Removal of Methylene Blue from Wastewater by Waste Roots from the Arsenic-Hyperaccumulator Pteris vittata: Fixed Bed Adsorption Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Bio-Adsorbent Preparation
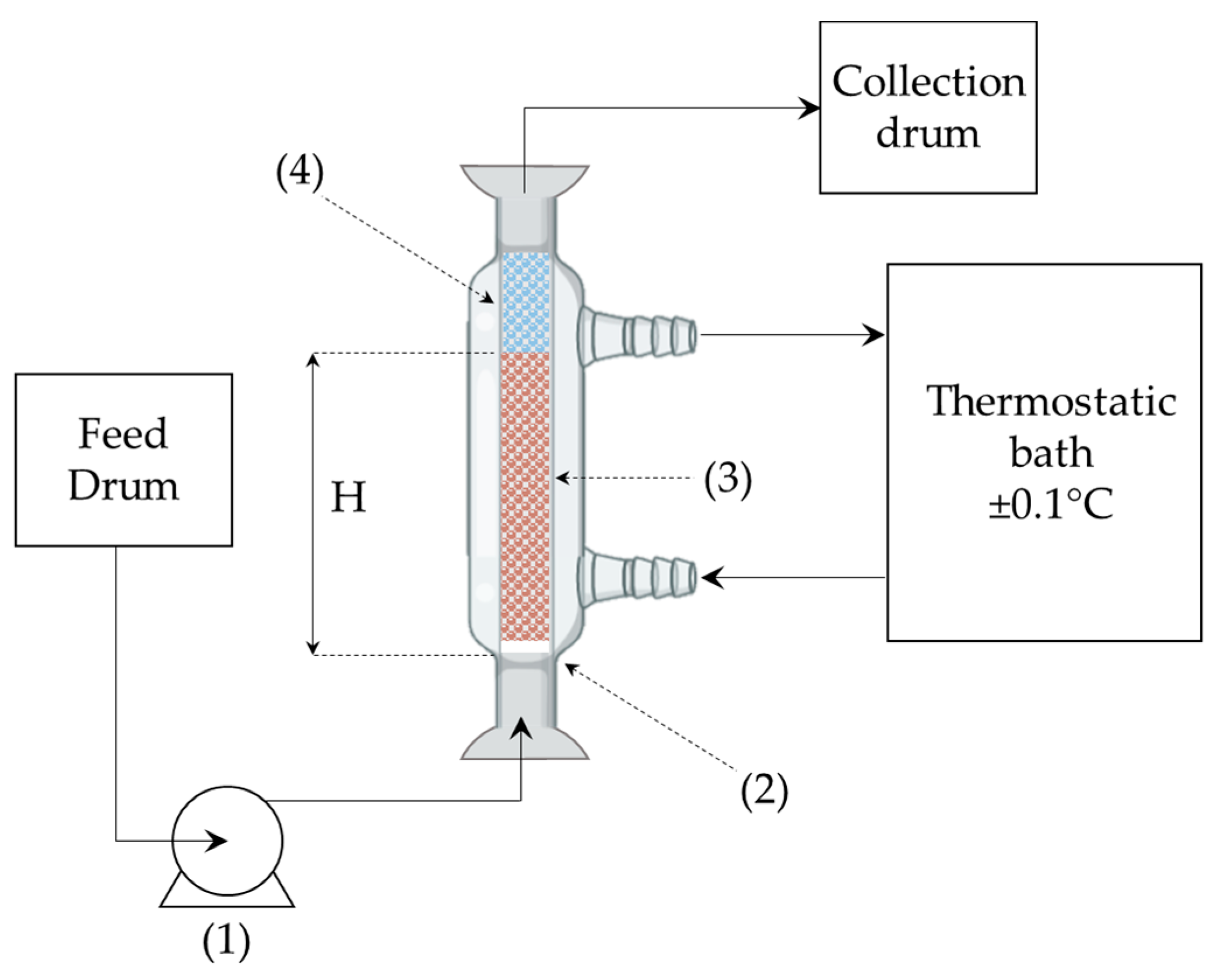
2.3. Experimental Set-Up and Column Tests
2.4. Bed Void Fraction and Solid Apparent Density Estimation
3. Results and Discussion
3.1. Bio-Adsorbent Preparation
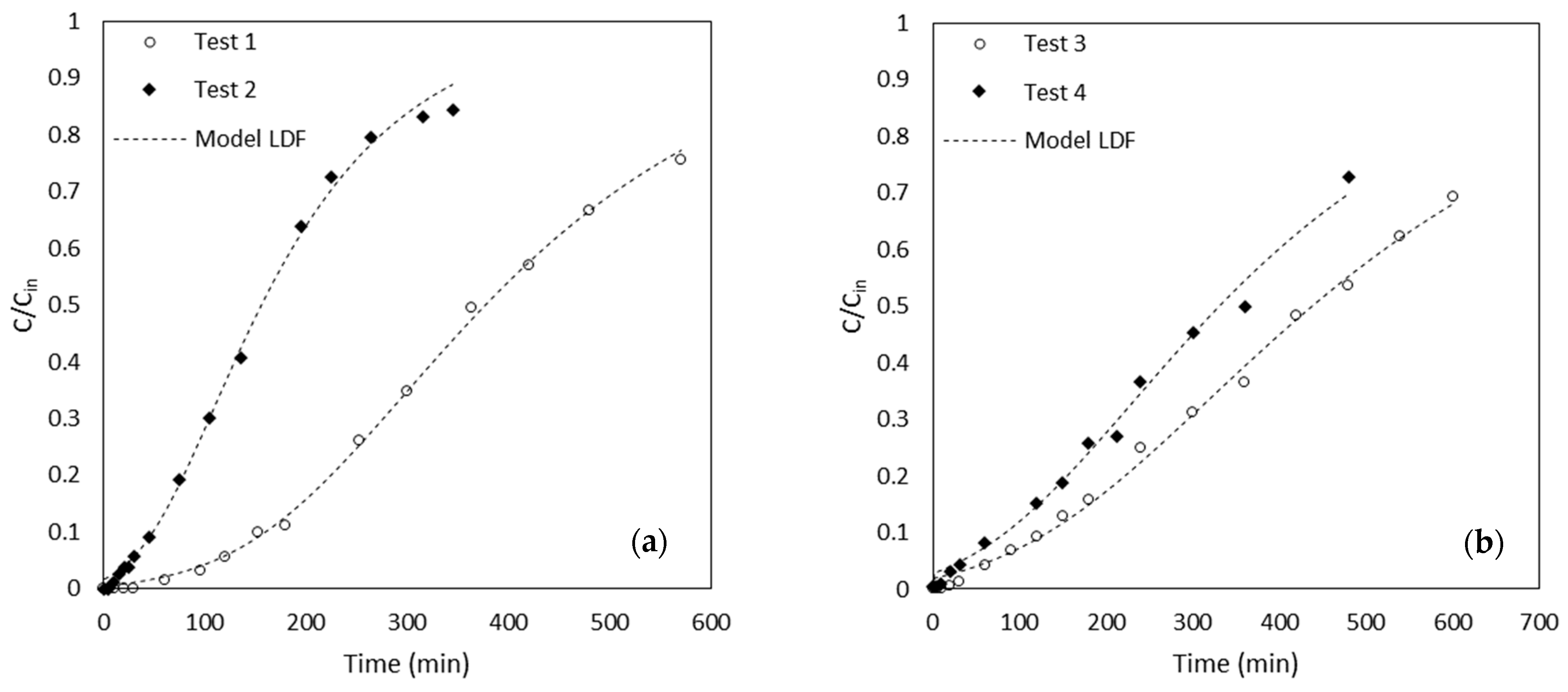
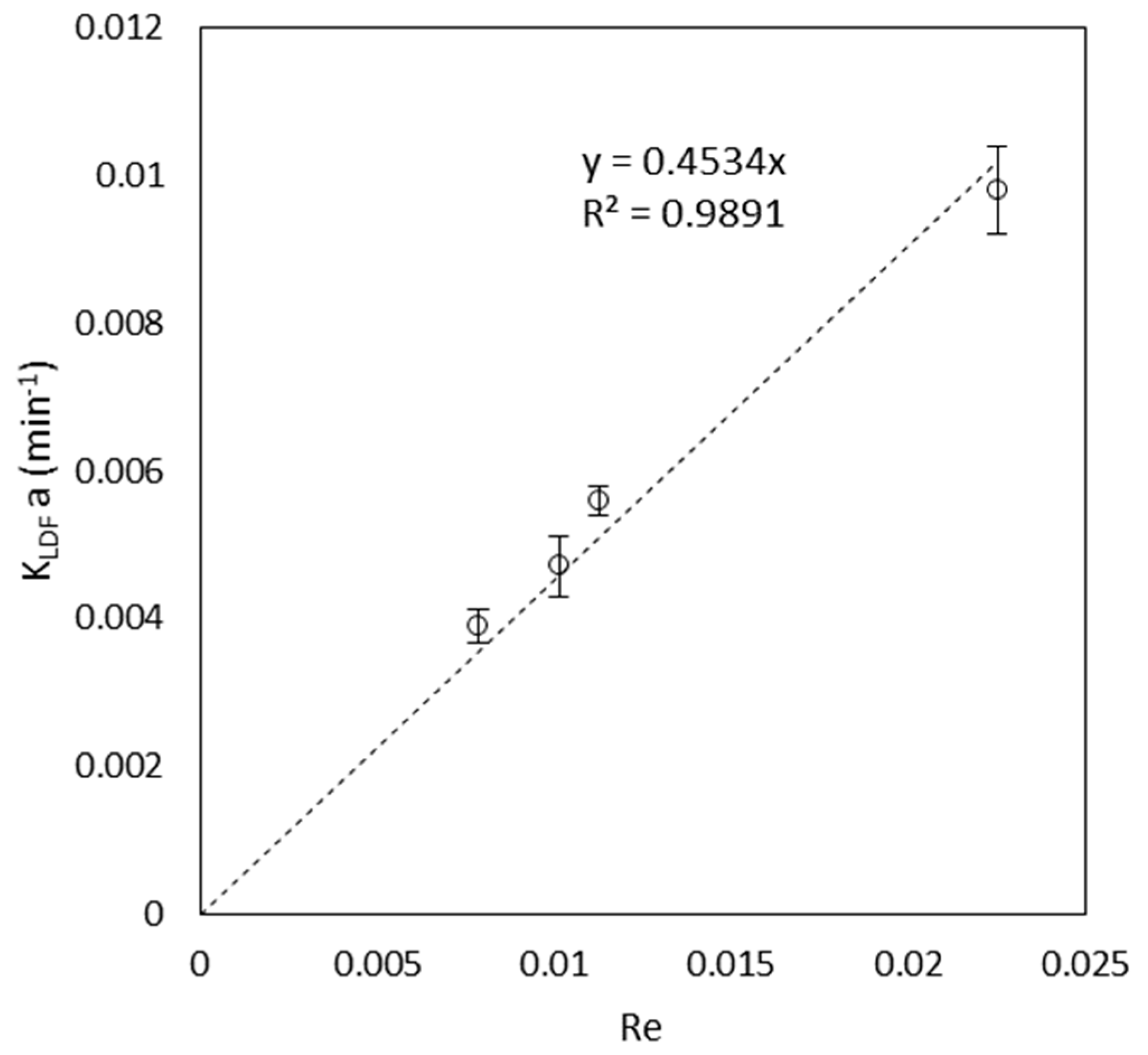
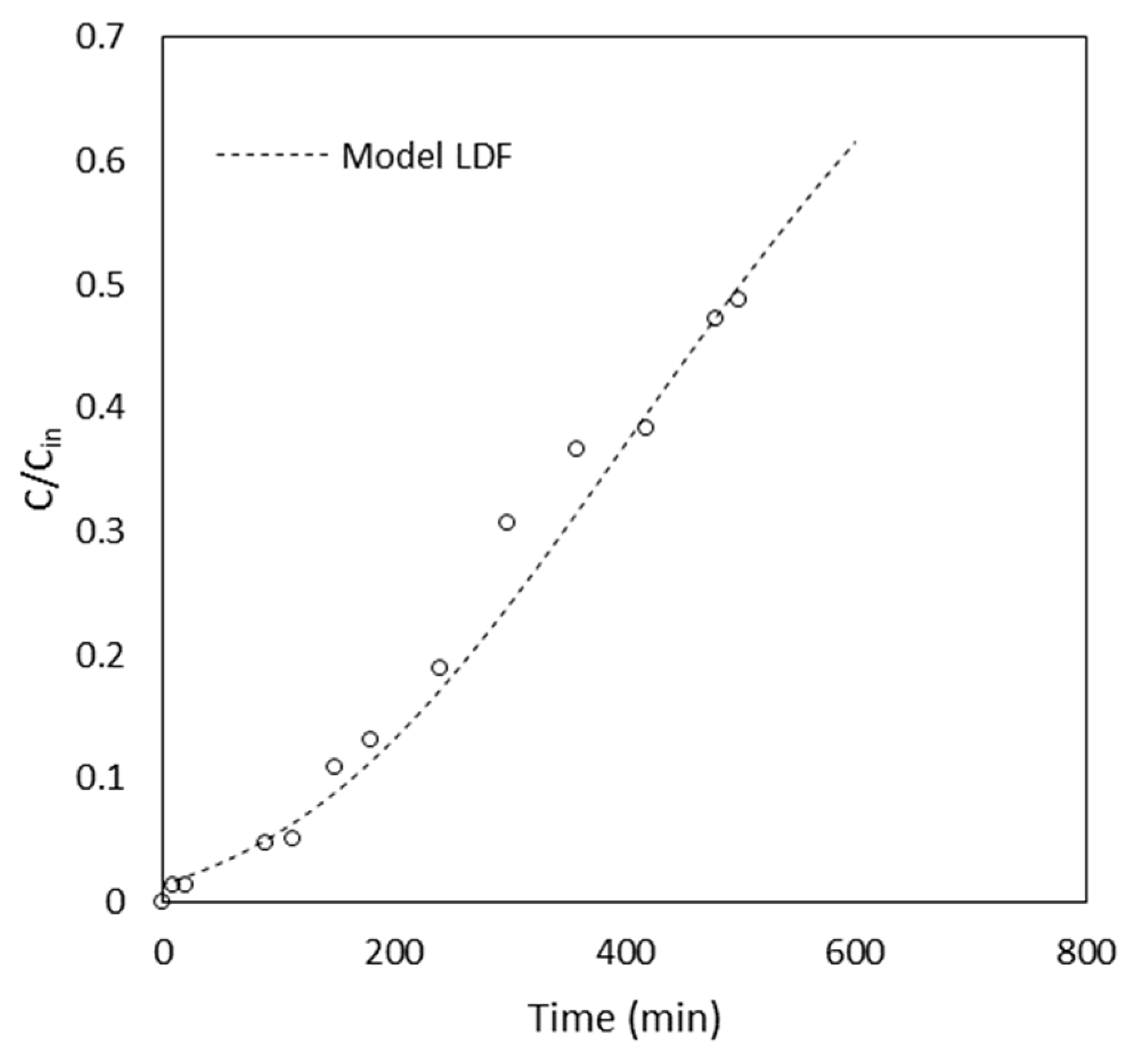
3.2. Column Tests
- the process is isothermal;
- both convection and axial dispersion were considered in the liquid phase: the well-known “longitudinal dispersed plug flow model” or simply the “dispersion model” [32] was adopted to take into account the fluid-dynamic deviations from an ideal plug-flow motion;
- the mass transfer of MB from the liquid to the solid phase was described by means of the well-established [33] linear driving force (LDF) approximation.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNESCO. Water Reuse: Potential for Expanding the Nation’s Water Supply through Reuse of Municipal Wastewater; The National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Capocelli, M.; Prisciandaro, M.; Piemonte, V.; Barba, D. A technical-economical approach to promote the water treatment & reuse processes. J. Clean. Prod. 2019, 207, 85–96. [Google Scholar] [CrossRef]
- Sardar, M.; Manna, M.; Maharana, M.; Sen, S. Remediation of Dyes from Industrial Wastewater Using Low-Cost Adsorbents; Springer: Berlin/Heidelberg, Germany, 2021; pp. 377–403. [Google Scholar] [CrossRef]
- Husain, Q. Potential applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: A review. Crit. Rev. Biotechnol. 2006, 26, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Gupta Suhas, V.K. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Malik, A.; Grohmann, E. Environmental Protection Strategies for Sustainable Development; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye removal from water and wastewater using various physical, chemical, and biological processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Yamamoto, K.; Fukushi, K. Hybrid treatment systems for dye wastewater. Crit. Rev. Environ. Sci. Technol. 2007, 37, 315–377. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Isa, A.C.; Affam, M.H.; Aminu, N.; Abubakar, S.; Noor, A.; Lawal, I.M.; Umaru, I.; Hassan, I. Effect of Environmental and Operational Parameters on Sequential Batch Reactor Systems in Dye Degradation. In Dye Biodegradation, Mechanisms and Techniques: Recent Advances; Muthu, S.S., Khadir, A., Eds.; Springer: Singapore, 2022; pp. 193–225. [Google Scholar] [CrossRef]
- Jamshaid, M.; ur Rehman, A.; Kumar, O.P.; Iqbal, S.; Nazir, M.A.; Anum, A.; Khan, H. Design of dielectric and photocatalytic properties of Dy–Ni substituted Ca0.5 Pb0.5−xFe12−yO19 M-type hexaferrites. J. Mater. Sci. Mater. Electron. 2021, 32, 16255–16268. [Google Scholar] [CrossRef]
- Mazzeo, L.; Rosa, D.; Bavasso, I.; Di Palma, L. Entrapped zinc oxide and titania nanoparticles in calcium alginate beads for the removal of Methylene Blue (MB): Adsorption properties and photocatalytic stability. Chem. Eng. Trans. 2021, 84, 181–186. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.J. Environmental impacts of desalination and brine treatment—Challenges and mitigation measures. Mar. Pollut. Bull. 2020, 161, 111773. [Google Scholar] [CrossRef]
- Panagopoulos, A. Water-energy nexus: Desalination technologies and renewable energy sources. Environ. Sci. Pollut. Res. 2021, 28, 21009–21022. [Google Scholar] [CrossRef]
- Almahbashi, N.M.Y.; Kutty, S.R.M.; Ayoub, M.; Noor, A.; Salihi, I.V.; Al-Nini, A.H.; Jagaba, A.; Aldhawi, B.N.S.; Ghaleb, A.A.S. Optimization of Preparation Conditions of Sewage sludge based Activated Carbon. Ain Shams Eng. J. 2021, 12, 1175–1182. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 411, 438. [Google Scholar] [CrossRef]
- Fayiga, A.O.; Ma, L.Q.; Santos, J.; Rathinasabapathi, B.; Stamps, B.; Littell, R.C. Effects of arsenic species and concentrations on arsenic accumulation by different fern species in a hydroponic system. Int. J. Phytoremediation 2005, 7, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Miyauchi, K.; Inoze, C.; Endo, G. Development of suitable hydroponics system for phytoremediation of arseniccontaminated water using an arsenic hyperaccumulator plant Pteris vittate. Biosci. Biotechnol. Biochem. 2016, 80, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Marzi, D.; Antenozio, M.; Vernazzaro, S.; Sette, C.; Veschetti, E.; Lucentini, L.; Daniele, G.; Brunetti, P.; Cardarelli, M. Advanced drinking groundwater as phytofiltration by the hyperaccumulating fern pteris vittate. Water 2021, 13, 2187. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Cost–benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci. Total Environ. 2016, 563, 796–802. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Chen, S. Supercritical water treatment of heavy metal and arsenic metalloid-bioaccumulating-biomass. Ecotoxicol. Environ. Saf. 2018, 157, 102–110. [Google Scholar] [CrossRef]
- Chen, J. Bio-oil production from hydrothermal liquefaction of Pteris vittata L.: Effects of operating temperatures and energy recovery. Bioresour. Technol. 2018, 265, 320–327. [Google Scholar] [CrossRef]
- Song, Y.; Liu, J.; Evrendilek, F.; Kuo, J.; Buyukada, M. Combustion behaviors of Pteris vittata using thermogravimetric, kinetic, emission and optimization analyses. J. Clean. Prod. 2019, 237, 117772. [Google Scholar] [CrossRef]
- Cai, W.; Chen, T.; Lei, M.; Wan, X. Effective strategy to recycle arsenic-accumulated biomass of Pteris vittata with high benefits. Sci. Total Environ. 2021, 756, 143890. [Google Scholar] [CrossRef]
- Bavasso, I.; Marzi, D.; Bracciale, M.P.; Di Palma, L.; Tirillò, J.; Sarasini, F. Plant Waste as Green Reinforcement for Polymer Composites: A Case Study of Pteris Vittata Roots. J. Nat. Fibers. 2022, 20, 2135669. [Google Scholar] [CrossRef]
- Mazzeo, L.; Marzi, D.; Irene, B.; Bracciale, M.P.; Piemonte, V.; Di Palma, L. Characterization of waste roots from the As hyperaccumulator Pteris vittata as low-cost adsorbent for Methylene Blue removal. Chem. Eng. Res. Des. 2022, 186, 13–21. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, L.; Bavasso, I.; Spallieri, M.; Bracciale, M.P.; Piemonte, V.; Di Palma, L. Effect of Water–Ethanol Extraction as Pre-Treatment on the Adsorption Properties of Aloe vera Waste. Materials 2022, 15, 5566. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, L.; Bavasso, I.; Bracciale, M.P.; Cocchi, M.; Di Palma, L.; Piemonte, V. Yerba Mate (Ilex paraguarensis) as Bio-Adsorbent for the Removal of Methylene Blue, Remazol Brilliant Blue and Chromium Hexavalent: Thermodynamic and Kinetic Studies. Water 2020, 12, 2016. [Google Scholar]
- Shahzad, K.; Nazir, M.A.; Jamshaid, M.; Kumar, O.P.; Najam, T.; Shah, S.S.A.; Rehman, A. Synthesis of nanoadsorbent entailed mesoporous organosilica for decontamination of methylene blue and methyl orange from water. Int. J. Environ. Anal. Chem. 2021, 1998471. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of adsorption and adsorption processes. React. Polym. Ion Exch. Sorbents. 1985, 4, 62. [Google Scholar] [CrossRef]
- Chung, S.F.; Wen, C.Y. Longitudinal dispersion of liquid flowing through fixed and fluidized beds. AIChE J. 1968, 14, 857–866. [Google Scholar] [CrossRef]
- Sircar, S.; Hufton, J.R. Intraparticle adsorbate concentration profile for linear driving force model. AIChE J. 2000, 46, 659–660. [Google Scholar] [CrossRef]
- de Araujo, C.M.B.; Ghislandi, M.G.; Rios, A.G.; da Costa, G.R.B.; Nascimento, B.F.; Ferreira, A.F.P.; da Motta Sobrinho, M.A.; Rodrigues, A.E. Wastewater treatment using recyclable agar-graphene oxide biocomposite hydrogel in batch and fixed-bed adsorption column: Bench experiments and modeling for the selective removal of organics. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 639, 128357. [Google Scholar] [CrossRef]
- Wilson, E.J.; Geankoplis, C.J. Liquid mass transfer at very low reynolds numbers in packed beds. Ind. Eng. Chem. Fundam. 1966, 5, 9–14. [Google Scholar] [CrossRef]
- Kumar, S.; Upadhyay, S.N.; Mathur, V.K. Low Reynolds Number Mass Transfer in Packed Beds of Cylindrical Particles. Ind. Eng. Chem. Process Des. Dev. 1977, 16, 1–8. [Google Scholar] [CrossRef]
- Nelson, P.A.; Galloway, T.R. Particle-to-fluid heat and mass transfer in dense systems of fine particles. Chem. Eng. Sci. 1975, 30, 1–6. [Google Scholar] [CrossRef]
- Leitão, A.; Carlos, P.; Santos, S.; Rodrigues, A. Tracer experiments in fixed beds: Effects of flow maldistribution on the estimation of transport kinetic parameters. Chem. Eng. J. Biochem. Eng. J. 1994, 53, 193–199. [Google Scholar] [CrossRef]
- Cecen, O.A. Activated Carbon for Water and Wastewater Treatment Integration of Adsorption and Biological Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]

| Test | |||
|---|---|---|---|
| T1 | 4.5 | 6.8 | 6.5 |
| T2 | 4.5 | 13.6 | 7.8 |
| T3 | 3 | 5.9 | 6.1 |
| T4 | 3 | 7.7 | 5.9 |
| Test | |||||
|---|---|---|---|---|---|
| T1 | 4.5 | 6.90 | 24 | 0.781 | 300.3 |
| T2 | 4.5 | 7.50 | 22 | 0.778 | 296.5 |
| T3 | 3 | 7.07 | 13.3 | 0.665 | 294.4 |
| T4 | 3 | 7.95 | 12 | 0.675 | 303.2 |
| T5 | 3 | 7.05 | 13.5 | 0.674 | 301.7 |
| Average ± DS | 299.2 ± 3.6 |
| Parameter | Description | Value (UOM) | Reference |
|---|---|---|---|
| Solid apparent density | 299.2 g/L | [This work] | |
| Maximum adsorption capacity | 112.34 mg/g | [25] | |
| Langmuir constant | 0.62 L/mg | [25] |
| Parameter | Description | Value (UOM) | Notes |
|---|---|---|---|
| Mass of solid | 0.228 g | - | |
| Flow rate | 6.3 mL/min | - | |
| Inlet MB concentration | 4.1 mg/L | - | |
| Bed height | 2.7 cm | - | |
| Equivalent particle diameter | 0.078 cm | - | |
| Bed void fraction | 0.664 | Equation (1) | |
| Reynolds number | 0.0104 | Equation (8) | |
| Product of LDF mass transfer coefficient with the specific surface area of PV roots | 4.68 × 10−3 1/min | Equation (10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzeo, L.; Marzi, D.; Bavasso, I.; Piemonte, V.; Di Palma, L. Removal of Methylene Blue from Wastewater by Waste Roots from the Arsenic-Hyperaccumulator Pteris vittata: Fixed Bed Adsorption Kinetics. Materials 2023, 16, 1450. https://doi.org/10.3390/ma16041450
Mazzeo L, Marzi D, Bavasso I, Piemonte V, Di Palma L. Removal of Methylene Blue from Wastewater by Waste Roots from the Arsenic-Hyperaccumulator Pteris vittata: Fixed Bed Adsorption Kinetics. Materials. 2023; 16(4):1450. https://doi.org/10.3390/ma16041450
Chicago/Turabian StyleMazzeo, Leone, Davide Marzi, Irene Bavasso, Vincenzo Piemonte, and Luca Di Palma. 2023. "Removal of Methylene Blue from Wastewater by Waste Roots from the Arsenic-Hyperaccumulator Pteris vittata: Fixed Bed Adsorption Kinetics" Materials 16, no. 4: 1450. https://doi.org/10.3390/ma16041450





