The Selection of the Best Derivatization Reagents for the Determination of Polyamines in Home-Made Wine Samples
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Apparatus
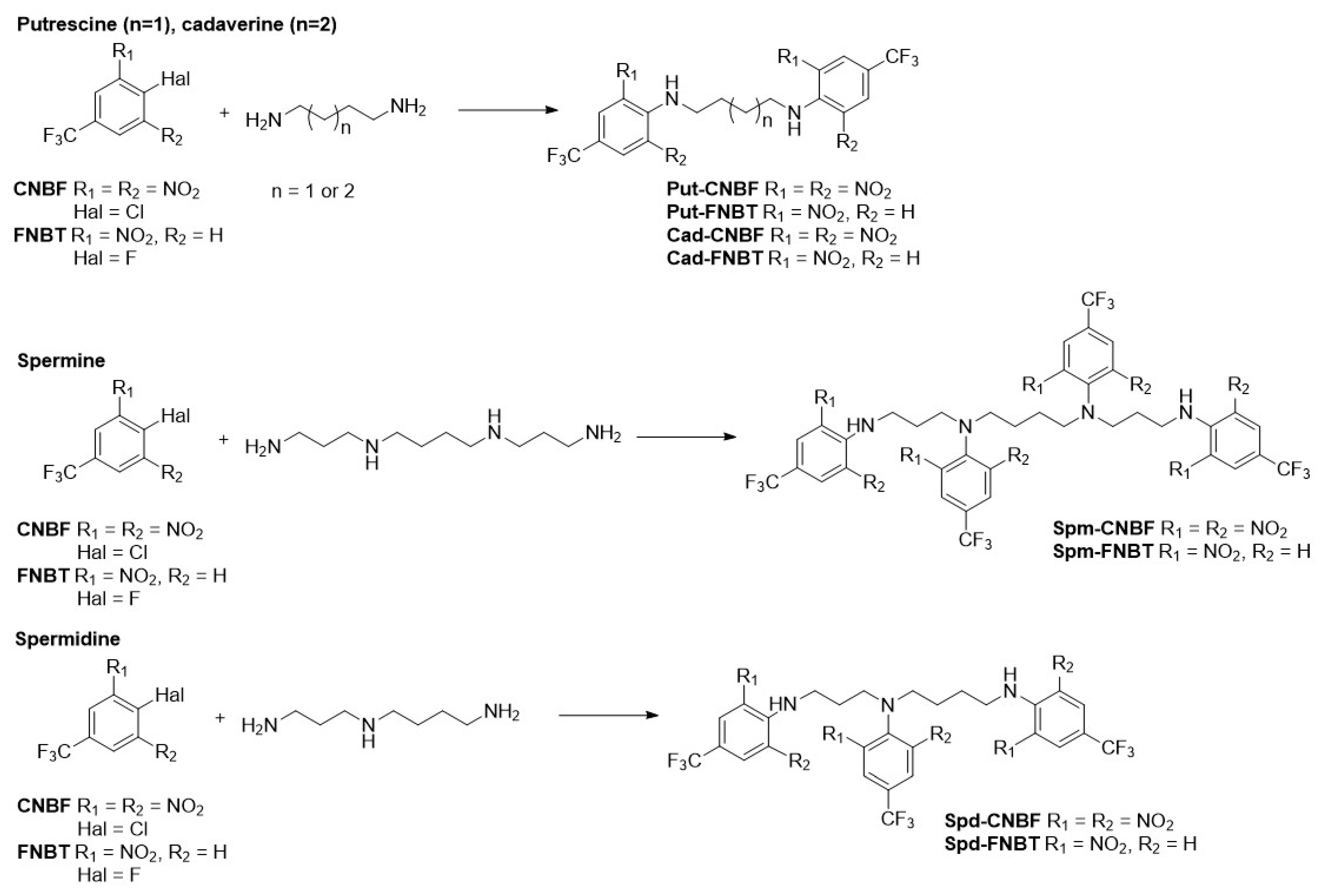
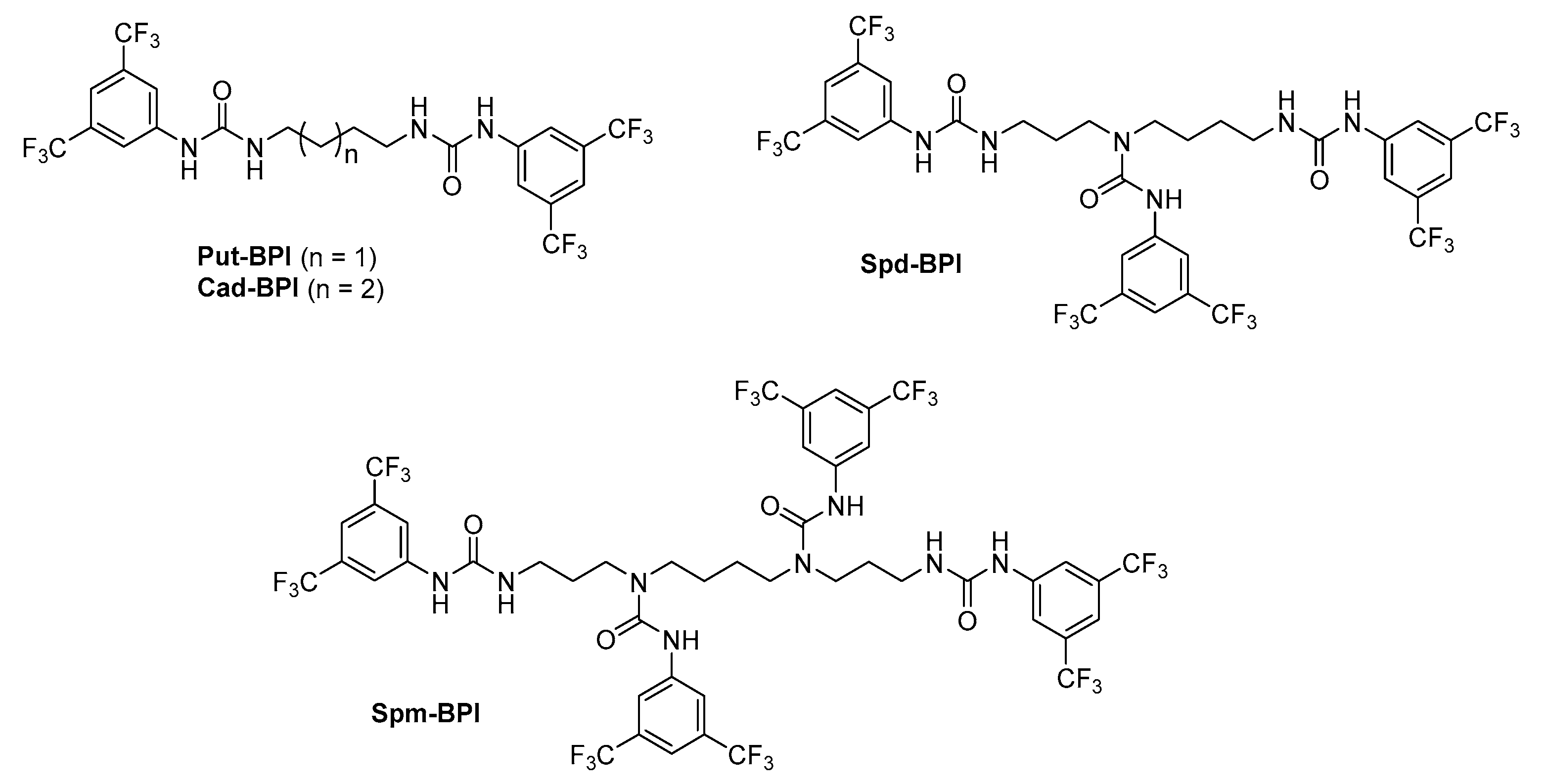
2.2. Selection of Molar Ratio for Polyamines’ Derivatization
2.3. Derivatization Process—General Procedures
2.4. 1H, 13C, 19F NMR, IR, and XRD Studies
2.5. Chromatographic Analysis
2.6. Samples Preparation
2.7. Selection of Sample Preparation Method
2.8. Statistical Evaluation of Proposed Procedures
3. Results and Discussion
3.1. Structural Characteristics of the Obtained Derivatives
3.2. RP-HPLC Method Development and Validation
3.3. The Determination of the PA Derivatives in Home-Made Wine Samples
3.3.1. Sample Preparation
3.3.2. Wine Sample Analysis
3.4. The Determination of the BAs in Home-Made Wine Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalač, P. Health effects and occurrence of dietary polyamines: A review for the period 2005–mid 2013. Food Chem. 2014, 161, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Teratani, T.; Kasahara, N.; Ijichi, T.; Fujimoto, Y.; Sakuma, Y.; Sata, N.; Kitayama, J. Activation of whole body by high levels of polyamine intake in rats. Amino Acids 2021, 53, 1695–1703. [Google Scholar] [CrossRef]
- Ibarra, A.A.G.; Wróbel, K.; Escobosa, A.R.C.; Elguera, J.C.T.; Garay-Sevilla, M.E.; Wróbel, K. Determination of putrescine, cadaverine, spermidine and spermine in different chemical matrices by high performance liquid chromatography–electrospray ionization–ion trap tandem mass spectrometry (HPLC–ESI–ITMS/MS). J. Chromatogr. B 2015, 1002, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Baratella, D.; Bonaiuto, E.; Magro, M.; de Almeida Roger, J.; Kanamori, Y.; Lima, G.P.P.; Agostinelli, E.; Vianello, F. Endogenous and food-derived polyamines: Determination by electrochemical sensing. Amino Acids 2018, 50, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Esparza, N.C.; Costa-Catala, J.; Comas-Basté, O.; Toro-Funes, N.; Latorre-Moratalla, M.L.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Occurrence of polyamines in foods and the influence of cooking processes. Foods 2021, 10, 1752. [Google Scholar] [CrossRef]
- Filipe-Ribeiro, L.; Milheiro, J.; Ferreira, L.C.; Correia, E.; Cosme, F.; Nunes, F.M. Biogenic amines and polyamines in wines: Does Dekkera/Brettanomyces red wine spoilage increases the risk of intake by consumers? LWT- Food Sci. Technol. 2019, 115, 108488–108495. [Google Scholar] [CrossRef]
- Jaguey-Hernández, Y.; Aguilar-Arteaga, K.; Ojeda-Ramirez, D.; Anorve-Morga, J.; González-Olivares, L.G.; Castaneda-Ovando, A. Biogenic amines levels in food processing: Efforts for their control in foodstuffs. Food Res. Int. 2021, 144, 110341–110355. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M. Update on biogenic amines in fermented and non-fermented beverages. Foods 2022, 11, 353. [Google Scholar] [CrossRef]
- Li, H.; Gan, J.; Yang, Q.; Fu, L.; Wang, Y. Colorimetric detection of food freshness based on amine-responsive dopamine polymerization on gold nanoparticles. Talanta 2021, 234, 22706–22714. [Google Scholar] [CrossRef]
- Ahangari, H.; Kurbanoglu, S.; Ehsani, A.; Uslu, B. Latest trends for biogenic amines detection in foods: Enzymatic biosensors and nanozymes applications. Trends Food Sci. Technol. 2021, 112, 75–87. [Google Scholar] [CrossRef]
- Michalski, R.; Pecyna-Utylska, P.; Kernert, J. Determination of ammonium and biogenic amines by ion chromatography. A review. J. Chromatogr. A 2021, 1651, 462319–462334. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, J.; Płotka-Wasylka, J. Literature update of analytical methods for biogenic amines determination in food and beverages. Trends Anal. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef]
- Vasconcelos, H.; de Almeida, J.M.M.M.; Matias, A.; Saraiva, C.; Jorge, P.A.S.; Coelho, L.C.C. Detection of biogenic amines in several foods with different sample treatments: An overview. Trends Food Sci. Technol. 2021, 113, 86–96. [Google Scholar] [CrossRef]
- Piasta, A.M.; Jastrzębska, A.; Krzemiński, M.P.; Muzioł, T.M.; Szłyk, E. New procedure of selected biogenic amines determination in wine samples by HPLC. Anal Chim. Acta 2014, 834, 58–66. [Google Scholar] [CrossRef]
- Jastrzębska, A.; Piasta, A.; Kowalska, S.; Krzemiński, M.; Szłyk, E. A new derivatization reagent for determination of biogenic amines in wines. J. Food Comp. Anal. 2016, 48, 111–119. [Google Scholar] [CrossRef]
- Jastrzębska, A.; Piasta, A.; Krzemiński, M.; Szłyk, E. Application of 3,5-bis-(trifluoromethyl)phenyl isothiocyanate for the determination of selected biogenic amines by LC-tandem mass spectrometry and 19F NMR. Food Chem. 2018, 239, 225–233. [Google Scholar] [CrossRef]
- Jastrzębska, A. Determination of home-made wine selected parameters and study of honey addition impact on pro-healthy components content. Eur. Food Res. Technol. 2022, 248, 963–973. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Simeonov, V.; Namieśnik, J. Characterization of home-made and regional fruit wines by evaluation of correlation between selected chemical parameters. Microchem. J. 2018, 140, 66–73. [Google Scholar] [CrossRef]
- CrysAlis Red and CrysAlis CCD; Oxford Diffraction Ltd.: Abingdon, UK, 2000.
- Sparta, K.M.; Krug, M.; Heinemann, U.; Mueller, U.; Weiss, M.S. XDSAPP2.0. J. Appl. Crystallogr. 2016, 49, 1085–1092. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer model energies and energy framworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Liu, S.-J.; Xu, J.-J.; Ma, C.-L.; Guo, C.-F. A comparative analysis of derivatization strategies for the determination of biogenic amines in sausage and cheese by HPLC. Food Chem. 2018, 266, 275–283. [Google Scholar] [CrossRef]
- Wang, Q.; Gallardo-Macias, R.; Rashmi; Golovko, M.Y.; Elsayed, A.A.R.; More, S.K.; Oncel, S.; Gurvich, V.J.; Basson, M.D. Discovery of Novel Small-Molecule FAK Activators Promoting Mucosal Healing. ACS Med. Chem. Lett. 2021, 12, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ollevier, T. Iron- or Zinc-Mediated Synthetic Approach to Enantiopure Dihydroquinoxalinones. Eur. J. Org. Chem. 2019, 1273–1280. [Google Scholar] [CrossRef]
- Gan, Z.; Li, G.; Yan, Q.; Deng, W.; Jiang, Y.-Y.; Yang, D. Visible-light-promoted oxidative desulphurisation: A strategy for the preparation of unsymmetrical ureas from isothiocyanates and amines Rusing molecular oxygen. Green Chem. 2020, 22, 2956–2962. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Julinová, M.; Vaňharová, L.; Jurča, M. Water-soluble polymeric xenobiotics—Polyvinyl alcohol and polyvinylpyrrolidon—And potential solutions to environmental issues: A brief review. J. Environ. Manag. 2018, 228, 213–222. [Google Scholar] [CrossRef]
- Costantini, A.; Vaudano, E.; Pulcini, L.; Carafa, T.; Garcia-Moruno, E. An Overview on Biogenic Amines in Wine. Beverages 2019, 5, 19. [Google Scholar] [CrossRef]
- Ancín-Azpilicueta, C.; Jiménez-Moreno, N.; Sola-Larrañaga, C. Chapter 9—Wine. In Innovations in Traditional Foods; Woodhead Publishing: Sawston, UK, 2019; pp. 221–256. [Google Scholar] [CrossRef]
- Gil, R.L.; Amorim, C.G.; Montenegro, M.C.B.S.M.; Araújo, A.N. HPLC-potentiometric method for determination of biogenic amines in alcoholic beverages: A reliable approach for food quality control. Food Chem. 2022, 372, 131288. [Google Scholar] [CrossRef]
- Moniente, M.; Botello-Morte, L.; García-Gonzalo, D.; Pagán, R.; Ontańón, I. Analytical strategies for the determination of biogenic amines in dairy products. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3612–3646. [Google Scholar] [CrossRef]
- Gao, X.; Li, C.; He, R.; Zhang, Y.; Wang, B.; Zhang, Z.H.; Ho, C.T. Research advances on biogenic amines in traditional fermented foods: Emphasis on formation mechanism, detection and control methods. Food Chem. 2023, 405, 134911. [Google Scholar] [CrossRef]
- Jain, A.; Verma, K.K. Strategies in liquid chromatographic methods for the analysis of biogenic amines without and with derivatization. TRAC 2018, 109, 62–82. [Google Scholar] [CrossRef]
- Hernández-Cassou, S.; Saurina, J. Derivatization strategies for the determination of biogenic amines in wines by chromatographic and electrophoretic techniques. J. Chromatogr. B 2011, 879, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Pravadali-Cekic, S.; Dennis, G.R.; Shalliker, R.A. Post column derivatisation analyses review. Is post-column derivatisation incompatible with modern HPLC columns? Anal. Chim Acta 2015, 889, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S.; Sato, Y.; Bounoshita, M.; Miyaji, T.; Tognarelli, D.J.; Saito, M. Optimization of an online post-column derivatization system for ultra high-performance liquid chromatography (UHPLC) and its applications to analysis of biogenic amines. Anal. Sci. 2013, 29, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Moratalla, M.L.; Bosch-Fusté, J.; Lavizzari, T.; Bover-Cid, S.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Validation of an ultra high pressure liquid chromatographic method for the determination of biologically active amines in food. J. Chromatogr. A 2009, 1216, 7715–7720. [Google Scholar] [CrossRef] [PubMed]
- Nalazek-Rudnicka, K.; Wasik, A. Development and validation of an LC–MS/MS method for the determination of biogenic amines in wines and beers. Mon. Chem. Chem. Mon. 2017, 148, 1685–1696. [Google Scholar] [CrossRef] [PubMed]

| Put | Cad | Spd | Spm | |
|---|---|---|---|---|
| CNBF | ||||
| Range [mg·L−1] | 4.00–31.00 | 2.00–22.00 | 2.00–20.00 | 2.00–24.00 |
| t [min] | 18.44 | 19.38 | 24.51 | 28.74 |
| Mean CVt [%] | 0.11 | 0.21 | 0.16 | 0.07 |
| R2 | 0.9997 | 0.9990 | 0.9992 | 0.9990 |
| DL [mg·L−1] | 0.10 | 0.12 | 0.15 | 0.12 |
| QL [mg·L−1] | 0.33 | 0.40 | 0.51 | 0.41 |
| Mean Rec [%] | 98.18 | 95.54 | 93.88 | 94.59 |
| Mean CV [%] | 1.09 | 2.57 | 1.65 | 3.27 |
| FNBT | ||||
| Range [mg·L−1] | 2.00–16.00 | 2.00–18.00 | 2.00– 0.00 | 2.00–18.00 |
| t [min] | 20.11 | 22.87 | 27.95 | 33.08 |
| Mean CVt [%] | 0.10 | 0.04 | 0.07 | 0.06 |
| R2 | 0.9999 | 0.9999 | 0.9990 | 0.09995 |
| DL [mg·L−1] | 0.10 | 0.17 | 0.15 | 0.19 |
| QL [mg·L−1] | 0.30 | 0.58 | 0.51 | 0.62 |
| Mean Rec [%] | 98.27 | 97.51 | 96.96 | 98.00 |
| Mean CV [%] | 1.22 | 1.15 | 1.08 | 1.29 |
| Put-CNBF | Put-FNBT | Cad-CNBF | Cad-FNBT | Spd-CNBF | Spd-FNBT | Spm-CNBF | Spm-FNBT | |
|---|---|---|---|---|---|---|---|---|
| sample 1 | 25.5 a, g ±0.3 | 26.4 b, i ±0.2 | 15.3 b, g ±0.4 | 14.9 a, g ±0.3 | 0.059 a, a ±0.005 | 0.076 b,a ±0.004 | 0.11 a, b ±0.02 | 0.13 b, a,b ±0.01 |
| sample 2 | 16.3 a, d ±0.1 | 17.3b, f ±0.2 | 5.75a,e ±0.03 | 6.30 b, f ±0.07 | nd | 0.03a ±0.01 | nd | nd |
| sample 3 | 21.1 a, f ±0.2 | 21.3 a, h ±0.1 | 14.5 a, f ±0.3 | 14.9b,g ±0.1 | 9.93 b,f ±0.04 | 9.80 a, f ±0.17 | 2.86 a,e ±0.05 | 2.85 a, c ±0.11 |
| sample 4 | 18.7 b, e ±0.1 | 18.2 a, g ±0.3 | 15.8 b, h ±0.1 | 14.7 a, g ±0.2 | 2.35 a,e ±0.04 | 2.79 b, e ±0.03 | nd | nd |
| sample 5 | 9.15 a, c ±0.20 | 10.6 b, e ±0.1 | 3.48 a, c ±0.18 | 3.86 b, d ±0.02 | 0.13 a,a ±0.02 | 0.26 b, b ±0.01 | 0.20 a, c ±0.02 | 0.20 a, b ±0.01 |
| sample 6 | 5.15 a, b ±0.13 | 5.36 b, c ±0.02 | 1.91 a, b ±0.01 | 2.07 a, c ±0.01 | 0.42 a, b ±0.02 | 0.66 b, c ±0.01 | 2.51 a, d ±0.02 | 2.82 b, c ±0.11 |
| sample 7 | 9.19 ±0.18 a, c | 9.85 ±0.04 b, d | 5.07 ±0.05 a, d | 5.37 ±0.04 b, e | nd | 0.02 ±0.01 a | nd | nd |
| sample 8 | 4.78 a, a ±0.02 | 5.10 b, a ±0.12 | 1.70 a, a,b ±0.01 | 1.82 b, b ±0.01 | 1.67 a, c ±0.09 | 2.04 b, d ±0.02 | 0.094 a, a ±0.007 | 0.095 a, a ±0.004 |
| sample 9 | 5.21 a, b ±0.03 | 5.50 b, b ±0.07 | 1.47 a, a ±0.01 | 1.55 b, a ±0.03 | 1.89 a, d ±0.01 | 1.97 b, d ±0.02 | 0.011 a, b ±0.001 | 0.014 b, a ±0.002 |
| PAs | Added [mg·L−1] | CNBF | FNBT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rec [%] | CV [%] | Mean Rec after 7 Days ±SD [%] | Mean Rec after 14 Days ±SD [%] | Rec [%] | CV [%] | Mean Rec after 7 Days ±SD [%] | Mean Rec after 14 Days ±SD | ||
| Put | 2.50 | 95.40 | 3.14 | 95.06 ± 4.16 | 94.11 ± 3.31 | 96.52 | 2.89 | 95.59 ± 3.13 | 95.34 ± 3.07 |
| 5.50 | 96.36 | 3.91 | 96.80 | 3.23 | |||||
| 10.50 | 97.21 | 1.53 | 98.25 | 1.26 | |||||
| Cad | 2.50 | 95.07 | 3.05 | 94.06 ± 3.08 | 92.07 ± 2.71 | 95.79 | 2.32 | 95.38 ± 3.03 | 95.16 ± 3.61 |
| 5.50 | 94.47 | 3.54 | 95.86 | 2.86 | |||||
| 10.50 | 95.76 | 2.96 | 97.22 | 1.72 | |||||
| Spd | 2.50 | 92.63 | 3.39 | 93.76 ± 3.72 | 92.60 ± 0.35 | 95.17 | 2.11 | 96.52 ± 2.89 | 95.30 ± 4.52 |
| 5.50 | 93.41 | 3.80 | 95.25 | 2.69 | |||||
| 10.50 | 94.53 | 3.27 | 96.80 | 2.36 | |||||
| Spm | 2.50 | 92.47 | 3.39 | 93.96 ± 3.81 | 92.63 ± 4.18 | 95.25 | 2.58 | 96.04 ± 2.55 | 95.23 ± 3.16 |
| 5.50 | 96.49 | 2.23 | 97.08 | 1.80 | |||||
| 10.50 | 94.67 | 4.07 | 96.24 | 1.99 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kmieciak, A.; Jastrzębska, A.; Szymańska, K.; Krzemiński, M.P.; Muzioł, T.M.; Kurzawa, M.; Szłyk, E. The Selection of the Best Derivatization Reagents for the Determination of Polyamines in Home-Made Wine Samples. Materials 2023, 16, 1474. https://doi.org/10.3390/ma16041474
Kmieciak A, Jastrzębska A, Szymańska K, Krzemiński MP, Muzioł TM, Kurzawa M, Szłyk E. The Selection of the Best Derivatization Reagents for the Determination of Polyamines in Home-Made Wine Samples. Materials. 2023; 16(4):1474. https://doi.org/10.3390/ma16041474
Chicago/Turabian StyleKmieciak, Anna, Aneta Jastrzębska, Karolina Szymańska, Marek P. Krzemiński, Tadeusz M. Muzioł, Marzanna Kurzawa, and Edward Szłyk. 2023. "The Selection of the Best Derivatization Reagents for the Determination of Polyamines in Home-Made Wine Samples" Materials 16, no. 4: 1474. https://doi.org/10.3390/ma16041474








