Effect of Annealing on the Interface and Properties of Pd/Al Composite Wires
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussion
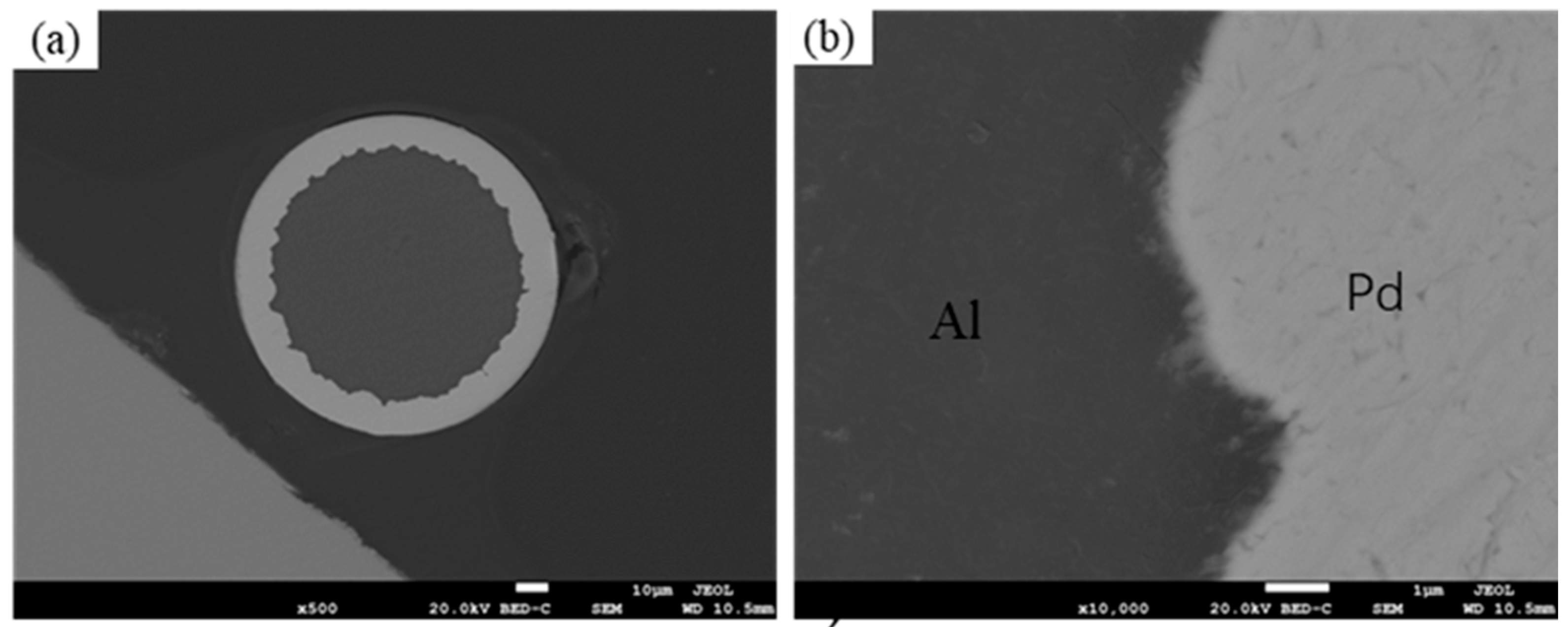
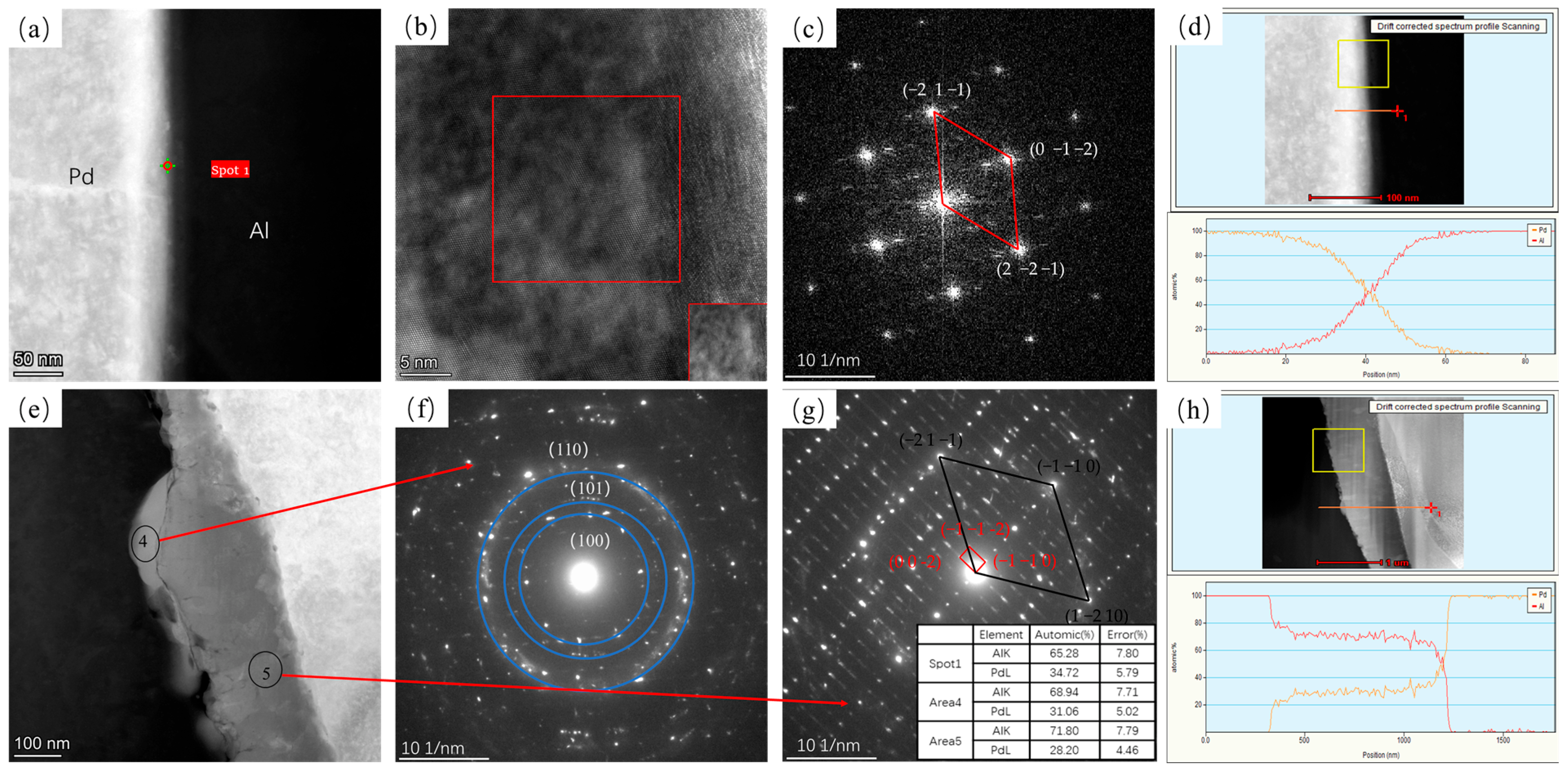
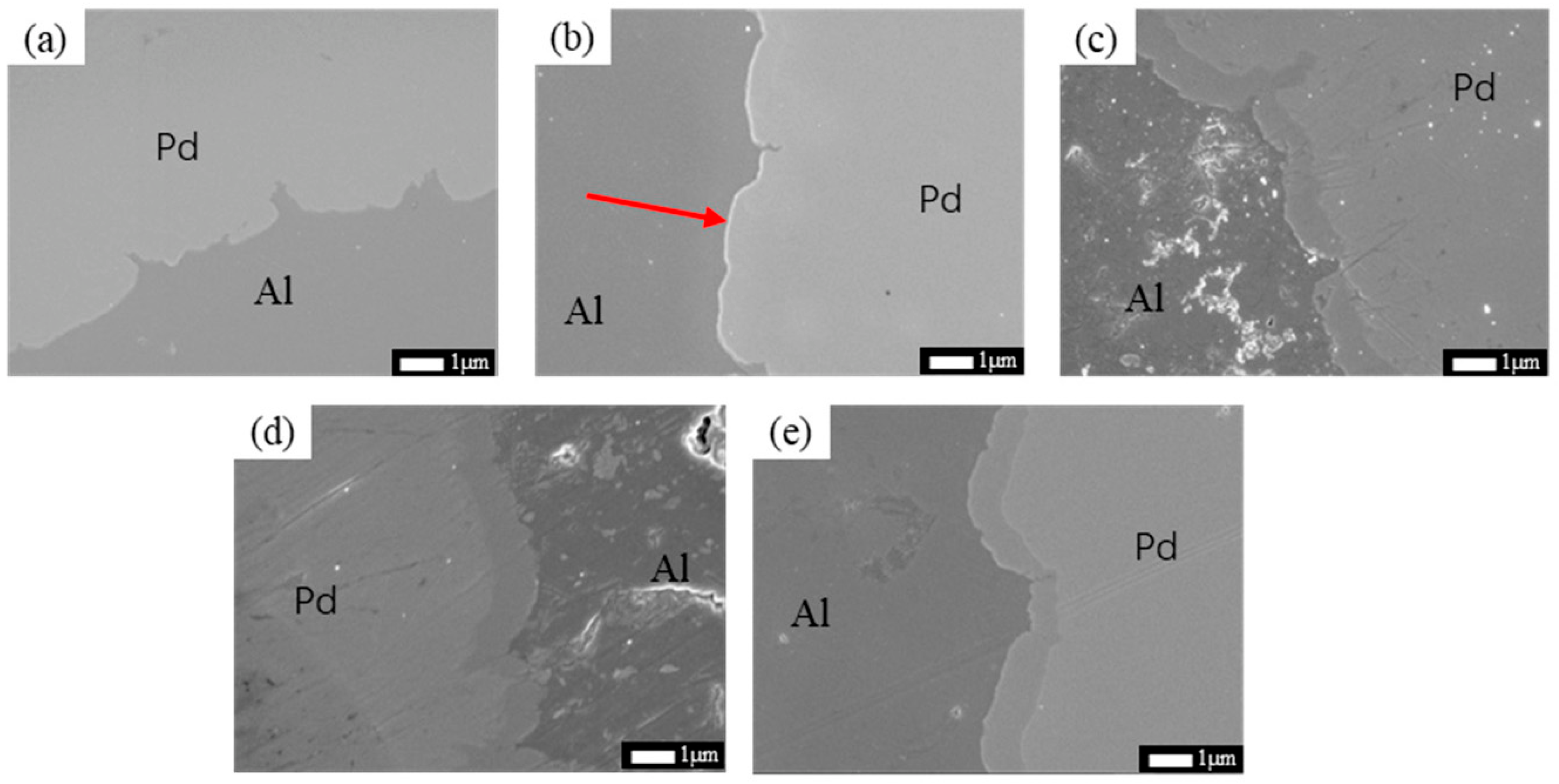
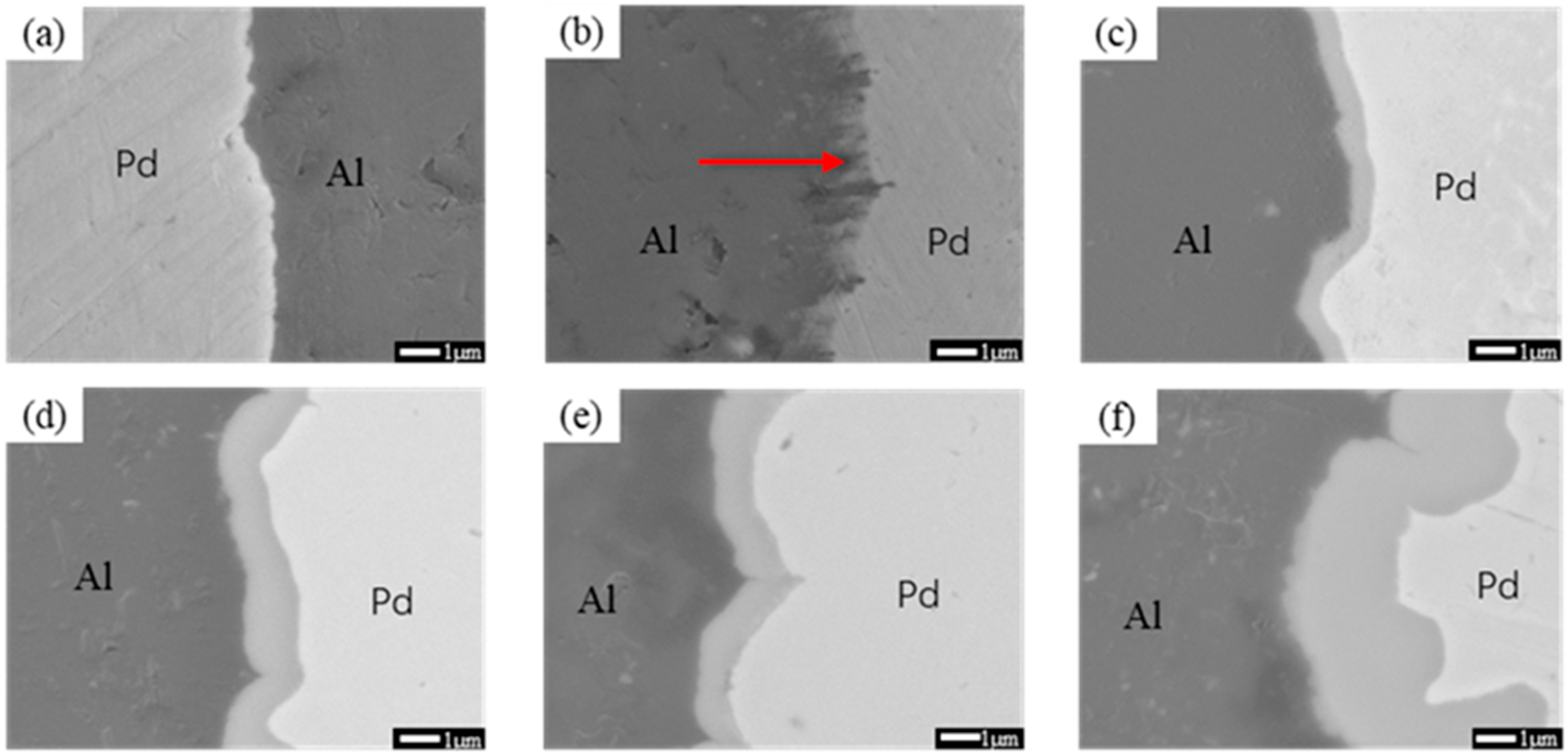
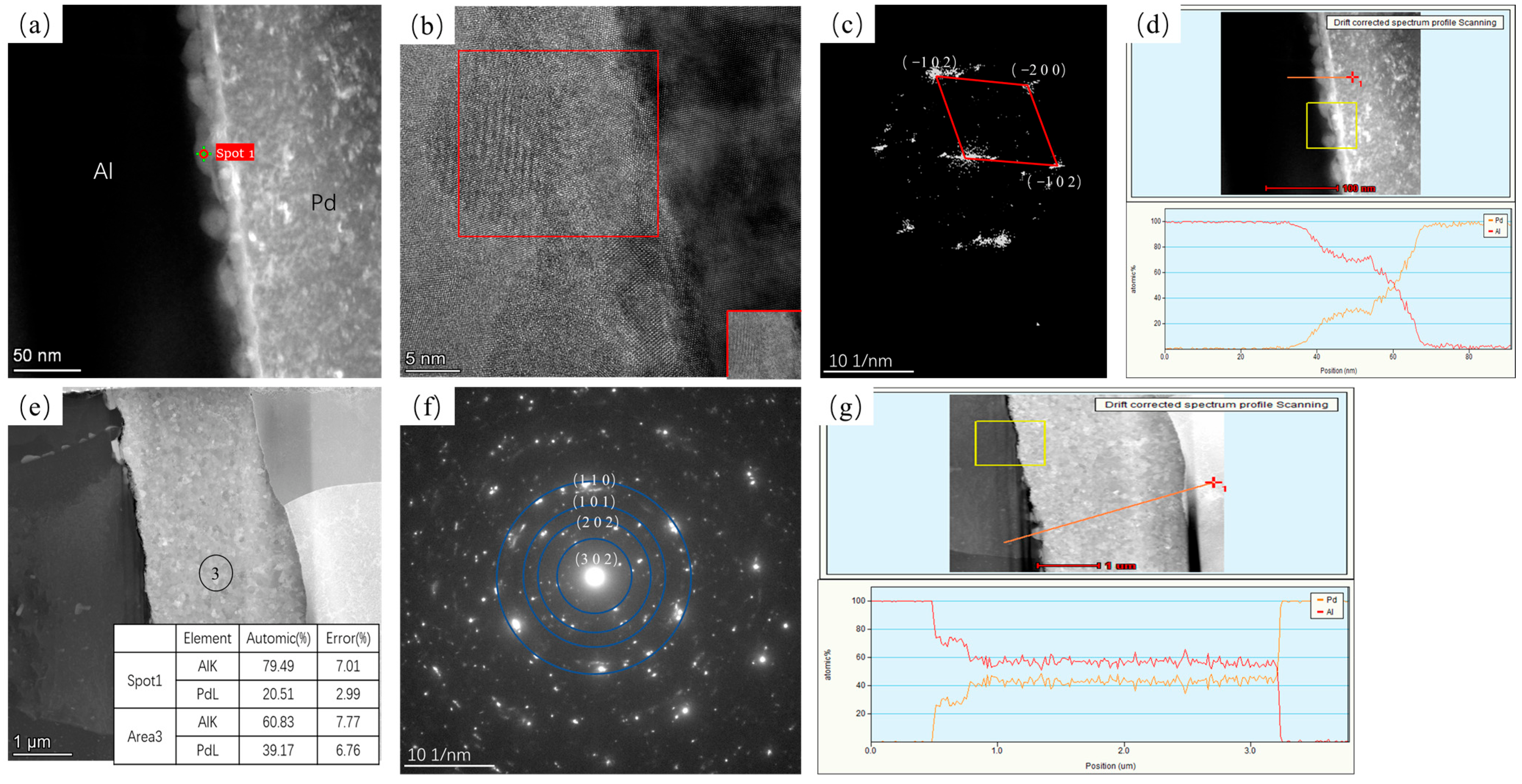
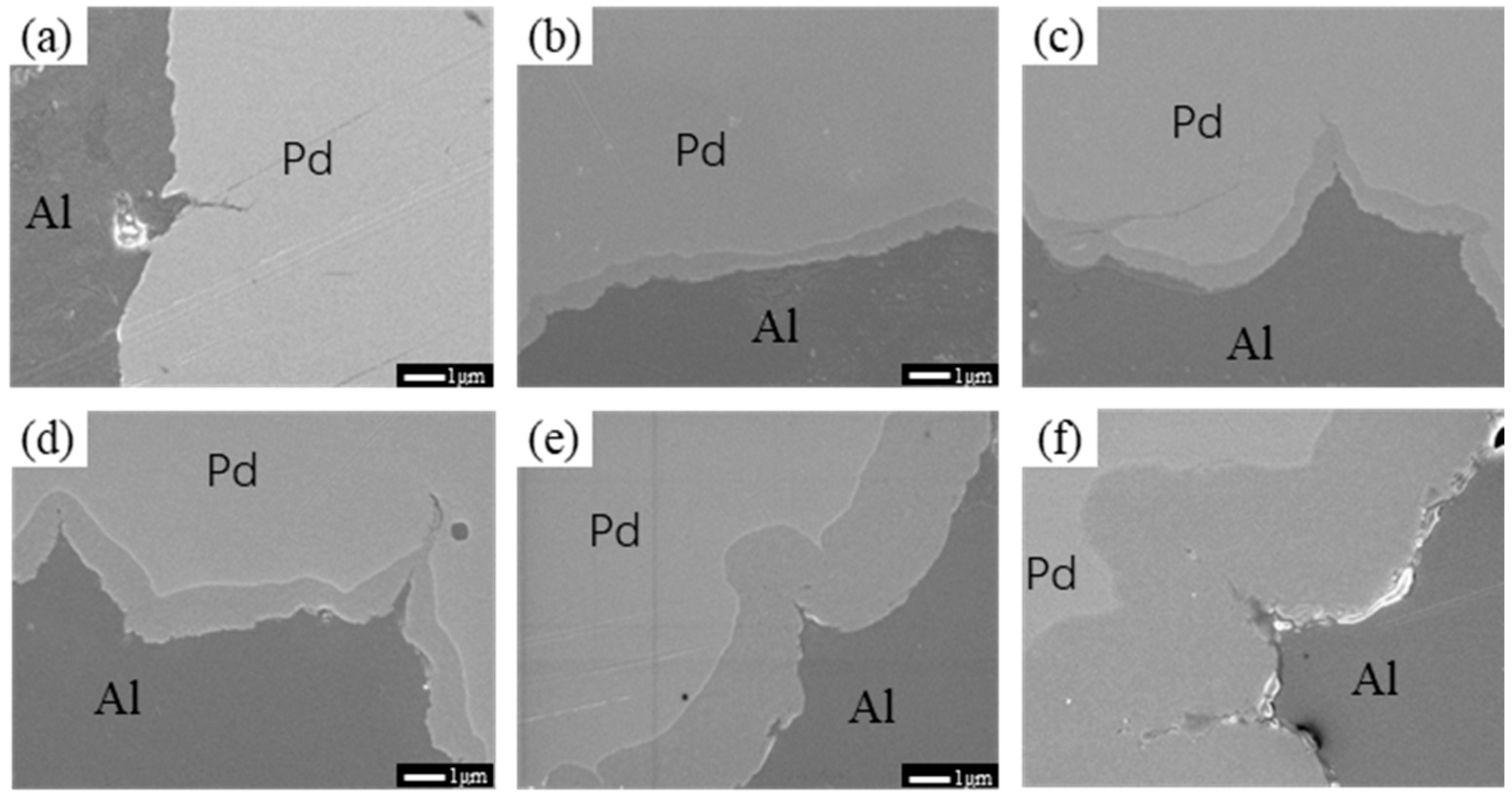
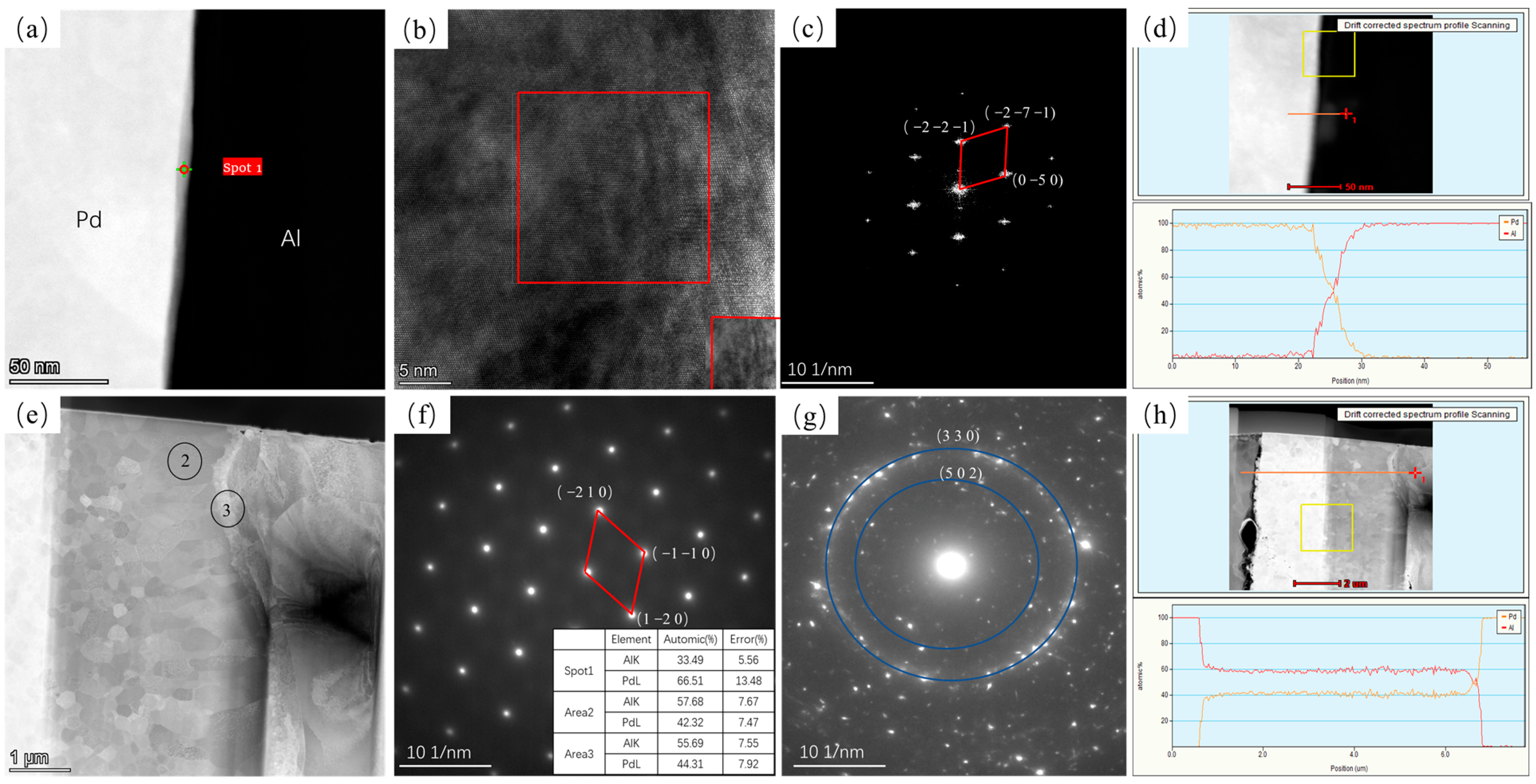
3.1. Interface Analysis
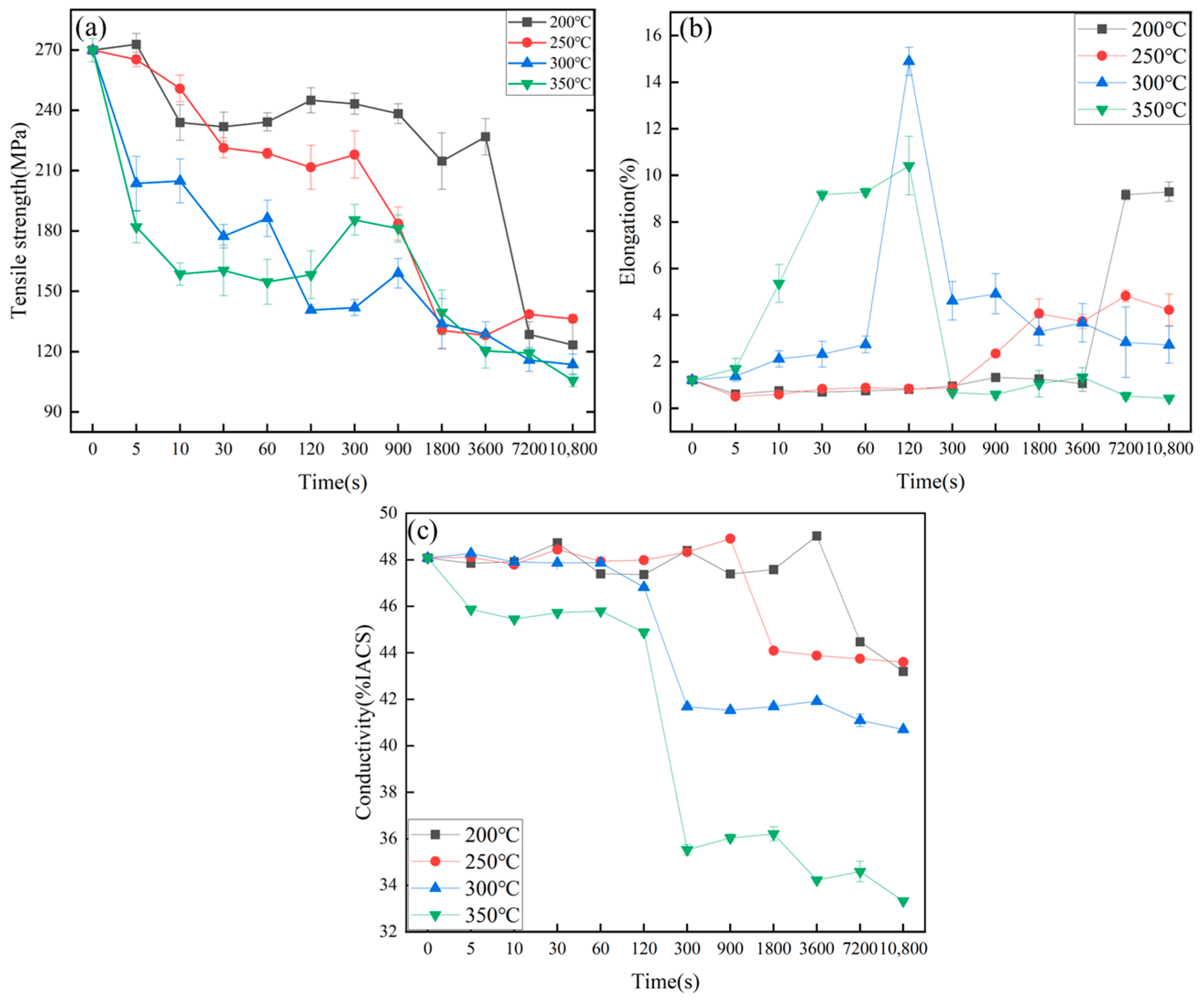
3.2. Mechanical and Conductive Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, Y.H.; Wu, B.; Lu, X.T. Effect of Post-Weld Annealing on Microstructure and Growth Behavior of Copper/Aluminum Friction Stir Welded Joint. Materials 2020, 13, 4591. [Google Scholar] [CrossRef]
- Camacho, A.M.; Rodríguez-Prieto, Á.; Herrero, J.M.; Aragón, A.M.; Bernal, C.; Lorenzo-Martin, C.; Yanguas-Gil, Á.; Martins, P.A. An Experimental and Numerical Analysis of the Compression of Bimetallic Cylinders. Materials 2019, 12, 4094. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Zhang, L.; Ma, S.N. Effects of Transition Layer on Bending Resistance in Copper-Clad Aluminum Composite Casting. J. Mater. Eng. Perform. 2019, 28, 3560–3566. [Google Scholar] [CrossRef]
- Lechner, S.; Rœmisch, U.; Nitschke, R.; Gensch, F.; Mueller, S. Optimization of the Indirect Extrusion Process of Copper-Clad Aluminum Rods by Methods of Statistical Experimental Designs and Numerical Analyses. Front. Mater. 2021, 8, 663306. [Google Scholar] [CrossRef]
- Dashti, A.; Keller, C.; Vieille, B.; Guillet, A.; Bouvet, C. Experimental and Finite Element Analysis of the Tensile Behavior of Architectured Cu-Al Composite Wires. Materials 2021, 14, 6305. [Google Scholar] [CrossRef]
- Liu, X.H.; Liu, X.F.; Xie, J.X. Effects of annealing process on interface and mechanical properties of silver clad aluminum wires. Procedia Eng. 2012, 27, 502–511. [Google Scholar] [CrossRef]
- Shi, B.B.; Liu, X.H.; Xie, J.X.; Xie, M. Preparation process of silver clad aluminum bars by vertical continuous casting composite forming. Chin. J. Eng. 2019, 41, 633–645. [Google Scholar]
- Pang, S.L.; Chen, X.; Xu, J.S. Analysis on damage characteristics and detonation performance of solid rocket engine charge subjected to jet. Def. Technol. 2022, 18, 1552–1562. [Google Scholar] [CrossRef]
- Boshra, I.K.; Ahmed, A. Composite Solid Rocket Propellant Based on GAP Polyurethane Matrix with Different Binder Contents. Chin. J. Explos. Propellents 2020, 43, 362–367. [Google Scholar]
- Cumming, D.R.S.; Thoms, S.; Weaver, J.M.R. 3 nm NiCr wires made using electron beam lithography and PMMA resist. Microelectron. Eng. 1996, 30, 423–425. [Google Scholar] [CrossRef]
- Chen, K.; Ren, Q.B.; Cheng, J.M. Structural response analysis of a solid rocket motor with HTPB propellant grain under vertical storage condition. J. Northwest. Polytech. Univ. 2022, 40, 56–61. [Google Scholar] [CrossRef]
- Caveny, L.H.; Kuo, K.; Shackelford, B.W. Thrust and ignition transients of the Space Shuttle solid rocket motor. J. Spacecr. Rocket. 1980, 17, 489–494. [Google Scholar] [CrossRef]
- Iwano, K.; Hashiba, K.; Nagae, J. Reduction of tunnel blasting induced ground vibrations using advanced electronic detonators. Tunn. Undergr. Space Technol. 2020, 105, 103556. [Google Scholar] [CrossRef]
- Shen, Q.F. Properties and applications of reactive PdRu-Al bimetallic fuses. Rare Met. Cem. Carbides 1980, 3, 14–16. [Google Scholar]
- Mcalister, A.J. The Al-Pd (Aluminum-Palladium) system. Bull. Alloy. Phase Diagr. 1986, 7, 368–374. [Google Scholar] [CrossRef]
- Ramos, A.S.; Vieira, M.T. Intermetallic compound formation in Pd/Al multilayer thin films. Intermetallics 2012, 25, 70–74. [Google Scholar] [CrossRef]
- Li, M.; Li, C.; Wang, F. Thermodynamic assessment of the Al–Pd system. Intermetallics 2006, 14, 39–46. [Google Scholar] [CrossRef]
- Guo, J.Y. Study of PdRu5-AlMg5 composite wire diffusion layers and ignition generators. Rare Met. Mater. Eng. 1982, 4, 29–37+120–122. [Google Scholar]
- Howard, J.K. Kinetics of compound formation in thin film couples of Al and transition metals. Vacuum 1976, 26, 68–71. [Google Scholar] [CrossRef]
- Köster, U.; Ho, P.S.; Ron, M. Thermal reactions between aluminum and palladium layered films. Thin Solid Films 1980, 67, 35–44. [Google Scholar] [CrossRef]
- Hung, L.S.; Nastasi, M.; Gyulai, J.; Mayer, J.W. Ioninduced amorphous and crystalline phase formation in Al/Ni, Al/Pd, and Al/Pt thin films. Appl. Phys. Lett. 1983, 42, 672. [Google Scholar] [CrossRef]
- Peng, X.K.; Heness, G.; Yeung, W.Y. Effect of rolling temperature on interface and bond strength development of roll bonded copper/aluminum metal laminates. J. Mater. Sci. 1999, 34, 277–281. [Google Scholar] [CrossRef]
- Peng, X.K.; Wuhrer, R.; Heness, G.; Yeung, W.Y. Rolling strain effects on the interlaminar properties of roll bonded copper/aluminum metal laminates. J. Mater. Sci. 2000, 35, 4357–4363. [Google Scholar] [CrossRef]
- Zhang, H.A.; Chen, G. Fabrication of Cu/Al compound material by solid-liquid bonding method and interface bonding mechanism. Chin. J. Nonferrous Met. 2008, 18, 414–419. [Google Scholar]
- Lee, W.B.; Bang, K.S.; Jung, S.B. Effects of intermetallic compound on the electrical and mechanical properties of friction welded Cu/Al bimetallic joints during annealing. J. Alloys Compd. 2005, 390, 212–219. [Google Scholar] [CrossRef]
- Hug, E.; Bellido, N. Brittleness study of intermetallic (Cu, Al) layers in copper-clad aluminium thin wires. Mater. Sci. Eng. A 2011, 528, 7103–7106. [Google Scholar] [CrossRef]
- Wang, Q.N.; Liu, X.H.; Liu, X.F. Study on Annealing process and microstructure properties of cold drawn copper clad Aluminum wire. Acta Metall. Sin. 2008, 44, 30–35. [Google Scholar]
- Abbasi, M.; Taheri, A.K.; Salehi, M.T. Growth rate of intermetallic compounds in Al/Cu bimetal produced by cold roll welding process. J. Alloys Compd. 2001, 319, 233–241. [Google Scholar] [CrossRef]
- Moisy, F.; Gueydan, A.; Sauvage, X.; Kaller, C.; Guilmeau, U. Influence of intermetallic compounds on the electrical resistivity of architectured copper clad aluminum composites elaborated by a restacking drawing method. Mater. Des. 2018, 155, 366–374. [Google Scholar] [CrossRef]


| T(°C) | Time(s) | |
|---|---|---|
| 5 | 10,800 | |
| 200 | Al3Pd2 | Al3Pd2, Al21Pd8 |
| 250 | Al3Pd2 | Al3Pd2, Al21Pd8 |
| 300 | Al3Pd2 | Al3Pd2 |
| 350 | Al3Pd5 | Al3Pd2, AlPd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gui, J.; Yang, Z.; Yin, X.; Xie, H.; Peng, L.; Zhang, W.; Mi, X. Effect of Annealing on the Interface and Properties of Pd/Al Composite Wires. Materials 2023, 16, 1545. https://doi.org/10.3390/ma16041545
Gui J, Yang Z, Yin X, Xie H, Peng L, Zhang W, Mi X. Effect of Annealing on the Interface and Properties of Pd/Al Composite Wires. Materials. 2023; 16(4):1545. https://doi.org/10.3390/ma16041545
Chicago/Turabian StyleGui, Jiabin, Zhen Yang, Xiangqian Yin, Haofeng Xie, Lijun Peng, Wenjing Zhang, and Xujun Mi. 2023. "Effect of Annealing on the Interface and Properties of Pd/Al Composite Wires" Materials 16, no. 4: 1545. https://doi.org/10.3390/ma16041545
APA StyleGui, J., Yang, Z., Yin, X., Xie, H., Peng, L., Zhang, W., & Mi, X. (2023). Effect of Annealing on the Interface and Properties of Pd/Al Composite Wires. Materials, 16(4), 1545. https://doi.org/10.3390/ma16041545






