Assessment of Intra-Oral Repair Systems for Veneered Zirconia and Zirconia Only
Abstract
1. Introduction
2. Material and Methods
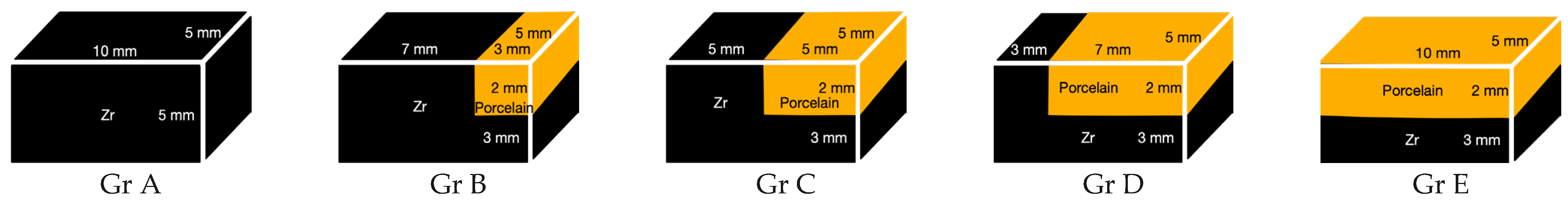
2.1. Specimen Preparation
| Laminate Veneer Repair | Composite Resin Repair | |
|---|---|---|
| Gr A = 100% Zr (n:20) | Gr A1 | Gr A2 |
| Gr B = 70% Zr (n:20) | Gr B1 | Gr B2 |
| Gr C = 50% Zr (n:20) | Gr C1 | Gr C2 |
| Gr D = 30% Zr (n:20) | Gr D1 | Gr D2 |
| Gr E = 0% Zr (n:20) | Gr E1 | Gr E2 |
2.1.1. Zirconia Preparation
2.1.2. Application of Suprastructure Ceramics
2.1.3. Surface Treatments of Specimens
2.1.4. Preparation of Laminate Veneers
2.2. Cementation of Laminate Veneers for Indirect Technique
2.3. Applying Composite Resins for Direct Technique
2.4. Aging
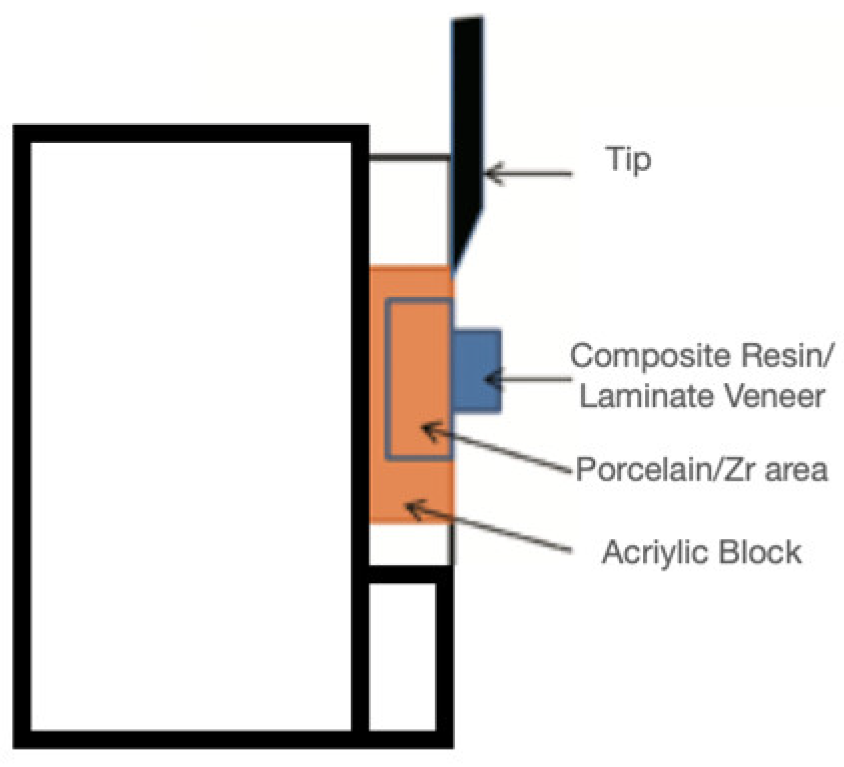
2.5. Adhesion Tests and Mode of Failure Analysis
2.6. Statistical Analysis
3. Results
3.1. Shear Bond Strength Results
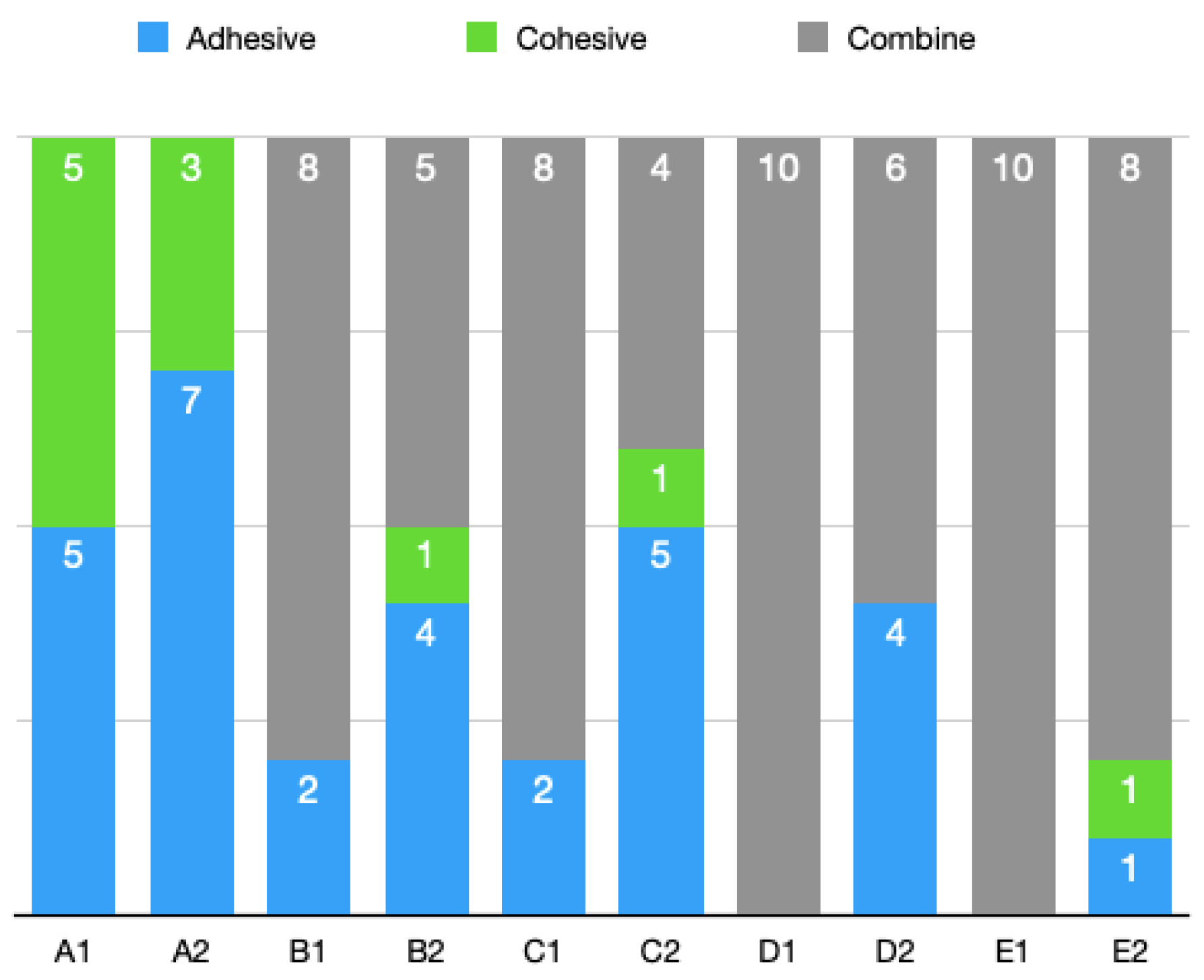
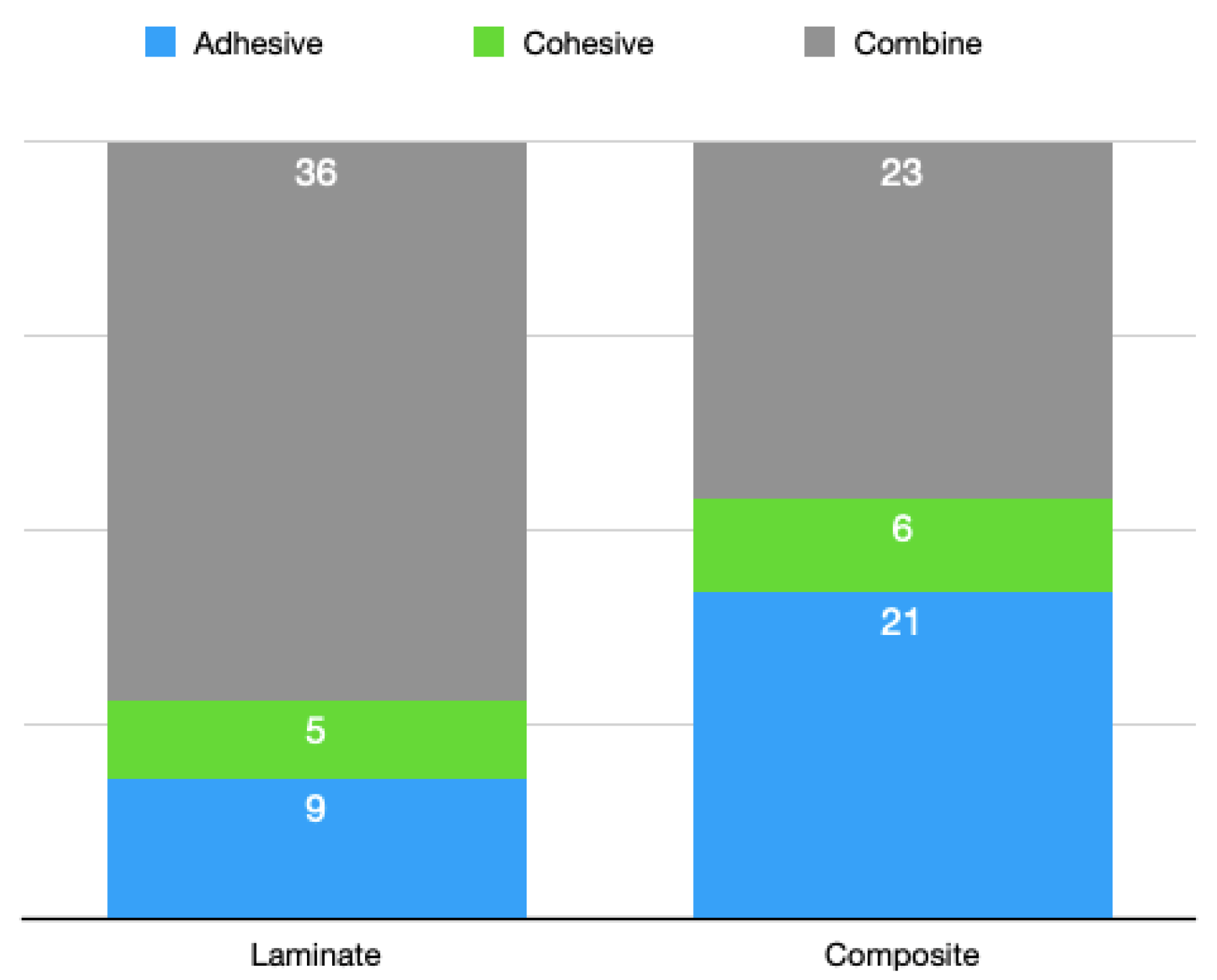
3.2. Stereomicroscopic Analysis Results
4. Discussion
5. Conclusions
- When entire zirconia substructure was exposed, the success of the porcelain repair made with laminate veneers was higher than that of the repair with the composite.
- In the group where most of the zirconia substructure was exposed, the success of the porcelain repair made with laminate veneers was higher than that of the repair with the composite.
- In cases where the zirconia and porcelain surfaces were at equal ratios, there was no statistically significant difference between the repair made with the composite or the repair made with the laminate veneers, but the laminate veneers had numerically higher bond strength values.
- In the group where a small part of the zirconia substructure was exposed, the success of the porcelain repair made with the laminate veneers was higher than that of the repair with the composite.
- When the zirconia substructure was not exposed, and the surface was completely made of porcelain, the bonding success of the porcelain repair made with the laminate veneers was higher than that made with the composite.
- The subgroups repaired with the composite showed more adhesive fractures than the subgroups repaired with the laminate veneers. This distribution was directly proportional to the bond strength values.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kartal, E. Evaluation of Shear Bond Strength of Porcelain Repair System after Different Surface Conditioning Methods in Zirconia Ceramics. Ph.D. Thesis, Necmettin Erbakan University, Konya, Turkey, 2017. [Google Scholar]
- Rosentiel, S.; Fujimoto, J. Contemporary Fixed Prosthodontics; Mosby Elsevier: Maryland Heights, MO, USA, 2006. [Google Scholar]
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Marx, R. Fracture toughness of dental ceramics: Comparison of bending and indentation method. Dent. Mater. 2002, 18, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G. Biological interactions of dental cast alloys with oral tissues. Dent. Mater. 2002, 18, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Schmitter, M.; Mueller, D.; Rues, S. Chipping behaviour of all-ceramic crowns with zirconia framework and CAD/CAM manufactured veneer. J. Dent. 2012, 40, 154–162. [Google Scholar] [CrossRef]
- Raigrodski, A.J.; Hillstead, M.B.; Meng, G.K.; Chung, K.H. Survival and Complications of Zirconia—Based Fixed Dental Prostheses: A Systematic Review. J. Prosthet. Dent. 2012, 107, 170–177. [Google Scholar] [CrossRef]
- Özcan, M.; Niedermier, W. Clinical study on the reasons for and location of failures of metal-ceramic restorations and survival of repairs. Int. J. Prosthodont. 2002, 15, 299–302. [Google Scholar]
- Hirschfeld, Z.; Rehany, A. Esthetic repair of porcelain in a complete-mouth reconstruction: A case report. Quintessence Int. 1991, 22, 945–947. [Google Scholar]
- Haselton, D.R.; Diaz-Arnold, A.M.; Dunne, J.T. Shear bond strengths of 2 intraoral porcelain repair systems to porcelain or metal substrates. J. Prosthet. Dent. 2001, 86, 526–531. [Google Scholar] [CrossRef]
- Beck, D.A.; Janus, C.E.; Douglas, H.B. Shear bond strength of composite resin porcelain repair materials bonded to metal and porcelain. J. Prosthet. Dent. 1990, 64, 529–533. [Google Scholar] [CrossRef]
- Dupont, R. Large ceramo-metallic restorations. Int. Dent. J. 1968, 18, 288–308. [Google Scholar]
- Aladağ, A.; Çömlekoğlu, M.E. Metal Destekli Seramik Faset ile Endirekt Seramik Kırığı Onarımı: Olgu Sunumu. EÜ Dişhek Fak. Derg. 2009, 30, 47–51. [Google Scholar]
- Cardoso, A.C.; Filho, P.S. Operative Dentistry Clinical and laboratory techniques for repair of fractured porcelain in fixed prostheses: A case report. Quintessence Int. 1994, 25, 835–838. [Google Scholar]
- Tsalouchou, E.; Cattell, M.J.; Knowles, J.C.; Pittayachawan, P.; McDonald, A. Fatigue and fracture properties of yttria partially stabilized zirconia crown systems. Dent. Mater. 2008, 24, 308–318. [Google Scholar] [CrossRef]
- Silva, N.R.F.A.; Sailer, I.; Zhang, Y.; Coelho, P.G.; Guess, P.C.; Zembic, A.; Kohal, R.J. Performance of Zirconia for Dental Healthcare. Materials 2010, 3, 863–896. [Google Scholar] [CrossRef]
- Sailer, I.; Pjetursson, B.E.; Zwahlen, M.; Hämmerle, C.H.F. A systematic review of the survival and complication rates of all-ceramic and metal-ceramic reconstructions after an observation period of at least 3 years. Part II: Fixed dental prostheses. Clin. Oral Implant. Res. 2007, 18, 86–96. [Google Scholar] [CrossRef]
- Özcan, M.; Valandro, L.F.; Amaral, R.; Leite, F.; Bottino, M.A. Bond strength durability of a resin composite on a reinforced ceramic using various repair systems. Dent. Mater. 2009, 25, 1477–1483. [Google Scholar] [CrossRef]
- Valandro, L.F.; Özcan, M.; Bottino, M.A.; Scotti, R.; della Bona, A. Bond strength of a resin cement to high-alumina and zirconia-reinforced ceramics: The effect of surface conditioning Article. Dent. Mater. 2006, 8, 175–181. [Google Scholar]
- Buonocore, M.; Matsui, A.; Gwinnett, A. Penetration of resin dental materials into enamel surfaces with reference to bonding. Arch. Oral Biol. 1968, 13, 61-IN20. [Google Scholar] [CrossRef]
- Nikaido, T.; Kunzelmann, K.-H.; Chen, H.; Ogata, M.; Harada, N.; Yamaguchi, S.; Cox, C.; Hickel, R.; Tagami, J. Evaluation of thermal cycling and mechanical loading on bond strength of a self-etching primer system to dentin. Dent. Mater. 2002, 18, 269–275. [Google Scholar] [CrossRef]
- ISO/TR 11405: 1994; Dental Materials—Guidance on Testing of Adhesion to Tooth Structure. ISO: Geneva, Switzerland, 1994. Available online: https://www.iso.org/standard/19347.html (accessed on 2 February 2023).
- Lee, S.J.; Cheong, C.W.; Wright, R.F.; Chang, B.M. Bond Strength of the Porcelain Repair System to All-Ceramic Copings and Porcelain. J. Prosthodont. 2013, 23, 112–116. [Google Scholar] [CrossRef]
- Braga, R.R.; Meira, J.B.; Boaro, L.C.; Xavier, T.A. Adhesion to tooth structure: A critical review of “macro” test methods. Dent. Mater. 2010, 26, e38–e49. [Google Scholar] [CrossRef] [PubMed]
- Ishibe, M.; Raigrodski, A.J.; Flinn, B.D.; Chung, K.-H.; Spiekerman, C.; Winter, R.R. Shear bond strengths of pressed and layered veneering ceramics to high-noble alloy and zirconia cores. J. Prosthet. Dent. 2011, 106, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Özcan, M.; Roos, M.; Trottmann, A.; Sailer, I.; Hämmerle, C.H. Load-bearing capacity and failure types of anterior zirconia crowns veneered with overpressing and layering techniques. Dent. Mater. 2011, 27, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Akyıl, M.Ş.; Uzun, I.H.; Bayindir, F. Bond Strength of Resin Cement to Yttrium-Stabilized Tetragonal Zirconia Ceramic Treated with Air Abrasion, Silica Coating, and Laser Irradiation. Photomed. Laser Surg. 2010, 28, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.-H.; Hwang, Y.-C. Bonding strengths of porcelain repair systems with various surface treatments. J. Prosthet. Dent. 1997, 78, 267–274. [Google Scholar] [CrossRef]
- Thurmond, J.W.; Barkmeier, W.W.; Wilwerding, T.M. Effect of porcelain surface treatments on bond strengths of composite resin bonded to porcelain. J. Prosthet. Dent. 1994, 72, 355–359. [Google Scholar] [CrossRef]
- Leibrock, A.; Degenhart, M.; Behr, M.; Rosentritt, M.; Handel, G. In vitro study of the effect of thermo- and load-cycling on the bond strength of porcelain repair systems. J. Oral Rehabil. 1999, 26, 130–137. [Google Scholar] [CrossRef]
- Jain, S.; Parkash, H.; Gupta, S.; Bhargava, A. To Evaluate the Effect of Various Surface Treatments on the Shear Bond Strength of Three Different Intraoral Ceramic Repair Systems: An In Vitro Study. J. Indian Prosthodont. Soc. 2013, 13, 315–320. [Google Scholar] [CrossRef]
- Mosharraf, R.; Rismanchian, M.; Savabi, O.; Ashtiani, A.H. Influence of surface modification techniques on shear bond strength between different zirconia cores and veneering ceramics. J. Adv. Prosthodont. 2011, 3, 221–228. [Google Scholar] [CrossRef]
- Qeblawi, D.M.; Muñoz, C.A.; Brewer, J.D.; Monaco, E.A. The effect of zirconia surface treatment on flexural strength and shear bond strength to a resin cement. J. Prosthet. Dent. 2010, 103, 210–220. [Google Scholar] [CrossRef]
- Usumez, A.; Hamdemirci, N.; Koroglu, B.Y.; Simsek, I.; Parlar, O.; Sari, T. Bond strength of resin cement to zirconia ceramic with different surface treatments. Lasers Med. Sci. 2013, 28, 259–266. [Google Scholar] [CrossRef]
- Attia, A. Influence of surface treatment and cyclic loading on the durability of repaired all-ceramic crowns. J. Appl. Oral Sci. 2010, 18, 194–200. [Google Scholar] [CrossRef]
- Özcan, M.; Vallittu, P.K. Effect of surface conditioning methods on the bond strength of luting cement to ceramics. Dent. Mater. 2003, 19, 725–731. [Google Scholar] [CrossRef]
- Guazzato, M.; Quach, L.; Albakry, M.; Swain, M.V. Influence of surface and heat treatments on the flexural strength of Y-TZP dental ceramic. J. Dent. 2005, 33, 9–18. [Google Scholar] [CrossRef]
- Sevmez, H.; Güngör, M.B.; Yılmaz, H. Surface Treatments of All Ceramic Restoration. J. Ege Univ. Sch. Dent. 2018, 39, 148–159. [Google Scholar] [CrossRef]
- Atsu, S.S.; Kilicarslan, M.A.; Kucukesmen, H.C.; Aka, P.S. Effect of zirconium-oxide ceramic surface treatments on the bond strength to adhesive resin. J. Prosthet. Dent. 2006, 95, 430–436. [Google Scholar] [CrossRef]
- Çevik, P.; Cengiz, D. Farklı yüzey işlemlerinin zirkonyanın ağız içi tamirine etkisi. Selcuk Dent. J. 2017, 4, 52–58. [Google Scholar] [CrossRef]
- Zogheib, L.V.; Della Bona, A.; Kimpara, E.T.; McCabe, J.F. Effect of hydrofluoric acid etching duration on the roughness and flexural strength of a lithium disilicate-based glass ceramic. Braz. Dent. J. 2011, 22, 45–50. [Google Scholar] [CrossRef]
- Özcan, M. Evaluation of alternative intra-oral repair techniques for fractured ceramic-fused-to-metal restorations. J. Oral Rehabil. 2003, 30, 194–203. [Google Scholar] [CrossRef]
- Thompson, J.Y.; Stoner, B.R.; Piascik, J.R.; Smith, R. Adhesion/cementation to zirconia and other non-silicate ceramics: Where are we now? Dent. Mater. 2011, 27, 71–82. [Google Scholar] [CrossRef]
- Dérand, P.; Dérand, T. Bond strength of luting cements to zirconium oxide ceramics. Int. J. Prosthodont. 2001, 13, 131–135. [Google Scholar]
- Özcan, M.; Allahbeickaraghi, A.; Dündar, M. Possible hazardous effects of hydrofluoric acid and recommendations for treatment approach: A review. Clin. Oral Investig. 2011, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Seabra, B.; Arantes-Oliveira, S.; Portugal, J. Influence of multimode universal adhesives and zirconia primer application techniques on zirconia repair. J. Prosthet. Dent. 2014, 112, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.A.; Valandro, L.F.; Scotti, R.; Buso, L. Effect of surface treatments on the resin bond to zirconium-based ceramic. Int. J. Prosthodont. 2005, 18, 60–65. [Google Scholar] [CrossRef]
- Matsumura, H.; Kawahara, M.; Tanaka, T.; Atsuta, M. A New Porcelain Repair System with a Silane Coupler, Ferric Chloride, and Adhesive Opaque Resin. J. Dent. Res. 1989, 68, 813–818. [Google Scholar] [CrossRef]
- Tokar, E.; Polat, S. Ağız İçi Tamir Yöntemlerinin Renk Açısından Değerlendirilmesi. Atatürk Üniv. Diş Hekim. Fak. Derg. 2019, 29, 26–32. [Google Scholar] [CrossRef]
- Kuraray Product Catalog n.d. Available online: https://www.kuraraynoritake.eu/pub/media/pdfs/product-catalog-chairside-en.pdf (accessed on 2 February 2023).
- Fan, P. Porcelain repair materials. Council on Dental Materials, Instruments and Equipment. J. Am. Dent. Assoc. 1991, 122, 124–130. [Google Scholar] [CrossRef]
- Lutz, F.; Phillips, R.W. A classification and evaluation of composite resin systems. J. Prosthet. Dent. 1983, 50, 480–488. [Google Scholar] [CrossRef]
- Lutz, F.; Setcos, J.C.; Phillips, R.W. New Finishing Instruments for Composite Resins. J. Am. Dent. Assoc. 1983, 107, 575–580. [Google Scholar] [CrossRef]
- Pratten, D.; Johnson, G. An evaluation of finishing instruments for an anterior and a posterior composite. J. Prosthet. Dent. 1988, 60, 154–158. [Google Scholar] [CrossRef]
- Horton, C.B.; Paulus, H.M.; Pelleu, G.B.; Rudolph, J.J. An evaluation of commercial pastes for finishing composite resin surfaces. J. Prosthet. Dent. 1977, 37, 674–679. [Google Scholar] [CrossRef]
- Stoddard, J.W.; Johnson, G.H. An evaluation of polishing agents for composite resins. J. Prosthet. Dent. 1991, 65, 491–495. [Google Scholar] [CrossRef]
- Szalewski, L.; Wójcik, D.; Bogucki, M.; Szkutnik, J.; Różyło-Kalinowska, I. The Influence of Popular Beverages on Mechanical Properties of Composite Resins. Materials 2021, 14, 3097. [Google Scholar] [CrossRef]
- Szalewski, L.; Wójcik, D.; Sofińska-Chmiel, W.; Kuśmierz, M.; Różyło-Kalinowska, I. How the Duration and Mode of Photopolymerization Affect the Mechanical Properties of a Dental Composite Resin. Materials 2022, 16, 113. [Google Scholar] [CrossRef]
- Kumchai, H.; Juntavee, P.; Sun, A.F.; Nathanson, D. Comparing the Repair of Veneered Zirconia Crowns with Ceramic or Composite Resin: An in Vitro Study. Dent. J. 2020, 8, 37. [Google Scholar] [CrossRef]
- Nejatidanesh, F.; Savabi, G.; Amjadi, M.; Abbasi, M.; Savabi, O. Five year clinical outcomes and survival of chairside CAD/CAM ceramic laminate veneers—a retrospective study. J. Prosthodont. Res. 2018, 62, 462–467. [Google Scholar] [CrossRef]
- Nejatidanesh, F.; Amjadi, M.; Akouchekian, M.; Savabi, O. Clinical performance of CEREC AC Bluecam conservative ceramic restorations after five years—A retrospective study. J. Dent. 2015, 43, 1076–1082. [Google Scholar] [CrossRef]
- Gurel, G.; Morimoto, S.; A Calamita, M.; Coachman, C.; Sesma, N. Clinical performance of porcelain laminate veneers: Outcomes of the aesthetic pre-evaluative temporary (APT) technique. Int. J. Periodontics Restor. Dent. 2012, 32, 625–635. [Google Scholar]
- Blatz, M.B.; Sadan, A.; Kern, M. Resin-ceramic bonding: A review of the literature. J. Prosthet. Dent. 2003, 89, 268–274. [Google Scholar] [CrossRef]
- Pisani-Proenca, J.; Erhardt, M.C.G.; Valandro, L.F.; Gutierrez-Aceves, G.; Bolanos-Carmona, M.V.; Del Castillo-Salmeron, R.; Bottino, M.A. Influence of ceramic surface conditioning and resin cements on microtensile bond strength to a glass ceramic. J. Prosthet. Dent. 2006, 96, 412–417. [Google Scholar] [CrossRef]
- Nothdurft, F.P.; Motter, P.J.; Pospiech, P.R. Effect of surface treatment on the initial bond strength of different luting cements to zirconium oxide ceramic. Clin. Oral Investig. 2008, 13, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Tanış, M.; Akçaboy, T.C. Effects of Different Surface Treatment Methods and MDP Monomer on Resin Cementation of Zirconia Ceramics an In Vitro Study. J. Lasers Med. Sci. 2015, 6, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.; Kisumbi, B.; Toworfe, G. Dimensional changes of resin/ionomer restoratives in aqueous and neutral media. Dent. Mater. 2000, 16, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Marocho, S.M.; Özcan, M.; Amaral, R.; Bottino, M.A.; Valandro, L.F. Effect of Resin Cement Type on the Microtensile Bond Strength to Lithium Disilicate Ceramic and Dentin Using Different Test Assemblies. J. Adhes. Dent. 2013, 15, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Zortuk, M.; Bolpaca, P.; Kilic, K.; Ozdemir, E.; Aguloglu, S. Effects of Finger Pressure Applied By Dentists during Cementation of All-Ceramic Crowns. Eur. J. Dent. 2010, 04, 383–388. [Google Scholar] [CrossRef]
- Miyazaki, M.; Iwasaki, K.; Onose, H.; Moore, B.K. Resin-modified glassionomers, effect of dentin primer application on the development of bond strength. Eur. J. Oral Sci. 1999, 107, 393–399. [Google Scholar] [CrossRef]
- Hara, A.T.; Pimenta, L.A.F.; Rodrigues-Júnior, A.L. Influence of cross-head speed on resin-dentin shear bond strength. Dent. Mater. 2001, 17, 165–169. [Google Scholar] [CrossRef]
- Panah, F.G.; Rezaei, S.M.M.; Ahmadian, L. The Influence of Ceramic Surface Treatments on the Micro-shear Bond Strength of Composite Resin to IPS Empress 2. J. Prosthodont. 2008, 17, 409–414. [Google Scholar] [CrossRef]
- Al-Dohan, H.M.; Yaman, P.; Dennison, J.B.; E Razzoog, M.; Lang, B.R. Shear strength of core-veneer interface in bi-layered ceramics. J. Prosthet. Dent. 2004, 91, 349–355. [Google Scholar] [CrossRef]
- Zaimoğlu, A.; Can, G.; Ersoy, A.; Aksu, L. Dişhekimliğinde Maddeler Bilgisi; Ankara Üniversitesi: Ankara, Turkey, 1993. [Google Scholar]
- Wolf, D.; Powers, J.; O’Keefe, K. Bond strength of composite to porcelain treated with new porcelain repair agents. Dent. Mater. 1992, 8, 158–161. [Google Scholar] [CrossRef]


| Product Name | Manufacturer | Chemical Composition |
|---|---|---|
| IPS e.max CAD ZirCad | Ivoclar Vivadent AG, Schaan, Liechtenstein | Zirconium dioxide (87–95), Yttrium oxide (4–6), Hafnium oxide (1–5), Aluminum oxide (0–1) |
| IPS e.max Ceram | Ivoclar Vivadent AG, Schaan, Liechtenstein | Silicon dioxide (60–65), Aluminium Oxide (9–11), Potassium oxide (7–8), Sodium oxide (7–8), Zinc Peroxide (2–3), Oxocalcium, Phosphorus pentoxide and Fluorine (2.5–7.5) |
| IPS Empress CAD | Ivoclar Vivadent AG, Schaan, Liechtenstein | Silicon dioxide (60–65), Aluminium Oxide (16–20), Potassium oxide (10–14), Sodium oxide (3.5–6.5), other oxides (0.5–7.0), pigments (0.2–1.0) |
| Panavia F 2.0 Paste A | Kuraray Medical Inc., Japan | 10-Methacryloyloxydecyl dihydrogen phosphate, hydrophobic aromatic dimethacrylate, hydrophobic aliphatic dimethacrylate, hydrophilic aliphatic dimethacrylate, silanized silica filler silanized colloidal silica, dl-camphorquinone, catalysts |
| Panavia F 2.0 Paste B | Kuraray Medical Inc., Japan | Hydrophobic aromatic dimethacrylate, hydrophobic aliphatic dimethacrylate, hydrophilic aliphatic dimethacrylate, silanized barium glass filler, catalysts, accelerators, pigments |
| Clearfil Ceramic Primer | Kuraray Medical Inc., Japan | 3-trimethoxysilylpropyl methacrylate (5), ethanol (80), other ingredients: 10-methacryloyloxydecyl dihydrogen phosphate |
| Clearfil SE Bond Primer | Kuraray Medical Inc., Japan | 2-hydroxyethyl methacrylate (10–30), other ingredients: 10-methacryloyloxydecyl dihydrogen phosphate Hydrophilic aliphatic dimethacrylate dl-camphorquinone, accelerators, water, dyes |
| Clearfil SE Bond, Bond | Kuraray Medical Inc., Japan | bisphenol A diglycidylmethacrylate (25–45), 22-hydroxyethyl methacrylate (20–40), other ingredients: 10-methacryloyloxydecyl dihydrogen phosphate, hydrophobic aliphatic dimethacrylate, colloidal silica, dl-camphorquinone initiators, accelerators |
| Hydrofluoric Acid | Ultradent, USA | 9% hydrofluoric acid |
| Clearfil Esthtetic Majesty Composite | Kuraray Medical Inc., Japan | bisphenol A diglycidylmethacrylate (Bis-GMA), other ingredients: silanized barium glass filler, pre-polymerized organic filler, hydrophobic aromatic dimethacrylate, hydrophobic aliphatic dimethacrylate, dl-camphorquinone, accelerators, initiators, pigments |
| Cojet Sand | 3M Espe, USA | Silica-coated sand |
| Silica Carbide Abrasives | English Abrasives | Cubiron and aluminum mineral |
| Mean ± S. Deviation (MPa) |
Median (Min–Max) (MPa) | Test Statistics | p | |
|---|---|---|---|---|
| A1 | 12.1 ± 4.4 | 11.6 (3.8–18.8) | t = 4.147 | 0.001 |
| A2 | 6.1 ± 1.4 | 6 (3.8–7.9) | ||
| B1 | 7.1 ± 3.1 | 7.4 (2.2–10.8) | t = 2.707 | 0.020 |
| B2 | 4.3 ± 1 | 4.4 (2.3–5.5) | ||
| C1 | 6.3 ± 3.6 | 5.4 (2.3–13.6) | t = 1.775 | 0.103 |
| C2 | 4.2 ± 1.3 | 4.1 (2–6.1) | ||
| D1 | 7.1 ± 2.2 | 6.5 (4.9–10.8) | t = 5.394 | <0.001 |
| D2 | 3.1 ± 0.8 | 3 (1.8–4.3) | ||
| E1 | 6.7 ± 3.1 | 6.2 (3.6–12.9) | t = 4.237 | 0.002 |
| E2 | 2.4 ± 0.8 | 2.6 (1.1–3.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordueri, T.M.; Ateş, M.M.; Özcan, M. Assessment of Intra-Oral Repair Systems for Veneered Zirconia and Zirconia Only. Materials 2023, 16, 1407. https://doi.org/10.3390/ma16041407
Ordueri TM, Ateş MM, Özcan M. Assessment of Intra-Oral Repair Systems for Veneered Zirconia and Zirconia Only. Materials. 2023; 16(4):1407. https://doi.org/10.3390/ma16041407
Chicago/Turabian StyleOrdueri, Tugçe Merve, Mehmet Muzaffer Ateş, and Mutlu Özcan. 2023. "Assessment of Intra-Oral Repair Systems for Veneered Zirconia and Zirconia Only" Materials 16, no. 4: 1407. https://doi.org/10.3390/ma16041407
APA StyleOrdueri, T. M., Ateş, M. M., & Özcan, M. (2023). Assessment of Intra-Oral Repair Systems for Veneered Zirconia and Zirconia Only. Materials, 16(4), 1407. https://doi.org/10.3390/ma16041407







