Molecular Mechanism of Conformational Crossover of Mefenamic Acid Molecules in scCO2
Abstract
:1. Introduction
- within the framework of quantum chemical calculations, using different functionals, to carry out conformational search for MA molecules, to determine the energy characteristics of the conformers and vibration frequencies of the key functional groups involved in conformational transitions, and to determine the parameters of probable intramolecular H-bonds in different conformers of MA molecules;
- within the framework of molecular dynamics simulation, using different force fields, to determine the effect of the medium (scCO2) and temperature on the energy characteristics and probability of formation of different MA conformers;
- using in situ IR spectroscopy, to conduct a detailed study of the conformational equilibrium of MA molecules in a true solution in scCO2 under isochoric heating conditions in the temperature range of 140−210 °C (along the isochore corresponding to the scCO2 density equal to 1.1 of its critical value);
- to compare the results obtained for the true solution with those obtained in [8] for a heterogeneous MA–scCO2 mixture.
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Quantum Chemical Calculations
2.2.2. Classical Molecular Dynamics Simulation
2.2.3. In Situ IR Spectroscopy
3. Results and Discussion
3.1. Conformational Analysis Quantum Chemical Calculations
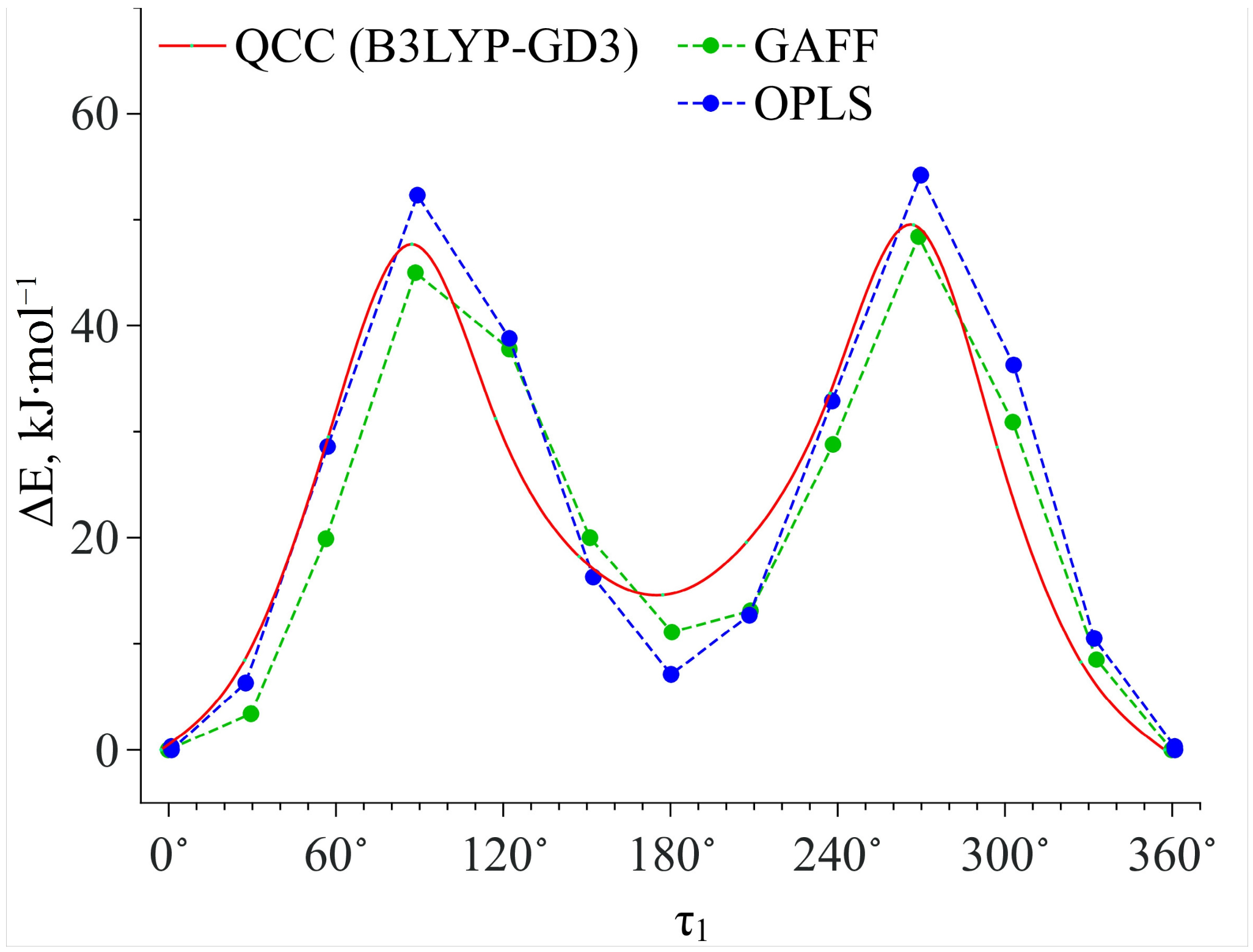
3.2. Conformational Analysis Molecular Dynamics Simulation
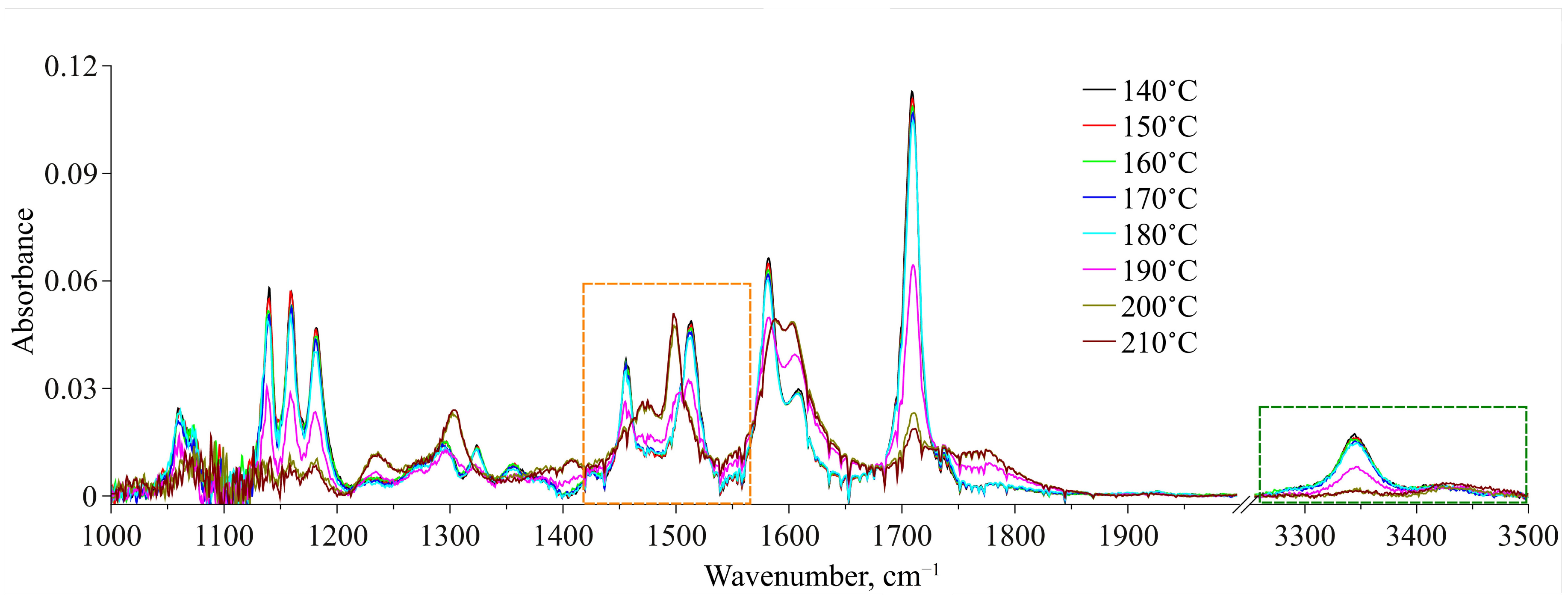
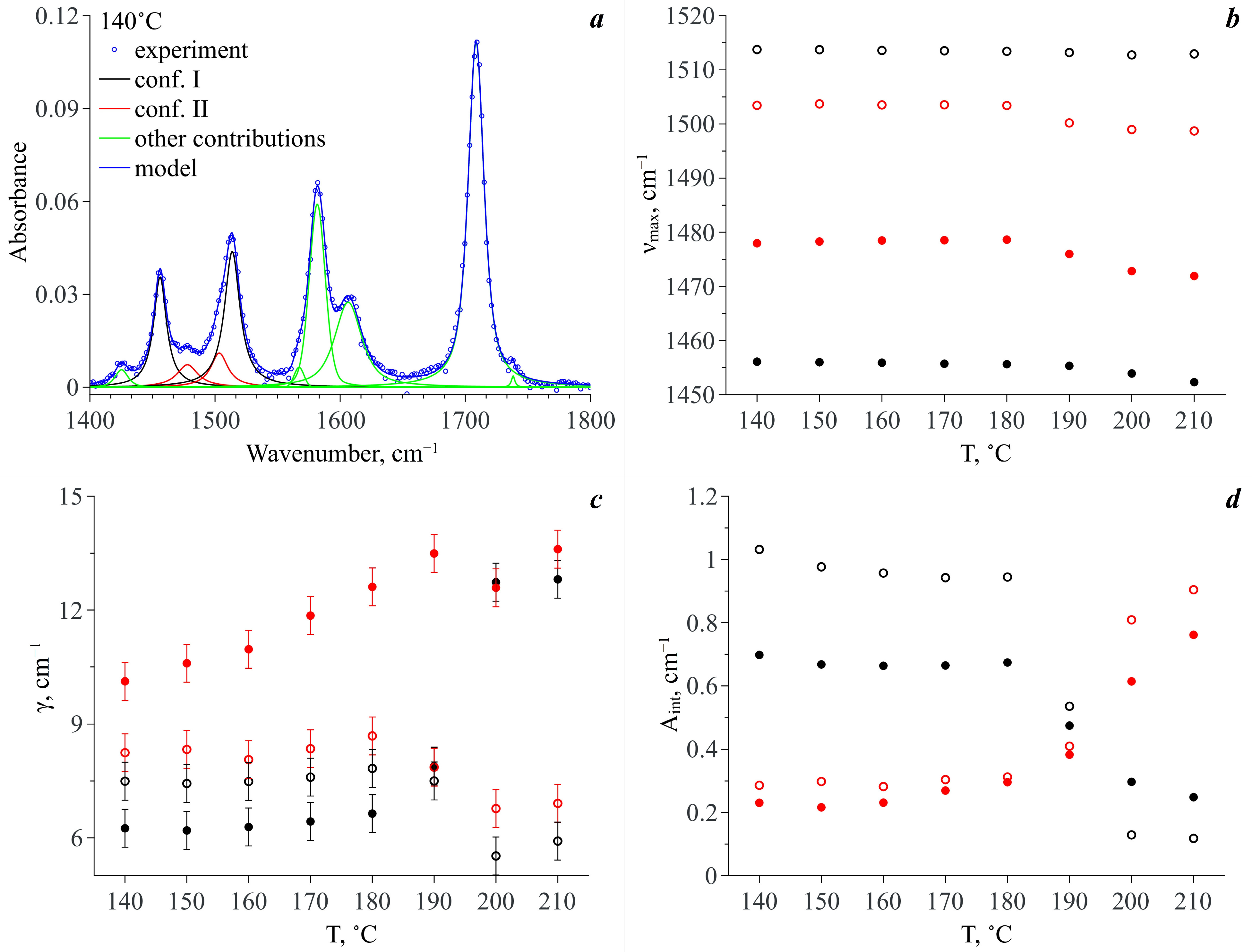
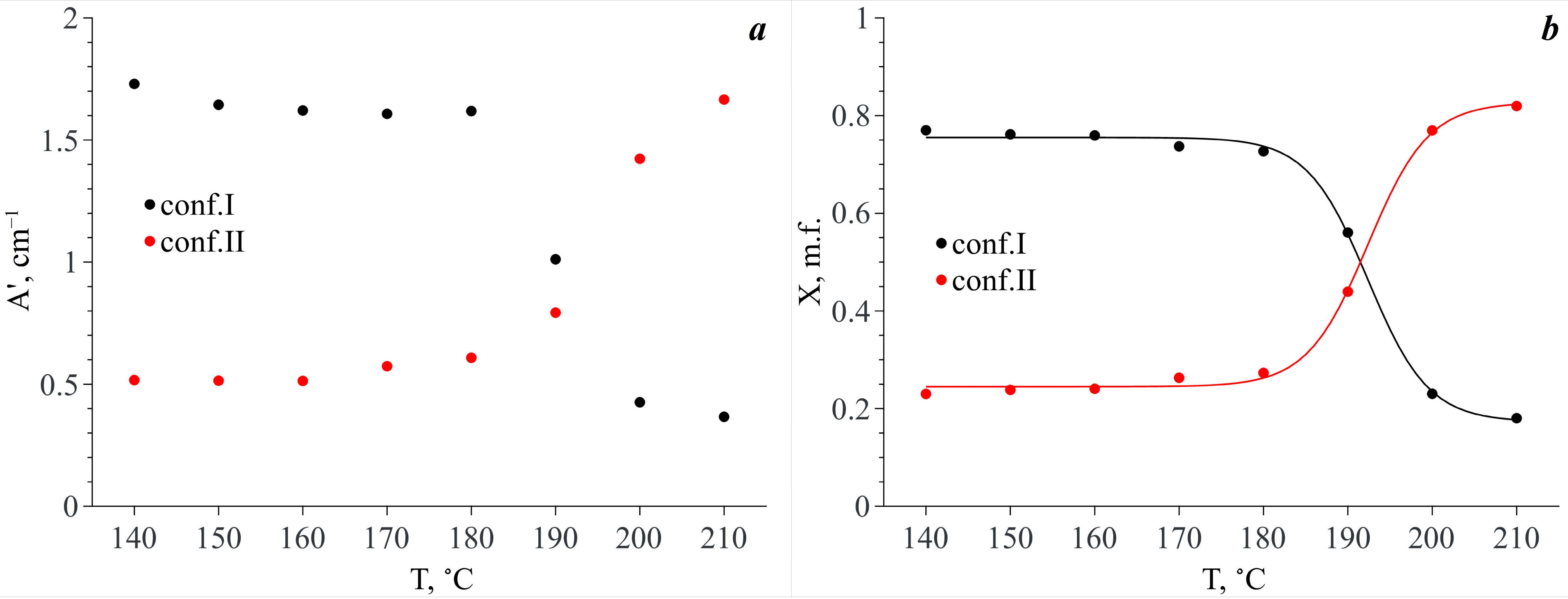
3.3. Conformational Analysis IR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SCF | supercritical fluid |
| MA | mefenamic acid |
| scCO2 | supercritical carbon dioxide |
| QCC | quantum chemical calculations |
| MD | molecular dynamics simulation |
References
- Nangia, A. Conformational Polymorphism in Organic Crystals. Acc. Chem. Res. 2008, 41, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cabeza, A.J.; Bernstein, J. Conformational Polymorphism. Chem. Rev. 2014, 114, 2170–2191. [Google Scholar] [CrossRef]
- Purohit, R.; Venugopalan, P. Polymorphism: An Overview. Reson. J. Sci. Educ. 2009, 14, 882–893. [Google Scholar] [CrossRef]
- Chieng, N.; Rades, T.; Aaltonen, J. An Overview of Recent Studies on the Analysis of Pharmaceutical Polymorphs. J. Pharm. Biomed. Anal. 2011, 55, 618–644. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.B.; Kumar, G.A.; Kumar, A.R.; Shavi, G.V.; Karthik, A.; Reddy, M.S.; Udupa, N. Supercritical Fluid Technology: Concepts and Pharmaceutical Applications. PDA J. Pharm. Sci. Technol. 2011, 65, 333–344. [Google Scholar] [CrossRef]
- Oparin, R.D.; Moreau, M.; De Walle, I.; Paolantoni, M.; Idrissi, A.; Kiselev, M.G. The Interplay between the Paracetamol Polymorphism and Its Molecular Structures Dissolved in Supercritical CO2 in Contact with the Solid Phase: In Situ Vibration Spectroscopy and Molecular Dynamics Simulation Analysis. Eur. J. Pharm. Sci. 2015, 77, 48–59. [Google Scholar] [CrossRef]
- Oparin, R.D.; Ivlev, D.V.; Vorobei, A.M.; Idrissi, A.; Kiselev, M.G. Screening of Conformational Polymorphism of Ibuprofen in Supercritical CO2. J. Mol. Liq. 2017, 239, 49–60. [Google Scholar] [CrossRef]
- Oparin, R.D.; Vaksler, Y.A.; Krestyaninov, M.A.; Idrissi, A.; Shishkina, S.V.; Kiselev, M.G. Polymorphism and Conformations of Mefenamic Acid in Supercritical Carbon Dioxide. J. Supercrit. Fluids 2019, 152, 104547–104561. [Google Scholar] [CrossRef]
- Oparin, R.D.; Ivlev, D.V.; Kiselev, M.G. Conformational Equilibria of Pharmaceuticals in Supercritical CO2, IR Spectroscopy and Quantum Chemical Calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 118072–118080. [Google Scholar] [CrossRef] [PubMed]
- Oparin, R.D.; Kurskaya, M.V.; Krestyaninov, M.A.; Idrissi, A.; Kiselev, M.G. Correlation between the Conformational Crossover of Carbamazepine and Its Polymorphic Transition in Supercritical CO2: On the Way to Polymorph Control. Eur. J. Pharm. Sci. 2020, 146, 105273–105280. [Google Scholar] [CrossRef]
- Oparin, R.D.; Vaksler, Y.A.Y.A.; Krestyaninov, M.A.M.A.; Idrissi, A.; Kiselev, M.G. High Temperature Polymorphic Conversion of Carbamazepine in Supercritical CO2: A Way to Obtain Pure Polymorph I. J. Mol. Liq. 2020, 323, 114630. [Google Scholar] [CrossRef]
- Oparin, R.D.; Krestyaninov, M.A.; Kiselev, M.G. Role of an Intramolecular H-Bond in Lidocaine Conformer Distribution and Polymorph Stability. J. Mol. Liq. 2022, 360, 119461. [Google Scholar] [CrossRef]
- Aguiar, A.J.; Zelmer, J.E. Dissolution Behavior of Polymorphs of Chloramphenicol Palmitate and Mefenamic Acid. J. Pharm. Sci. 1969, 58, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Seethalekshmi, S.; Row, T.N.G. Conformational Polymorphism in a Non-Steroidal Anti-Inflammatory Drug, Mefenamic Acid. Cryst. Growth Des. 2012, 12, 4283–4289. [Google Scholar] [CrossRef]
- McConnell, J.F.; Company, F.Z. N-(2, 3-Xylyl) Anthranilic Acid, C15H15NO2, Mefenamic Acid. Cryst. Struct. Commun. 1976, 5, 861–864. [Google Scholar]
- Yang, X.; Sarma, B.; Myerson, A.S. Polymorph Control of Micro/Nano-Sized Mefenamic Acid Crystals on Patterned Self-Assembled Monolayer Islands. Cryst. Growth Des. 2012, 12, 5521–5528. [Google Scholar] [CrossRef]
- Lee, E.H.; Byrn, S.R.; Carvajal, M.T. Additive-Induced Metastable Single Crystal of Mefenamic Acid. Pharm. Res. 2006, 23, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Oswald, I.D.H.; Pulham, C.R. Accessing Mefenamic Acid Form II through High-Pressure Recrystallisation. Pharmaceutics 2017, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, R.K.; Zhou, W. Infrared Studies of the Thermal Conversion of Mefenamic Acid between Polymorphic States. Vib. Spectrosc. 2005, 37, 53–59. [Google Scholar] [CrossRef]
- Belov, K.V.; Dyshin, A.A.; Krestyaninov, M.A.; Efimov, S.V.; Khodov, I.A.; Kiselev, M.G. Conformational Preferences of Tolfenamic Acid in DMSO-CO2 Solvent System by 2D NOESY. J. Mol. Liq. 2022, 367, 120481. [Google Scholar] [CrossRef]
- Belov, K.V.; Batista de Carvalho, L.A.E.; Dyshin, A.A.; Kiselev, M.G.; Sobornova, V.V.; Khodov, I.A. Conformational Analysis of Mefenamic Acid in ScCO2-DMSO by the 2D NOESY Method. Russ. J. Phys. Chem. B 2022, 16, 1191–1199. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, Inc., Wallingford CT. 2016. Available online: https://gaussian.com/g09citation/ (accessed on 16 May 2022).
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.; Petersson, G.A.; Frisch, M.J.; Dobek, F.J.; Scalmani, G.; Throssell, K. A Density Functional with Spherical Atom Dispersion Terms. J. Chem. Theory Comput. 2012, 8, 4989–5007. [Google Scholar] [CrossRef] [PubMed]
- Oparin, R.D.; Vaksler, Y.A.; Krestyaninov, M.A.; Idrissi, A.; Kiselev, M.G. Possibility of Dopant Morphology Control in the Process of Polymer Impregnation with Pharmaceuticals in a Supercritical CO2 Medium. J. Mol. Liq. 2021, 330, 115657. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian Basis Sets for Molecular Calculations. I. Second Row Atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Weinhold, F. Nature of H-Bonding in Clusters, Liquids, and Enzymes: An Ab Initio, Natural Bond Orbital Perspective. J. Mol. Struct. Theochem 1997, 398, 181–197. [Google Scholar] [CrossRef]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO 3.0 Program Manual; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 1990. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and Its Applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen Bond Strengths Revealed by Topological Analyses of Experimentally Observed Electron Densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Laio, A.; Parrinello, M. Escaping Free-Energy Minima. Proc. Natl. Acad. Sci. USA 2002, 99, 12562–12566. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Duan, Z. An Optimized Molecular Potential for Carbon Dioxide. J. Chem. Phys. 2005, 122, 214507. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Oparin, R.D.; Idrissi, A.; Fedorov, M.V.; Kiselev, M.G. Dynamic and Static Characteristics of Drug Dissolution in Supercritical CO2 By Infrared Spectroscopy: Measurements of Acetaminophen Solubility in a Wide Range of State Parameters. J. Chem. Eng. Data 2014, 59, 3517–3523. [Google Scholar] [CrossRef]
- Belov, K.V.; De Carvalho, L.A.E.B.; Dyshin, A.A.; Efimov, S.V.; Khodov, I.A. The Role of Hidden Conformers in Determination of Conformational Preferences of Mefenamic Acid by NOESY Spectroscopy. Pharmaceutics 2022, 14, 2276. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring Nature and Predicting Strength of Hydrogen Bonds: A Correlation Analysis Between Atoms-in-Molecules Descriptors, Binding Energies, and Energy Components of Symmetry-Adapted Perturbation Theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef] [PubMed]
- Krestyaninov, M.A.; Kolker, A.M. Quantum Chemical Analysis of Hydrogen Bonding in Pyrrolidone Dimers and Pyrrolidone–Water Complexes. J. Mol. Liq. 2021, 341, 116909. [Google Scholar] [CrossRef]
- Oparin, R.; Tassaing, T.; Danten, Y.; Besnard, M. Water-Carbon Dioxide Mixtures at High Temperatures and Pressures: Local Order in the Water Rich Phase Investigated by Vibrational Spectroscopy. J. Chem. Phys. 2005, 123, 224501. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A General-Purpose Peak Fitting Program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Oparin, R.D.; Vorobyev, E.A.; Kiselev, M.G. A New Method for Measuring the Solubility of Slightly Soluble Substances in Supercritical Carbon Dioxide. Russ. J. Phys. Chem. B 2016, 10, 1108–1115. [Google Scholar] [CrossRef]
- Kalikin, N.N.; Kurskaya, M.V.; Ivlev, D.V.; Krestyaninov, M.A.; Oparin, R.D.; Kolesnikov, A.L.; Budkov, Y.A.; Idrissi, A.; Kiselev, M.G. Carbamazepine Solubility in Supercritical CO2: A Comprehensive Study. J. Mol. Liq. 2020, 311, 113104–113110. [Google Scholar] [CrossRef]
- Kalikin, N.N.; Oparin, R.D.; Kolesnikov, A.L.; Budkov, Y.A.; Kiselev, M.G. A Crossover of the Solid Substances Solubility in Supercritical Fluids: What Is It in Fact? J. Mol. Liq. 2021, 334, 115997. [Google Scholar] [CrossRef]
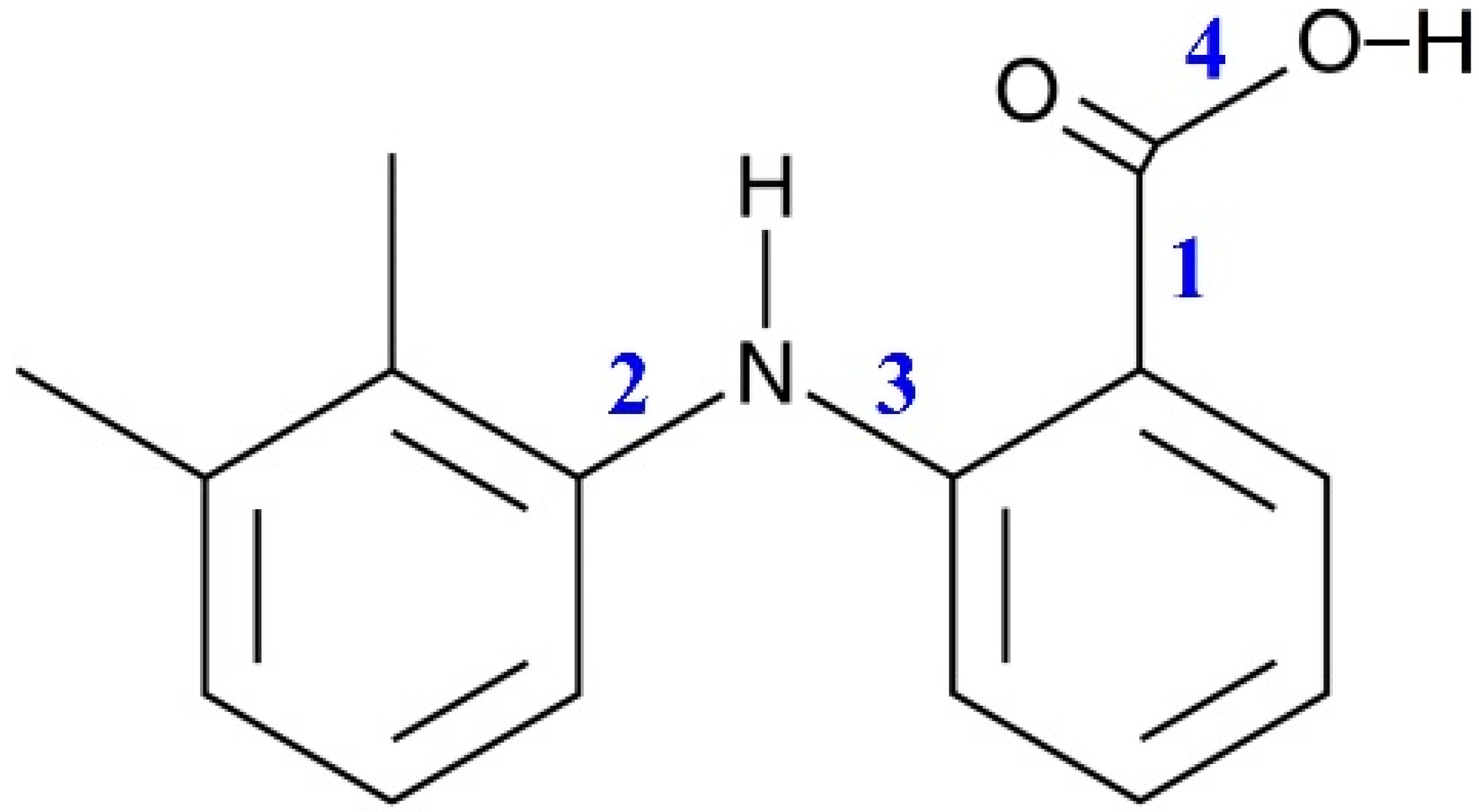

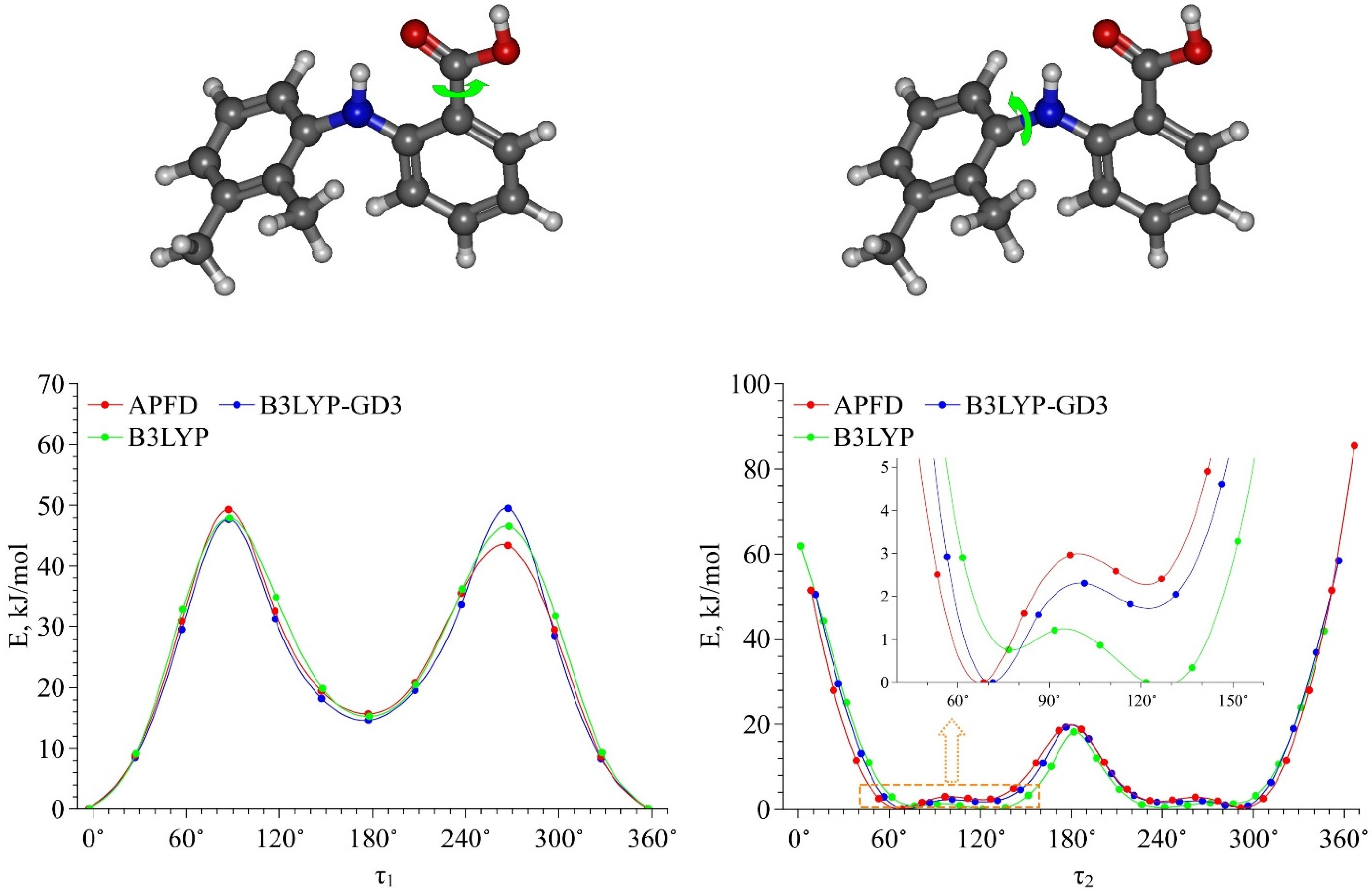
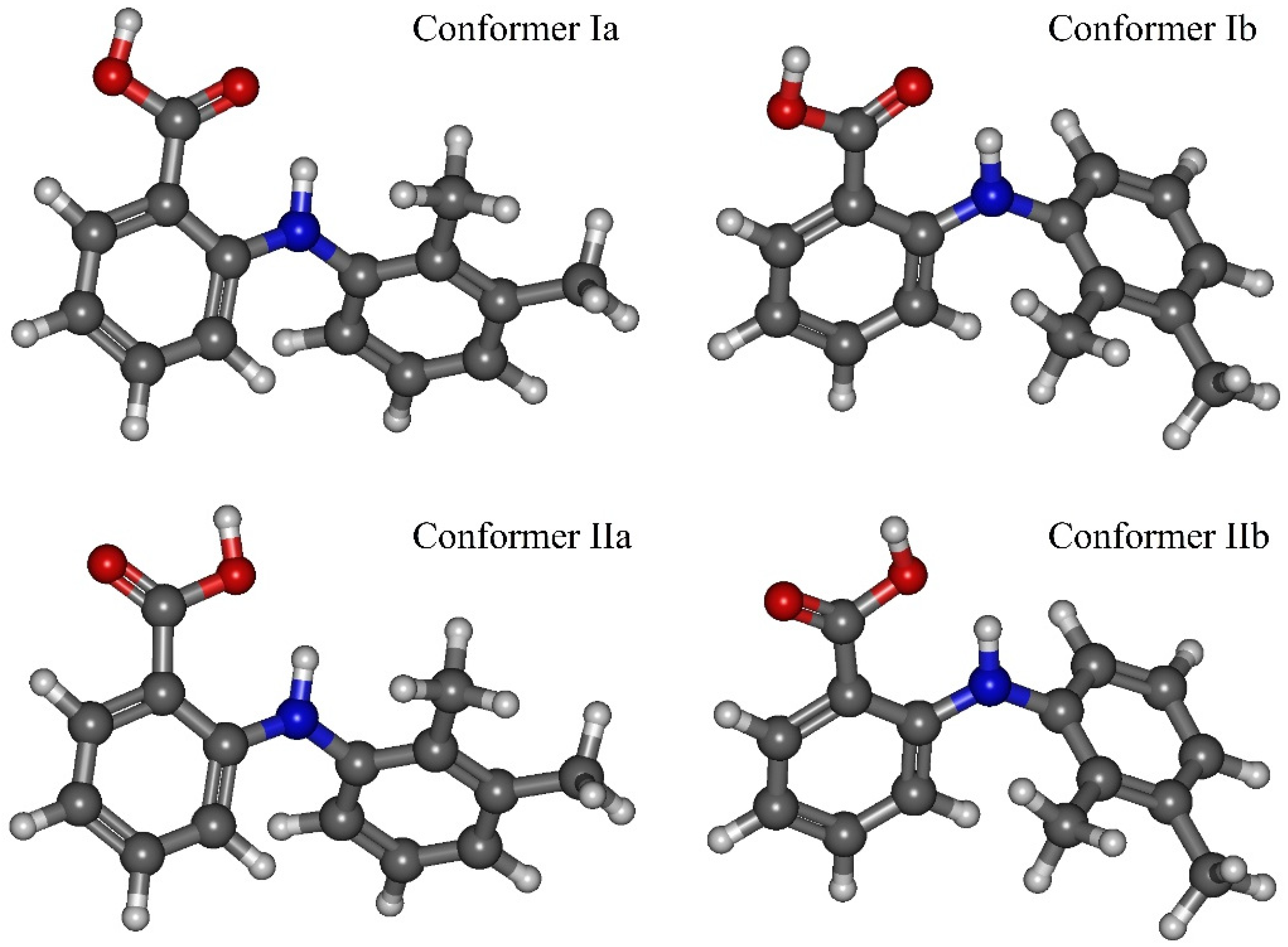
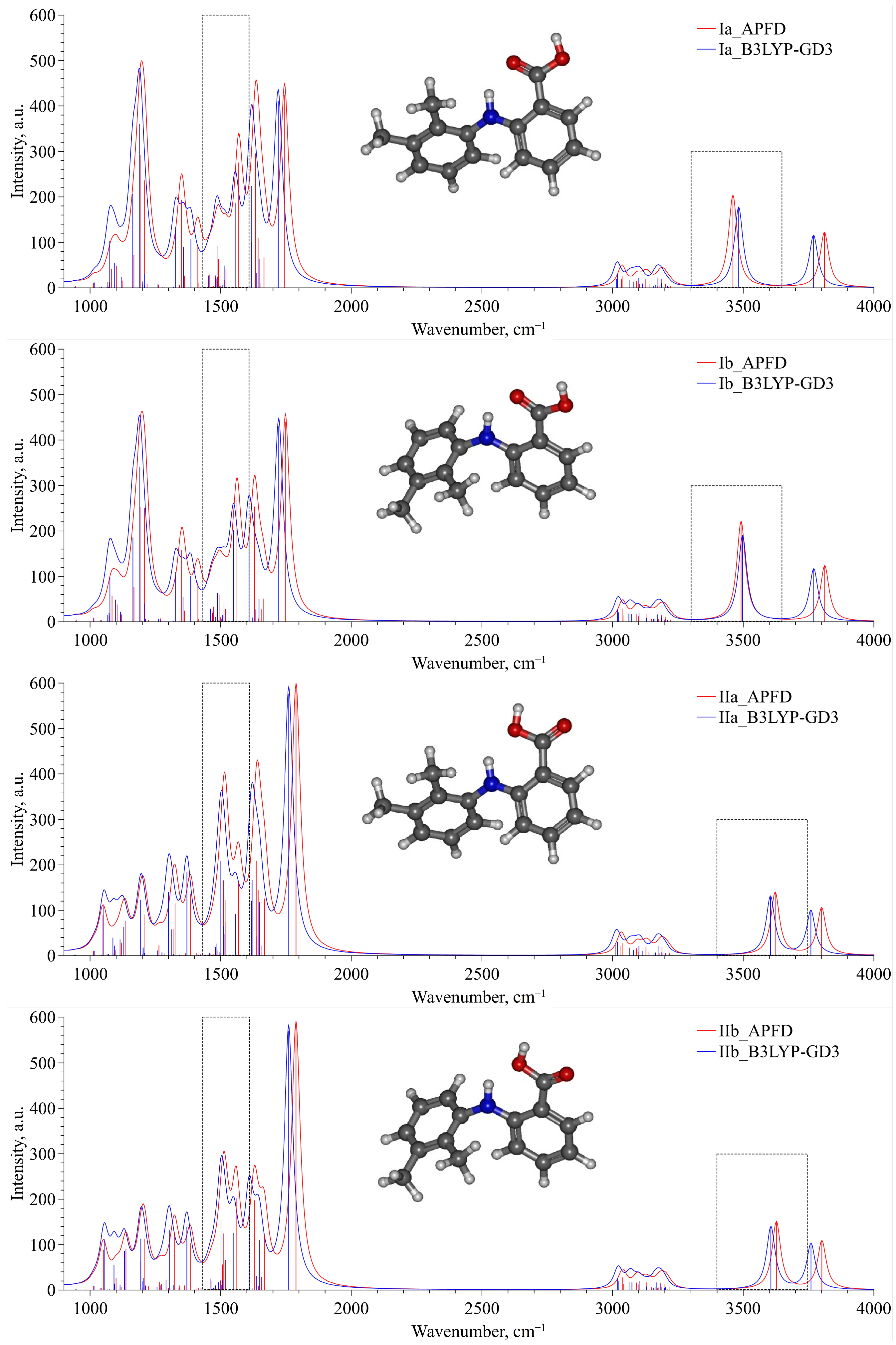

| T, °C | P, bar |
|---|---|
| 140.00 | 296.03 |
| 150.00 | 316.58 |
| 160.00 | 337.09 |
| 170.00 | 357.57 |
| 180.00 | 378.02 |
| 190.00 | 398.43 |
| 200.00 | 418.80 |
| 210.00 | 439.13 |
| Functional | Dihedral Angle | Conf. Ia | Conf. Ib | Conf. IIa | Conf. IIb |
|---|---|---|---|---|---|
| B3LYP | τ1 | 4.09° | −2.17° | −173.95° | 175.27° |
| τ2 | 135.10° | 76.64° | 135.65° | 77.39° | |
| ∆E, kJ·mol−1 | 0.00 | 1.48 | 16.05 | 16.66 | |
| B3LYP–GD3 | τ1 | 4.13° | −2.77° | −173.14° | 174.12° |
| τ2 | 132.06° | 71.47° | 132.16° | 72.39° | |
| ∆E, kJ·mol−1 | 1.14 | 0.00 | 16.65 | 14.56 | |
| APFD | τ1 | 4.34° | −2.87° | −173.04° | 174.23° |
| τ2 | 135.44° | 68.48° | 135.82° | 69.36° | |
| ∆E, kJ·mol−1 | 1.45 | 0.00 | 18.07 | 15.63 |
| Functional | Conformer | E2, kJ·mol−1 | q,e | ρ(N−H…O), a.u. | −½V(N−H…O), kJ·mol−1 |
|---|---|---|---|---|---|
| B3LYP–GD3 | Ia | 42.17 | 0.0219 | 0.03360 | 36.50758 |
| Ib | 39.37 | 0.0202 | 0.03183 | 33.85582 | |
| IIa | 28.49 | 0.0099 | 0.02835 | 29.85194 | |
| IIb | 22.72 | 0.0081 | 0.02712 | 28.13223 | |
| APFD | Ia | 72.51 | 0.0412 | 0.03676 | 41.65356 |
| Ib | 46.28 | 0.0224 | 0.03479 | 38.55547 | |
| IIa | 26.78 | 0.0092 | 0.02966 | 31.96542 | |
| IIb | 24.18 | 0.0082 | 0.02828 | 29.97008 |
| T = 160 °C | T = 190 °C | T = 220 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Conf. I | Barrier | Conf. II | Conf. I | Barrier | Conf. II | Conf. I | Barrier | Conf. II | |
| GAFF | |||||||||
| τ1, ° | −7.3 | −102.9 | 168.9 | 14.7 | 102.9 | −161.6 | 14.7 | 102.9 | −157.9 |
| τ2, ° | −146.9 | −139.6 | −154.3 | 150.6 | 161.6 | 154.3 | 154.3 | 150.6 | 150.6 |
| ΔG, kJ·mol−1 | 0.0 | 38.16 | 10.25 | 0.0 | 36.99 | 9.89 | 0.0 | 34.36 | 8.05 |
| OPLS | |||||||||
| τ1, ° | 3.7 | −95.5 | −176.3 | 0.0 | 95.5 | −172.6 | 3.7 | −91.8 | −180.0 |
| τ2, ° | 165.3 | −154.3 | 161.6 | −161.6 | 172.7 | 154.3 | 172.7 | −180.0 | 165.3 |
| ΔG, kJ·mol−1 | 0.0 | 47.54 | 3.88 | 0.0 | 45.31 | 3.95 | 0.0 | 44.74 | 2.51 |
| GAFF | OPLS | |||||
|---|---|---|---|---|---|---|
| T = 160 °C | T = 190 °C | T = 220 °C | T = 160 °C | T = 190 °C | T = 220 °C | |
| Conf. I | 0.9451 | 0.9288 | 0.8769 | 0.7428 | 0.7284 | 0.6919 |
| Conf. II | 0.0463 | 0.0375 | 0.0732 | 0.2572 | 0.2716 | 0.3081 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oparin, R.D.; Krestyaninov, M.A.; Ivlev, D.V.; Kiselev, M.G. Molecular Mechanism of Conformational Crossover of Mefenamic Acid Molecules in scCO2. Materials 2023, 16, 1403. https://doi.org/10.3390/ma16041403
Oparin RD, Krestyaninov MA, Ivlev DV, Kiselev MG. Molecular Mechanism of Conformational Crossover of Mefenamic Acid Molecules in scCO2. Materials. 2023; 16(4):1403. https://doi.org/10.3390/ma16041403
Chicago/Turabian StyleOparin, Roman D., Mikhail A. Krestyaninov, Dmitry V. Ivlev, and Michael G. Kiselev. 2023. "Molecular Mechanism of Conformational Crossover of Mefenamic Acid Molecules in scCO2" Materials 16, no. 4: 1403. https://doi.org/10.3390/ma16041403
APA StyleOparin, R. D., Krestyaninov, M. A., Ivlev, D. V., & Kiselev, M. G. (2023). Molecular Mechanism of Conformational Crossover of Mefenamic Acid Molecules in scCO2. Materials, 16(4), 1403. https://doi.org/10.3390/ma16041403







