Modification of Cotton and Leather Surfaces Using Cold Atmospheric Pressure Plasma and TiO2-SiO2-Reduced Graphene Oxide Nanopowders
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis of Graphene Oxide (GO)
2.3. Preparation of Mixed TiO2-SiO2 and TiO2-SiO2/Reduced Graphene Oxide Nanopowders
2.4. Preparation of Treated Cotton and Leather Samples
2.5. Characterization of Cotton and Leather Samples
2.6. Evaluation of Self-Cleaning Photocatalytic Activity
3. Results and Discussion
3.1. Scanning Electron Microscopy Analysis
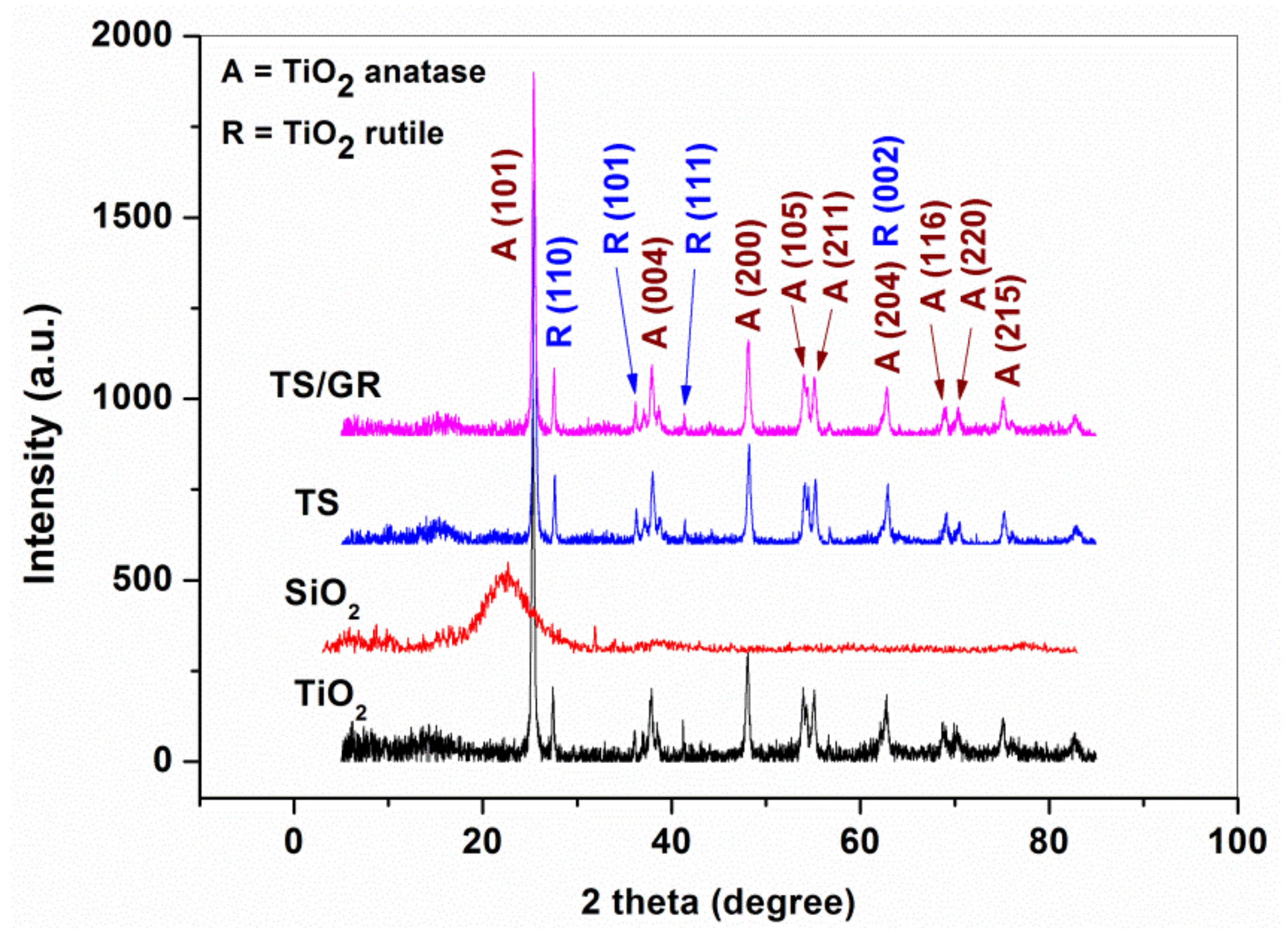
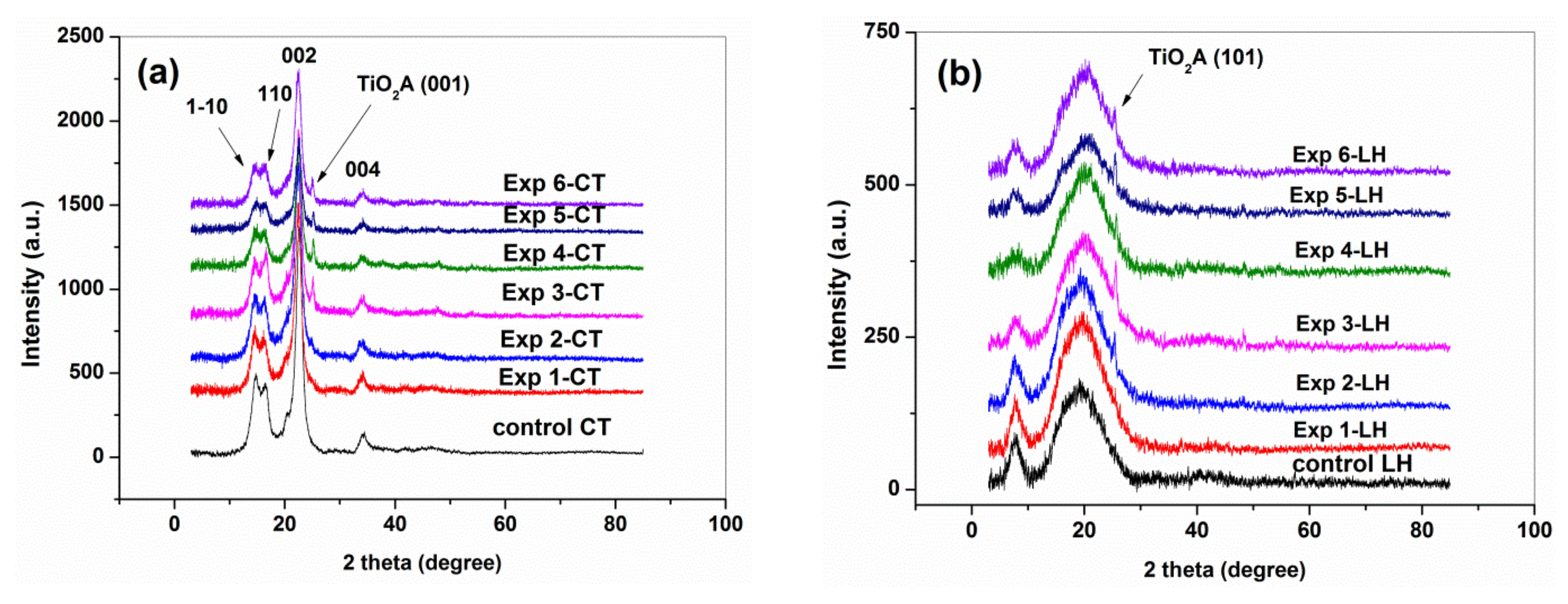
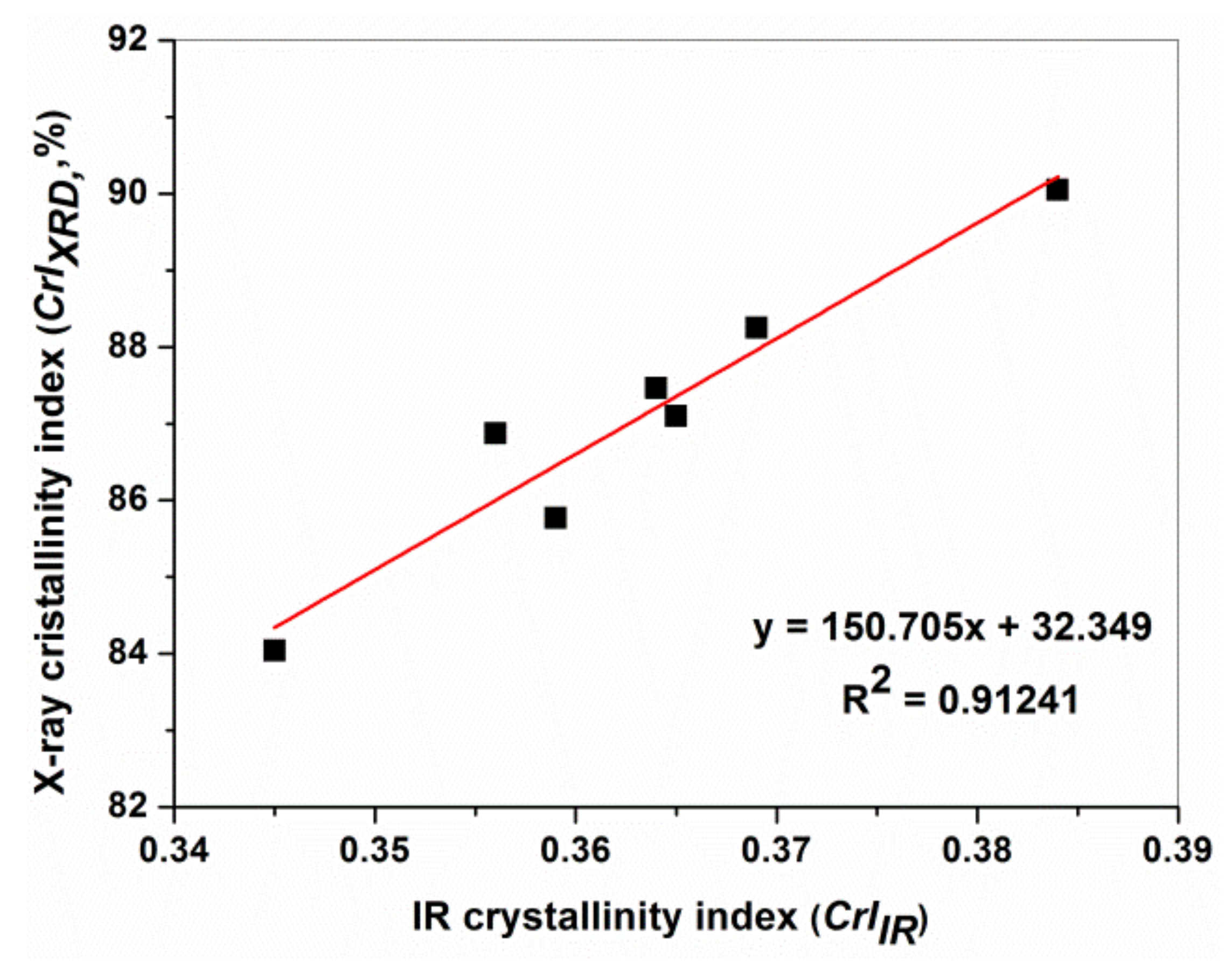
3.2. XRD Characterization
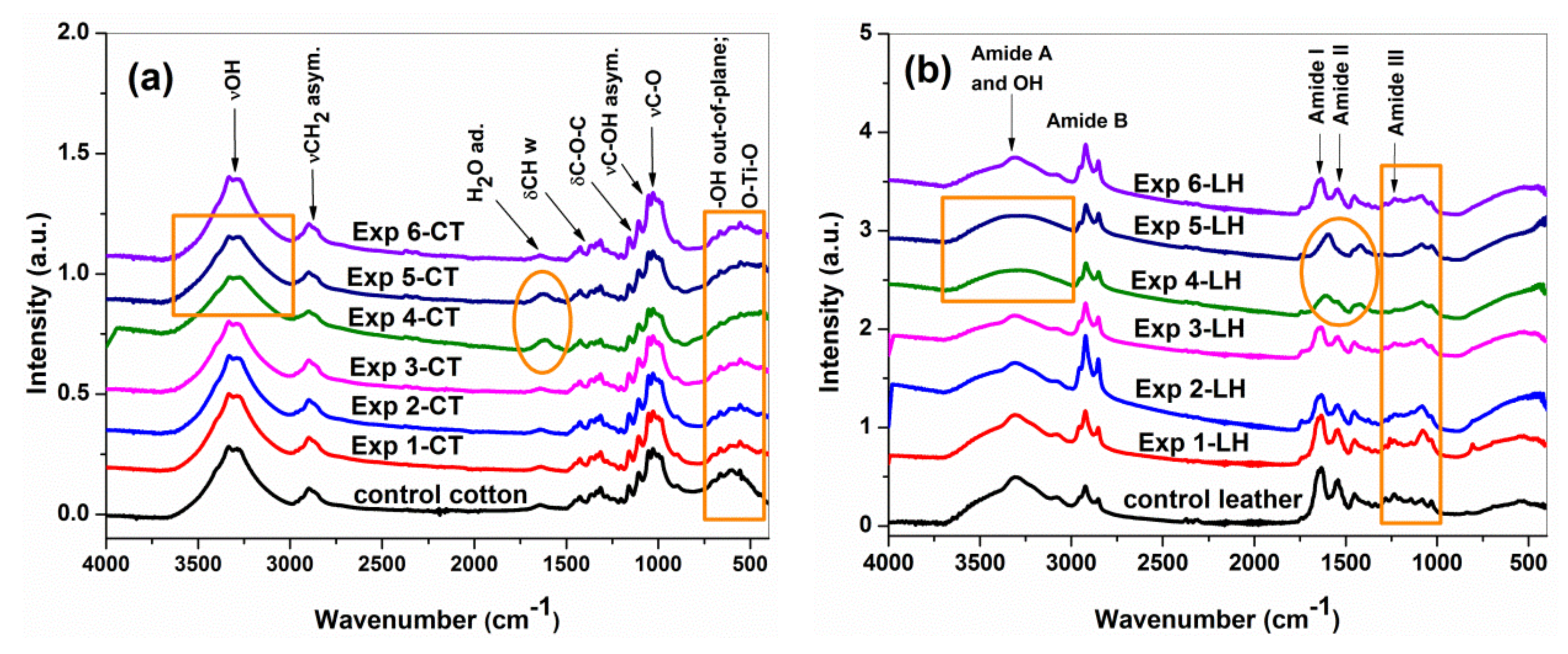
3.3. ATR FTIR Spectroscopy Characterization
3.4. UV–Vis Spectroscopy Analysis
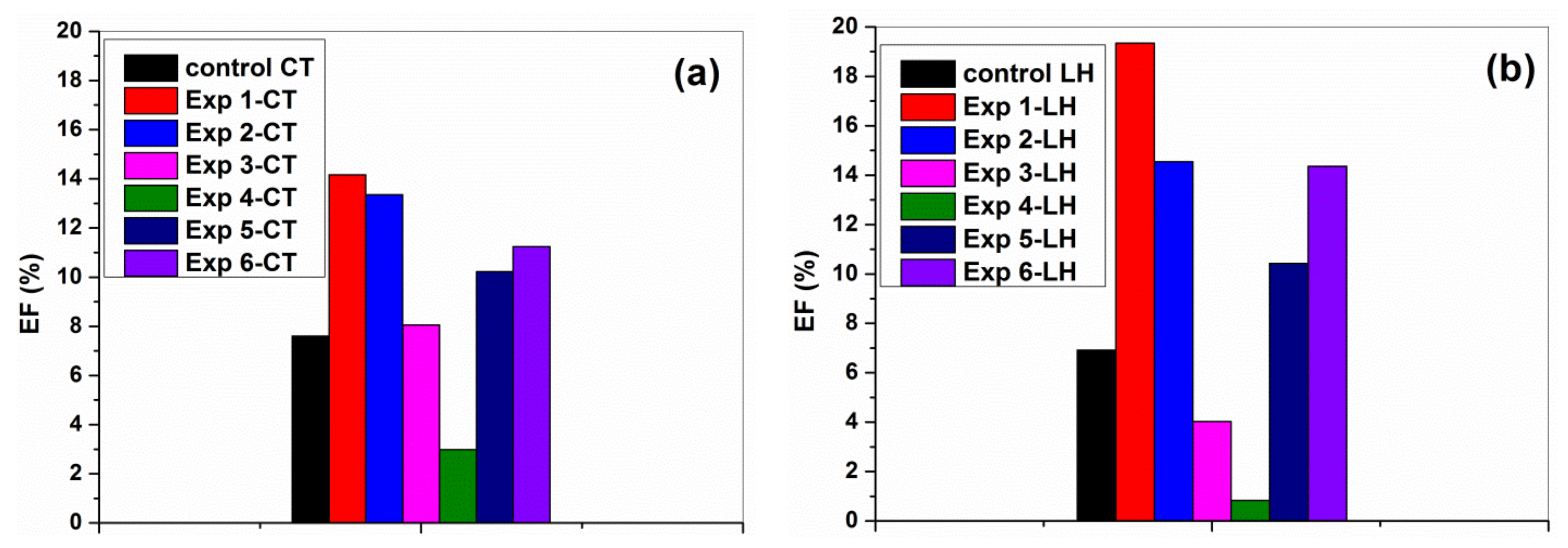
3.5. Self-Cleaning Properties of the Cotton and Leather Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miśkiewicz, P. Nanotechnology in textile industry. World Sci. News 2018, 100, 74–85. [Google Scholar]
- Haque, M. Nano Fabrics in the 21stcentury: A review. Asian J. Nanosci. Mater. 2019, 2, 131–148. [Google Scholar]
- Muthukrishnan, L. Nanotechnology for cleaner leather production: A review. Environ. Chem. Lett. 2021, 19, 2527–2549. [Google Scholar] [CrossRef]
- Shah, M.A.; Pirzada, B.M.; Price, G.; Shibiru, A.L.; Qurashi, A. Applications of nanotechnology in smart textile industry: A critical review. J. Adv. Res. 2022, 38, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.M.; Simončič, B.; Tomšič, B. Recent advances in TiO2-functionalized textile surfaces. Surf. Interfaces 2020, 22, 100890. [Google Scholar] [CrossRef]
- Molina, J. Graphene-based fabrics, and their applications: A review. RSC Adv. 2016, 6, 68261–68291. [Google Scholar] [CrossRef]
- Neves, A.I.S.; Saadi, Z. Challenges of Coating Textiles with Graphene. Johns. Matthey Technol. Rev. 2021, 66, 106–113. [Google Scholar] [CrossRef]
- Stanca, M.; Gaidau, C.; Alexe, C.-A.; Stanculescu, I.; Vasilca, S.; Matei, A.; Simion, D.; Constantinescu, R.-R. Multifunctional Leather Surface Design by Using Carbon Nanotube-Based Composites. Materials 2021, 14, 3003. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, M.; Liu, X.; Yue, O.; Wang, X.; Jiang, H. Advanced collagen nanofibers-based functional bio-composites for high-value utilization of leather: A review. J. Sci. Adv. Mater. Devices 2021, 6, 153–166. [Google Scholar] [CrossRef]
- Monteiro, W.A. Chapter 12. Radiation effects in textile materials, radiation effects in materials. In Radiation Effects in Materials; Shahidi, S., Wiener, J., Eds.; IntechOpen BoD–Books on Demand: London, UK, 2016; pp. 309–328. [Google Scholar]
- Chapter 13. Plasma and other irradiation technologies application in textile. In Frontiers of Textile Materials: Polymers, Nanomaterials, Enzymes, and Advanced Modification Techniques; Shabbir, M., Ahmed, S., Sheikh, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA; Scrivener Publishing LLC: Massauchets, MA, USA, 2020; pp. 309–333. [Google Scholar]
- Jelil, R.A. A review of Scrivener Publishing LLC: Beverly, MA, USAlow-temperature plasma treatment of textile materials. J. Mater. Sci. 2015, 50, 5913–5943. [Google Scholar] [CrossRef]
- Tudoran, C.; Roşu, M.; Coroş, M. A concise overview on plasma treatment for application on textile and leather materials. Plasma Process. Polym. 2020, 17, 2000046. [Google Scholar] [CrossRef]
- Yuranova, T.; Mosteo, R.; Bandara, J.; Laub, D.; Kiwi, J. Self-cleaning cotton textiles surfaces modified by photoactive SiO2/TiO2 coating. J. Mol. Catal. A Chem. 2006, 244, 160–167. [Google Scholar] [CrossRef]
- Liu, X.; Huang, F.; Yu, W. Preparation and property of TiO2/SiO2 multilayer film on the fabric by sol-gel process. Fibers Polym. 2013, 14, 1101–1106. [Google Scholar] [CrossRef]
- Molina, J.; Fernandes, F.; Fernández, J.; Pastorc, M.; Correia, A.; Souto, A.; Carneiro, J.; Teixeira, V.; Cases, F. Photocatalytic fabrics based on reduced graphene oxide and TiO2 coatings. Mater. Sci. Eng. B 2015, 199, 62–76. [Google Scholar] [CrossRef]
- Dhineshbabu, N.; Arunmetha, S.; Manivasakan, P.; Karunakaran, G.; Rajendran, V. Enhanced functional properties of cotton fabrics using TiO2/SiO2 nanocomposites. J. Ind. Text. 2016, 45, 674–692. [Google Scholar] [CrossRef]
- Farouk, A.; Sharaf, S. Sol-gel hybrid nanomaterials based on TiO2/SiO2 as multifunctional finishing for cotton fabric. Egypt. J. Chem. 2016, 59, 407–427. [Google Scholar]
- Rilda, Y.; Fadhli, F.; Syukri, S.; Alif, A.; Aziz, H.; Chandren, S.; Nur, H. Self-Cleaning TiO2-SiO2 clusters on cotton textile prepared by dip-spin coating process. J. Teknol. 2016, 78, 113–120. [Google Scholar] [CrossRef]
- Landi, S., Jr.; Carneiro, J.; Ferdov, S.; Fonseca, A.; Neves, I.; Ferreira, M.; Parpot, P.; Soares, O.; Pereira, M. Photocatalytic degradation of Rhodamine B dye by cotton textile coated with SiO2-TiO2 and SiO2-TiO2-HY composites. J. Photochem. Photobiol. A Chem. 2017, 346, 60–69. [Google Scholar] [CrossRef]
- Li, W.; Gao, J.; Wang, L. Enhancement of durable photocatalytic properties of cotton/polyester fabrics using TiO2/SiO2 via one step sonosynthesis. J. Ind. Text. 2017, 46, 1633–1655. [Google Scholar] [CrossRef]
- Deshpande, R.H.; Wasif, A.I.; Shah, K. Multifinishing of cotton using reduced graphene oxide. Int. Res. J. Eng. Technol. 2019, 6, 3. [Google Scholar]
- Shaheen, T.; Zaghloul, S.; Salem, S. A new facile strategy for multifunctional textiles development through in situ deposition of SiO2/TiO2 nanosols hybrid. Ind. Eng. Chem. Res. 2019, 58, 20203–20212. [Google Scholar] [CrossRef]
- Trang, P.T.T.; Giang, L.H.; Manh, N.B.; Cong, T.D.; Tung, N.T.; Anh, V.T. High Flame Retardant Performance of SiO2-TiO2 Sol Coated on Polyester/Cotton Fabrics. VNU J. Sci. Nat. Sci. Technol. 2021, 37, 11–18. [Google Scholar] [CrossRef]
- Gaidau, C.; Petica, A.; Ignat, M.; Popescu, L.M.; Piticescu, R.M.; Tudor, I.A.; Piticescu, R.R. Preparation of silica doped titania nanoparticles with thermal stability and photocatalytic properties and their application for leather surface functionalization. Arab. J. Chem. 2017, 10, 985–1000. [Google Scholar] [CrossRef]
- Kaygusuz, M.K.; Meyer, M.; Aslan, A. The Effect of TiO2-SiO2 Nanocomposite on the Performance Characteristics of Leather. Mater. Res. 2017, 20, 1103–1110. [Google Scholar] [CrossRef]
- Kaygusuz, M.; Ide, S.; Karaarslan, D. Nanoscopic investigation on TiO2-SiO2-GLYMO nanocomposite coated and plasma treated leathers. Polym. Technol. Mater. 2020, 59, 966–974. [Google Scholar] [CrossRef]
- Kale, M.B.; Luo, Z.; Zhang, X.; Dhamodharan, D.; Divakaran, N.; Mubarak, S.; Wu, L.; Xu, Y. Waterborne polyurethane/graphene oxide-silica nanocomposites with improved mechanical and thermal properties for leather coatings using screen printing. Polymer 2019, 170, 43–53. [Google Scholar] [CrossRef]
- Gao, J.; Li, W.; Zhao, X.; Wang, L.; Pan, N. Durable visible light self-cleaning surfaces imparted by TiO2/SiO2/GO photocatalyst. Text. Res. J. 2019, 89, 517–527. [Google Scholar] [CrossRef]
- Kaygusuz, M.; Meyer, M.; Junghans, F.; Aslan, A. Modification of leather surface with atmospheric pressure plasma and nano-finishing. Polym. Plast. Technol. Eng. 2018, 57, 260–268. [Google Scholar] [CrossRef]
- Bolboacă, S.D.; Jäntschi, L. Design of Experiments: Useful Orthogonal Arrays for Number of Experiments from 4 to 16. Entropy 2007, 9, 198–232. [Google Scholar] [CrossRef]
- Pogacean, F.; Socaci, C.; Pruneanu, S.; Biris, A.; Coros, M.; Magerusan, L.; Katona, G.; Turcu, R.; Borodi, G. Graphene based nanomaterials as chemical sensors for hydrogen peroxide—A comparison study of their intrinsic peroxidase catalytic behavior. Sens. Actuators B Chem. 2015, 213, 474–483. [Google Scholar] [CrossRef]
- Tudoran, C. Universal Cold Plasma Applicator to Be Used in Surface Engineering. Romanian Patent RO133593A0/RO201800648A, 30 August 2019. [Google Scholar]
- Bhat, N.; Netravali, A.; Gore, A.; Sathianarayanan, M.; Arolkar, G.; Deshmukh, R. Surface modification of cotton fabrics using plasma technology. Text. Res. J. 2011, 81, 1014–1026. [Google Scholar] [CrossRef]
- Idris, A.; Majidnia, Z.; Valipour, P. Antibacterial improvement of leather by surface modification using corona discharge and silver nanoparticles application. J. Sci. Technol. 2013, 5, 15. [Google Scholar]
- Silvestre, C.R.; Blasco, M.P.C.; López, S.R.; Aguilar, H.P.; Limiñana, M.P.; Gil, E.B.; Calpena, E.O.; Ais, F.A. Hydrophobic Leather Coating for Footwear Applications by a Low-Pressure Plasma Polymerisation Process. Polymers 2021, 13, 3549. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, J.; Liu, F.; Zhang, Z.; Li, Z.; Yang, M.; Li, L. The effects of surface modification using O2 low temperature plasma on chrome tanning properties of natural leather. J. Ind. Text. 2018, 49, 534–547. [Google Scholar] [CrossRef]
- Martínez, J.; Palomares-Sánchez, S.; Ortega-Zarzosa, G.; Ruiz, F.; Chumakov, Y. Rietveld refinement of amorphous SiO2 prepared via sol–gel method. Mater. Lett. 2006, 60, 3526–3529. [Google Scholar] [CrossRef]
- Il’Ves, V.G.; Zuev, M.G.; Sokovnin, S.Y. Properties of Silicon Dioxide Amorphous Nanopowder Produced by Pulsed Electron Beam Evaporation. J. Nanotechnol. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.; Himmel, M.; Parilla, P.; Johnson, D. Cellulose lose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Abidi, N.; Manike, M. X-ray diffraction and FTIR investigations of cellulose deposition during cotton fiber development. Text. Res. J. 2017, 88, 719–730. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Cabrales, L.; Abidi, N. Kinetics of Cellulose Deposition in Developing Cotton Fibers Studied by Thermogravimetric Analysis. Fibers 2019, 7, 78. [Google Scholar] [CrossRef]
- Parikh, D.; Thibodeaux, D.; Condon, B. X-ray crystallinity of bleached and cross linked cottons. Text. Res. J. 2007, 77, 612–616. [Google Scholar] [CrossRef]
- Karahan, H.; Özdo, E. Improvements of surface functionality of cotton fibers by atmospheric plasma treatment. Fiber. Polym. 2008, 9, 21–26. [Google Scholar] [CrossRef]
- Liu, Y.; Thibodeaux, D.; Bauer, P.; Van Derveer, D. Comparative investigation of Fourier Transform Infrared (FT-IR) spectroscopy and X-ray Diffraction (XRD) in the determination of cotton fiber crystallinity. Appl. Spectrosc. 2012, 66, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Andonegi, M.; Irastorza, A.; Izeta, A.; Cabezudo, S.; De La Caba, K.; Guerrero, P. A Green Approach towards Native Collagen Scaffolds: Environmental and Physicochemical Assessment. Polymers 2020, 12, 1597. [Google Scholar] [CrossRef]
- Gao, D.; Cheng, Y.; Wang, P.; Li, F.; Wu, Y.; Lyu, B.; Ma, J.; Qin, J. An eco-friendly approach for leather manufacture based on P(POSS-MAA)-aluminum tanning agent combination tannage. J. Clean. Prod. 2020, 257, 120546. [Google Scholar] [CrossRef]
- Sun, L.; Hou, H.; Li, B.; Zhang, Y. Characterization of acid- and pepsin-soluble collagen extracted from the skin of Nile tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef]
- Xu, Z.; Guan, X.; Liu, J.; Fan, H.; Chen, Y. Improving collagen extraction through an alternative strategy based on succinic anhydride pretreatment to retain collagen’s triple-helix structure. J. Appl. Polym. Sci. 2017, 134, 45424. [Google Scholar] [CrossRef]
- Orgel, J.P.R.O.; Antonio, J.D.S.; Antipova, O. Molecular and structural mapping of collagen fibril interactions. Connect. Tissue Res. 2010, 52, 2–17. [Google Scholar] [CrossRef]
- Ahmad, I.; Kan, C.-W.; Yao, Z. Photoactive cotton fabric for UV protection and self-cleaning. RSC Adv. 2019, 9, 18106–18114. [Google Scholar] [CrossRef]
- Stanisławska, A.; Staroszczyk, H.; Szkodo, M. The effect of dehydration/rehydration of bacterial nanocellulose on its tensile strength and physicochemical properties. Carbohydr. Polym. 2020, 236, 116023. [Google Scholar] [CrossRef]
- da Silva Fernandes, R.; Moura, M.; Glenn, G.; Aouada, F. Thermal, microstructural, and spectroscopic analysis of Ca2+ alginate/clay nanocomposite hydrogel beads. J. Mol. Liq. 2018, 265, 327–336. [Google Scholar] [CrossRef]
- Nelson, M.; O’Connor, R. Relationship of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimating of crystallinity in cellulose I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- León-López, A.; Fuentes-Jiménez, L.; Hernández-Fuentes, A.; Campos-Montiel, R.; Aguirre-Álvarez, G. Hydrolysed collagen from sheepskins as a source of functional peptides with antioxidant activity. Int. J. Mol. Sci. 2019, 20, 3931. [Google Scholar] [CrossRef]
- Albu, M.G.; Ghica, M.V.; Leca, M.; Popa, L.; Borlescu, C.; Cremenescu, E.; Giurginca, M.; Trandafir, V. Doxycycline Delivery From Collagen Matrices Crosslinked With Tannic Acid. Mol. Cryst. Liq. Cryst. 2010, 523, 97–105. [Google Scholar] [CrossRef]
- Júnior, Z.S.S.; Botta, S.B.; Ana, P.A.; Franca, C.M.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Deana, A.; Bussadori, S.K. Effect of papain-based gel on type I collagen—spectroscopy applied for microstructural analysis. Sci. Rep. 2015, 5, 11448. [Google Scholar] [CrossRef]
- Siqueira, P.; Siqueira, E.; De Lima, A.E.; Siqueira, G.; Pinzón-Garcia, A.D.; Lopes, A.P.; Segura, M.E.C.; Isaac, A.; Pereira, F.V.; Botaro, V.R. Three-Dimensional Stable Alginate-Nanocellulose Gels for Biomedical Applications: Towards Tunable Mechanical Properties and Cell Growing. Nanomaterials 2019, 9, 78. [Google Scholar] [CrossRef]
- Ojstršek, A.; Fakin, D. Washing durability and photo-stability of nano TiO2-SiO2 coatings exhausted onto cotton and cotton/polyester fabrics. Coatings 2019, 9, 545. [Google Scholar] [CrossRef]
- Samouillan, V.; Merbahi, N.; Yousfi, M.; Gardou, J.-P.; Delaunay, F.; Dandurand, J.; Lacabanne, C. Effect of low-temperature plasma jet on thermalstability and physical structure of type I collagen. IEEE Trans. Plasma Sci. 2012, 40, 1688–1695. [Google Scholar] [CrossRef]
- Zhou, K.; Zhu, Y.; Yang, X.; Jiang, X.; Li, C. Preparation of graphene-TiO2 composites with enhanced photocatalytic activity. New J. Chem. 2011, 35, 353–359. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.-L.; Zhang, Q.; Peng, J.; Li, J.; Zhai, M.; Yu, Z.-Z. Facile synthesis of well-dispersed graphene by γ-ray induced reduction of graphene oxide. J. Mater. Chem. 2012, 22, 13064–13069. [Google Scholar] [CrossRef]
- Abdelrahman, M.E.-N.T.K.M.; Hatshan, M. Development of antimicrobial, UV blocked and photocatalytic self-cleanable cotton fibers decorated with silver nanoparticles using silver carbamate and plasma activation. Cellulose 2021, 28, 1105–1121. [Google Scholar]
- Ju, H.; Liu, X.; Zhang, G.; Liu, D.; Yang, Y. Comparison of the Structural Characteristics of Native Collagen Fibrils Derived from Bovine Tendons Using Two Different Methods: Modified Acid-Solubilized and Pepsin-Aided Extraction. Materials 2020, 13, 358. [Google Scholar] [CrossRef] [PubMed]
- Zyoud, A.H.; Saleh, F.; Helal, M.H.; Shawahna, R.; Hilal, H.S. Anthocyanin-Sensitized TiO2 Nanoparticles for Phenazopyridine Photodegradation under Solar Simulated Light. J. Nanomater. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Verduin, J.; den Uijl, M.; Peters, R.; van Bommel, M. Photodegradation products and their analysis in food. Food Sci. Nutr. 2020, 6, 16. [Google Scholar] [CrossRef]
- Laleh, G.; Frydoonfar, H.; Heidary, R.; Jameei, R.; Zare, S. The Effect of Light, Temperature, pH and Species on Stability of Anthocyanin Pigments in Four Berberis Species. Pak. J. Nutr. 2006, 5, 90–92. [Google Scholar]
- Bozzi, A.; Yuranova, T.; Guasaquillo, I.; Laub, D.; Kiwi, J. Self-cleaning of modified cotton textiles by TiO2 at low temperatures under daylight irradiation. J. Photochem. Photobiol. A Chem. 2005, 174, 156–164. [Google Scholar] [CrossRef]
- Radetić, M. Functionalization of textile materials with TiO2 nanoparticles. J. Photochem. Photobiol. C Photochem. Rev. 2013, 16, 62–76. [Google Scholar] [CrossRef]
- Zhang, Q.; Bao, N.; Wang, X.; Hu, X.; Miao, X.; Chaker, M.; Ma, D. Advanced Fabrication of Chemically Bonded Graphene/TiO2 Continuous Fibers with Enhanced Broadband Photocatalytic Properties and Involved Mechanisms Exploration. Sci. Rep. 2016, 6, 38066. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; Thomas, N.; Louis, J.; Mathew, D.T.; Ganguly, P.; John, H.; Pillai, S.C. Graphene coupled TiO2 photocatalysts for environmental applications: A review. Chemosphere 2021, 271, 129506. [Google Scholar] [CrossRef] [PubMed]
- Babyszko, A.; Wanag, A.; Sadłowski, M.; Kusiak-Nejman, E.; Morawski, A.W. Synthesis and Characterization of SiO2/TiO2 as Photocatalyst on Methylene Blue Degradation. Catalysts 2022, 12, 1372. [Google Scholar] [CrossRef]
- Mihailović, D.; Šaponjić, Z.; Molina, R.; Puač, N.; Jovančić, P.; Nedeljković, J.; Radetić, M. Improved Properties of Oxygen and Argon RF Plasma-Activated Polyester Fabrics Loaded with TiO2 Nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Mihailović, D.; Šaponjić, Z.; Radoičić, M.; Lazović, S.; Jovančić, C.J.B.P.; Nedeljković, J.; Radetić, M. Functionalization of cotton fabrics with corona/air RF plasma and colloidal TiO2 nanoparticles. Cellulose 2011, 18, 811–825. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, L.; Sun, R. Structure and Properties of Cotton Fibers Modified with Titanium Sulfate and Urea under Hydrothermal Conditions. J. Eng. Fibers Fabr. 2014, 9, 67–75. [Google Scholar] [CrossRef]
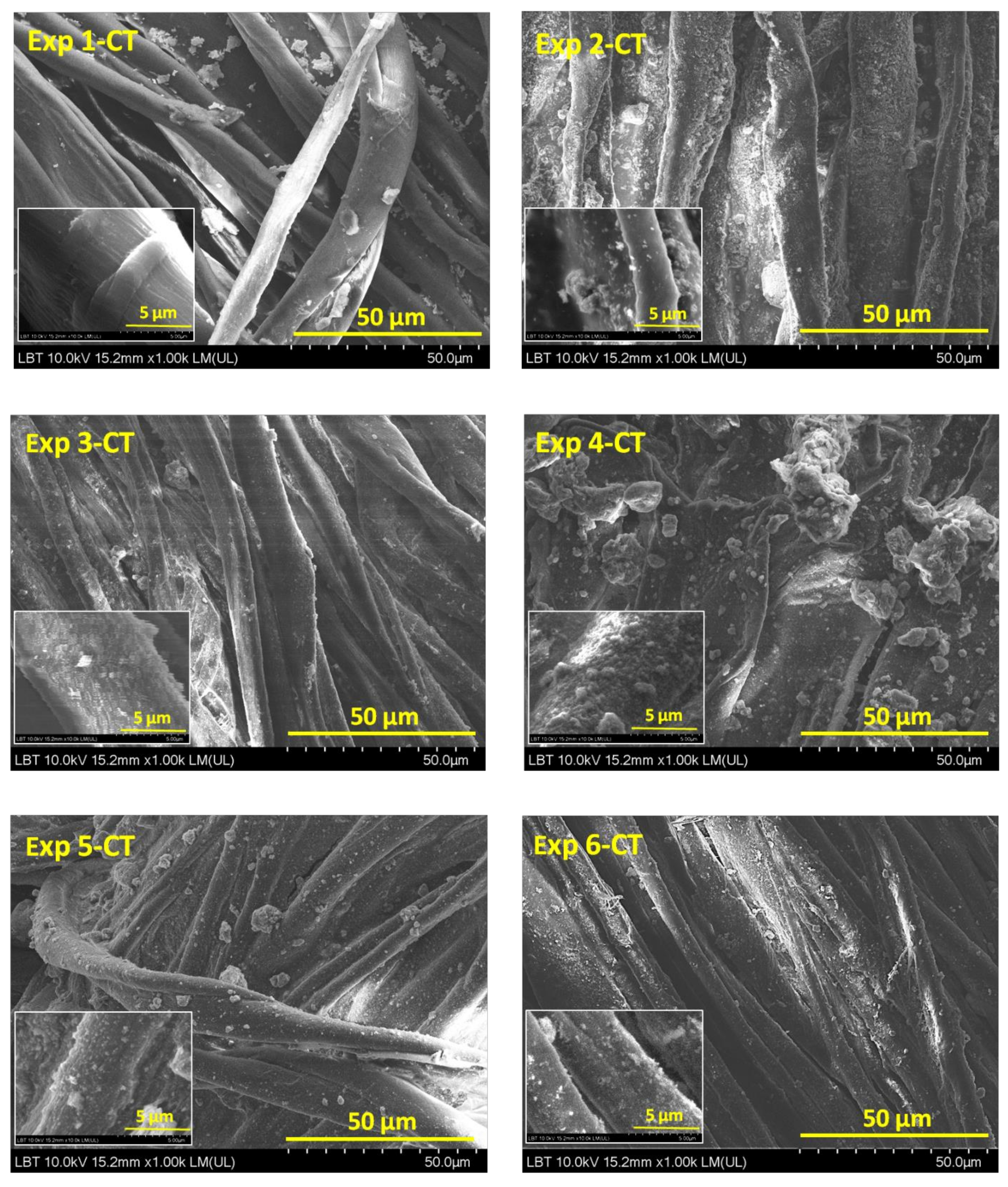
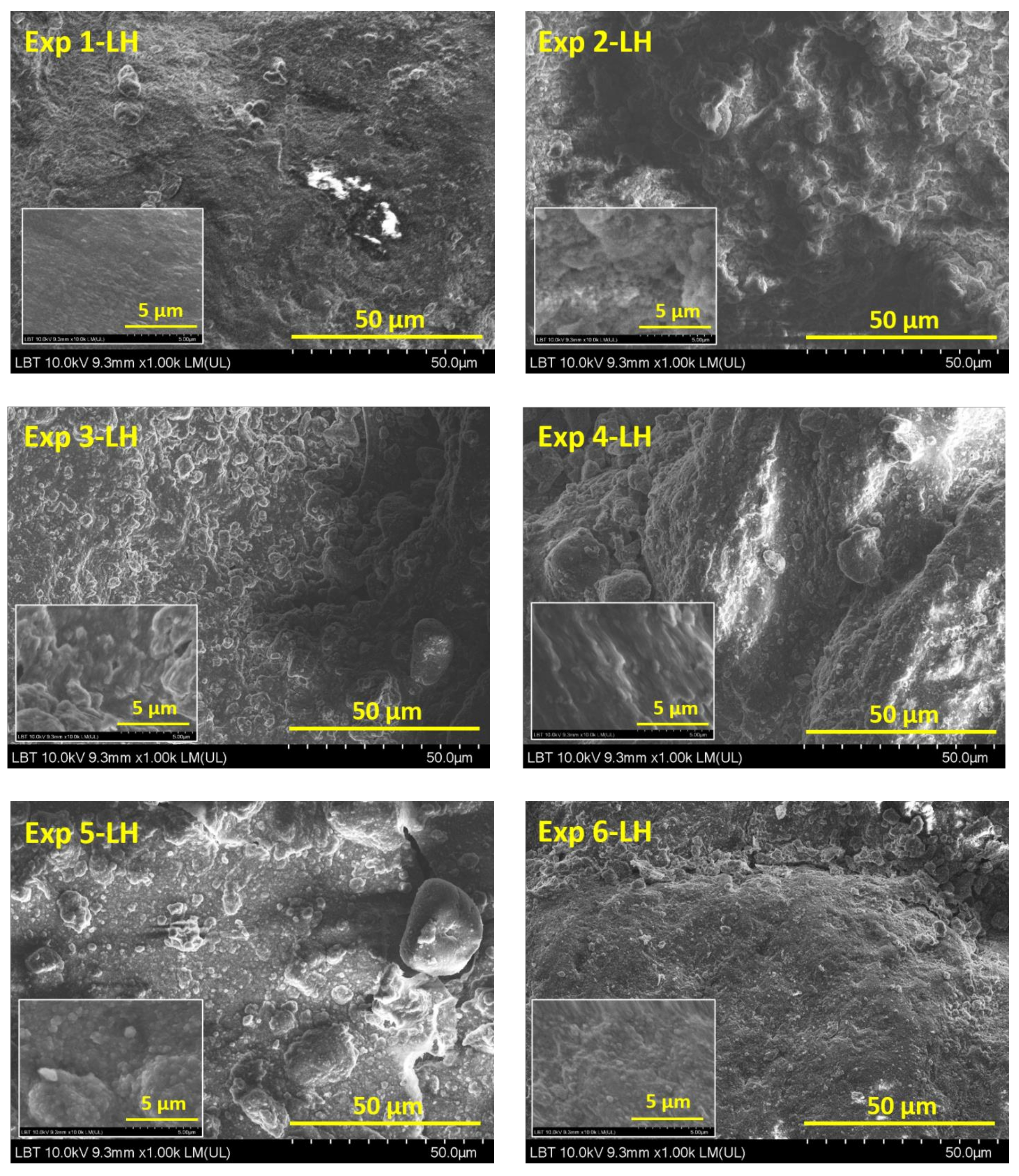
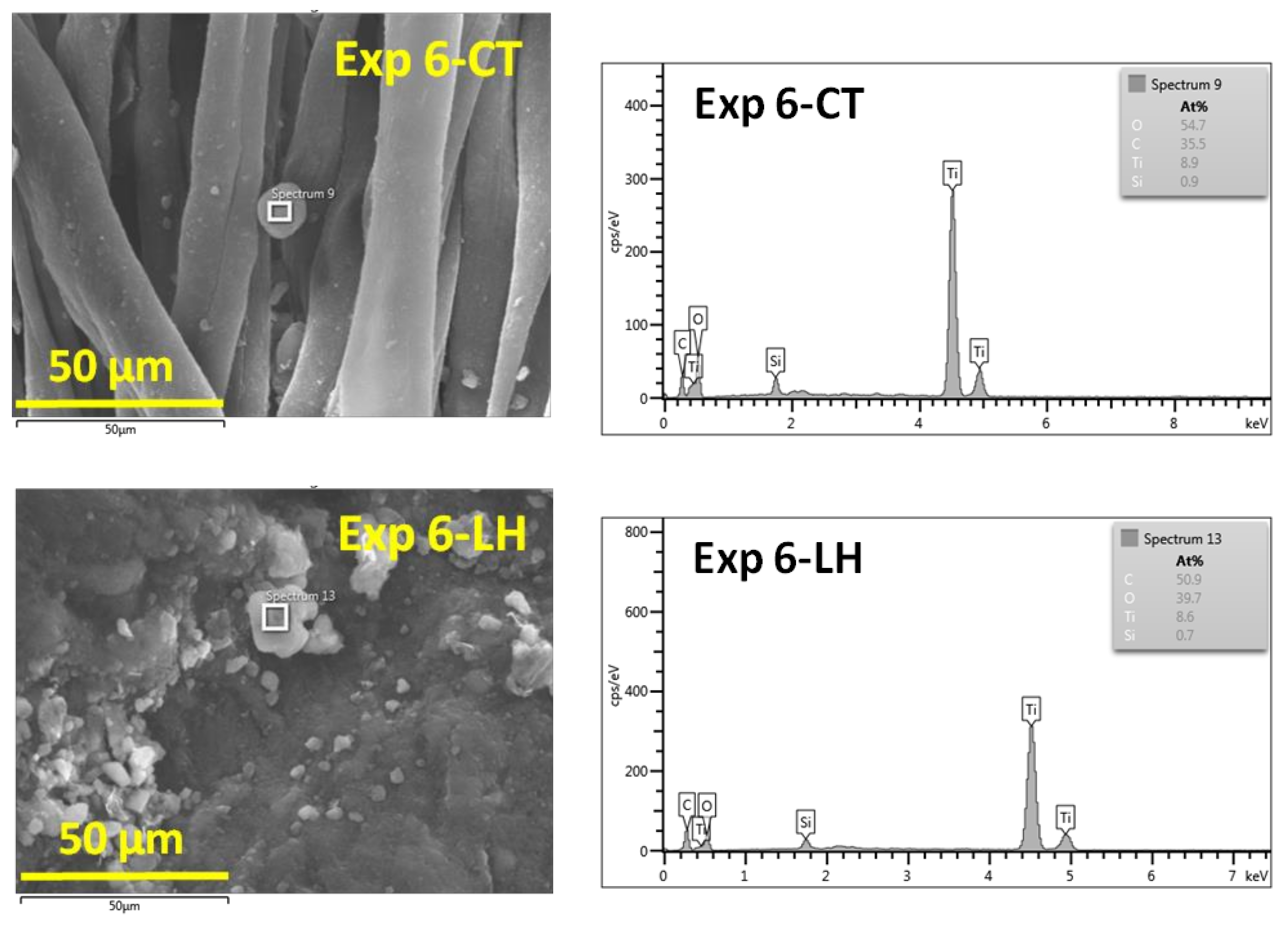
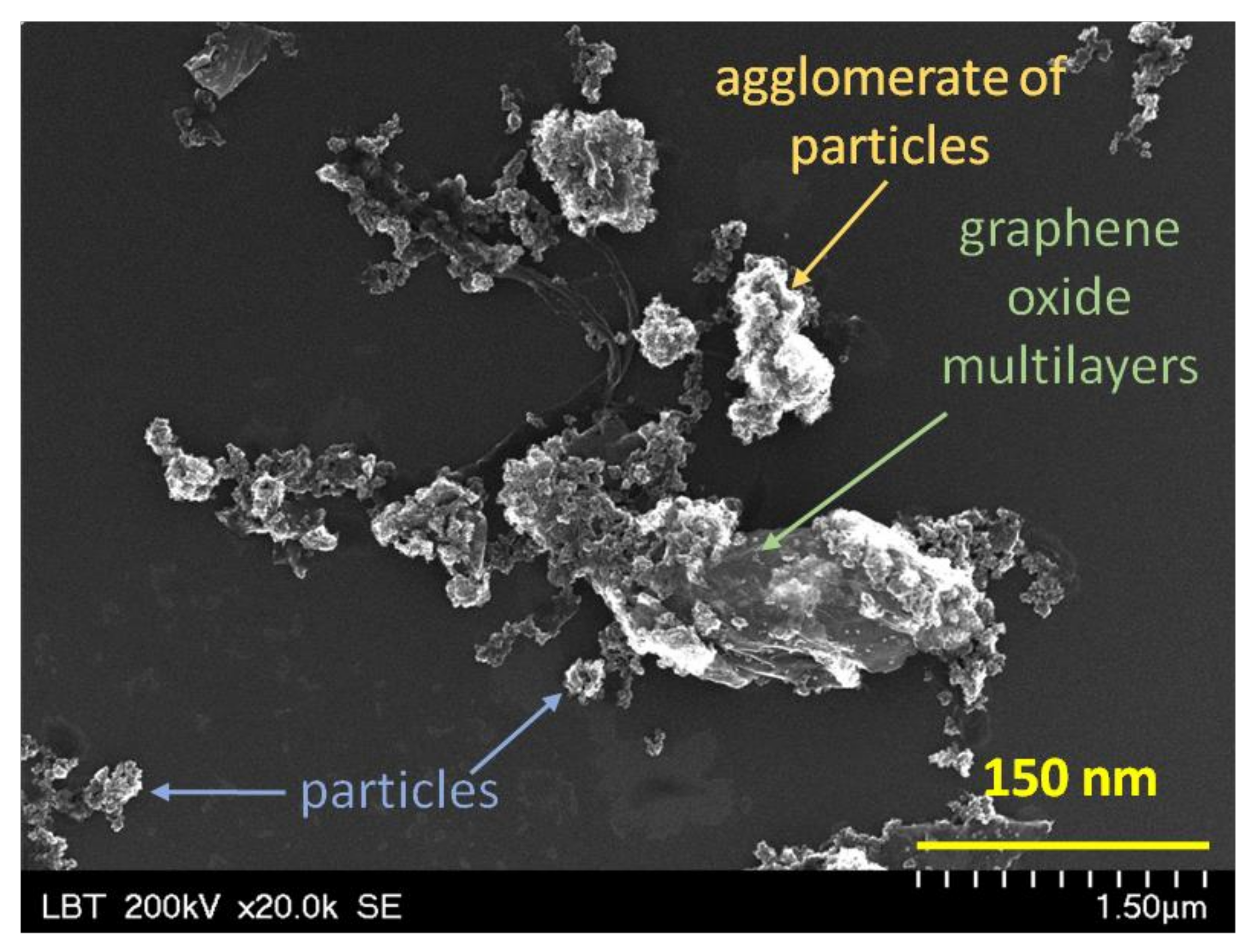



| Experimental Runs | Factor A Gas Plasma, 5 L·min−1 | Factor B Organic Dispersion Medium, 5 mL | Factor C Nanopowder, 0.01 g·mL−1 |
|---|---|---|---|
| 1 | Ar | D4/D5/Ethyl L-lactate | TS |
| 2 | Ar | D4/D5/Ethyl L-lactate | TS/GR |
| 3 | Ar/O3 | Ethyl L-lactate | TS |
| 4 | Ar/O3 | GRos/Ethyl L-lactate and NaAlg/H2O | TS/GR |
| 5 | No plasma | GRos/Ethyl L-lactate and NaAlg/H2O | TS |
| 6 | No plasma | Ethyl L-lactate | TS/GR |
| Sample | CT | Exp 1-CT | Exp 2-CT | Exp 3-CT | Exp 4-CT | Exp 5-CT | Exp 6-CT |
|---|---|---|---|---|---|---|---|
| CrIXRD (%) | 90.05 | 87.10 | 85.77 | 88.25 | 87.46 | 84.04 | 86.87 |
| Sample | LH | Exp 1-LH | Exp 2-LH | Exp 3-LH | Exp 4-LH | Exp 5-LH | Exp 6-LH |
|---|---|---|---|---|---|---|---|
| d7.7° (nm) | 1.14 | 1.13 | 1.14 | 1.13 | 1.09 | 1.02 | 1.14 |
| d20.3° (Å) | 4.58 | 4.52 | 4.52 | 4.42 | 4.37 | 4.35 | 4.40 |
| Sample | AIII/A1450 | ∆υ = υAI − υAII (cm−1) |
|---|---|---|
| LH | 1.01 | 90 |
| Exp 1-LH | 0.99 | 91 |
| Exp 2-LH | 0.88 | 90 |
| Exp 3-LH | 0.95 | 93 |
| Exp 4-LH | 0.67 | 62 |
| Exp 5-LH | 0.49 | 58 |
| Exp 6-LH | 0.87 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosma, D.-V.; Tudoran, C.; Coroș, M.; Socaci, C.; Urda, A.; Turza, A.; Roșu, M.-C.; Barbu-Tudoran, L.; Stanculescu, I. Modification of Cotton and Leather Surfaces Using Cold Atmospheric Pressure Plasma and TiO2-SiO2-Reduced Graphene Oxide Nanopowders. Materials 2023, 16, 1397. https://doi.org/10.3390/ma16041397
Cosma D-V, Tudoran C, Coroș M, Socaci C, Urda A, Turza A, Roșu M-C, Barbu-Tudoran L, Stanculescu I. Modification of Cotton and Leather Surfaces Using Cold Atmospheric Pressure Plasma and TiO2-SiO2-Reduced Graphene Oxide Nanopowders. Materials. 2023; 16(4):1397. https://doi.org/10.3390/ma16041397
Chicago/Turabian StyleCosma, Dragoș-Viorel, Cristian Tudoran, Maria Coroș, Crina Socaci, Alexandra Urda, Alexandru Turza, Marcela-Corina Roșu, Lucian Barbu-Tudoran, and Ioana Stanculescu. 2023. "Modification of Cotton and Leather Surfaces Using Cold Atmospheric Pressure Plasma and TiO2-SiO2-Reduced Graphene Oxide Nanopowders" Materials 16, no. 4: 1397. https://doi.org/10.3390/ma16041397
APA StyleCosma, D.-V., Tudoran, C., Coroș, M., Socaci, C., Urda, A., Turza, A., Roșu, M.-C., Barbu-Tudoran, L., & Stanculescu, I. (2023). Modification of Cotton and Leather Surfaces Using Cold Atmospheric Pressure Plasma and TiO2-SiO2-Reduced Graphene Oxide Nanopowders. Materials, 16(4), 1397. https://doi.org/10.3390/ma16041397









