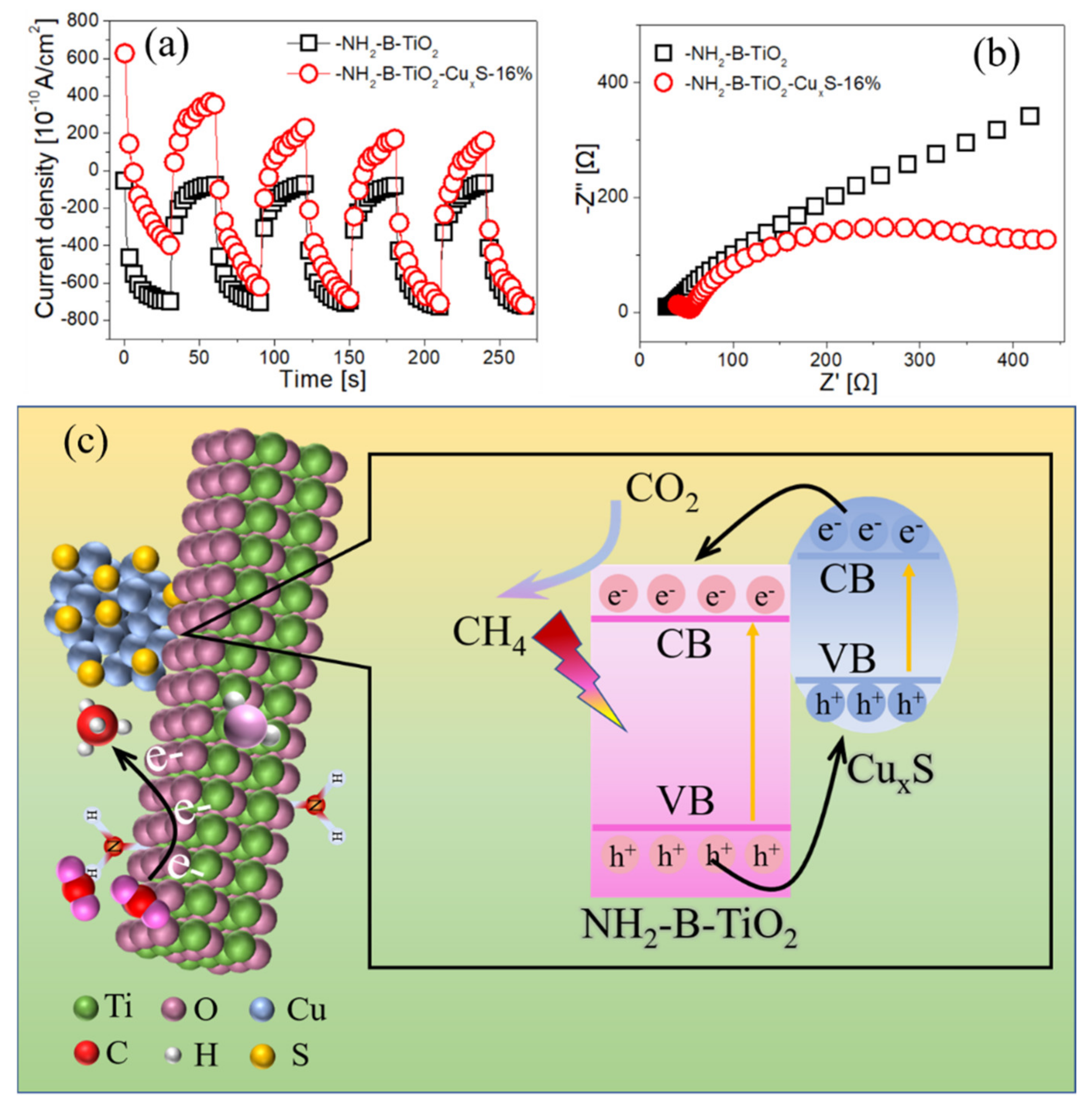
3. Results and Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, T.; Liu, S.; Sajid, M.; Li, K.; Ali, M.; Liu, L.; Chen, W. Electrochemical CO2 reduction to C2+ products using Cu-based electrocatalysts: A review. Nano Res. Energy 2022, 1, e9120021. [Google Scholar] [CrossRef]
- Lu, K.-Q.; Li, Y.-H.; Zhang, F.; Qi, M.-Y.; Chen, X.; Tang, Z.-R.; Yamada, Y.M.A.; Anpo, M.; Conte, M.; Xu, Y.-J. Rationally designed transition metal hydroxide nanosheet arrays on graphene for artificial CO2 reduction. Nat. Commun. 2020, 11, 5181. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Jiang, Y.; Xiong, Y. Photocatalytic CO2 conversion: What can we learn from conventional COx hydrogenation? Chem. Soc. Rev. 2020, 49, 6579–6591. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zhao, X.; Jiang, E.; Yan, Y.; Zhou, P.; Huo, P. Boosting water decomposition by sulfur vacancies for efficient CO2 photoreduction. Energy Environ. Sci. 2022, 15, 1556–1562. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Huang, H.; Zhang, Y.; Ma, T. Surface sites engineering on semiconductors to boost photocatalytic CO2 reduction. Nano Energy 2020, 75, 104959. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Yu, Y.; Dong, X.; Chen, P.; Geng, Q.; Wang, H.; Li, J.; Zhou, Y.; Dong, F. Synergistic Effect of Cu Single Atoms and Au–Cu Alloy Nanoparticles on TiO2 for Efficient CO2 Photoreduction. ACS Nano 2021, 15, 14453–14464. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Z.; Wu, S.; Zhang, J. Advances for CO2 Photocatalytic Reduction in Porous Ti-Based Photocatalysts. ACS EST Eng. 2022, 2, 942–956. [Google Scholar] [CrossRef]
- Qiu, P.; Lu, M.; Cheng, G.; Li, W.; Liu, L.; Xiong, J. Co-implantation of oxygen vacancy and well-dispersed Cu cocatalyst into TiO2 nanoparticles for promoting solar-to-hydrogen evolution. Int. J. Hydrog. Energy 2023, 48, 933–942. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, K.; Wu, X.; Zhang, G. Highly Enhanced Full Solar Spectrum-Driven Photocatalytic CO2 Reduction Performance in Cu2–xS/g-C3N4 Composite: Efficient Charge Transfer and Mechanism Insight. Sol. RRL 2021, 5, 2000326. [Google Scholar] [CrossRef]
- Qiu, P.; Xiong, J.; Lu, M.; Liu, L.; Li, W.; Wen, Z.; Li, W.; Chen, R.; Cheng, G. Integrated p-n/Schottky junctions for efficient photocatalytic hydrogen evolution upon Cu@TiO2-Cu2O ternary hybrids with steering charge transfer. J. Colloid Interface Sci. 2022, 622, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Qiu, P.; Xiong, J.; Zhu, X.; Cheng, G. Facilely anchoring Cu2O nanoparticles on mesoporous TiO2 nanorods for enhanced photocatalytic CO2 reduction through efficient charge transfer. Chin. Chem. Lett. 2022, 33, 3709–3712. [Google Scholar] [CrossRef]
- Ávila-López, M.A.; Tan, J.Z.Y.; Luévano-Hipólito, E.; Torres-Martínez, L.M.; Maroto-Valer, M.M. Production of CH4 and CO on CuxO and NixOy coatings through CO2 photoreduction. J. Environ. Chem. Eng. 2022, 10, 108199. [Google Scholar] [CrossRef]
- Fan, H.-T.; Wu, Z.; Liu, K.-C.; Liu, W.-S. Fabrication of 3D CuS@ZnIn2S4 hierarchical nanocages with 2D/2D nanosheet subunits p-n heterojunctions for improved photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 433, 134474. [Google Scholar] [CrossRef]
- Yu, D.; Xu, L.; Zhang, H.; Li, J.; Wang, W.; Yang, L.; Jiang, X.; Zhao, B. A new semiconductor-based SERS substrate with enhanced charge collection and improved carrier separation: CuO/TiO2 p-n heterojunction. Chin. Chem. Lett. 2022, 107771. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. Folic acid and CuS conjugated graphene oxide: An efficient photocatalyst for explicit degradation of toxic dyes. Appl. Surf. Sci. 2021, 566, 150648. [Google Scholar] [CrossRef]
- Rameshbabu, R.; Ravi, P.; Sathish, M. Cauliflower-like CuS/ZnS nanocomposites decorated g-C3N4 nanosheets as noble metal-free photocatalyst for superior photocatalytic water splitting. Chem. Eng. J. 2019, 360, 1277–1286. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, X.; Yu, C.; Li, F.; Zhou, W.; Wei, L. Construct interesting CuS/TiO2 architectures for effective removal of Cr(VI) in simulated wastewater via the strong synergistic adsorption and photocatalytic process. Sci. Total Environ. 2021, 796, 148941. [Google Scholar] [CrossRef]
- Chandra, M.; Bhunia, K.; Pradhan, D. Controlled Synthesis of CuS/TiO2 Heterostructured Nanocomposites for Enhanced Photocatalytic Hydrogen Generation through Water Splitting. Inorg. Chem. 2018, 57, 4524–4533. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Y.; Zhang, Y.Y.; Zhang, J.; Shi, Y.; Li, Z.; Feng, Z.C.; Li, C. In situ loading of CuS nanoflowers on rutile TiO2 surface and their improved photocatalytic performance. Appl. Surf. Sci. 2016, 370, 312–319. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, M.; Li, J.; Zhang, G.; Xin, Y.; Chai, C. Construction of immobilized 0D/1D heterostructure photocatalyst Au/CuS/CdS/TiO2 NBs with enhanced photocatalytic activity towards moxifloxacin degradation. Chem. Eng. J. 2020, 389, 124476. [Google Scholar] [CrossRef]
- He, H.Y. Facile synthesis of ultrafine CuS nanocrystalline/TiO2: Fe nanotubes hybrids and their photocatalytic and Fenton-like photocatalytic activities in the dye degradation. Microporous Mesoporous Mater. 2016, 227, 31–38. [Google Scholar] [CrossRef]
- Lee, H.; Kwak, B.S.; Park, N.-K.; Baek, J.-I.; Ryu, H.-J.; Kang, M. Assembly of a check-patterned CuSx–TiO2 film with an electron-rich pool and its application for the photoreduction of carbon dioxide to methane. Appl. Surf. Sci. 2017, 393, 385–396. [Google Scholar] [CrossRef]
- Lin, H.; Li, L.; Zhao, M.; Huang, X.; Chen, X.; Li, G.; Yu, R. Synthesis of High-Quality Brookite TiO2 Single-Crystalline Nanosheets with Specific Facets Exposed: Tuning Catalysts from Inert to Highly Reactive. J. Am. Chem. Soc. 2012, 134, 8328–8331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lkhamjav, S.; Qiu, B.; Dong, C.; Dong, C.; Zhou, Y.; Shen, B.; Xing, M.; Zhang, J. A facile strategy to prepare Fe3+ modified brookite TiO2 with high photocatalytic activity under ultraviolet light and visible light. Res. Chem. Intermed. 2017, 43, 2055–2066. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.; Andino, J.M.; Li, Y. Photocatalytic CO2 Reduction with H2O on TiO2 Nanocrystals: Comparison of Anatase, Rutile, and Brookite Polymorphs and Exploration of Surface Chemistry. ACS Catal. 2012, 2, 1817–1828. [Google Scholar] [CrossRef]
- Li, K.; Peng, T.; Ying, Z.; Song, S.; Zhang, J. Ag-loading on brookite TiO2 quasi nanocubes with exposed {210} and {001} facets: Activity and selectivity of CO2 photoreduction to CO/CH4. Appl. Catal. B 2016, 180, 130–138. [Google Scholar] [CrossRef]
- Li, K.; Peng, B.; Jin, J.; Zan, L.; Peng, T. Carbon nitride nanodots decorated brookite TiO2 quasi nanocubes for enhanced activity and selectivity of visible-light-driven CO2 reduction. Appl. Catal. B 2017, 203, 910–916. [Google Scholar] [CrossRef]
- Jin, J.; Luo, J.; Zan, L.; Peng, T. One-Pot Synthesis of Cu-Nanocluster-Decorated Brookite TiO2 Quasi-Nanocubes for Enhanced Activity and Selectivity of CO2 Photoreduction to CH4. ChemPhysChem 2017, 18, 3230–3239. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, S.; Jin, J.; Li, R.; Zhang, J.; Peng, T. Brookite TiO2 Nanoparticles Decorated with Ag/MnOx Dual Cocatalysts for Remarkably Boosted Photocatalytic Performance of the CO2 Reduction Reaction. Langmuir 2021, 37, 12487–12500. [Google Scholar] [CrossRef]
- Cho, K.M.; Kim, K.H.; Park, K.; Kim, C.; Kim, S.; Al-Saggaf, A.; Gereige, I.; Jung, H.-T. Amine-Functionalized Graphene/CdS Composite for Photocatalytic Reduction of CO2. ACS Catal. 2017, 7, 7064–7069. [Google Scholar] [CrossRef]
- Jin, L.; Shaaban, E.; Bamonte, S.; Cintron, D.; Shuster, S.; Zhang, L.; Li, G.; He, J. Surface Basicity of Metal@TiO2 to Enhance Photocatalytic Efficiency for CO2 Reduction. ACS Appl. Mater. Interfaces 2021, 13, 38595–38603. [Google Scholar] [CrossRef]
- Yang, G.; Xiong, J.; Lu, M.; Wang, W.; Li, W.; Wen, Z.; Li, S.; Li, W.; Chen, R.; Cheng, G. Co-embedding oxygen vacancy and copper particles into titanium-based oxides (TiO2, BaTiO3, and SrTiO3) nanoassembly for enhanced CO2 photoreduction through surface/interface synergy. J. Colloid Interface Sci. 2022, 624, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiong, J.; Cheng, G.; Li, W.; Dou, S. Promoting solar-to-hydrogen evolution on Schottky interface with mesoporous TiO2-Cu hybrid nanostructures. J. Colloid Interface Sci. 2019, 545, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Wei, Y.; Xiong, J.; Cheng, G. Impact of post-processing modes of precursor on adsorption and photocatalytic capability of mesoporous TiO2 nanocrystallite aggregates towards ciprofloxacin removal. Chem. Eng. J. 2018, 349, 1–16. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, G.; Xiong, J.; Zhu, J.; Gan, Y.; Zhang, M.; Li, Z.; Dou, S. Synergistic impact of cocatalysts and hole scavenger for promoted photocatalytic H2 evolution in mesoporous TiO2-NiSx hybrid. J. Energy Chem. 2019, 32, 45–56. [Google Scholar] [CrossRef]
- Zhu, Q.; Cao, Y.; Tao, Y.; Li, T.; Zhang, Y.; Shang, H.; Song, J.; Li, G. CO2 reduction to formic acid via NH2-C@Cu2O photocatalyst in situ derived from amino modified Cu-MOF. J. CO2 Util. 2021, 54, 101781. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Muhammad Adeel Sharif, H.; Chen, Z.; Chen, Y.; Zhou, B.; Xiao, K.; Yang, B.; Duan, Q. Amine-CdS for exfoliating and distributing bulk MoO3 for photocatalytic hydrogen evolution and Cr(VI) reduction. Chem. Eng. J. 2021, 406, 126849. [Google Scholar] [CrossRef]
- Shao, Y.; Dou, Z.; Liang, X.; Zhang, X.; Ji, M.; Pang, M.; Wang, M.; Wang, X. ZnIn2S4 nanosheet growth on amine-functionalized SiO2 for the photocatalytic reduction of CO2. Catal. Sci. Technol. 2022, 12, 606–612. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Ke, J.; Wang, S.; Wang, L.; Xiao, H. Black NiO-TiO2 nanorods for solar photocatalysis: Recognition of electronic structure and reaction mechanism. Appl. Catal. B 2018, 224, 705–714. [Google Scholar] [CrossRef]
- Torres, J.A.; da Cruz, J.C.; Nogueira, A.E.; da Silva, G.T.S.T.; de Oliveira, J.A.; Ribeiro, C. Role of Cu0-TiO2 interaction in catalyst stability in CO2 photoreduction process. J. Environ. Chem. Eng. 2022, 10, 107291. [Google Scholar] [CrossRef]
- Fu, J.; Cao, S.; Yu, J. Dual Z-scheme charge transfer in TiO2–Ag–Cu2O composite for enhanced photocatalytic hydrogen generation. J. Mater. 2015, 1, 124–133. [Google Scholar] [CrossRef]
- Yuan, L.; Hung, S.-F.; Tang, Z.-R.; Chen, H.M.; Xiong, Y.; Xu, Y.-J. Dynamic Evolution of Atomically Dispersed Cu Species for CO2 Photoreduction to Solar Fuels. ACS Catal. 2019, 9, 4824–4833. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.-H.; Qi, M.-Y.; Tang, Z.-R.; Xu, Y.-J. Boosting the activity and stability of Ag-Cu2O/ZnO nanorods for photocatalytic CO2 reduction. Appl. Catal. B 2020, 268, 118380. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Shen, X.; Peng, P.; Xiong, L.; Yu, Y. Octahedral Cu2O-modified TiO2 nanotube arrays for efficient photocatalytic reduction of CO2. Chin. J. Catal. 2015, 36, 2229–2236. [Google Scholar] [CrossRef]
- Liang, T.-Y.; Chan, S.-J.; Patra, A.S.; Hsieh, P.-L.; Chen, Y.-A.; Ma, H.-H.; Huang, M.H. Inactive Cu2O Cubes Become Highly Photocatalytically Active with Ag2S Deposition. ACS Appl. Mater. Interfaces 2021, 13, 11515–11523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, M.; Yang, S.; Hu, Y.; Mu, B.; Tie, Z.; Jin, Z. In-situ grown CuOx nanowire forest on copper foam: A 3D hierarchical and freestanding electrocatalyst with enhanced carbonaceous product selectivity in CO2 reduction. Nano Res. Energy 2022, 1, e9120033. [Google Scholar] [CrossRef]
- Zhang, F.; Zhuang, H.-Q.; Zhang, W.; Yin, J.; Cao, F.-H.; Pan, Y.-X. Noble-metal-free CuS/CdS photocatalyst for efficient visible-light-driven photocatalytic H2 production from water. Catal. Today 2019, 330, 203–208. [Google Scholar] [CrossRef]
- Wang, Q.; Yun, G.; Bai, Y.; An, N.; Chen, Y.; Wang, R.; Lei, Z.; Shangguan, W. CuS, NiS as co-catalyst for enhanced photocatalytic hydrogen evolution over TiO2. Int. J. Hydrogen Energy 2014, 39, 13421–13428. [Google Scholar] [CrossRef]
- Zhang, L.J.; Xie, T.F.; Wang, D.J.; Li, S.; Wang, L.L.; Chen, L.P.; Lu, Y.C. Noble-metal-free CuS/CdS composites for photocatalytic H2 evolution and its photogenerated charge transfer properties. Int. J. Hydrogen Energy 2013, 38, 11811–11817. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, M.; Xin, Y.; Chai, C.; Chen, Q. Construction of immobilized CuS/TiO2 nanobelts heterojunction photocatalyst for photocatalytic degradation of enrofloxacin: Synthesis, characterization, influencing factors and mechanism insight. J. Chem. Technol. Biotechnol. 2019, 94, 2219–2228. [Google Scholar]
- Wang, L.; Dong, Y.; Zhang, J.; Tao, F.; Xu, J. Construction of NiO/g-C3N4 p-n heterojunctions for enhanced photocatalytic CO2 reduction. J. Solid State Chem. 2022, 308, 122878. [Google Scholar] [CrossRef]
- Li, R.; Ma, H.; Shu, J.; Lian, Z.; Chen, N.; Ou, S.; Jin, R.; Li, S.; Yang, H. Surface engineering of copper sulfide-titania-graphitic carbon nitride ternary nanohybrid as an efficient visible-light photocatalyst for pollutant photodegradation. J. Colloid Interface Sci. 2021, 604, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, D.; Qin, Y.; Xie, Y.; Ling, Y.; Ye, H.; Zhang, Y. Facile construction of BiOBr/CoAl-LDH heterojunctions with suppressed Z-axis growth for efficient photoreduction of CO2. Sep. Purif. Technol. 2022, 302, 122090. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Chen, Y.; Yao, L.; Zhao, X.; Shi, H.; Cao, M.; Liang, Y. Heterogeneous p–n Junction CdS/Cu2O Nanorod Arrays: Synthesis and Superior Visible-Light-Driven Photoelectrochemical Performance for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2018, 10, 11652–11662. [Google Scholar] [CrossRef]
- Meng, F.; Li, J.; Cushing, S.K.; Zhi, M.; Wu, N. Solar Hydrogen Generation by Nanoscale p–n Junction of p-type Molybdenum Disulfide/n-type Nitrogen-Doped Reduced Graphene Oxide. J. Am. Chem. Soc. 2013, 135, 10286–10289. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: Protecting Cu2O from photocorrosion. Appl. Catal. B 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Yang, Q.; Qin, W.; Xie, Y.; Zong, K.; Guo, Y.; Song, Z.; Luo, G.; Raza, W.; Hussain, A.; Ling, Y.; et al. Constructing 2D/1D heterostructural BiOBr/CdS composites to promote CO2 photoreduction. Sep. Purif. Technol. 2022, 298, 121603. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, M.; Rashid, R.; Shafique, S.; Akhter, P.; Yang, W.; Ahmed, A.; Nawaz, Z.; Park, Y.-K. Development of hierarchically porous LaVO4 for efficient visible-light-driven photocatalytic desulfurization of diesel. Chem. Eng. J. 2021, 420, 130529. [Google Scholar] [CrossRef]
- Cai, W.; Yu, X.; Cao, Y.; Hu, C.; Wang, Y.; Zhao, Y.; Bu, Y. Electron-coupled enhanced interfacial interaction of Ce-MOF/Bi2MoO6 heterostructure for boosted photoreduction CO2. J. Environ. Chem. Eng. 2022, 10, 107461. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, C.; He, W.; Wei, Y.; Zhao, Z.; Liu, J. The Z-scheme g-C3N4/3DOM-WO3 photocatalysts with enhanced activity for CO2 photoreduction into CO. Chin. Chem. Lett. 2022, 33, 939–942. [Google Scholar] [CrossRef]
- Wu, X.; Wang, C.; Wei, Y.; Xiong, J.; Zhao, Y.; Zhao, Z.; Liu, J.; Li, J. Multifunctional photocatalysts of Pt-decorated 3DOM perovskite-type SrTiO3 with enhanced CO2 adsorption and photoelectron enrichment for selective CO2 reduction with H2O to CH4. J. Catal. 2019, 377, 309–321. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, M.; Lu, M.; Zhao, K.; Han, C.; Cheng, G.; Wen, Z. Achieving simultaneous Cu particles anchoring in meso-porous TiO2 nanofabrication for enhancing photo-catalytic CO2 reduction through rapid charge separation. Chin. Chem. Lett. 2022, 33, 1313–1316. [Google Scholar] [CrossRef]
- Xiong, Z.; Lei, Z.; Kuang, C.-C.; Chen, X.; Gong, B.; Zhao, Y.; Zhang, J.; Zheng, C.; Wu, J.C.S. Selective photocatalytic reduction of CO2 into CH4 over Pt-Cu2O TiO2 nanocrystals: The interaction between Pt and Cu2O cocatalysts. Appl. Catal. B 2017, 202, 695–703. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Li, H.; Li, P.; Zou, Z. Au@TiO2 yolk–shell hollow spheres for plasmon-induced photocatalytic reduction of CO2 to solar fuel via a local electromagnetic field. Nanoscale 2015, 7, 14232–14236. [Google Scholar] [CrossRef]
- Kuai, L.; Zhou, Y.; Tu, W.; Li, P.; Li, H.; Xu, Q.; Tang, L.; Wang, X.; Xiao, M.; Zou, Z. Rational construction of a CdS/reduced graphene oxide/TiO2 core–shell nanostructure as an all-solid-state Z-scheme system for CO2 photoreduction into solar fuels. RSC Adv. 2015, 5, 88409–88413. [Google Scholar] [CrossRef]
- Manzanares, M.; Fàbrega, C.; Oriol Ossó, J.; Vega, L.F.; Andreu, T.; Morante, J.R. Engineering the TiO2 outermost layers using magnesium for carbon dioxide photoreduction. Appl. Catal. B 2014, 150–151, 57–62. [Google Scholar] [CrossRef]


| Photocatalyst | Light Source | Reaction Condition | CH4 Production Rate [μmol/g/h] | References |
|---|---|---|---|---|
| NH2-B-TiO2-CuxS | 300 W Xe lamp | H2O vapor | 3.34 | This work |
| Pt-Cu2O/TiO2 | 300 W Xe lamp | H2O vapor | 1.42 | [64] |
| TiO2/g-C3N4 | 300 W Xe lamp | H2O vapor | 2.50 | [28] |
| Au@TiO2 | 300 W Xe lamp | H2O vapor | 2.52 | [65] |
| CdS/rGO/TiO2 | 300 W Xe lamp | H2O vapor | 0.063 | [66] |
| Mg-TiO2 | 300 W Xe lamp | H2O vapor | 1.0 | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhu, X.; Xiong, J.; Wen, Z.; Cheng, G. A p–n Junction by Coupling Amine-Enriched Brookite–TiO2 Nanorods with CuxS Nanoparticles for Improved Photocatalytic CO2 Reduction. Materials 2023, 16, 960. https://doi.org/10.3390/ma16030960
Chen Z, Zhu X, Xiong J, Wen Z, Cheng G. A p–n Junction by Coupling Amine-Enriched Brookite–TiO2 Nanorods with CuxS Nanoparticles for Improved Photocatalytic CO2 Reduction. Materials. 2023; 16(3):960. https://doi.org/10.3390/ma16030960
Chicago/Turabian StyleChen, Zhangjing, Xueteng Zhu, Jinyan Xiong, Zhipan Wen, and Gang Cheng. 2023. "A p–n Junction by Coupling Amine-Enriched Brookite–TiO2 Nanorods with CuxS Nanoparticles for Improved Photocatalytic CO2 Reduction" Materials 16, no. 3: 960. https://doi.org/10.3390/ma16030960
APA StyleChen, Z., Zhu, X., Xiong, J., Wen, Z., & Cheng, G. (2023). A p–n Junction by Coupling Amine-Enriched Brookite–TiO2 Nanorods with CuxS Nanoparticles for Improved Photocatalytic CO2 Reduction. Materials, 16(3), 960. https://doi.org/10.3390/ma16030960








