Improving the Dispersibility of TiO2 in the Colloidal System Using Trifunctional Spherosilicates
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Trifunctional Octasubstituted Spherosilicates
- (1)
- allyl cinnamate (22.20 g), hexene (7.45 g), and diethylene glycol monovinyl ether (3.90 g) in molar ratio 4:3:1
- (2)
- allyl cinnamate (16.65 g), hexene (9.93 g), and allyl glycidyl ether (3.37 g) in molar ratio 3:4:1
- (3)
- allyl cinnamate (22.20 g), octene (9.93 g), and vinyltrimethoxysilane (4.37 g) in molar ratio 4:3:1
- (4)
- eugenol (14.53 g), octene (9.93 g), and vinyltrimethoxysilane (8.74 g) in molar ratio 3:3:2 was added.
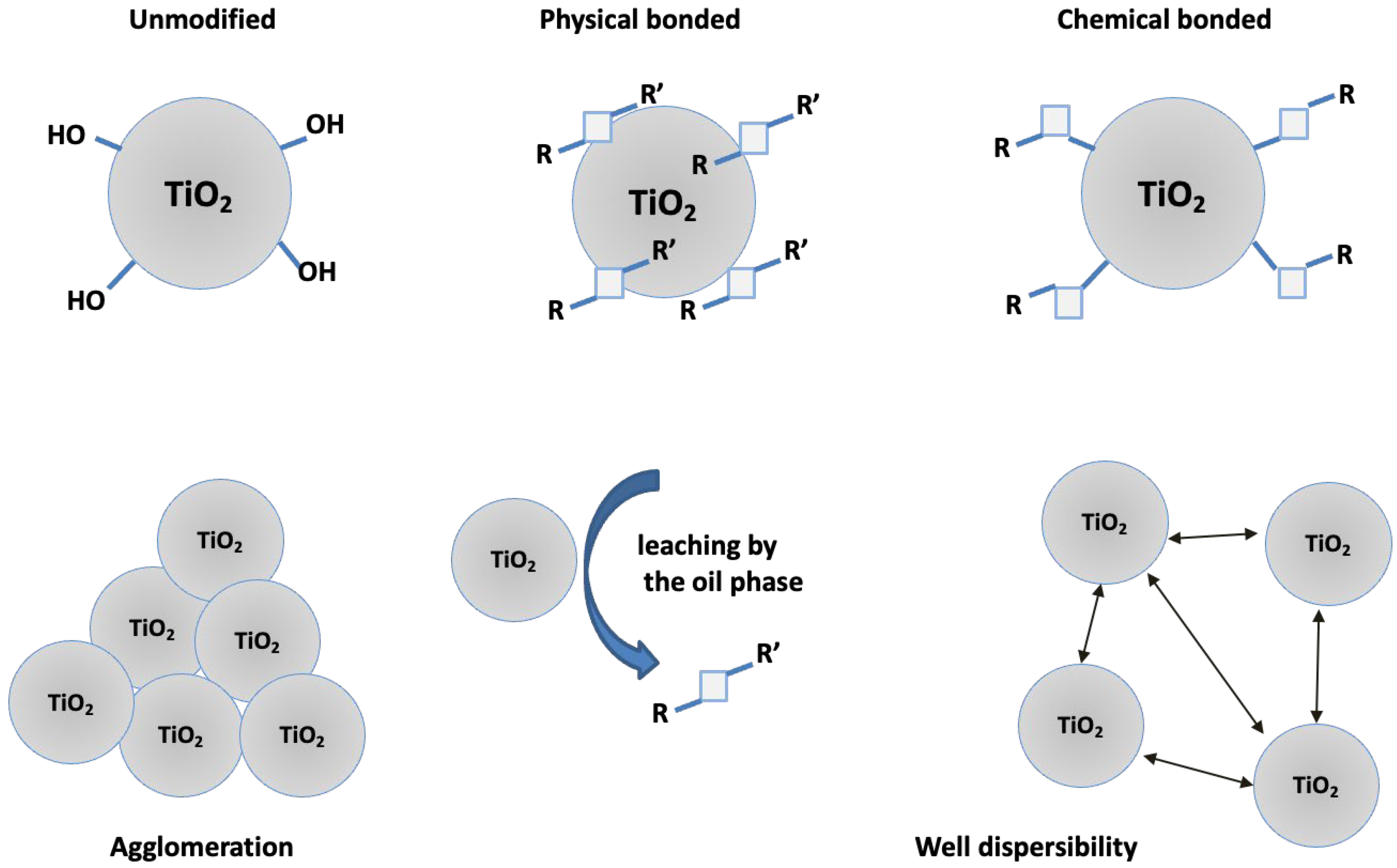
2.2. Modification of Titanium Dioxide with Octaspherosilicate Derivatives
2.3. Preparation of Emulsions with Modified Titanium Dioxide
2.4. Analytical Methods
3. Results and Discussion
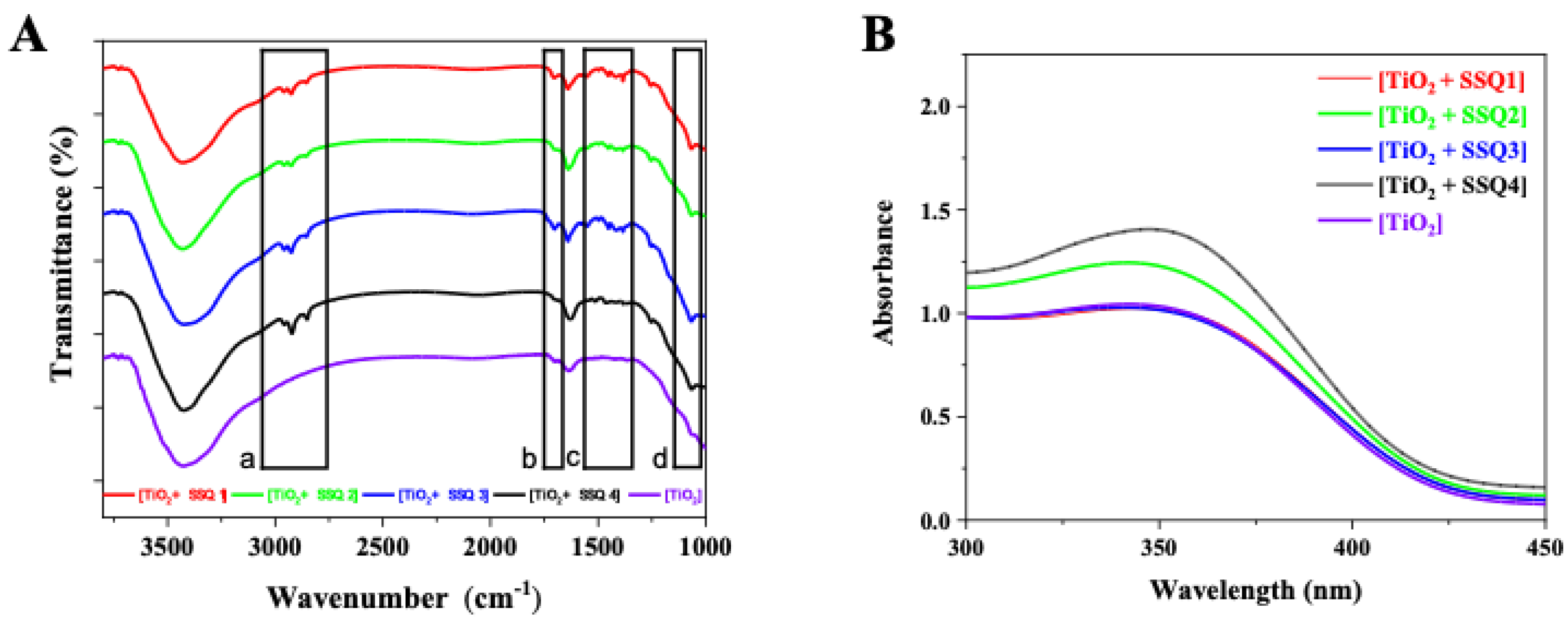
3.1. Characterization of Organosilicon Modifiers
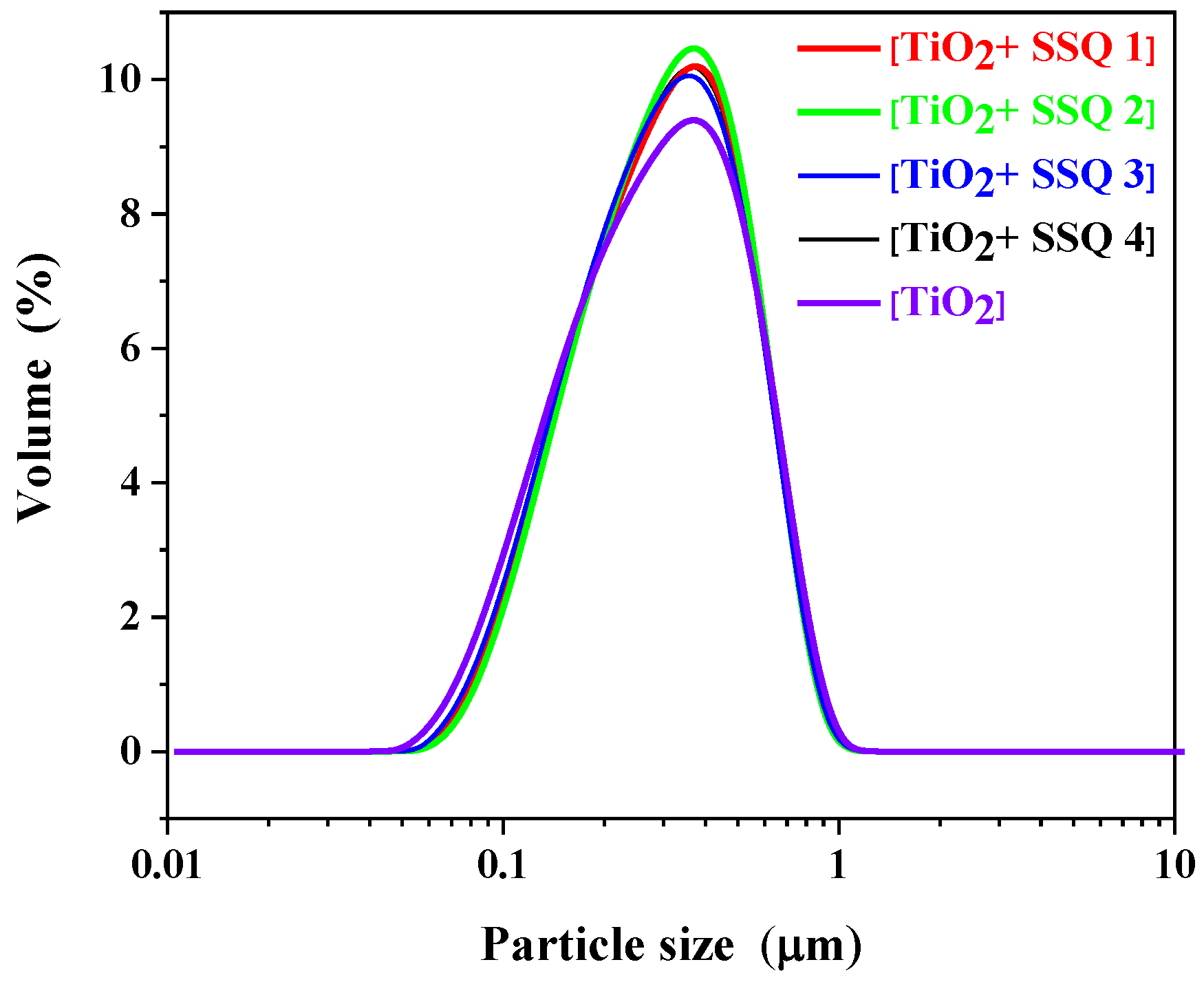
3.2. Characterization of Modified Titanium Dioxide
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korotcenkov, G. Titanium Dioxide (TiO2) and Its Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Bordes, C.; Bolzinger, M.; El Achak, M.; Pirot, F.; Arquier, D.; Agusti, G.; Chevalier, Y. Formulation of Pickering emulsions for the development of surfactant-free sunscreen creams. Int. J. Cosmet. Sci. 2021, 43, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Glówczyk-Zubek, J. Cosmetic application of micro-fine titanium dioxide. J. Appl. Cosmetol. 2004, 22, 143–153. [Google Scholar]
- Nunes, S.M.; Josende, M.E.; González-Durruthy, M.; Ruas, C.P.; Gelesky, M.A.; Romano, L.A.; Fattorini, D.; Regoli, F.; Monserrat, J.M.; Ventura-Lima, J. Different crystalline forms of titanium dioxide nanomaterial (rutile and anatase) can influence the toxicity of copper in golden mussel Limnoperna fortunei? Aquatic. Toxicol. 2018, 205, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, G.L.; Paola, A.D.; Palmisano, L.; Selli, E. Effect of titanium dioxide crystalline structure on the pho-tocatalytic production of hydrogen. Photochem. Photobiol. Sci. 2011, 10, 355–360. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.; Popov, A. Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding polymorphic phase transformation behavior during growth of nano-crystalline aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Trestsova, M.A.; Utepova, I.A.; Chupakhin, O.N.; Semenov, M.V.; Pevtsov, D.N.; Nikolenko, L.M.; Tovstun, S.A.; Gadomska, A.; Shchepochkin, A.; Kim, G.A. Oxidative CH/CH Coupling of Dipyrromethanes with Azines by TiO2-Based Photocatalytic System. Synthesis of New BODIPY Dyes and Their Photophysical and Electro-chemical Properties. Molecules 2021, 26, 5549. [Google Scholar] [CrossRef]
- Tsebriienko, T.; Popov, A.I. Effect of Poly(Titanium Oxide) on the Viscoelastic and Thermophysical Properties of Interpenetrating Polymer Networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Nam, Y.; Lim, J.H.; Ko, K.C.; Lee, J.Y. Photocatalytic activity of TiO2 nanoparticles: A theoretical aspect. J. Mater. Chem. A 2019, 7, 13833–13859. [Google Scholar] [CrossRef]
- Xue, C.; Wu, J.; Lan, F.; Liu, W.; Yang, X.; Zeng, F.; Xu, H. Nano Titanium Dioxide Induces the Generation of ROS and Potential Damage in HaCaT Cells Under UVA Irradiation. J. Nanosci. Nanotechnol. 2010, 10, 8500–8507. [Google Scholar] [CrossRef]
- Morsella, M.; D’Alessandro, N.; Lanterna, A.E.; Scaiano, J.C. Improving the Sunscreen Properties of TiO2 through an Understanding of Its Catalytic Properties. ACS Omega 2016, 1, 464–469. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Krysztafkiewicz, A.; Jesionowski, T. Adsorption of organic dyes on the aminosilane modi-fied TiO2 surface. Dye. Pigment. 2004, 62, 121–130. [Google Scholar] [CrossRef]
- Siddiquey, I.A.; Ukaji, E.; Furusawa, T.; Sato, M.; Suzuki, N. The effects of organic surface treatment by meth-acryloxypropyltrimethoxysilane on the photostability of TiO2. Mater. Chem. Phys. 2007, 105, 162–168. [Google Scholar] [CrossRef]
- Yao, C.; Gao, G.S.; Lin, X.P.; Yang, X.J.; Lu, L.D.; Wang, X. Surface modification of nanosized TiO2 with silane coupling reagent. J. Inorg. Mater. 2006, 21, 315–321. [Google Scholar]
- Zhou, H.; Sun, S.; Ding, H. Surface Organic Modification of TiO2 Powder and Relevant Characterization. Adv. Mater. Sci. Eng. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Brząkalski, D.; Przekop, R.E.; Frydrych, M.; Pakuła, D.; Dobrosielska, M.; Sztorch, B.; Marciniec, B. Where ppm Quantities of Silsesquioxanes Make a Difference—Silanes and Cage Siloxanes as TiO2 Dispersants and Stabi-lizers for Pigmented Epoxy Resins. Materials 2022, 15, 494. [Google Scholar] [CrossRef]
- Chua, M.H.; Zhou, H.; Xu, J. Polymer-POSS hybrid materials as fire retardants. In Polyhedral Oligomeric Silsesquioxane (POSS) Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 305–332. [Google Scholar]
- Niemczyk, A.; Dziubek, K.; Sacher-Majewska, B.; Czaja, K.; Czech-Polak, J.; Oliwa, R.; Lenża, J.; Szołyga, M. Thermal Stability and Flame Retardancy of Polypropylene Composites Containing Siloxane-Silsesquioxane Resins. Polymers 2018, 10, 1019. [Google Scholar] [CrossRef]
- Szołyga, M.; Dutkiewicz, M.; Nowicki, M.; Sałasińska, K.; Celiński, M.; Marciniec, B. Phosphorus-Containing Silsesquioxane Derivatives as Additive or Reactive Components of Epoxy Resins. Materials 2020, 13, 5373. [Google Scholar] [CrossRef]
- Sztorch, B.; Pakuła, D.; Kustosz, M.; Romanczuk-Ruszuk, E.; Gabriel, E.; Przekop, R.E. The Influence of Orga-nofunctional Substituents of Spherosilicates on the Functional Properties of PLA/TiO2 Composites Used in 3D Printing (FDM/FFF). Polymers 2022, 14, 5493. [Google Scholar] [CrossRef]
- Mittal, K.L. Silanes and Other Coupling Agents; CRC Press: Boka Raton, FL, USA, 2007; Volume 4. [Google Scholar]
- Nowacka, M.; Ambrożewicz, D.; Jesionowski, T. TiO2-SiO2/Ph-POSS functional hybrids: Preparation and characterisation. J. Nanomater. 2013, 2013, 19. [Google Scholar] [CrossRef]
- Szwarc, K.; Siwinska-Stefanska, K.; Marciniec, B.; Jesionowski, T. Synthesis and characterisation of SiO2/POSS hybrid systems obtained using the mechanical method. Physicochem. Probl. Miner. Process. 2012, 48, 181–192. [Google Scholar]
- Filho, N.L.D.; De Aquino, H.A.; Pires, G.; Caetano, L. Relationship between the dielectric and mechanical properties and the ratio of epoxy resin to hardener of the hybrid thermosetting polymers. J. Braz. Chem. Soc. 2006, 17, 533–541. [Google Scholar] [CrossRef]
- Madhurambal, G.; Ravindran, B.; Mariappan, M.; Mojumdar, S. Thermal, UV and FTIR spectral studies in al-kali metal cinnamates. J. Therm. Anal Calorim. 2010, 100, 811–815. [Google Scholar] [CrossRef]
- Foudah, A.I.; Shakeel, F.; Alqarni, M.H.; Ross, S.A.; Salkini, M.A.; Alam, P. Simultaneous estimation of cin-namaldehyde and eugenol in essential oils and traditional and ultrasound-assisted extracts of different spe-cies of cinnamon using a sustainable/green HPTLC technique. Molecules 2021, 26, 2054. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.C.; Tavares, T.D.; Teixeira, M.A.; Teixeira, M.O.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Eu-genol-containing essential oils loaded onto chitosan/polyvinyl alcohol blended films and their ability to eradicate Staphylococcus aureus or Pseudomonas aeruginosa from infected microenvironments. Pharmaceutics 2012, 13, 195. [Google Scholar] [CrossRef]
- Joshi, M.; Bhattacharyya, A. Nanotechnology—A new route to high-performance functional textiles. Text. Prog. 2011, 43, 155–233. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Wang, A.; Wu, L.; Wang, Q. Facile synthesis and photocatalytic activity of a novel titanium dioxide nanocomposite coupled with zinc porphyrin. Nanomater. Nanotechnol. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Song, J.; Qin, J.; Qu, J.; Song, Z.; Zhang, W.; Xue, X.; Shi, Y.; Zhang, T.; Ji, W.; Zhang, R.; et al. The effects of particle size distribution on the optical properties of titanium dioxide rutile pigments and their applications in cool non-white coatings. Sol. Energy Mater. Sol. Cells 2014, 130, 42–50. [Google Scholar] [CrossRef]
- Ridley, M.K.; Hackley, V.A.; Machesky, M.L. Characterization and surface-reactivity of nanocrystalline ana-tase in aqueous solutions. Langmuir 2006, 22, 10972–10982. [Google Scholar] [CrossRef]
- Halimi, S.U.; Abu Bakar, N.F.; Ismail, S.N.; Hashib, S.A.; Naim, M.N. Electrospray deposition of titanium dioxide (TiO2) nanoparticles. In Proceedings of the AIP Conference Proceedings, Surabaya, Indonesia, 23–25 October 2014; Volume 1586, pp. 57–62. [Google Scholar] [CrossRef]
- Olejnik, A.; Panek, R.; Madej, J.; Franus, W.; Goscianska, J. Low-cost zeolitic carriers for delivery of hy-droxychloroquine immunomodulatory agent with antiviral activity. Microporous Mesoporous Mater. 2022, 346, 112315. [Google Scholar] [CrossRef]
- Olejnik, A.; Glowka, A.; Nowak, I. Release studies of undecylenoyl phenylalanine from topical formulations. Saudi Pharm. J. 2018, 26, 709–718. [Google Scholar] [CrossRef]
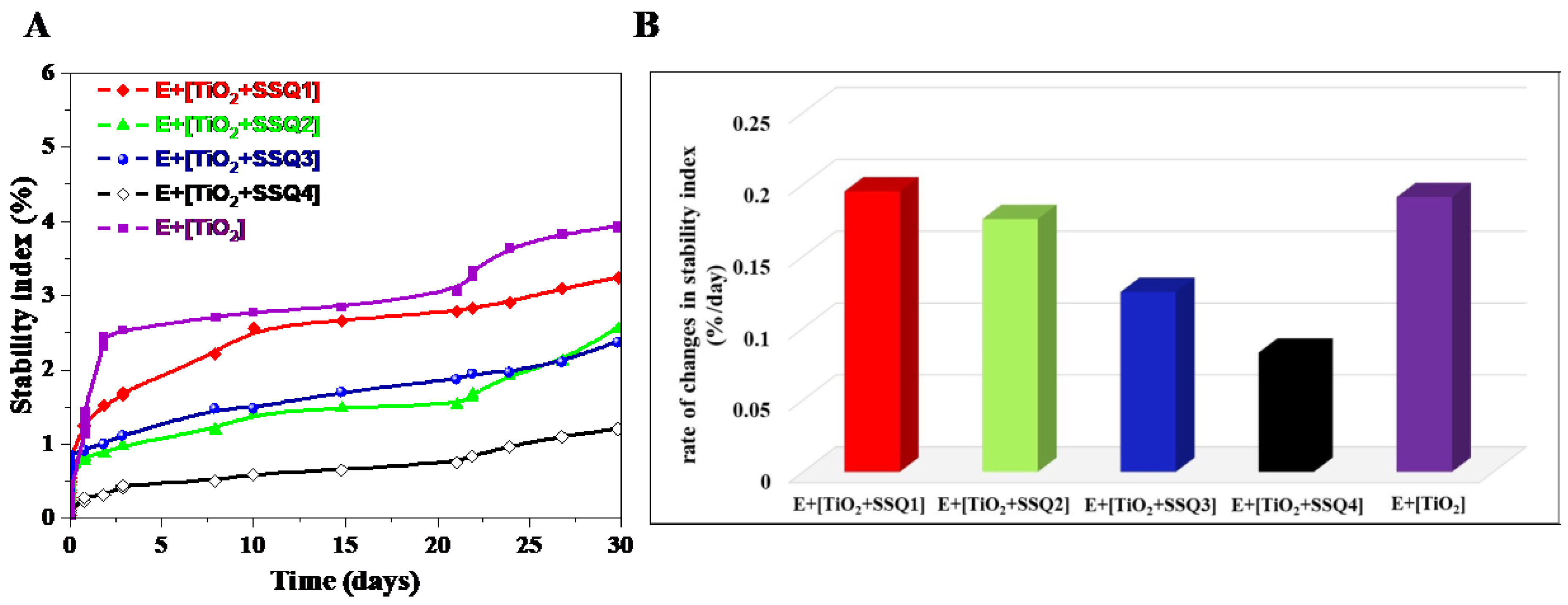
- Santos, J.; Trujillo, L.A.; Calero, N.; Alfaro, M.C.; Munoz, J. Physical Characterization of a Commercial Suspo-emulsion as a Reference for the Development of Suspoemulsions. Chem. Eng. Technol. 2013, 36, 1883–1890. [Google Scholar] [CrossRef]
- Mengual, O.; Meunier, G.; Cayré, I.; Puech, K.; Snabre, P. TURBISCAN MA 2000: Multiple light scattering measurement for concentrated emulsion and suspension instability analysis. Talanta 1999, 50, 445–456. [Google Scholar] [CrossRef]
- Olejnik, A.; Kapuscinska, A.; Schroeder, G.; Nowak, I. Physico-chemical characterization of formulations containing endomorphin-2 derivatives. Amino Acids 2017, 49, 1719–1731. [Google Scholar] [CrossRef]
- Olejnik, A.; Schroeder, G.; Nowak, I. The tetrapeptide N-acetyl-Pro-Pro-Tyr-Leu in skin care formulations—Physicochemical and release studies. Int. J. Pharm. 2015, 492, 161–168. [Google Scholar] [CrossRef]
- Zalewska, A.; Kowalik, J.; Grubecki, I. Application of turbiscan lab to study the effect of emulsifier content on the stability of plant origin dispersion. Chem. Process Eng. 2019, 40, 399–409. [Google Scholar]
- Santos, J.; Calero, N.; Trujillo-Cayado, L.; Garcia, M.; Muñoz, J. Assessing differences between Ostwald ripening and coalescence by rheology, laser diffraction and multiple light scattering. Colloids Surf. B Biointerfaces 2017, 159, 405–411. [Google Scholar] [CrossRef]
- Kim, J.-H.; Hossain, S.M.; Kang, H.-J.; Park, H.; Tijing, L.; Park, G.W.; Suzuki, N.; Fujishima, A.; Jun, Y.-S.; Shon, H.K.; et al. Hydrophilic/Hydrophobic Silane Grafting on TiO2 Nanoparticles: Photocatalytic Paint for Atmospheric Cleaning. Catalysts 2021, 11, 193. [Google Scholar] [CrossRef]
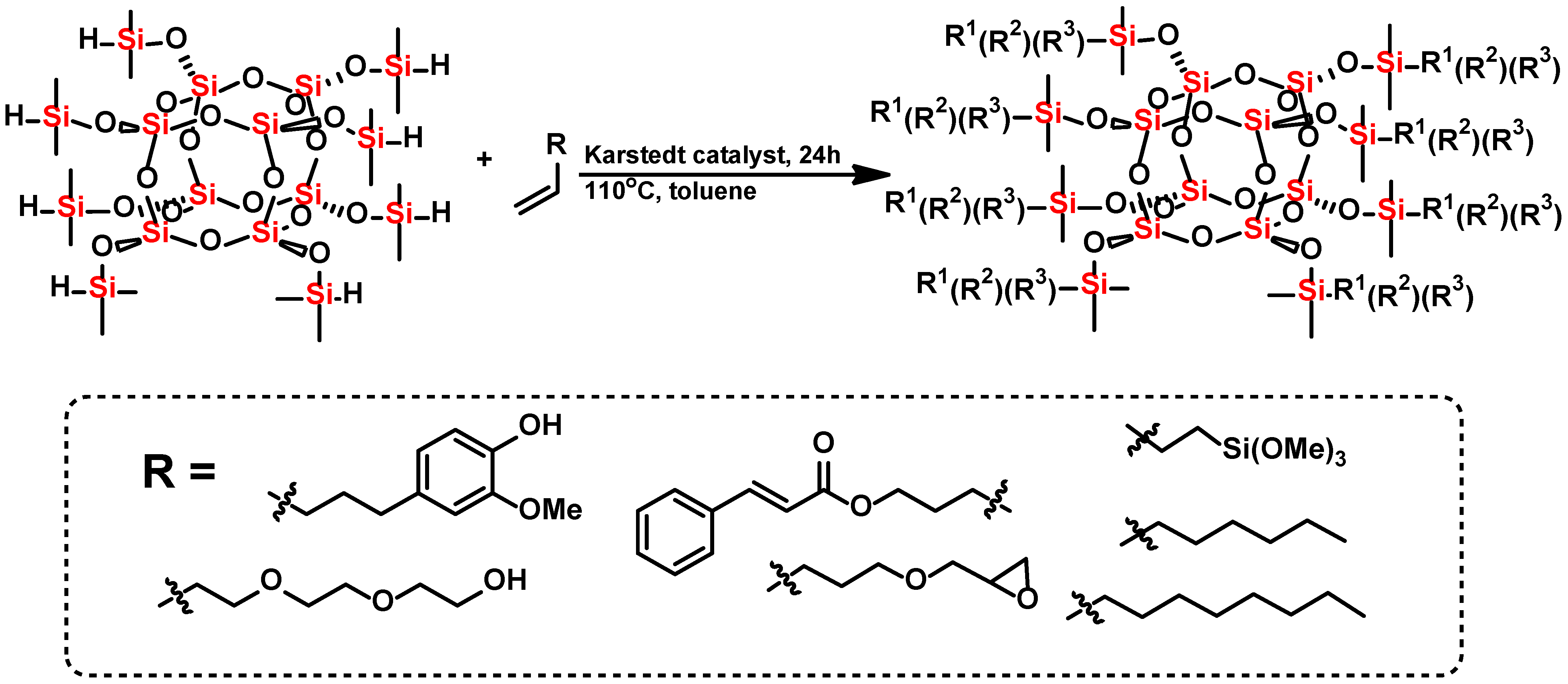
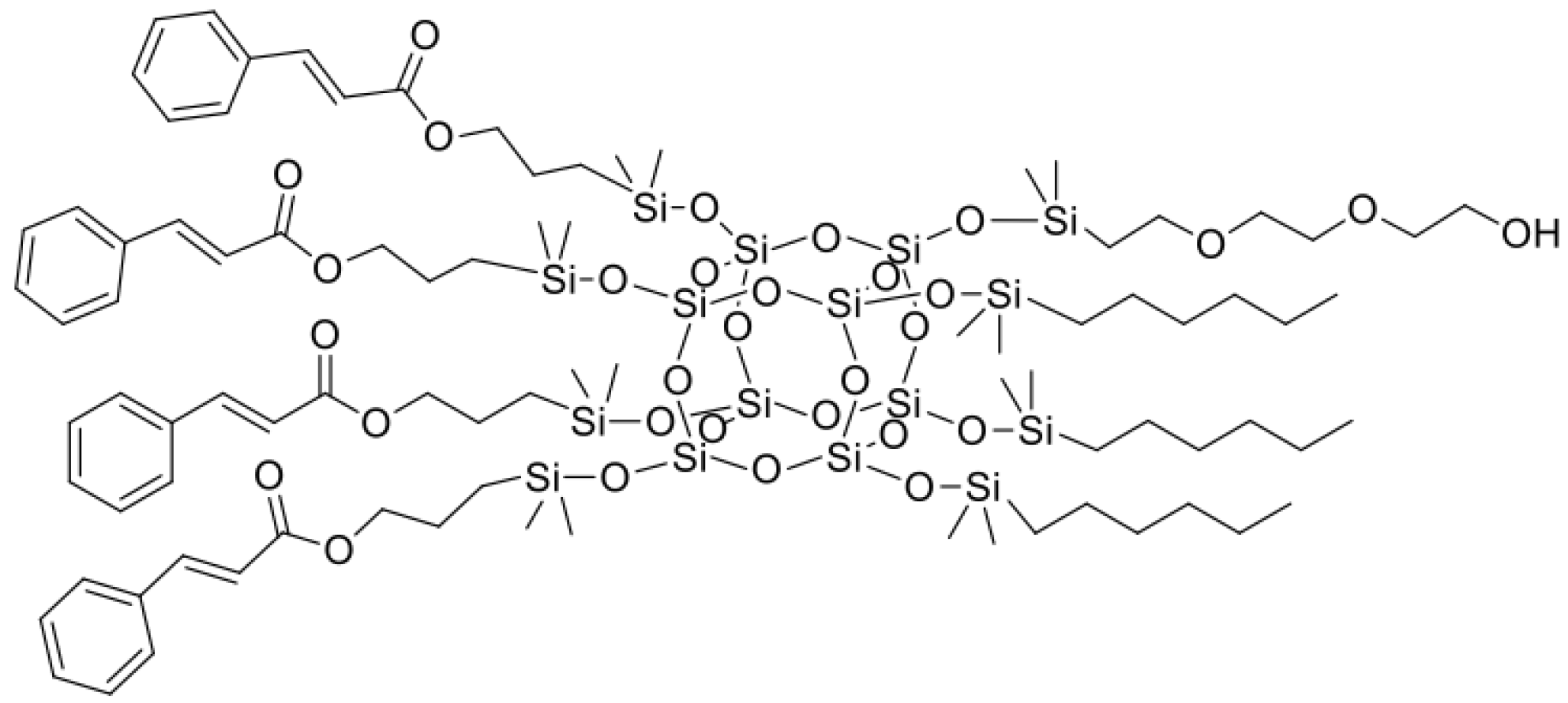
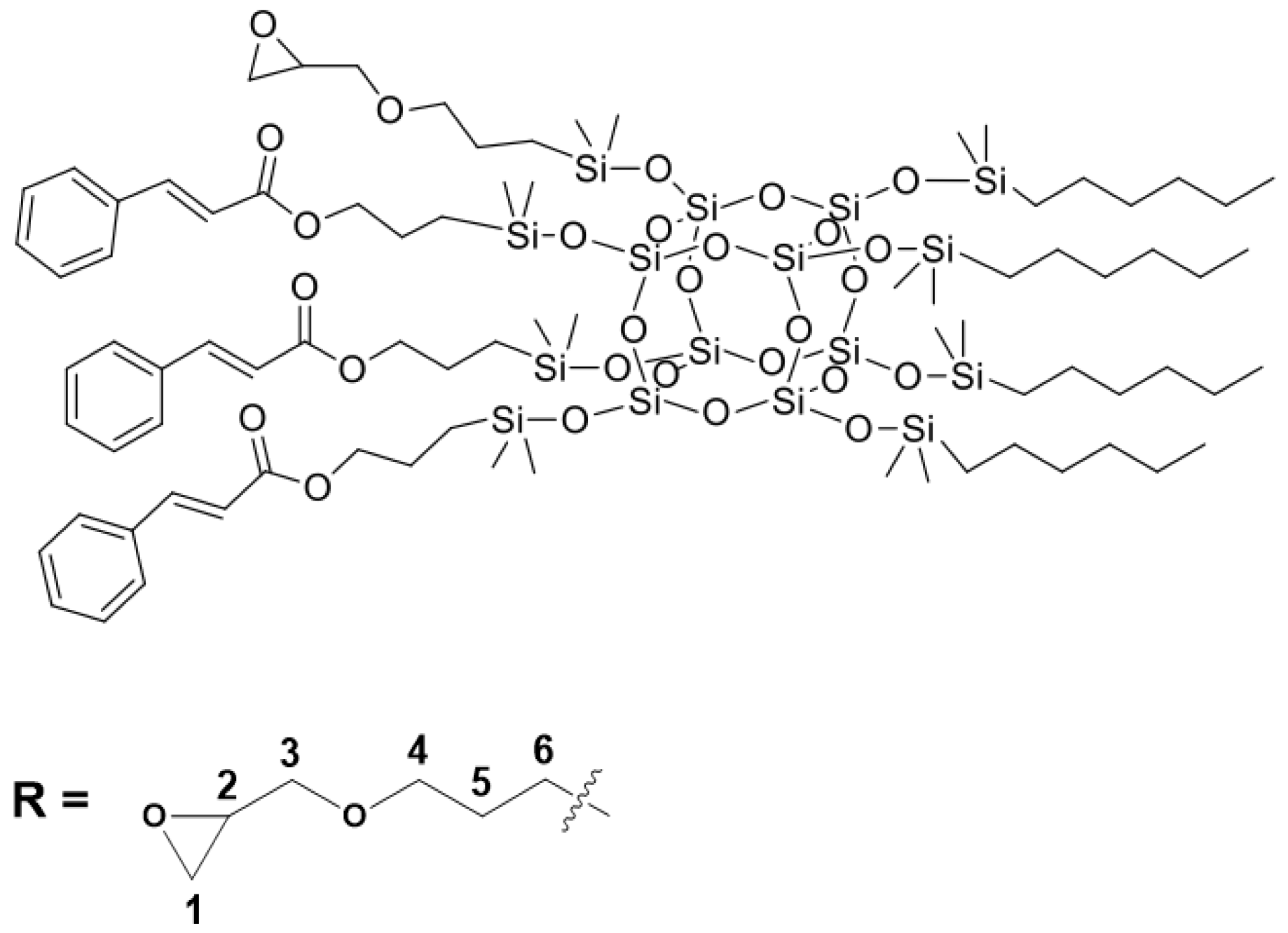
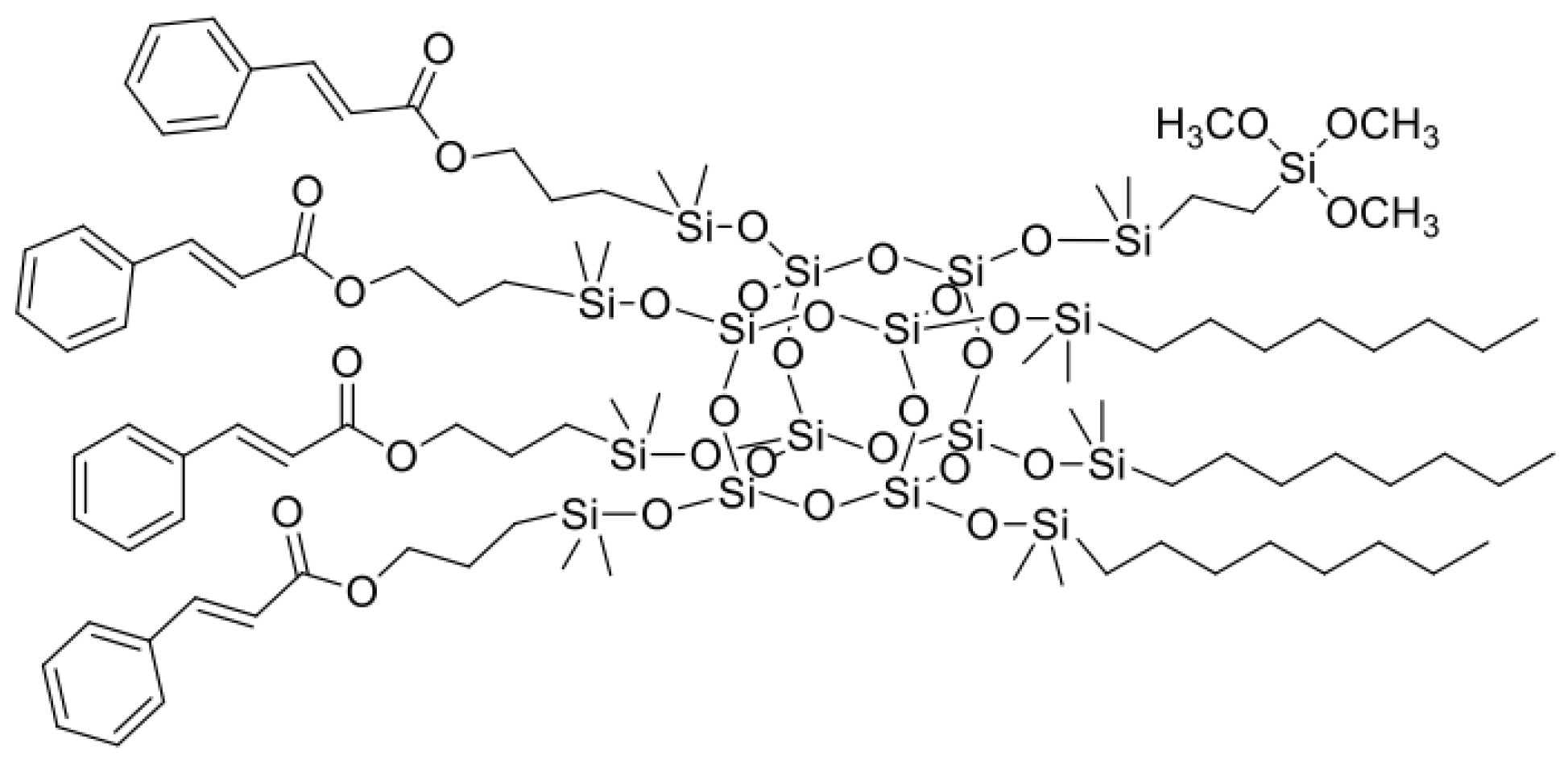
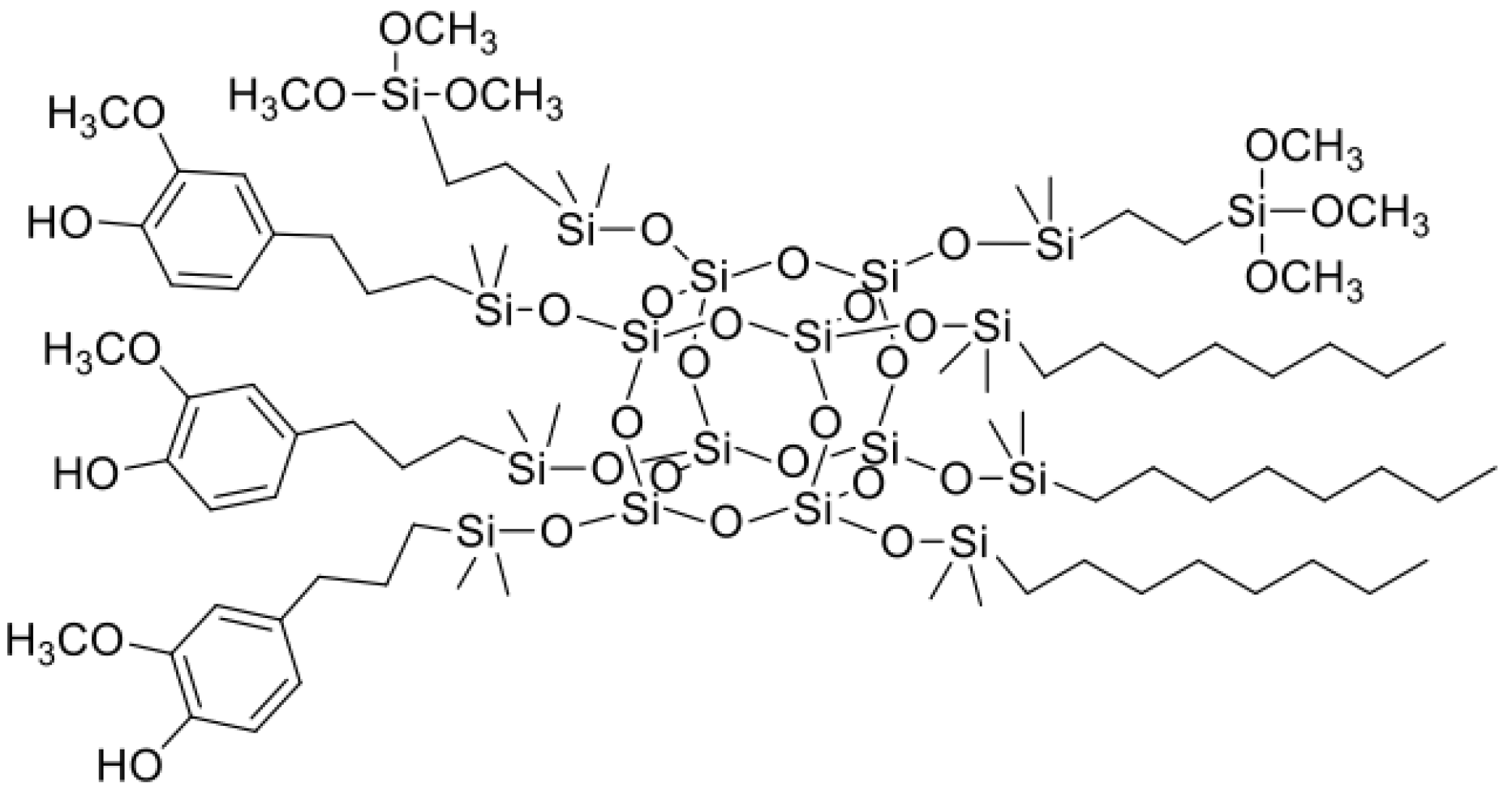



| Sample Name | Core | Olefin 1 | Olefin 2 | Olefin 3 | Molar Ratios |
|---|---|---|---|---|---|
| SSQ1 | octaspherosilicate | allyl cinnamate | hexene | DGME * | 4:3:1 |
| SSQ2 | octaspherosilicate | allyl cinnamate | hexene | allyl glycidyl ether | 3:4:1 |
| SSQ3 | octaspherosilicate | allyl cinnamate | octene | VTMOS ** | 4:3:1 |
| SSQ4 | octaspherosilicate | eugenol | octene | VTMOS ** | 3:3:2 |
| Phases | Components | Amount |
|---|---|---|
| Water | 64.30% | |
| Water phase | Glycerin | 7.50% |
| Xanthan gum | 0.30% | |
| Glycerol stearate | 2.00% | |
| Polyglyceryl-3-methylglucose distearate | 2.00% | |
| Oil phase | Cetyl alcohol | 3.00% |
| Butyrospermum parkii butter | 7.50% | |
| Vegetable oil | 7.50% | |
| Modified TiO2/TiO2 | 5.00% | |
| Phenoxyethanol | 0.90% |
| Name | Density (g/mL) | Refractive Index | Molecular Weight (g/mol) |
|---|---|---|---|
| SSQ1 | 1.1119 ± 0.0002 | 1.4934 ± 0.0002 | 2152.66 |
| SSQ2 | 1.0953 ± 0.0002 | 1.4832 ± 0.0003 | 2030.66 |
| SSQ3 | 1.1077 ± 0.0001 | 1.4888 ± 0.0002 | 2252.73 |
| SSQ4 | 1.0929 ± 0.0003 | 1.4740 ± 0.0003 | 2140.70 |
| Name | d (0.1) | d (0.5) | d (0.9) | Zeta Potential (mV) |
|---|---|---|---|---|
| [TiO2+SSQ1] | 0.134 ± 0.004 μm | 0.303 ± 0.014 μm | 0.577 ± 0.020 μm | −34.2 ± 0.9 |
| [TiO2+SSQ2] | 0.139 ± 0.002 μm | 0.307 ± 0.010 μm | 0.576 ± 0.024 μm | −34.9 ± 4.3 |
| [TiO2+SSQ3] | 0.132 ± 0.008 μm | 0.297 ± 0.007 μm | 0.574 ± 0.032 μm | −35.5 ± 4.7 |
| [TiO2+SSQ4] | 0.134 ± 0.005 μm | 0.303 ± 0.012 μm | 0.579 ± 0.032 μm | −39.5 ± 0.9 |
| [TiO2] | 0.123 ± 0.002 μm | 0.293 ± 0.009 μm | 0.588 ± 0.018 μm | 24.5 ± 0.7 |
| Name | Contact Angle |
|---|---|
| [TiO2+SSQ1] | 138° |
| [TiO2+SSQ2] | 142° |
| [TiO2+SSQ3] | 130° |
| [TiO2+SSQ4] | 140° |
| [TiO2] | 0° |
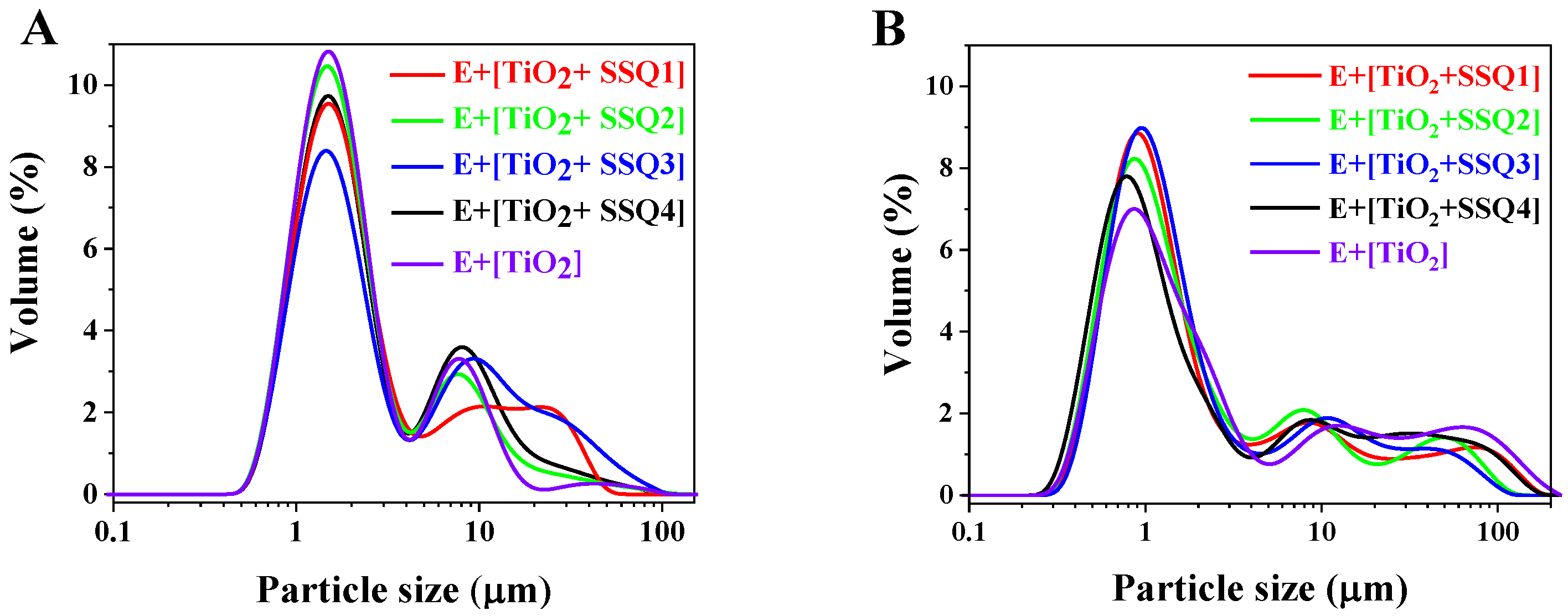
| Name | d (0.1) | d (0.5) | d (0.9) |
|---|---|---|---|
| After Preparation | |||
| E [TiO2+SSQ1] | 0.961 ± 0.008 μm | 1.94 ± 0.024 μm | 18.0 ± 0.042 μm |
| E [TiO2+SSQ2] | 0.932 ± 0.010 μm | 1.78 ± 0.031 μm | 9.60 ± 0.035 μm |
| E [TiO2+SSQ3] | 0.973 ± 0.012 μm | 2.18 ± 0.028 μm | 22.7 ± 0.032 μm |
| E [TiO2+SSQ4] | 0.964 ± 0.014 μm | 1.92 ± 0.023 μm | 11.6 ± 0.018 μm |
| E [TiO2] | 0.939 ± 0.007 μm | 1.76 ± 0.018 μm | 8.55 ± 0.029 μm |
| After 3 months | |||
| E [TiO2+SSQ1] | 0.594 ± 0.011 μm | 1.26 ± 0.017 μm | 31.3 ± 0.019 μm |
| E [TiO2+SSQ2] | 0.574 ± 0.008 μm | 1.30 ± 0.014 μm | 25.5 ± 0.038 μm |
| E [TiO2+SSQ3] | 0.614 ± 0.012 μm | 1.29 ± 0.026 μm | 20.5 ± 0.028 μm |
| E [TiO2+SSQ4] | 0.531 ± 0.009 μm | 1.28 ± 0.032 μm | 38.1 ± 0.030 μm |
| E [TiO2] | 0.591 ± 0.013 μm | 1.58 ± 0.019 μm | 52.6 ± 0.045 μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sztorch, B.; Nowak, K.; Frydrych, M.; Leśniewska, J.; Krysiak, K.; Przekop, R.E.; Olejnik, A. Improving the Dispersibility of TiO2 in the Colloidal System Using Trifunctional Spherosilicates. Materials 2023, 16, 1442. https://doi.org/10.3390/ma16041442
Sztorch B, Nowak K, Frydrych M, Leśniewska J, Krysiak K, Przekop RE, Olejnik A. Improving the Dispersibility of TiO2 in the Colloidal System Using Trifunctional Spherosilicates. Materials. 2023; 16(4):1442. https://doi.org/10.3390/ma16041442
Chicago/Turabian StyleSztorch, Bogna, Krzysztof Nowak, Miłosz Frydrych, Julia Leśniewska, Klaudia Krysiak, Robert E. Przekop, and Anna Olejnik. 2023. "Improving the Dispersibility of TiO2 in the Colloidal System Using Trifunctional Spherosilicates" Materials 16, no. 4: 1442. https://doi.org/10.3390/ma16041442
APA StyleSztorch, B., Nowak, K., Frydrych, M., Leśniewska, J., Krysiak, K., Przekop, R. E., & Olejnik, A. (2023). Improving the Dispersibility of TiO2 in the Colloidal System Using Trifunctional Spherosilicates. Materials, 16(4), 1442. https://doi.org/10.3390/ma16041442








