Abstract
Due to its high hydrogen storage efficiency and safety, Mg/MgH2 stands out from many solid hydrogen storage materials and is considered as one of the most promising solid hydrogen storage materials. However, thermodynamic/kinetic deficiencies of the performance of Mg/MgH2 limit its practical applications for which a series of improvements have been carried out by scholars. This paper summarizes, analyzes and organizes the current research status of the hydrogen storage performance of Mg/MgH2 and its improvement measures, discusses in detail the hot studies on improving the hydrogen storage performance of Mg/MgH2 (improvement measures, such as alloying treatment, nano-treatment and catalyst doping), and focuses on the discussion and in-depth analysis of the catalytic effects and mechanisms of various metal-based catalysts on the kinetic and cyclic performance of Mg/MgH2. Finally, the challenges and opportunities faced by Mg/MgH2 are discussed, and strategies to improve its hydrogen storage performance are proposed to provide ideas and help for the next research in Mg/MgH2 and the whole field of hydrogen storage.
1. Introduction
Currently, hydrogen storage technologies can be divided into two categories: physical storage and chemical storage. The former includes liquefied hydrogen storage, compressed gas storage, cryo-compression and hydrogen storage by solid-state physisorption materials. The latter includes hydrogen storage by solid or liquid state chemisorption materials [1,2,3]. Among all hydrogen storage technologies, solid-state hydrogen storage technology has received a lot of attention, because it not only offers high safety but also high hydrogen storage density [4]. In the past, scholars have studied a variety of solid-state hydrogen storage materials, including physisorption materials, such as carbon-based materials, metal organic framework (MOFs) and zeolites, as well as chemisorption materials, such as hydrogen storage alloys, complex metal hydrides and lightweight binary metal hydrides. The U.S. Department of Energy (DOE) specifies that the on-board hydrogen storage system should have a hydrogen storage capacity of 5.5 wt.% at a hydrogen pressure of 5–12 bar and a temperature of about 85 °C, and the number of cycles of hydrogen storage materials should not be less than 1000 [5,6]. Figure 1 shows not only the different hydrogen storage technologies and their hydrogen storage capacities but also DOE’s goals for hydrogen storage systems for comparison [7]. In general, volumetric density (kg H2/m3) and gravimetric H2 density (wt.%) are commonly used to measure the hydrogen storage capacity of a hydrogen storage system, the former being the mass of hydrogen stored per unit volume of the system, and the latter being the ratio of the mass of hydrogen stored in the system to the mass of the system. However, no hydrogen storage material has been found so far that meets all the requirements proposed by DOE. Therefore, the research of high-capacity and high-performance hydrogen storage materials is the key and the most challenging aspect of the large-scale application of solid-state hydrogen storage technology.
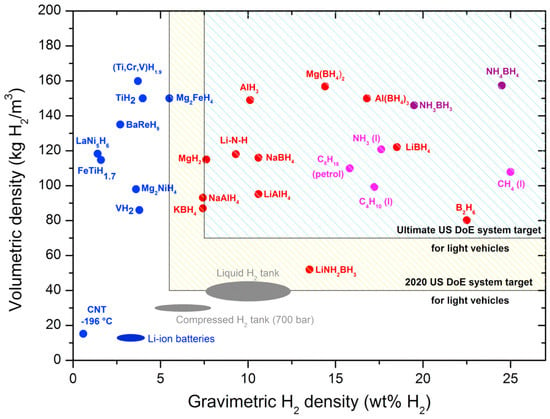
Figure 1.
Overview of different hydrogen storage systems and their volumetric and gravimetric hydrogen density. The U.S. Department of Energy targets for the hydrogen storage system are also shown for comparison. Copyright 2017, Elsevier. Reproduced with permission from [7].
Physisorption materials, such as carbon materials, MOFs and zeolites, rely on weak Vander Waals forces, electrostatic, orbital interactions and other weaker effects for hydrogen adsorption [8,9]. Although the gravimetric H2 density (wt.%) DOE requirements are met by some materials, such as activated carbon (~9.0 wt.%) [10,11], microporous porous carbon (~11.2 wt.%) [12] and MOFs (~10.6 wt.%) [13], these materials are difficult to be widely used because of the low binding energy (4–10 kJ/mol) between hydrogen molecules and the material, which leads to the limitation of the hydrogen storage capacity at room temperature and makes it difficult to hold the hydrogen to the surface [3]. Although modification of such hydrogen storage materials with metals can enhance the binding energy, it may tend to create discrete metallic nanoclusters in the materials, thereby hindering their hydrogen storage capacity [2,14,15].
In contrast, chemisorption materials, such as metal hydrides, have strong binding energy (40–80 kJ/mol) between hydrogen atoms and the material, which helps hydrogen storage materials to store hydrogen under ambient conditions [3]. The hydrogen storage capacity, thermodynamic/kinetic performance and cycling performance of metal hydrides (e.g., hydrogen storage alloys, complex metal hydrides, light binary metal hydrides, etc.) have received a lot of attention.
There are abundant types of hydrogen storage alloys, including AB5, AB, AB2, A2B, AB3 alloys, etc. In fact, catalyst doping and partial substitution of A or B by metallic elements are widely used to improve the hydrogen storage performance of hydrogen storage alloys. As early as 1976, Bronoel et al. [16] reported the hydrogen storage capacity of LaNi5 of type AB5. Later studies showed that LaNi5 had potential exploitability [17,18,19]. Singh et al. [18] found that the doping of graphite enhanced the hydrogen storage performance of LaNi5, especially the reversibility. In addition to the introduction of catalysts, Dashbabu et al. [19] found some improvement in the hydrogen storage performance of LaNi5 after partial substitution of Ni by Al, and the degree of modification was influenced by the amount of aluminum added. More efforts are needed to enhance the performance of hydrogen storage alloys and make them applicable to a wider range of fields.
Similar to hydrogen storage alloys, complex metal hydrides are also a “family”, which includes alanates, borohydrides, nitrides, etc. [2,20,21]. Complex metal hydrides have considerable hydrogen storage capacity, such as LiBH4 with a theoretical hydrogen storage capacity of 18.4 wt.% [22]. Nevertheless, the insufficient thermodynamic/kinetic performance and irreversibility of such hydrogen storage materials limit their practical applications [23,24,25]. In response, scholars have taken measures, such as catalyst doping, to improve them. Different catalysts have emerged, including metal-based catalysts [26,27,28,29,30] and nonmetallic catalysts [31,32]. Recently, Ismail et al. [26] found that the initial dehydrogenation temperature of NaAlH4 was reduced by about 100 °C after the introduction of CoFe2O4. In addition, Li et al. [33] found that composites formed by NaAlH4 with nanoporous material (Raney nickel) reduced the initial dehydrogenation temperature by about 125 °C compared to pure NaAlH4.
MgH2, a lightweight binary metal hydride, has been widely investigated as one of the most promising solid hydrogen storage materials due to its low cost, abundant resources, high hydrogen storage capacity and good reversibility. The reversible hydrogen storage capacity of Mg/MgH2 can reach approximately 7.6 wt.%, which satisfies DOE’s regulations [1,5,6,34]. However, the hydrogen storage performance of Mg/MgH2 has certain drawbacks, such as high thermodynamic stability (enthalpy ~76 kJ/mol and entropy ~130 kJ/mol [35]), slow hydrogen absorption/desorption kinetics and high temperature of hydrogen absorption/desorption, which are the main reasons why Mg/MgH2 is difficult to be used on a large scale. Under atmospheric pressure, the dehydrogenation temperature of MgH2 is over 300 °C; the temperature required for the reaction of magnesium with hydrogen to form MgH2 exceeds 300 °C when the hydrogen pressure exceeds 3 MPa. In addition, the chemical bond of MgH2 (Mg-H) is too stable resulting in an activation energy (Ea) of about 160 kJ/mol for the dehydrogenation reaction of MgH2. Excitingly, due to the good exploitability of Mg/MgH2, scholars have been able to significantly improve its hydrogen storage performance through a series of measures, mainly including alloying treatment, nano-treatment and catalyst doping [34,35,36,37]. Alloying treatment and nanosizing treatment can effectively reduce the thermodynamic stability of Mg/MgH2, while both have very limited contribution to the kinetic performance of Mg/MgH2. Catalyst doping, with its relatively powerful modification ability, is the best option used to compensate for the lack of kinetic performance of Mg/MgH2 and, therefore, has received wide attention as one of the most convenient and feasible methods to improve the kinetics of Mg/MgH2.
In this paper, alloying treatment, nano-treatment and catalyst doping measures to improve the hydrogen storage performance of Mg/MgH2 are discussed in detail, and the catalytic performance and mechanism of various metal-based catalysts are discussed and analyzed in depth. Finally, the challenges and opportunities faced by Mg/MgH2 are discussed, and strategies to improve its hydrogen storage performance are proposed to provide ideas and help for the next research in Mg/MgH2 and the whole field of hydrogen storage.
2. Improvement of Hydrogen Storage Performance of Mg/MgH2 by Alloying Treatment
It is well known that the high stability of Mg/MgH2 originates from the bond strength between the Mg-H bonds, which can be effectively mitigated by alloying treatments, due to the structural and compositional adjustments that destabilize Mg/MgH2 [38]. In general, the alloying treatment means that additional metallic elements are used to form new alloys with Mg elements. The alloying treatment based on Mg/MgH2 and its improvement of hydrogen storage performance are summarized in Table 1.

Table 1.
Improvement of hydrogen storage performance of Mg/MgH2 by alloying treatment.
Alloying treatment, not only for the modification of alloying materials, is also helpful for the modification of Mg/MgH2. Back in 2011, Mg0.95In0.05 solid solution, was found to have a smaller enthalpy of hydrogen absorption compared to pure Mg, which was about 68.1 kJ/mol [47]. Similarly, later, alloy materials, such as 0.75Mg-0.25Ti and 0.75Mg-0.25V were also found to have reduced enthalpy of hydrogen absorption/dehydrogenation of Mg/MgH2 [39]. Recently, Lu et al. [48] explored the hydrogen storage performance of MgxMn1−x and found that the introduction of Mn elements destabilized Mg/MgH2. At higher Mn content, the thermodynamic destabilization of Mg/MgH2 makes it reversible for hydrogen absorption and dehydrogenation even at room temperature. Furthermore, Khan et al. [40] found that the Mg2Ni alloy had a low enthalpy of hydrogen absorption (−57.47 kJ/mol) and dehydrogenation (61.26 kJ/mol). The thermodynamic performance of the Mg/MgH2 was improved; however, there was a considerable loss of hydrogen storage capacity, with a maximum storage capacity of only about 3.44 wt.%. As early as the 1980s, Mg2Ni and Mg2Cu have been documented [49,50]. Not only that, many metallic elements were found to form different types of alloys with Mg. Recent studies have shown that metals such as Al [41], Ce [42], Pr [43] and Gd [44] could also be alloyed with Mg and the resulting alloys possessed good hydrogen storage performance.
Passing et al. [41] found that Mg-Al alloys possessed good thermodynamic/kinetic performance and at the same time, the alloy could store about 5.8 wt.% of hydrogen. However, Mg90Ce3Ni7 alloy [42], Pr-Mg-Ni-based alloy [43] and Gd5Mg95-xNix type alloy [44] did not exhibit good hydrogen storage capacity. Notably, the hydrogen absorption and desorption performance of these hydrogen storage materials were enhanced due to the addition of different metallic elements. Song et al. [42] found that Mg90Ce3Ni7 alloy had good hydrogen absorption performance at low temperature. The alloy could absorb more than 3.5 wt.% H2 within 30 min at 100 °C. In addition, Bu et al. [43] found that the hydrogen diffusion capacity of Pr-Mg-Ni-based alloys increased with the increase of Ni content. However, at 200 °C the hydrogen absorption capacity of the alloys decreased from 5.41 wt.% of Pr5Mg90Ni5 to 4.49 wt.% of Pr5Mg80Ni15 within 60 min. Bu’s team [44] also found that the hydrogen absorption capacity of Gd5Mg95-xNix-type alloys decreased with the increase of Ni content, while the hydrogen absorption rate was affected by temperature. Among them, Gd5Mg80Ni15 had the fastest dehydrogenation rate and smaller dehydrogenation activation energy. In addition, the thermomechanical performance of the alloy due to the addition of Gd and the variation of Ni content was superior compared to those of pure Mg. It may not be a coincidence that the higher hydrogen storage capacity of Mg-Al alloys is due to the fact that Mg-Y-Zn [45] and Mg-Ni-Y [46] alloys were also found to have high hydrogen storage capacity.
These specific examples mentioned above demonstrate that alloying treatment is an effective means used to improve the hydrogen storage performance of Mg/MgH2 in different ways. However, some of these examples also exemplify the very important point that alloying treatments can effectively alleviate the thermodynamic performance of Mg/MgH2 but tend to lead to the sacrifice of some of the considerable hydrogen storage capacity of the hydrogen storage material. Therefore, there is still room for further development of alloying, and in order to achieve better improvement, it is necessary to optimize the alloy preparation technology and control the type and content of doping elements, etc.
3. Improvement of Hydrogen Storage Performance of Mg/MgH2 by Nanosizing
Nanosizing treatment means reducing the particle size or the crystallite size of Mg/MgH2 down to the nanometer level. As nanosizing usually allows Mg/MgH2 to have small size and great activity, it therefore enhances its thermodynamic/kinetic performance. The commonly used nanomaterial preparation methods can be divided into direct preparation, which mainly includes high energy milling, gas-phase reaction, chemical reduction, etc., and non-direct preparation, which is to limit the particle or crystallite size of Mg/MgH2 by scaffolding materials. Among them, high energy milling is traditional and simple, which has been favored by many scholars. Examples of successful nanosizing of Mg/MgH2 systems has been summarized in Table 2.

Table 2.
Improvement of hydrogen storage performance of Mg/MgH2 by nanosizing.
Scholars have prepared Mg/MgH2 with different particle or crystallite sizes by ball milling and found that the dehydrogenation temperature decreased as the particle or crystallite size decreased. In 2006, Varin et al. [51] prepared MgH2 with particle sizes in the range of 500–600 nm by ball milling and found that peak hydrogen desorption temperature was effectively reduced by about 40–60 °C (from about 414 °C to about 370 °C). In fact, this is not really a nanoparticle (truly nanometric size is about 100 nm or below), but the study showed that the reduction in particle size contributed to the reduction of the hydrogen desorption temperature of MgH2. Notably, increasing the amount of γ-MgH2 might effectively reduce the hydrogen desorption temperature of MgH2. Although, the simple and convenient ball milling method to prepare nanoscale MgH2 effectively alleviated the barrier of material dehydrogenation, the size of nanomaterials prepared by this method was still not small enough and prone to agglomeration and impurity incorporation [57]. In order to obtain smaller nanoparticles or crystallites, Zhang et al. [58] prepared Mg particles of about 40 nm using (gas-phase reaction method) an acetylene plasma metal reaction. It was shown that the treated nanomaterials possessed low activation energies for hydrogen absorption and dehydrogenation, 61.6 kJ /mol and 114 kJ/ mol, respectively. In the same year, Norberg et al. [53] prepared three different sizes of Mg nanocrystals using a chemical reduction method. It was shown that the kinetics of hydrogen absorption increased with decreasing size of the nanocrystals. This huge rate enhancement was not only due to the reduction in particle size but was likely due to an increase in the defect density present in smaller nanocrystals. Later studies focused on reducing the particle or crystallite size, in which Liu et al. [58] found that Mg/MgH2 particles with sizes of about 8 nm and 25 nm, respectively, prepared by the same chemical reduction method had better kinetic/thermodynamic performance. It is noteworthy that the nanomaterials prepared by the above method are prone to particle aggregation and lack cyclic stability [59]. Therefore, both smaller size nanomaterials and the control of the particle aggregation phenomenon should be of concern, so attaching nanomaterials to a suitable carrier is a good option.
In order to obtain Mg/MgH2 with smaller particle or crystallite size and better hydrogen absorption/dehydrogenation cycling stability, the size of Mg/MgH2 can be limited to the nanometer level by inert support materials. In recent years, scaffold materials, such as mesoporous materials, carbon nanotubes and porous carbon materials, have received a lot of attention. As early as 2007, Jongh et al. [60] used porous carbon materials for the first time to constrain magnesium melt so as to prepare nanomaterials. This study prepared magnesium nanocrystals in the range of 2–5 nm or even smaller by controlling the pore size of the carbon material. In a later study, Au et al. [54] used carbon aerogels to load MgH2 nanoparticles in the range of 6–20 nm. It was observed that the carbon material as a carrier effectively inhibited the growth of nanoparticles, thus promoting the cycling performance of the nanomaterials. An experiment by Liu et al. [55] on nanoscale MgH2 encapsulated with carbon nanotubes demonstrated again the good binding of the scaffold material to MgH2, which was prepared by the route shown in Figure 2. The study showed that the hydrogen desorption rate of carbon nanotube-supported nano-MgH2 at 275 °C and within 1 h was rapid, and the dehydrogenation amount reached 5.70 wt.%. In addition, the carbon material as a carrier effectively improved the cycling performance of MgH2. Recently, Zhang et al. [56] explored the hydrogen storage performance of MgH2 supported by different carbon materials, including coconut shell carbon (CSC), multi-walled carbon nanotubes (CNT), graphite (G) and activated carbon (AC). It was shown that all these different types of carbon materials led to different degrees of improvement in the kinetic performance of dehydrogenation/rehydrogenation of Mg/MgH2. Among them, CSC as a template can effectively inhibit the growth and agglomeration of MgH2. In addition, the layered structure of carbon materials helps to maintain the high specific surface area and high dispersion of nano-Mg/MgH2, which leads to the continuous improvement of its hydrogen absorption and desorption performance.
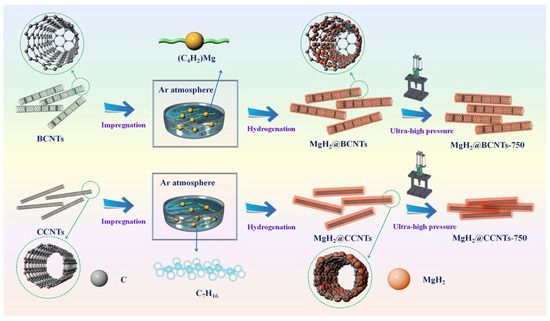
Figure 2.
Schematic illustration of the self-assembly of MgH2 NPs on the BCNTs and CCNTs by impregnation and hydrogenation and densification under ultra-high pressure of 750 MPa. Copyright 2019, Elsevier. Reproduced with permission from [55].
Nanosizing techniques, which improve the thermodynamic/kinetic performance of Mg/MgH2 by reducing its particle or crystallite size, are still limited for the improvement of kinetic and cycling performance, and there is still room for development.
4. Improvement of Hydrogen Storage Performance of Mg/MgH2 by Catalyst Doping
Catalyst doping can effectively alleviate the dehydrogenation/rehydrogenation energy barrier of Mg/MgH2 and improve the kinetic performance of Mg/MgH2. A large number of different types of catalysts have been investigated by scholars. It is noteworthy that many nanocatalysts have better catalytic effects because they provide more active catalytic sites and closer contact with Mg/MgH2.
In the late 1990s, some scholars used transition metals to improve the kinetics of MgH2. In 1999, Liang et al. [61] found that MgH2 started to dehydrogenate at a temperature of 200 °C due to the introduction of 5 at.%V, followed by rehydrogenation at a high rate at room temperature. In the same year, Liang’s group [62] also found that Ti, V, Mn, Fe and Ni metal catalysts all showed good catalytic performance for the hydrogen absorption and desorption process of Mg/MgH2. Among them, V showed the most significant catalytic effect on the rate of dehydrogenation of MgH2, while Ti was the most effective in promoting its hydrogen absorption kinetics. In addition, the activation energy of MgH2 dehydrogenation was significantly decreased by the nanosized catalyst. Later, the good catalytic effect of metallic elements, such as Ni, Ti and Fe for MgH2 was investigated again [63]. Moreover, this study also showed that the effect of Ti, Fe and Ni co-catalysis would be better, which resulted in a 35.71 kJ/mol decrease in the dehydrogenation activation energy of MgH2.
In the next chapters, based on several widely used metal elements, the catalytic performance and mechanism of these metal-based catalysts are discussed and analyzed in detail. Monometals, metal alloys, compounds and metal-based composites formed by transition metal elements, such as nickel (Ni), iron (Fe), titanium (Ti), vanadium (V) and manganese (Mn) in the past ten years, are mainly discussed and analyzed, organized and summarized in Tables 3–7. Among them, the composites are dominated by carbon-supported metal-based catalysts. In addition, many other metals are also mentioned in these metal-based catalysts, such as niobium (Nb), cobalt (Co), zirconium (Zr), etc.
4.1. Nickel (Ni)-Based Catalysts
For a long time, nickel (Ni)-based catalysts have been studied to improve the hydrogen storage performance of Mg/MgH2; Table 3 summarizes the improvement of the hydrogen storage performance of Mg/MgH2 by nickel-based catalysts in the past decade.

Table 3.
Hydrogen storage performance of Mg/MgH2 system doped with nickel-based catalysts.
In 2013, it was shown that Ni doping not only improved the dehydrogenation ability of MgH2 but also enhanced its rehydrogenation kinetics [63]. Later, El-Eskandarany et al. [78] found that the ball milling time affected the catalytic effect. At 50 °C within 300 min, the sample milled for 25 h absorbed about 3.9 wt.% of H2, while the sample milled for 50 h absorbed only 3.6 wt.% of H2. Compared to the former, the considerable decrease in hydrogen storage capacity of the latter was due to its higher Ni concentration. Recently, Yang et al. [64] demonstrated that the flaky nickel nanocatalysts could effectively improve the kinetic performance of MgH2. However, the cycling performance of MgH2 was not enhanced, and there was a significant weakening of the hydrogen storage capacity within 10 cycles. It is worth mentioning that the formation of Mg2Ni/Mg2NiH4 on the surface of Mg/MgH2 in the hydrogen absorption and dehydrogenation reaction is an important factor in promoting the kinetics, as shown in Figure 3. Later, Dan et al. [79] introduced 2–6 nm nickel nanoparticles into MgH2. It was shown that the effective improvement in the kinetic performance of MgH2 was attributed to the in situ formation of reactive species, such as Mg2NiH0.3 during the hydrogen absorption and dehydrogenation process. Various reactive substances (e.g., Mg2Ni/Mg2NiH4, Mg2NiH0.3) formed in situ between metallic nickel and Mg/MgH2 during hydrogen absorption and dehydrogenation play an important role in the hydrogen storage performance of Mg/MgH2.
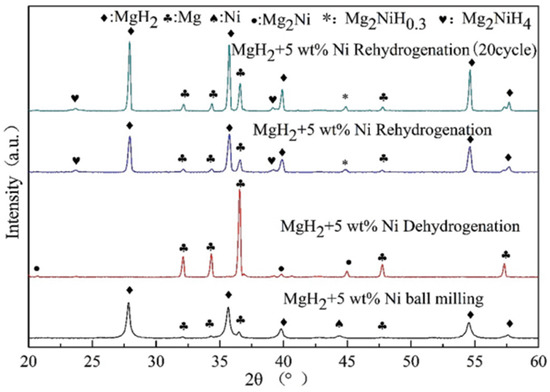
Figure 3.
XRD patterns after ball milling, the hydrogen dehydrogenation, rehydrogenation and after the 20th cycle for MgH2 + 5 wt.% Ni. Copyright 2021, The Royal Society of Chemistry. Reproduced with permission from [64].
On the basis of metallic nickel, scholars have developed nickel-based alloy catalysts. In 2014, Motavalli and Rajabi [80] prepared two different forms of Ni3FeMn, a cast alloy and a melt-spun powder, respectively. It was shown that the latter, i.e., melt-spun powder, was harder than the former and had better catalytic effect. Later, El-Eskandarany’s group [65,66,81] successively found that the introduction of Zr2Ni and ZrNi5 led to a significant enhancement of the kinetics and cycling performance of MgH2. At a temperature of 250 °C, MgH2-Zr2Ni could cycle 2546 hydrogen absorption/dehydrogenation experiments within 1250 h [65]; the MgH2-ZrNi5 composite system maintained high hydrogen storage performance after 600 cycles within 568 h at 275 °C [66]. In recent years, Ding et al. [67] prepared MgCCo1.5Ni1.5 by combining various catalytic elements and then introduced it into MgH2. It was shown that the alloy material effectively enhanced the kinetic performance of the Mg/MgH2 system. Recently, Zhang et al. [68,69] studied a series of Ni-based solid solutions, including Ni-Cu, Ni-Fe and Ni-Co. It was shown that all these Ni-based solid solutions effectively improved the kinetic performance of MgH2. Among them, Ni-50%Cu had a better catalytic effect on the dehydrogenation of MgH2 than Ni-25%Cu, Ni-75%Cu, Cu and Ni. More importantly, NiCu was uniformly distributed on the surface of MgH2, which enhanced the activity of MgH2 and also limited the growth of MgH2 particles [68]. Moreover, among Ni-25%X (X = Fe, Co, Cu), Ni-25%Co showed the most excellent catalytic effect, and the dehydrogenated MgH2 could absorb 5.39 wt.% of hydrogen within 3 min at 275 °C. The excellent hydrogen absorption kinetics can be attributed to the catalytic effect of the in situ formed Mg2Ni(Co) [69].
On the basis of metallic nickel, scholars have also developed nickel-based compound catalysts, including NiB [70], NiO [71], NiS [72], Ni3S2 [77], NiMoO4 [73], etc. Liu et al. [70] found that the kinetic performance of 10 wt.% NiB-doped MgH2 was greatly improved, because the doping of the catalyst reduced the barrier and the driving force of nucleation. Later, Zhang et al. [71] conducted a series of experiments on the catalysis of nickel-based compounds, including not only NiO as mentioned above, but also Ni3C, Ni3N, Ni2P, etc. It was shown that all these Ni-based catalysts exhibited good catalytic effects, and the dehydrogenation ability of MgH2-Ni3C was the most remarkable. The introduction of Ni3C allowed MgH2 to reduce the most dehydrogenation temperature and obtain the highest dehydrogenation amount. Xie et al. [72] found that the dehydrogenation activation energy of MgH2 doped with 5 wt.% flower-like NiS particles could be reduced to 64.71 kJ/mol, and the remarkable catalytic performance of NiS can be attributed to the in situ formation of multiphase catalytic substances. Recently, Zeng et al. [77] and Hou et al. [73] reported the catalytic effect of Ni3S2 and NiMoO4 on the hydrogen storage performance of MgH2, respectively. Zeng et al. [77] found that Mg2Ni and MgS were formed during the first dehydrogenation of Ni3S2@C with MgH2; Hou et al. [73] found that NiMoO4 and MgH2 produced Mg2Ni and Mo after the first dehydrogenation reaction. Therefore, their study showed that the catalytic effect of Ni3S2 and NiMoO4 is equally attributed to the multiple active substances produced during the hydrogen absorption and desorption process.
The multiple elements contained in Ni-based alloys and Ni-based compounds usually contribute to the improved kinetic performance of Mg/MgH2; however, the defects of Mg/MgH2 in cyclic performance gradually emerge. Scholars have found that the use of carbon materials to support nickel-based catalysts not only helps to further improve the kinetic performance of Mg/MgH2, but also enhances the stability of Mg/MgH2 during cycling. Recently, Meng et al. [74] introduced an electrospinning-based reduction method to generate nickel nanoparticles in situ in carbon nanofibers and investigated the catalytic effect of this composite (Ni@C) on the hydrogen storage performance of MgH2. Figure 4 shows the brief synthesis steps of Ni@C. It was shown that the nickel nanoparticles were protected from irreversible fusion and aggregation in subsequent high-temperature pyrolysis because of the presence of carbon nanofibers, leading to the excellent kinetic and cycling performance of MgH2. Later, Duan et al. [75] and Hou et al. [76] investigated the catalytic performance of carbon nanotube-supported nickel (CNTs-Ni) and biomass charcoal material-supported nickel (Ni/BC) for MgH2, respectively. The study reconfirmed that the introduction of carbon materials was a better choice for improving the hydrogen storage performance of MgH2. In fact, Zeng et al. [77] and Hou et al. [73] also focused on the catalytic performance of carbon-supported Ni3S2 and NiMoO4, respectively. Zeng et al. [77] found that the introduction of carbon provided additional catalysis for Mg/MgH2 on top of Mg2Ni and MgS. To some extent, the catalytic performance of Ni3S2@C was superior to that of Ni3S2. Hou et al. [73] showed that the introduction of rGO on top of Mg2Ni and Mo effectively inhibited the growth and agglomeration of Mg/MgH2 particles during hydrogen absorption and desorption, thus promoting their cyclic stability. Figure 5 shows the synergistic catalytic mechanism of rGO and NiMoO4 for Mg/MgH2 particles. Carbon-supported nickel-based catalysts not only provide more active catalytic substances to promote the kinetic properties of Mg/MgH2, but also enhance the cycling performance of Mg/MgH2 by the unique properties of carbon materials.
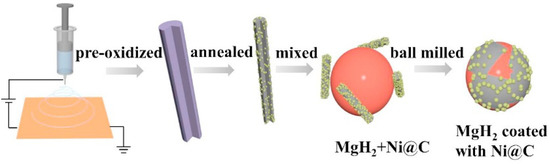
Figure 4.
Schematic illustration for the Ni@C formation process and the synergetic catalytic effect on MgH2. Copyright 2021, Elsevier. Reproduced with permission from [74].
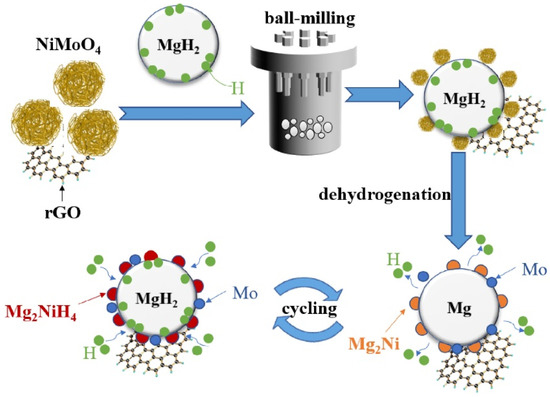
Figure 5.
Schematic diagram of the action mechanism of NiMoO4 and rGO co-catalyzing MgH2 particles. Copyright 2022, Elsevier. Reproduced with permission from [73].
A series of nickel-based catalysts have been systematically investigated to improve the hydrogen storage performance of Mg/MgH2. These Ni-based catalysts all have different degrees of catalytic effects on the hydrogen storage performance of Mg/MgH2, and the following points are worth mentioning: (1) The different active substances (e.g., Mg2Ni/Mg2NiH4, etc.) formed between Mg/MgH2 and nickel-based catalysts during the hydrogen absorption and desorption cycle have important positive effects on the improvement of the hydrogen storage performance of Mg/MgH2. (2) It is difficult to enhance the cycling performance of Mg/MgH2 by a single Ni-based catalyst, which can be effectively compensated by the combination of carbon materials with Ni-based catalysts.
4.2. Iron (Fe)-Based Catalysts
As early as 1999, iron (Fe) catalysts have been documented to improve the hydrogen storage performance of MgH2 [62]. Table 4 summarizes the improvement of the hydrogen storage performance of Mg/MgH2 by Fe-based catalysts in the last decade.

Table 4.
Hydrogen storage performance of Mg/MgH2 system doped with iron-based catalysts.
In 2013, it was shown that Fe-doped Mg/MgH2 had good dehydrogenation/rehydrogenation performance. Mg doped with 5 wt.% Fe could absorb 4.98 wt.% of hydrogen after 15 min of hydrogen absorption at 270 °C, which was second only to the Mg/MgH2 system doped with 5 wt.% Ni [63]. Later, Zhang et al. [82] found that iron nanosheets (Fe NS) led to better dehydrogenation/rehydrogenation kinetics of Mg/MgH2 compared to iron particles. Recently, Song et al. [93] introduced Fe nanocatalysts into MgH2 and found that the operating temperature and activation energy of MgH2 was significantly reduced. However, the growth of grains in Mg/MgH2-Fe composites during the dehydrogenation/rehydrogenation cycle resulted in capacity loss and kinetic degradation. Nano-Fe can better contribute to the improvement of the kinetic performance of MgH2; however, the problem of an unstable cycling performance of MgH2 is unavoidable.
Based on metallic iron, scholars have investigated the catalytic performance of iron-based alloys for MgH2. Santos et al. [83] found that FeNb alloys had good catalytic performance in the hydrogen absorption and desorption process, while pure metals Fe, Nb and Fe+Nb showed better activity than FeNb alloys in terms of hydrogen absorption and desorption kinetics. FeNb is at a disadvantage because of the low chemical interface energy of the nanointerfaces between MgH2/FeNb alloys. It is worth mentioning that the pure metal Fe exhibited significant catalytic performance in this study. In recent years, iron-based alloys, such as Ni3Fe [84,91], FeCoNi [85] and CoFeB [86], have been reported, and all these iron-based catalysts exhibited good catalytic performance that can be attributed to the synergistic catalytic effect of multiple active catalytic substances and Fe formed in situ during the hydrogen absorption and desorption process. The presence of multiple catalytic elements together improve the hydrogen absorption and desorption performance of Mg/MgH2, but the catalytic performance of the Fe-based alloys still needs to be improved in order to promote the cycle performance of Mg/MgH2.
On the basis of metallic iron, scholars have also studied iron-based compound catalysts, such as CoFe2O4 [94] and FeCl3 [87], which showed good catalytic performance. The doping of 7 mol% CoFe2O4 and 5 wt.% FeCl3 led to the reduction of the initial dehydrogenation temperature of MgH2 to 260 °C and 290 °C, respectively. Further studies showed that 10 wt.% Fe has a slight advantage over 10 wt.% FeCl3 in terms of kinetic catalytic performance under the same conditions of the dehydrogenation process in a short time [87]. Later, Gattia et al. [95] confirmed that the adoption of iron oxides (Fe2O3 and Fe3O4), which are less costly and abundant raw materials, as catalysts was also a good choice. Recently, Fu et al. [88] found that MgH2-FeNi2S4 composites had good kinetic performance and the synergistic effect of in situ generated Mg2Ni/Mg2NiH4, MgS and Fe is an important factor to enhance the hydrogen storage performance of Mg/MgH2. Recently, Song et al. [89] found that FeOOH was also a good catalyst, which led to a low operating temperature of Mg/MgH2 and good hydrogen absorption and desorption kinetics. The MgH2-5 wt.% FeOOH composite began to release hydrogen at about 230 °C. In addition, the composite could reversibly absorb 4.4 wt.% hydrogen at 200 °C under a 3.2 MPa hydrogen pressure within 60 min. However, it is difficult for FeOOH to enhance the cycle stability of Mg/MgH2, so the catalytic performance of FeOOH needs to be improved. In addition, in this study Song et al. also provided a report on enhancing the catalytic performance of FeOOH, which will be elaborated later. Taken together, the iron-based compound catalysts exhibit good catalytic performance that can be attributed to the active substances in the process of hydrogen absorption and desorption. However, the cycling performance of Mg/MgH2 is a difficult point that needs to be improved.
To improve the cycling performance of Mg/MgH2, scholars have adopted carbon-supported iron-based catalysts or used special core-shell structures. Liu et al. [84] investigated the catalytic performance of graphene-supported Ni3Fe alloy (Ni3Fe/rGO) and comprehensively compared the catalytic performance of Ni3Fe/rGO, Ni3Fe, Fe/rGO and Ni/rGO. It was shown that the comprehensive catalytic performance of these four catalysts was graded in descending order of Ni3Fe/rGO, Ni/rGO, Ni3Fe and Fe/rGO. Later, catalysts, such as graphene templated FeCoNi (FeCoNi@GS) [85], carbon nanotubes decorated with CoFeB (CoFeB/CNTs) [86] and Fe–Ni catalyst modified three-dimensional graphene (Fe–Ni@3DG) [90], were developed, and the development of such catalysts demonstrated that the introduction of carbon materials could promote the catalytic effect of iron-based catalysts and enhance the cycling performance of Mg/MgH2. Recently, Hou et al. [91] prepared Ni3Fe/BC nanocatalysts by the solid-phase reduction method using low-cost biomass carbon (BC) as a carrier and comprehensively compared the catalytic performance of different catalysts, and the catalyst types and performance are shown in Figure 6 and Figure 7. From the two figures, it can be seen that the comprehensive hydrogen storage performance of MgH2-10 wt.%Ni3Fe/BC is excellent. Notably, the composite could reversibly absorb 5.35 wt.% hydrogen at 150 °C under a 3 MPa hydrogen pressure within 10 min. More importantly, the synergistic catalysis of Mg2Ni/Mg2NiH4 and Fe formed in situ jointly improved the dehydrogenation/rehydrogenation capacity of Mg/MgH2. At the same time, iron could also accelerate the mutual conversion of Mg2Ni/Mg2NiH4 to achieve a double promoting effect.
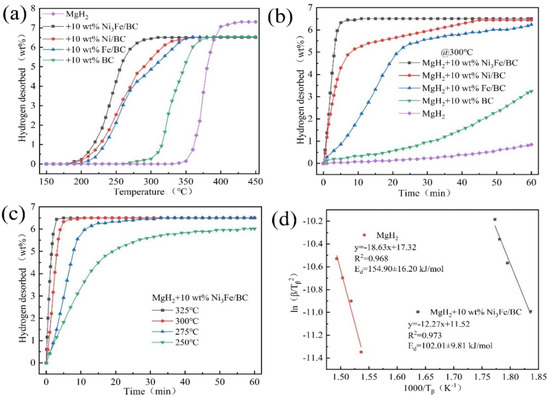
Figure 6.
Rising temperature dehydrogenation (a) and isothermal dehydrogenation (b) curves of pure MgH2 and MgH2 with 10 wt.% of different catalyst samples, isothermal dehydrogenation curves of MgH2 + 10 wt.% Ni3Fe/BC composites at different temperatures (c), and Kissinger plots of MgH2 and MgH2 + 10 wt.% Ni3Fe/BC (d). Copyright 2022, Royal Society of Chemistry. Reproduced with permission from [91].
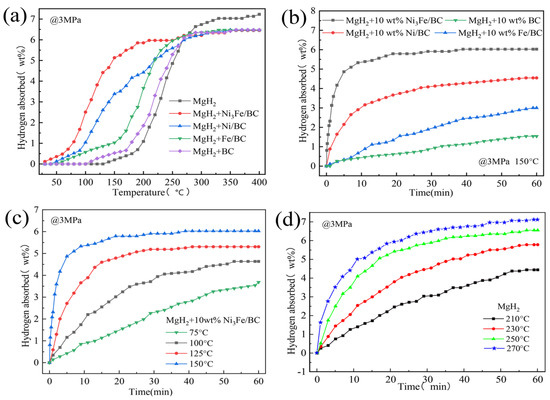
Figure 7.
Isothermal hydrogen uptake curves of pure MgH2 and MgH2 with 10 wt.% of different catalyst samples (a), isothermal hydrogen uptake curves of MgH2 with 10 wt.% of different catalyst samples under 3 MPa H2 pressure at 150 °C (b), isothermal hydrogen uptake curves of MgH2 + 10 wt.% Ni3Fe/BC composite (c) and MgH2 (d) at different temperatures. Copyright 2022, Royal Society of Chemistry. Reproduced with permission from [91].
Based on the FeOOH mentioned above, Song et al. [89] also prepared novel graphene-supported FeOOH nanodots (FeOOH NDs@G) and found that MgH2-10 wt.% FeOOH NDs@G had excellent hydrogen absorption and desorption kinetics. In addition, the hydrogen capacity of the composite exhibited good cycling stability by maintaining 98.5% of the initial capacity after 20 cycles. The catalytic effect of FeOOH NDs@G could be attributed to the synergistic effect between the graphene nanosheets and the in situ formed Fe. The introduction of carbon materials provides a large number of loading sites for MgH2 and catalyst particles, which inhibits their agglomeration and growth, promotes the dissociation and diffusion of hydrogen atoms and ultimately leads to further improvement of MgH2 kinetic performance and enhanced cycling performance. In a recent study, Ren et al. [92] prepared a core-shell catalyst, Ni/Fe3O4@MIL (MIL: metal-organic framework), by wet chemical method, and the morphology and elemental distribution of this structure can be referred to Figure 8. It was shown that this special structure not only provided a suitable reaction site for the catalyst and MgH2, but also inhibited the nanoparticle aggregation and the stability of MgH2 was maintained, which also provided an important idea for the study of enhancing the hydrogen storage performance of MgH2.
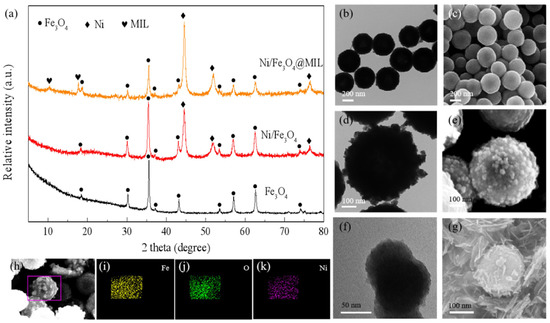
Figure 8.
(a) XRD patterns of the Fe3O4, Ni/Fe3O4 and Ni/Fe3O4@MIL. TEM and SEM images of Fe3O4 (b,c), Ni/Fe3O4 (d,e) and Ni/Fe3O4@MIL (f,g). EDS mapping of the elemental distribution of (i) Fe, (j) O and (k) Ni corresponding to (h) Ni/Fe3O4. Copyright 2022, Elsevier. Reproduced with permission from [92].
A series of iron-based catalysts have been investigated to improve the hydrogen storage performance of Mg/MgH2. However, the doping of Mg/MgH2 with a single iron-based catalyst does not lead to good cycling performance, while the introduction of carbon materials or the synthesis of special structures effectively enhances the cycling stability of Mg/MgH2.
4.3. Titanium (Ti)-Based Catalysts
Back in 1999, there was a successful example of titanium (Ti) catalyzing MgH2 [62]. Table 5 summarizes the improvement of hydrogen storage performance of Mg/MgH2 by titanium-based catalysts in the past decade.

Table 5.
Hydrogen storage performance of Mg/MgH2 system doped with titanium-based catalysts.
In 2013, it was shown that 5 wt.% Ti led to a hydrogen absorption of 4.3 wt.% for Mg after hydrogenation at 270 °C for 15 min, slightly inferior to Mg/MgH2 system doped with the same amount of Ni and Fe catalysts, respectively [63]. Later, both Shahi et al. [96] and Wang et al. [108] found that TiH2 was formed when metal Ti was ball-milled with MgH2, and the catalytic effect of TiH2 promoted the dehydrogenation/rehydrogenation reaction of MgH2. In addition, Wang et al. [108] further found that TiH1.971 was also formed during co-milling of MgH2 with Ti. Active substances, such as TiH2 and TiH1.97, are important factors for the enhancement of the hydrogen storage performance of MgH2. Recently, the above phenomenon was discovered again when Pukazhselvan et al. [109] found that Ti/MgH2 generated TiH2-x after intense mechanical grinding, which was converted to TiH2 in the subsequent hydrogen absorption and desorption reactions. It is not difficult to see that the titanium has a good catalytic effect, which can be attributed to the active substances TiH1.971 and TiH2 produced by Ti and MgH2 in a series of reactions.
On the basis of titanium metal, titanium-based alloy catalysts were used to improve the hydrogen storage performance of Mg/MgH2. Ren et al. [110] found that TiMn2 could effectively improve the hydrogen storage performance of MgH2, and no phase change was found in the catalyst during the experiments. Later, catalysts, such as Ti-Mn-Cr [111] and Ti-Cr-Mn-Fe-V [112], were found to produce finer particles after mechanical alloying and, therefore, had greater activity. In addition, the improved dehydrogenation performance of MgH2 may be related to a more uniform distribution of alloying elements [111,112]. Recently, Hu et al. [98] found that when MgH2−10 wt.%TiMgVNi3 was ball-milled in a hydrogen atmosphere, Ti, Mg, V, and Ni formed corresponding hydrides. In further reactions, highly dispersed (Ti,V)H2 and in situ formed Mg2NiH4 nanoparticles were uniformly distributed on the surface of MgH2 powder, thus MgH2 exhibited excellent kinetic performance. Generally, the polymetallic elements in titanium-based alloys each have a certain catalytic effect, and in the alloying treatment and further reaction, these metal elements can be converted into metals or metal hydrides and other active substances that enhance the hydrogen storage performance of Mg/MgH2.
Based on titanium metal, scholars have also developed various titanium-based compound catalysts. Shahi et al. [96] and Wang et al. [108] also explored the catalytic performance of titanium-based compounds. Shahi et al. [96] found that Ti, TiCl3, TiO2 and TiF3 catalysts all improved the Mg/MgH2 rehydrogenation kinetics, while the catalytic effect of TiF3 was particularly prominent. Nevertheless, the cycling performance of MgH2-TiF3 composites was deficient, for which the introduction of single-walled carbon nanotubes (SWCNTs) effectively remedied this aspect. Later, the catalytic performance of TiF3 was again demonstrated by Wang et al. [108]. Furthermore, by comparing the catalytic performance of TiN, TiO2, Ti and TiF3, Wang’s team found that the comprehensive performance of these catalysts decreased according to TiF3, Ti, TiO2 and TiN. More importantly, TiF3 and MgH2 also formed TiH1.971 and TiH2 during the co-milling process, and similar phenomena also occurred during the decomposition of MgH2-TiO2, while TiN’s performance was too stable to produce active substances.
The TiH1.971 or TiH2 generated by some titanium-based compounds with Mg/MgH2 in the process of ball milling and hydrogen absorption and desorption provided great help to the improvement of Mg/MgH2 hydrogen storage performance. Recently, many scholars have conducted related studies, especially for TiO2, to further explore its catalytic performance and mechanism. Pandey et al. [99] found that nanosized titanium dioxide quantum dots (TiO2:QDs) had good catalytic effects on the hydrogen absorption and desorption performance and cyclic stability of MgH2. In addition, multiple valence states of the Ti were found. As shown in Figure 9, during the dehydrogenation process, Ti4+ was reduced to Ti3+ and Ti3+ was reduced to Ti2+, and in this case, the Mg-H bond was unstable and broken, and electrons flowed from Mg-H to Ti. During the rehydrogenation process, Ti3+ and Ti2+ were converted to Ti4+, and these multiple changes of valence states led to a significant increase in the hydrogen absorption performance of Mg/MgH2. The dehydrogenated Mg/MgH2 system could absorb about 6.10 wt.% hydrogen within 77 s at 280 °C.
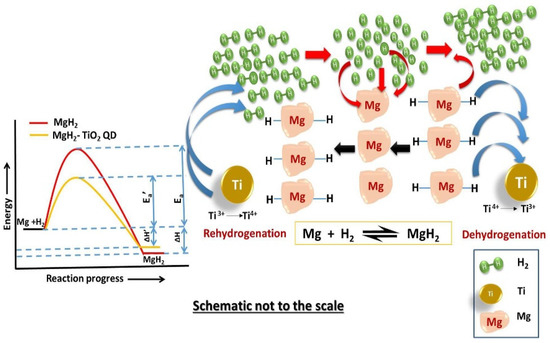
Figure 9.
Schematic diagram depicting multiple valencies of Ti in TiO2:QDs during dehydrogenation and rehydrogenation of MgH2+TiO2:QDs sample. Copyright 2021, Elsevier. Reproduced with permission from [99].
Later, the positive effect of multivalent Ti species in MgH2-TiO2 on the hydrogen storage performance of MgH2 was explored several times. Zou et al. [113] found that the synergistic effect of microwave irradiation and heating contributed to the homogeneous dispersion of defective TiO2−x species around Mg/MgH2 and promoted the reduction of Ti4+ to lower valence states. TiO2−x and multivalent Ti species are catalysts for electron transfer between Mg2+ and H−, thus promoting the diffusion of hydrogen. In the same year, Ren et al. [114] explored the hydrogen storage performance of MgH2/TiO2 heterostructures, and Figure 10 shows the synthesis of two-dimensional TiO2 nanosheets (2D TiO2 NS), the impregnation of MgBu2 and the self-assembly of MgH2 nanoparticles on TiO2 NS. It was shown that the superior hydrogen storage performance of MgH2/TiO2 was attributed to a synergistic effect in two aspects. The high specific surface area of 2D TiO2 NS provided a channel for the rapid diffusion of hydrogen and inhibited the growth and aggregation of MgH2 nanoparticles, thus improving their cyclic stability. On the other hand, the multiphase interface composed of Mg2+ and multivalent Ti species provided more diffusion pathways for hydrogen.
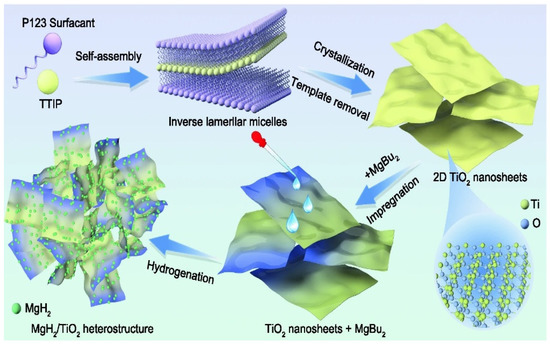
Figure 10.
Synthesis process illustration of the MgH2/TiO2 heterostructure. Copyright 2022, Springer Singapore. Reproduced with permission from [114].
It can be seen that the above titanium-based compounds are good catalysts for the challenge of insufficient hydrogen storage performance of Mg/MgH2. In general, catalysts that provide more titanium-based active substances can better enhance the hydrogen storage performance of Mg/MgH2. TiF3, TiO2 and other titanium-based compounds can generate titanium hydride, TiO2-x and multivalent titanium species with Mg/MgH2, and the synergistic effect of these active substances can enhance the hydrogen storage performance of Mg/MgH2.
In the last five years, a range of new titanium-based compound catalysts (MXenes) have been developed [100,101,102,115,116,117,118]. Shen et al. [100] and Zhang et al. [101] developed (Ti0.5V0.5)3C2 and TiVO3.5, respectively. (Ti0.5V0.5)3C2 not only significantly improved the dehydrogenation/rehydrogenation of Mg/MgH2, but also effectively enhanced its cycling performance [100]. Later TiVO3.5 was synthesized under an oxygen atmosphere at 300 °C by using a solid solution (Ti0.5V0.5)3C2 as a precursor. In terms of hydrogen absorption kinetics, TiVO3.5 possessed better catalytic performance than (Ti0.5V0.5)3C2 [101]. It is worth mentioning that during the ball milling process, both of the above-mentioned catalysts generated the metals Ti and V, thus promoting the dissociation and reorganization of hydrogen molecules.
Since the successive appearance of (Ti0.5V0.5)3C2 and TiVO3.5, a large number of MXenes catalysts have been reported later, such as Ti2C [115], Ti2CT2 [116], Ti3C2 [117] and Ti3C2Tx [102,118], etc. The study of Li et al. [115] showed that two-dimensional Ti2C had a good catalytic effect on the dehydrogenation process of MgH2, as shown in Figure 11, which could be summarized into two points. Ti2C MXene itself had good hydrogen adsorption ability and thermal conductivity. On the other hand, the surface Ti atoms with multivalence served as the intermediate for electrons shifting between H− and Mg2+. Recently, the catalytic effect of Ti2C on MgH2 was demonstrated again. Huang et al. [116] found that this catalytic effect was not only due to the Ti atoms serving as an intermediary for electron transfer between Mg2+ and H−, but also due to the catalytic effect of TiH2 formed in situ at the Ti2C/MgH2 interface, which together promoted the dehydrogenation reaction of MgH2. In addition, Ti2C could enhance the dehydrogenation reaction of MgH2 more effectively than Ti2CT2 (T = O, F, OH). The different surface functional groups in Ti2CT2 also had a significant effect on the dehydrogenation performance of MgH2, and Ti2C(OH)2 had a better catalytic performance than Ti2CF2.
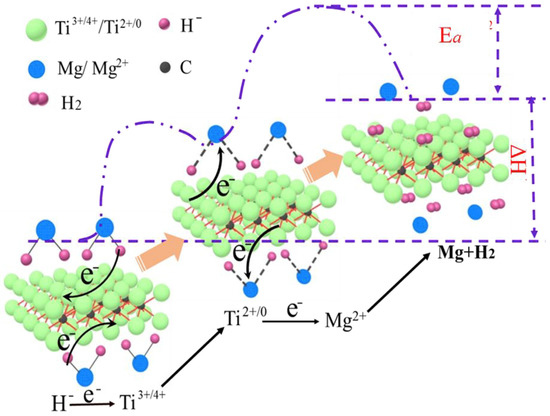
Figure 11.
Schematic of the mechanism of Ti2C in catalyzing the dehydrogenation of MgH2. Copyright 2019, Elsevier. Reproduced with permission from [115].
Recently, Wu et al. [117] synthesized a composite hydrogen storage system of MgH2 with multilayer Ti3C2 (ML-Ti3C2). It was shown that the electron transfer generated by Ti and multivalent Ti in this hydrogen storage system promoted the dissociation or recombination of hydrogen molecules. Later, Gao et al. [102] prepared Ti3C2Tx catalysts in a similar way to the previous (Ti0.5V0.5)3C2 [100]. The accordion-like Ti3C2Tx (F-Ti3C2Tx) was obtained by removing the Al layer in Ti3AlC2 first, and then the paper-like Ti3C2Tx (E-F-Ti3C2Tx) was obtained by ultrasonic stripping and filtration. Finally, the two different morphologies of Ti3C2Tx were introduced into MgH2 separately. Among them, F-Ti3C2Tx has more edge surfaces in contact with MgH2, and E-F-Ti3C2Tx has more base surfaces in contact with MgH2. It was shown that different exposure surfaces were the dominant factors affecting the catalytic activity of Ti3C2Tx, and F-Ti3C2Tx with more exposed edge surfaces showed better catalytic activity promoting more in situ formation of metallic Ti and thus better MgH2 kinetics. In the same year, Gao’s group [118] again reported the catalytic performance of Ti3C2Tx, which was the first report of Ti3C2Tx with different residual Al. It was shown that the residual Al in Ti3C2Tx contributed to its catalytic activity. The unique Ti-Al metal bond could change the electronic structure of Al, which contributed to the desorption and absorption of H atoms. Invariably, multivalent Ti can further enhance the kinetic performance of MgH2.
Among the many titanium-based catalysts, titanium-based MXenes have been widely used to improve the hydrogen storage performance of Mg/MgH2 only in recent years. Titanium-based MXenes have good hydrogen storage capacity and thermal conductivity by themselves. On the other hand, the in situ generation of various active substances is an important factor to promote the dissociation or recombination of hydrogen molecules. Among them, Ti and multivalent Ti are in a very special position, as they are the intermediaries of electron transfer between H− and Mg2+, which are the key factors to enhance the hydrogen storage performance of Mg/MgH2. Therefore, making the in situ formation of more metallic Ti and multivalent Ti species in titanium-based MXenes is the focus of promoting the performance enhancement of Mg/MgH2.
As mentioned previously, carbon nanotube-supported titanium-based catalyst [96] enhanced the kinetic and cycling performance of MgH2. In recent years, carbon- supported titanium-based catalysts have maintained a high degree of heat, including not only carbon-supported alloys, but also carbon-supported compounds, and the carbon materials used include carbon nanotubes (CNTs), graphene (Gr), and so on. Zr0.4Ti0.6Co-CNTs [119], TiFe/CNTs [97] and Ti-Ni-Fe@Gr [103] have excellent catalytic performance: on top of the multi-element co-catalysis of MgH2 by the alloy, the introduction of carbon materials can inhibit the aggregation of nanoparticles, which further enhances not only the kinetics but also the cycling performance. In addition, scholars used different types of carbon materials to support titanium-based compounds, and by this method, catalysts, such as TiH2@Gr [104], TiO2@Gr [104], TiO2@C [105] and TiO2@rGO [120], were prepared. Among them, Verma et al. [104] found that TiH2@Gr was more effective than Ti@Gr and TiO2@Gr for the catalysis of MgH2. The excellent catalytic performance of these carbon-supported titanium-based compound catalysts is mainly due to the addition of carbon materials as co-catalysts based on the catalysis of titanium-based active substances, such as polyvalent titanium, which inhibits the aggregation of nanoparticles, thereby further enhancing the kinetic performance of MgH2 and also its cycling performance.
Recently, Ti-based MXenes, not only as catalysts but also as carriers of various active catalysts (similar to the loading role of carbon materials), have been focused on by scholars with most studies on Ti3C2 and Ti3C2Tx. Gao et al. [106] first removed Al from Ti3AlC2 to obtain Ti3C2 and then reduced Ni nanoparticles to the surface of Ti3C2 matrix by chemical reduction, thereby preparing a sandwich-like Ni/Ti3C2 catalyst, and its synthesis process and morphology are shown in Figure 12. It was shown that the Ni nanoparticles with the smallest size and the best dispersion on the surface of Ti3C2 substrate had the best catalytic activity, and the electronic interactions from the rich interface between Ni and Ti3C2 could greatly improve the hydrogen storage performance of MgH2. In addition, the electron transfer of multivalent Ti and the unique structure of Ni/Ti3C2 were also important factors for the catalytic performance. In the same year, Gao’s group [107] self-assembled TiO2 nanoparticles (M-TiO2) on several layers of Ti3C2Tx (FL-Ti3C2Tx) by a one-step sonication method, which could alleviate the heavy accumulation of FL-Ti3C2Tx and the agglomeration of M-TiO2 nanoparticles, resulting in a large number of interfaces between them. The abundant interface not only serves as a hydrogen diffusion channel, but also the electron transfer at the interface can enhance the catalytic activity of the whole heterogeneous structure. In addition, multivalent Ti can effectively enhance the reversible hydrogen storage performance of MgH2. Recently, Ti3C2 and FL-Ti3C2Tx have also been used to load catalytic substances, such as PrF3 nanoparticles [121] and Ni@C nanosheets [122], respectively. PrF3/Ti3C2 prepared by Wang et al. [121] exhibited excellent catalytic activity for hydrogen storage of MgH2, not only because of the significant enhancement of Ti3C2 MXene by PrF3, but more so because of the facilitation effect of multivalent Ti-species. The Ni@C/FL-Ti3C2Tx prepared by Peng et al. [122] had efficient catalytic performance, not only due to the catalytic performance of FL-Ti3C2Tx itself, but also because of the active substances (Mg2NiH4 and small-size, highly dispersible Ti nanoparticles) formed in situ during the reaction of MgH2-Ni@C/FL-Ti3C2Tx.
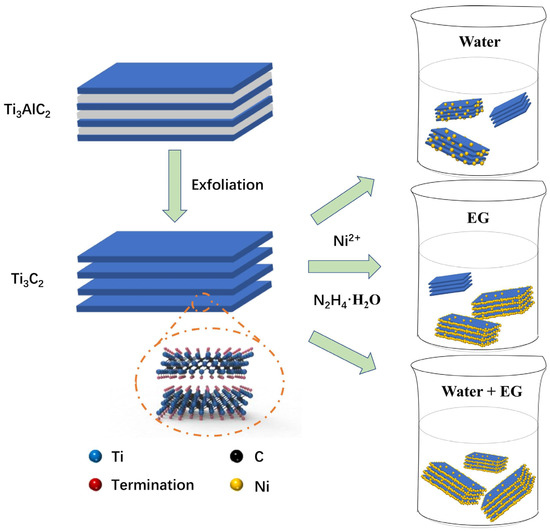
Figure 12.
The synthesis procedures of sandwich-like Ni/Ti3C2 catalysts. Copyright 2021, Elsevier. Reproduced with permission from [106].
Titanium-based MXenes, as carriers of active substances, share similarities with carbon materials both acting as carriers and co-catalysts to improve the kinetic and cycling performance of Mg/MgH2. However, the difference with carbon materials is that instead of carbon, titanium-based MXenes provide highly dispersed Ti and multivalent Ti species during the reaction with Mg/MgH2.
The catalytic performance of titanium-based catalysts has been systematically studied by scholars, and the following points are worth mentioning in the comprehensive development history and trends of titanium-based catalysts: (1) Titanium metal, some titanium-based compounds (TiF3, TiO2, etc.) and titanium-based alloys generate a variety of titanium-based active substances (TiO2−x, TiH1.971 and multivalent titanium species, etc.) or active substances formed by other elements in the reaction with Mg/MgH2 have good catalytic effects on Mg/MgH2. Therefore, the introduction of these titanium-based catalysts, which are easy to generate active substances, is more helpful to enhance the kinetic performance of Mg/MgH2. (2) Carbon-supported titanium-based alloys or carbon-supported titanium-based compounds have further catalytic performance. Based on the original catalyst, the carbon material acts as a co-catalyst, which not only enhances the kinetic performance of Mg/MgH2, but also strengthens its cycling performance. (3) Titanium-based MXenes can be used to significantly improve the hydrogen storage performance of Mg/MgH2, mainly because of two aspects: as catalysts and as carriers of catalytic substances. As catalysts, the in situ generation of a variety of active substances (Ti and multivalent Ti, etc.) from titanium-based MXenes is an important factor in enhancing the kinetics. As catalysts, similar to carbon materials, they can be used to load active substances and act as co-catalysts to improve the kinetics and cycling performance of Mg/MgH2. Unlike carbon materials, titanium-based MXenes provide highly dispersed Ti as well as multivalent titanium during the reaction with Mg/MgH2. Therefore, titanium-based MXenes, which will be one of the main titanium-based catalysts to be studied in the future, have good prospects.
4.4. Vanadium(V)-Based Catalysts
In 1999, the study of vanadium (V) catalyzing MgH2 has been reported [61]. Overall, vanadium possesses a good catalytic effect and can rank well among many metal catalysts [62]. Later, vanadium-based catalysts were gradually investigated, and Table 6 summarizes the improvement of the hydrogen storage performance of Mg/MgH2 by vanadium-based catalysts in the past decade.

Table 6.
Hydrogen storage performance of Mg/MgH2 system doped with vanadium-based catalysts.
In the last decade, da Conceição et al. [123] found that V, VC and VCl3 all showed good catalytic effects, among which, VC and VCl3 better enhanced the kinetic performance of MgH2. It is worth mentioning that MgH2 doped with just 5 wt.% VCl3 exhibited rapid kinetics and good hydrogen capacity. The Mg/MgH2-5 wt.% VCl3 system could absorb about 5.4 wt.% hydrogen within 2.5 min at 300 °C. Thus, VCl3 has a significant catalytic effect and can reduce the use of pure V to reduce the cost. Later, Milošević et al. [124] explored the hydrogen storage performance of MgH2-VO2(B) and found that during hydrogen absorption and desorption, part of VO2(B) was reduced to V at high temperatures and a VH2 phase appeared. The vanadium metal together with multivalent vanadium (VO2/VH2 system) contributed to the kinetic performance of MgH2. Later, many multi-element vanadium-based catalysts were developed, such as the alloy V45Zr20Ni20Cu10Al3Pd2 [125], compounds VB2 [126], V4Nb18O55 [127] and Ni3(VO4)2 [129].
El-Eskandarany et al. [125] investigated the catalytic performance of two different forms of V45Zr20Ni20Cu10Al3Pd2 (intermetallic compound powders and metallic glassy powders) for MgH2. It was shown that the latter, compared to the former, exhibited better catalytic performance for the kinetics of the Mg/MgH2. MgH2 doped with 10 wt.% metallic glassy V45Zr20Ni20Cu10Al3Pd2 could desorb hydrogen about 5.5 wt.% hydrogen within 3 min at 180 °C. Recently, Zang et al. [129] synthesized Ni3(VO4)2 and introduced it into MgH2. It was shown that the hydrogen storage performance of MgH2 performed well based on the dual catalysis of Mg2Ni and V generated by the reaction. In addition, this study identified the intermediate active species NiV2O4 for the first time. Later, Pang et al. [126] found that VB2 nanoparticles with dual catalytic function could significantly enhance the kinetics of MgH2. It was shown that during ball milling and dehydrogenation, VB2 reacted with MgH2 to form V and MgB2 in situ. V acted as the active species, providing the nucleation site and reducing the apparent activation energy, while MgB2 had some hydrogen absorption capacity. Therefore, the synergistic effect of V and MgB2 is an important factor for VB2 to improve the hydrogen storage performance of MgH2. In a recent study, Meng et al. [127] prepared V4Nb18O55 microspheres as a modified catalyst for MgH2. It was shown that the synergistic effect of V5+ and Nb5+ was clear, and V4Nb18O55 composed of V5+ and Nb5+ had a better catalytic effect than V2O5 and Nb2O5. More importantly, the homogeneous construction of the Nb/V interface not only preserved the ability of Nb to weaken the Mg-H bond, but also alleviated the strong adsorption ability of metal Nb on hydrogen atoms resulting in a relative energy barrier of only 0.5 eV for the whole dehydrogenation process of MgH2, which was 0.22 and 0.43 eV lower than that of Nb and V, respectively, as shown in Figure 13. Therefore, V4Nb18O55 effectively plays the advantages of V and Nb elements and significantly improves the hydrogen storage performance of MgH2.
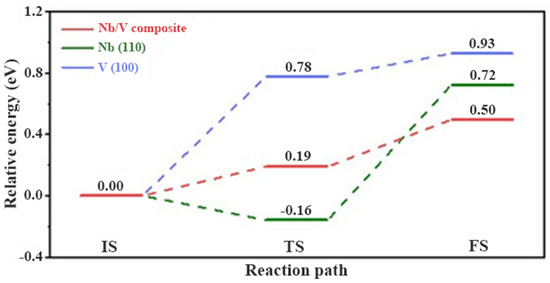
Figure 13.
Calculated energy profiles for the H2 desorption of MgH2 on V (100), Nb (110) and Nb/V composites, respectively. Copyright 2022, Wiley Online Library. Reproduced with permission from [127].
The catalytic effect of multiple elements in the above vanadium-based catalysts effectively improves the hydrogen storage performance of Mg/MgH2, which is better reflected by the carbon-supported vanadium-based catalysts, mainly due to the synergistic effect of the V-based active elements with carbon. Wang et al. [128] synthesized nano-V2O3@C, in which V2O3 nanoparticles were loaded on cubic carbon nanoboxes. It was shown that the doping of 9 wt.% nano-V2O3@C resulted in a significant decrease in the operating temperature and a significant improvement in the kinetic performance of MgH2. It is worth mentioning that V2O3 was reduced to metal V during ball milling and initial heating, and V remained unchanged during hydrogen absorption and desorption, thus promoting the breakage of Mg-H bonds and improvement of kinetic performance.
Many vanadium-based catalysts have been used to improve the hydrogen storage performance of Mg/MgH2; the following points are worth mentioning: (1) Considering the high cost of pure V, vanadium-based compounds or alloys with better catalytic performance can be adopted to reduce the cost. (2) Multi-element vanadium-based alloys or compounds generally have better catalytic performance due to the synergistic catalytic performance of in situ generated V metal, multivalent V and other active substances that can complement each other and together contribute to the kinetic performance of Mg/MgH2. (3) Carbon-supported vanadium-based catalysts can achieve better multi-catalytic effects due to the synergistic effect of vanadium-based active elements with carbon. However, the combination of vanadium-based catalysts with carbon materials has been relatively little studied, and it would be good to try to combine more vanadium-based catalysts with carrier materials, such as carbon materials, in the future development of vanadium-based catalysts.
4.5. Manganese (Mn)-Based Catalyst
In the late 1990s, Liang’s group [62] conducted research on a Mn catalyst. Later, although manganese-based catalysts were studied, they were still relatively rare compared to Ti, Fe and Ni. Table 7 summarizes the improvement of hydrogen storage performance of Mg/MgH2 by manganese-based catalysts in recent years.

Table 7.
Hydrogen storage performance of Mg/MgH2 system doped with manganese-based catalysts.
In recent years, Sun et al. [130] found that doping of 10 wt.% submicron-Mn could achieve good catalytic effect. At this time, MgH2 was rapidly dehydrogenated by 6.6 wt.% within 8 min at 300 °C. At a temperature of 100 °C and a hydrogen pressure of 3 MPa, the fully dehydrogenated MgH2 reabsorbed nearly 3.0 wt.% of hydrogen within 30 min. More importantly, the submicron-Mn-doped MgH2 exhibited good cyclic stability. Recently, Chen et al. [131] found that the initial dehydrogenation temperature of MgH2 was reduced from 355 °C to 175 °C after the introduction of 10 wt.% nano-Mn, which was better than that of 10 wt.% submicron-Mn. Moreover, the dehydrogenated material could absorb hydrogen even at a low temperature of 50 °C. It is worth mentioning that in addition to the kinetic performance, the cyclic performance of MgH2 is also improved.
Based on manganese metal, scholars have developed some manganese-based alloy catalysts. Meena et al. [137] found that MgH2 with the introduction of NiMn9.3Al4.0Co14.1Fe3.6 obtained a lower working temperature. Later, Zhang et al. [132] used LaNi4.5Mn0.5 to improve the hydrogen storage performance of MgH2 and found that LaNi4.5Mn0.5 submicro-particles had a significant catalytic effect. It was shown that this catalyst could enable MgH2 to start dehydrogenation at a lower temperature (175 °C) and enhance its kinetic performance. At 300 °C, MgH2 doped with 10 wt.% submicronLaNi4.5Mn0.5 could desorb 6.6 wt% H2 within 6 min. Moreover, the fully dehydrogenated system could absorb 4.1 wt.% H2 within 10 min at 150 °C. It is worth mentioning that the synergistic effect between the in situ formed Mg2Ni/Mg2NiH4, Mn and LaH3 is the key to enhance the hydrogen storage performance.
Based on manganese metal, scholars have also explored the catalytic performance of manganese-based compounds. Sun’s team [130] found that MnCl2 could reduce the initial dehydrogenation temperature of MgH2 from 315 °C to 225 °C. Later, Zhang et al. [133] found good kinetic performance of MgH2 with the addition of 10 wt.% Mn3O4 nanoparticles. In addition, the cycling stability of MgH2-Mn3O4 was good and its hydrogen storage performance could be well maintained in 20 cycles. More importantly, Mn3O4 with MgH2 was reduced to metal Mn in the reaction, Mg0.9Mn0.1O was also generated along with it and the Mg-H bond was subsequently weakened. Therefore, active substances, such as Mn and Mg0.9Mn0.1O are the key for Mn3O4 to improve the hydrogen storage performance of MgH2. Recently, MnO achieved a catalytic effect similar to that of Mn3O4. Fu et al. [134] found that MgH2-MnO generated Mn and Mg0.9Mn0.1O after the first dehydrogenation, which promoted the breakage of the Mg-H bond and improved the reversible hydrogen storage performance of MgH2. Wang et al. [135] found that MnS could significantly improve the hydrogen absorption and desorption kinetics of Mg/MgH2, and its catalytic effect could be attributed to the in-situ formation of the active substance metal Mn. The catalytic performance of MnMoO4 was demonstrated in a recent study in which Zhang et al. [136] showed that MnMoO4 rod catalysts could effectively improve the hydrogen storage performance of MgH2. In this study, MnMoO4 reduced the initial dehydrogenation temperature of MgH2 and enhanced the kinetic and cyclic performance of MgH2. It is worth mentioning that the synergistic effect of the in situ generated Mn and MgMo2O7 is the key to improve the hydrogen storage performance of MgH2.
In fact, Fu’s team [134] synthesized carbon-supported MnO nanocomposites (MnO@C) on the basis of MnO. It was shown that MgH2-10 wt.%MnO@C had superior reversible hydrogen storage performance compared to MgH2 and MgH2-10 wt.%MnO due to the co-catalytic effect of Mn, Mg0.9Mn0.1O and C during the reaction.
Combining the above manganese-based catalysts, the following points can be derived: (1) Multi-element manganese-based alloys or compounds have good catalytic performance because of the active materials formed by different elements in the process of hydrogen absorption and desorption (such as Mn, Mg0.9Mn0.1O and LaH3, etc.), and their synergistic effect is the key to enhance the hydrogen storage performance of Mg/MgH2. (2) Carbon materials have a certain gain for Mn-based catalysts, and the reversible hydrogen storage performance of Mg/MgH2 can be further improved under the catalytic effect of carbon materials. However, the study of carbon-supported Mn-based catalysts is still relatively small and inconvenient to draw systematic conclusions, which can be extended in this direction to expand more possibilities of Mn-based catalysts.
4.6. Summary of Catalytic Approach
Facing the challenge of insufficient kinetic performance and cycling performance of Mg/MgH2, scholars have studied a large number of metal-based catalysts, including nickel-based, iron-based, titanium-based, vanadium-based and manganese-based catalysts, which have all enhanced the kinetic performance and cycling performance of Mg/MgH2 to different degrees. The following points can be derived from the above studies.
- (1)
- Catalyst particle size, doping amount and ball milling time are important factors to improve the hydrogen storage performance of Mg/MgH2; however, catalyst materials with small particle size, high doping amount and long ball milling time should not be pursued blindly, and the pros and cons need to be weighed otherwise it may be counterproductive.
- (2)
- For the enhancement of the hydrogen storage performance of Mg/MgH2, the active substances (Mg2Ni/Mg2NiH4, Fe, multivalent titanium, V, Mg0.9Mn0.1O, etc.) generated in the reaction between metal-based catalysts and Mg/MgH2 have an important positive impact. Usually, multi-element catalysts have better catalytic effects than single metal catalysts because the synergy between multiple active substances formed by multiple elements can complement each other and together enhance the kinetic performance of Mg/MgH2.
- (3)
- A single metal-based catalyst is certainly excellent, but it is difficult to enhance the cycling performance of Mg/MgH2, while the combination of carbon material and catalyst can effectively limit the particle size and inhibit its growth and agglomeration, thus further enhancing the kinetic and cycling performance of Mg/MgH2. Here, titanium-based MXenes have to be mentioned, which can be used not only as catalysts to improve the kinetic performance of Mg/MgH2, but also as carriers of catalytic substances to improve the kinetic and cyclic performance of Mg/MgH2. Regardless of the use, the catalytic effect of titanium-based MXenes on Mg/MgH2 comes mainly from the in situ generation of a variety of active substances, such as metal Ti and multivalent Ti species. Therefore, titanium-based MXenes are of great significance for the improvement of hydrogen storage performance of Mg/MgH2, which will be one of the main research titanium-based catalysts and materials for supporting active substances in the future.
5. Conclusions and Prospects
For the sake of sustainable development and the development of hydrogen energy, scholars have been working hard to explore and obtain high-efficiency hydrogen storage materials. Among a large number of hydrogen storage materials, Mg/MgH2 stands out because of its unique advantages of high reversible hydrogen storage capacity, high reliability and high exploitability. However, Mg/MgH2 has problems, such as over-stable thermodynamic performance, high temperature of hydrogen desorption and slow kinetic performance. Research on the modification of Mg/MgH2 is advancing at high speed and high quality.
Enhancing the comprehensive hydrogen storage performance of Mg/MgH2 is a long and arduous challenge, and the success of modification measures, such as alloying, nanosizing and catalyst doping, has added much hope to this challenge. Scholars have been able to effectively improve the thermodynamic performance of Mg/MgH2 through alloying and nanosizing, while catalyst doping can largely enhance its kinetic and cyclic performance. In the future, catalyst doping is likely to remain the main improvement technique and alloying and nanosizing can assist it to better improve the hydrogen storage performance of Mg/MgH2. Based on the current hot research, the following points are worth mentioning: (1) In order to ensure that the catalysts can fully play their roles, the morphology, size and other indicators of the catalysts are very important. Based on this, nanoscale catalysts would be a better choice. (2) The active material generated during the reaction between the catalyst and Mg/MgH2 is very important; therefore, a multi-element catalyst that can generate more active material would be a better choice. (3) A single catalyst, which does not necessarily improve the cycling performance of Mg/MgH2, can be assisted by the introduction of carrier materials such as carbon materials to enhance the cycling stability of Mg/MgH2. Based on this, titanium-based MXenes would be an additional good choice, which is not only an excellent catalyst but also a good carrier material. Moreover, if more carrier-based materials can be developed and active substances with excellent catalytic ability can be combined with them, it will help the advancement of research in Mg/MgH2 and even the whole field of hydrogen storage. (4) Last but not least, in order to systematically enhance the hydrogen storage performance of Mg/MgH2 in future studies, on the basis of alloying or nanosizing to improve the thermodynamic performance of Mg/MgH2, catalysts with superior performance can be adopted to substantially enhance the kinetic and cycling performance of Mg/MgH2.
This paper mainly discusses the research progress and trends of hydrogen storage performance of Mg/MgH2 in the past decade and its improvement measures, hoping to provide ideas and help for future research on Mg/MgH2 and even many hydrogen storage materials. It is believed that with continued efforts in the right research direction and a good combination of the advantages of Mg/MgH2 and improvement measures, the production of Mg/MgH2 with superior hydrogen storage performance will not be far away.
Author Contributions
Conceptualization, X.Y., J.Z. and Q.H.; methodology, X.Y. and W.L.; data curation, W.L. and Q.H.; writing—original draft preparation, W.L., X.Y. and J.Z.; writing—review and editing, X.Y., J.Z. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Postgraduate Research & Practice Innovation Program of Jiangsu Province grant number No. KYCX21_3504, No. KYCX 20_3100.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge the Postgraduate Research & Practice Innovation Program of Jiangsu Province (Grant No. KYCX21_3504, Grant No. KYCX 20_3100).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Q.; Lu, Y.; Luo, Q.; Yang, X.; Yang, Y.; Tan, J.; Dong, Z.; Dang, J.; Li, J.; Chen, Y.; et al. Thermodynamics and kinetics of hydriding and dehydriding reactions in Mg-based hydrogen storage materials. J. Magnes. Alloys 2021, 9, 1922–1941. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Hashmi, S.A.R.; Kim, K.-H. MXenes: Emerging 2D materials for hydrogen storage. Nano Energy 2021, 85, 105989. [Google Scholar] [CrossRef]
- Hoang, T.K.A.; Antonelli, D.M. Exploiting the Kubas Interaction in the Design of Hydrogen Storage Materials. Adv. Mater. 2009, 21, 1787–1800. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Ma, H.; Lu, C.; Luo, H.; Wang, X.; Huang, X.; Lan, Z.; Guo, J. Aluminum hydride for solid-state hydrogen storage: Structure, synthesis, thermodynamics, kinetics, and regeneration. J. Energy Chem. 2021, 52, 428–440. [Google Scholar] [CrossRef]
- Satyapal, S.; Petrovic, J.; Read, C.; Thomas, G.; Ordaz, G. The U.S. Department of Energy’s National Hydrogen Storage Project: Progress towards meeting hydrogen-powered vehicle requirements. Catal. Today 2007, 120, 246–256. [Google Scholar] [CrossRef]
- Klebanoff, L.E.; Ott, K.C.; Simpson, L.J.; O’Malley, K.; Stetson, N.T. Accelerating the Understanding and Development of Hydrogen Storage Materials: A Review of the Five-Year Efforts of the Three DOE Hydrogen Storage Materials Centers of Excellence. Metall. Mater. Trans. E 2014, 1, 81–117. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H.-w. Hydrogen—A sustainable energy carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Lochan, R.C.; Head-Gordon, M. Computational studies of molecular hydrogen binding affinities: The role of dispersion forces, electrostatics, and orbital interactions. Phys. Chem. Chem. Phys. 2006, 8, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Klontzas, E.; Tylianakis, E.; Froudakis, G.E. On the Enhancement of Molecular Hydrogen Interactions in Nanoporous Solids for Improved Hydrogen Storage. J. Phys. Chem. Lett. 2011, 2, 1824–1830. [Google Scholar] [CrossRef]
- Romanos, J.; Beckner, M.; Prosniewski, M.; Rash, T.; Lee, M.; Robertson, J.D.; Firlej, L.; Kuchta, B.; Pfeifer, P. Boron-neutron Capture on Activated Carbon for Hydrogen Storage. Sci. Rep. 2019, 9, 2971. [Google Scholar] [CrossRef]
- Blankenship, T.S.; Balahmar, N.; Mokaya, R. Oxygen-rich microporous carbons with exceptional hydrogen storage capacity. Nat. Commun. 2017, 8, 1545. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, L.S.; Mokaya, R. Cigarette butt-derived carbons have ultra-high surface area and unprecedented hydrogen storage capacity. Energy Environ. Sci. 2017, 10, 2552–2562. [Google Scholar] [CrossRef]
- Ahmed, A.; Liu, Y.; Purewal, J.; Tran, L.D.; Wong-Foy, A.G.; Veenstra, M.; Matzger, A.J.; Siegel, D.J. Balancing gravimetric and volumetric hydrogen density in MOFs. Energy Environ. Sci. 2017, 10, 2459–2471. [Google Scholar] [CrossRef]
- Sigal, A.; Rojas, M.I.; Leiva, E.P. Is hydrogen storage possible in metal-doped graphite 2D systems in conditions found on Earth? Phys. Rev. Lett. 2011, 107, 158701. [Google Scholar] [CrossRef]
- Lee, H.; Ihm, J.; Cohen, M.L.; Louie, S.G. Calcium-decorated graphene-based nanostructures for hydrogen storage. Nano Lett. 2010, 10, 793–798. [Google Scholar] [CrossRef]
- Bronoel, G.; Sarradin, J.; Bonnemay, M.; Percheron, A.; Achard, J.C.; Schlapbach, L. A new hydrogen storage electrode. Int. J. Hydrogen Energy 1976, 1, 251–254. [Google Scholar] [CrossRef]
- Georgiadis, M.C.; Kikkinides, E.S.; Makridis, S.S.; Kouramas, K.; Pistikopoulos, E.N. Design and optimization of advanced materials and processes for efficient hydrogen storage. Comput. Chem. Eng. 2009, 33, 1077–1090. [Google Scholar] [CrossRef]
- Singh, U.R.; Bhogilla, S. Performance analysis of LaNi5 added with expanded natural graphite for hydrogen storage system. Int. J. Hydrogen Energy 2022, in press. [CrossRef]
- Dashbabu, D.; Kumar, E.A.; Jain, I.P. Thermodynamic analysis of a metal hydride hydrogen compressor with aluminium substituted LaNi5 hydrides. Int. J. Hydrogen Energy 2022, in press. [CrossRef]
- Rusman, N.A.A.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Z.; Zheng, J.; Li, X.; Akiba, E.; Li, H.-W. Perspectives and challenges of hydrogen storage in solid-state hydrides. Chinese J. Chem. Eng. 2021, 29, 1–12. [Google Scholar] [CrossRef]
- Li, C.; Peng, P.; Zhou, D.W.; Wan, L. Research progress in LiBH4 for hydrogen storage: A review. Int. J. Hydrogen Energy 2011, 36, 14512–14526. [Google Scholar] [CrossRef]
- Ali, N.A.; Sazelee, N.A.; Ismail, M. An overview of reactive hydride composite (RHC) for solid-state hydrogen storage materials. Int. J. Hydrogen Energy 2021, 46, 31674–31698. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Gao, M.; Pan, H. Tailoring Thermodynamics and Kinetics for Hydrogen Storage in Complex Hydrides towards Applications. Chem. Rec. 2016, 16, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Ismail, M.; Ali, N.A.; Sazelee, N.A.; Muhamad, S.U.; Suwarno, S.; Idris, N.H. CoFe2O4 synthesized via a solvothermal method for improved dehydrogenation of NaAlH4. Int. J. Hydrogen Energy 2022, 47, 41320–41328. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, D.; Fan, G.; Chen, Y.; Fan, Y.; Liu, B. N-doped carbon coated Ti3C2 MXene as a high-efficiency catalyst for improving hydrogen storage kinetics and stability of NaAlH4. Renew. Energy 2022, 188, 778–787. [Google Scholar] [CrossRef]
- Bogdanović, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials1Invited paper presented at the International Symposium on Metal–Hydrogen Systems. J. Alloys Compd. 1997, 253–254, 1–9. [Google Scholar] [CrossRef]
- Ahmad, M.A.N.; Sazelee, N.A.; Ali, N.A.; Ismail, M. Enhancing the dehydrogenation properties of LiAlH4 using K2NiF6 as additive. Int. J. Hydrogen Energy 2022, 47, 24843–24851. [Google Scholar] [CrossRef]
- Kytsya, A.; Berezovets, V.; Verbovytskyy, Y.; Bazylyak, L.; Kordan, V.; Zavaliy, I.; Yartys, V.A. Bimetallic Ni-Co nanoparticles as an efficient catalyst of hydrogen generation via hydrolysis of NaBH4. J. Alloys Compd. 2022, 908, 164484. [Google Scholar] [CrossRef]
- Samatya Ölmez, S.; Balbay, A.; Saka, C. Phosphorus doped carbon nanodots particles based on pomegranate peels for highly active dehydrogenation of sodium borohydride in methanol. Int. J. Hydrogen Energy 2022, 47, 31647–31655. [Google Scholar] [CrossRef]
- Ye, J.; Xia, G.; Yu, X. In-situ constructed destabilization reaction of LiBH4 wrapped with graphene toward stable hydrogen storage reversibility. Mater. Today Energy 2021, 22, 100885. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.Z.; Zhang, Y.; Liu, D.M.; Wang, C.Y.; Si, T.Z.; Li, Y.T.; Zhang, Q.A. Coupling of nanoconfinement with metallic catalysis in supported NaAlH4 for low-temperature hydrogen storage. J. Power Sources 2021, 491, 229611. [Google Scholar] [CrossRef]
- Sui, Y.; Yuan, Z.; Zhou, D.; Zhai, T.; Li, X.; Feng, D.; Li, Y.; Zhang, Y. Recent progress of nanotechnology in enhancing hydrogen storage performance of magnesium-based materials: A review. Int. J. Hydrogen Energy 2022, 47, 30546–30566. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Hou, Q.; Guo, X.; Xu, G. Improvement of Mg-Based Hydrogen Storage Materials by Metal Catalysts: Review and Summary. ChemistrySelect 2021, 6, 8809–8829. [Google Scholar] [CrossRef]
- Ding, X.; Chen, R.; Zhang, J.; Cao, W.; Su, Y.; Guo, J. Recent progress on enhancing the hydrogen storage properties of Mg-based materials via fabricating nanostructures: A critical review. J. Alloys Compd. 2022, 897, 163137. [Google Scholar] [CrossRef]
- Hou, Q.; Yang, X.; Zhang, J. Review on Hydrogen Storage Performance of MgH2: Development and Trends. ChemistrySelect 2021, 6, 1589–1606. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Aziz, M.A.A.; Ruhaimi, A.H.; Taib, M.R. Magnesium-based alloys for solid-state hydrogen storage applications: A review. Int. J. Hydrogen Energy 2021, 46, 31067–31083. [Google Scholar] [CrossRef]
- Korablov, D.; Besenbacher, F.; Jensen, T.R. Kinetics and thermodynamics of hydrogenation-dehydrogenation for Mg-25%TM (TM = Ti, Nb or V) composites synthesized by reactive ball milling in hydrogen. Int. J. Hydrogen Energy 2018, 43, 16804–16814. [Google Scholar] [CrossRef]
- Khan, D.; Zou, J.; Zeng, X.; Ding, W. Hydrogen storage properties of nanocrystalline Mg2Ni prepared from compressed 2MgH2Ni powder. Int. J. Hydrogen Energy 2018, 43, 22391–22400. [Google Scholar] [CrossRef]
- Passing, M.; Pistidda, C.; Capurso, G.; Jepsen, J.; Metz, O.; Dornheim, M.; Klassen, T. Development and experimental validation of kinetic models for the hydrogenation/dehydrogenation of Mg/Al based metal waste for energy storage. J. Magnes. Alloys 2022, 10, 2761–2774. [Google Scholar] [CrossRef]
- Song, F.; Yao, J.; Yong, H.; Wang, S.; Xu, X.; Chen, Y.; Zhang, L.; Hu, J. Investigation of ball-milling process on microstructure, thermodynamics and kinetics of Ce–Mg–Ni-based hydrogen storage alloy. Int. J. Hydrogen Energy 2022, in press. [CrossRef]
- Bu, W.; Peng, W.; Liu, Q.; Luo, J.; Dai, X. Hydrogen storage properties of Pr-Mg-Ni- based alloys prepared by vacuum induction melting. Vacuum 2022, 197, 110865. [Google Scholar] [CrossRef]
- Bu, W.; Liu, Q.; Peng, W.; Zhang, T.; Dai, X. Hydrogen storage characteristics, kinetics and thermodynamics of Gd-Mg-Ni-based alloys. Int. J. Hydrogen Energy 2022, in press. [CrossRef]
- Pan, S.X.; Zhang, J.; Zhou, X.J.; Jin, R.S.; He, J.H.; Chen, J.N.; Lu, X.Z.; Chen, X.M. Investigation on hydrogen storage properties of as-cast, extruded and swaged Mg–Y–Zn alloys. Int. J. Hydrogen Energy 2022, 47, 34545–34554. [Google Scholar] [CrossRef]
- Yu, Y.; Ji, Y.; Zhang, S.; Wang, S.; Chen, Y.; yong, H.; Liu, B.; Zhang, Y. Microstructure characteristics, hydrogen storage thermodynamic and kinetic properties of Mg–Ni–Y based hydrogen storage alloys. Int. J. Hydrogen Energy 2022, 47, 27059–27070. [Google Scholar] [CrossRef]
- Zhong, H.C.; Wang, H.; Liu, J.W.; Sun, D.L.; Zhu, M. Altered desorption enthalpy of MgH2 by the reversible formation of Mg(In) solid solution. Scr. Mater. 2011, 65, 285–287. [Google Scholar] [CrossRef]
- Lu, Y.; Asano, K.; Schreuders, H.; Kim, H.; Sakaki, K.; Machida, A.; Watanuki, T.; Dam, B. Nanostructural Perspective for Destabilization of Mg Hydride Using the Immiscible Transition Metal Mn. Inorg. Chem. 2021, 60, 15024–15030. [Google Scholar] [CrossRef]
- Schefer, J.; Fischer, P.; Hälg, W.; Stucki, F.; Schlapbach, L.; Didisheim, J.J.; Yvon, K.; Andresen, A.F. New structure results for hydrides and deuterides of the hydrogen storage material Mg2Ni. J. Less Common Met. 1980, 74, 65–73. [Google Scholar] [CrossRef]
- von Waldkirch, T.; Seiler, A.; Zürcher, P.; Mathieu, H.J. Mg-based hydrogen storage materials: Surface segregation in Mg2Cu and related catalytic effects. Mater. Res. Bull. 1980, 15, 353–362. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T.; Wronski, Z. Particle size, grain size and γ-MgH2 effects on the desorption properties of nanocrystalline commercial magnesium hydride processed by controlled mechanical milling. Nanotechnology 2006, 17, 3856–3865. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, R.; Yang, J.; Zhao, W.; Zheng, J.; Tian, W.; Li, X. Synthesis of magnesium nanoparticles with superior hydrogen storage properties by acetylene plasma metal reaction. Int. J. Hydrogen Energy 2011, 36, 4967–4975. [Google Scholar] [CrossRef]
- Norberg, N.S.; Arthur, T.S.; Fredrick, S.J.; Prieto, A.L. Size-Dependent Hydrogen Storage Properties of Mg Nanocrystals Prepared from Solution. J. Am. Chem. Soc. 2011, 133, 10679–10681. [Google Scholar] [CrossRef]
- Au, Y.S.; Obbink, M.K.; Srinivasan, S.; Magusin, P.C.M.M.; de Jong, K.P.; de Jongh, P.E. The Size Dependence of Hydrogen Mobility and Sorption Kinetics for Carbon-Supported MgH2 Particles. Adv. Funct. Mater. 2014, 24, 3604–3611. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, S.; Xiao, X.; Chen, M.; Sun, C.; Yao, Z.; Hu, Z.; Chen, L. Novel 1D carbon nanotubes uniformly wrapped nanoscale MgH2 for efficient hydrogen storage cycling performances with extreme high gravimetric and volumetric capacities. Nano Energy 2019, 61, 540–549. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Ma, T.; Li, K.; Ye, F.; Wang, X.; Jiao, L.; Yuan, H.; Wang, Y. Facile synthesis of small MgH2 nanoparticles confined in different carbon materials for hydrogen storage. J. Alloys Compd. 2020, 825, 153953. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.F.; Ares Fernandez, J.R.; Klassen, T.; Bormann, R. Using MgO to improve the (de)hydriding properties of magnesium. Mater. Res. Bull. 2006, 41, 1118–1126. [Google Scholar] [CrossRef]
- Liu, W.; Aguey-Zinsou, K.-F. Size effects and hydrogen storage properties of Mg nanoparticles synthesised by an electroless reduction method. J. Mater. Chem. A 2014, 2, 9718–9726. [Google Scholar] [CrossRef]
- Lu, Y.; Li, J.; Ye, T.-N.; Kobayashi, Y.; Sasase, M.; Kitano, M.; Hosono, H. Synthesis of Rare-Earth-Based Metallic Electride Nanoparticles and Their Catalytic Applications to Selective Hydrogenation and Ammonia Synthesis. ACS Catal. 2018, 8, 11054–11058. [Google Scholar] [CrossRef]
- de Jongh, P.E.; Wagemans, R.W.P.; Eggenhuisen, T.M.; Dauvillier, B.S.; Radstake, P.B.; Meeldijk, J.D.; Geus, J.W.; de Jong, K.P. The Preparation of Carbon-Supported Magnesium Nanoparticles using Melt Infiltration. Chem. Mater. 2007, 19, 6052–6057. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Hydrogen storage properties of the mechanically milled MgH2–V nanocomposite. J. Alloys Compd. 1999, 291, 295–299. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2–Tm (Tm=Ti, V, Mn, Fe and Ni) systems. J. Alloys Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- Shahi, R.R.; Tiwari, A.P.; Shaz, M.A.; Srivastava, O.N. Studies on de/rehydrogenation characteristics of nanocrystalline MgH2 co-catalyzed with Ti, Fe and Ni. Int. J. Hydrogen Energy 2013, 38, 2778–2784. [Google Scholar] [CrossRef]
- Yang, X.; Hou, Q.; Yu, L.; Zhang, J. Improvement of the hydrogen storage characteristics of MgH2 with a flake Ni nano-catalyst composite. Dalton Trans. 2021, 50, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- El-Eskandarany, M.S.; Al-Matrouk, H.; Shaban, E.; Al-Duweesh, A. Superior catalytic effect of nanocrystalline big-cube Zr2Ni metastable phase for improving the hydrogen sorption/desorption kinetics and cyclability of MgH2 powders. Energy 2015, 91, 274–282. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Shaban, E.; Al-Matrouk, H.; Behbehani, M.; Alkandary, A.; Aldakheel, F.; Ali, N.; Ahmed, S.A. Structure, morphology and hydrogen storage kinetics of nanocomposite MgH2/10 wt% ZrNi5 powders. Mater. Today Energy 2017, 3, 60–71. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, L.; Fu, Y.; Wang, W.; Wang, Y.; Bi, J.; Li, Y.; Han, S. Enhanced kinetics of MgH2 via in situ formed catalysts derived from MgCCo1.5Ni1.5. J. Alloys Compd. 2020, 822, 153621. [Google Scholar] [CrossRef]
- Zhang, J.; He, L.; Yao, Y.; Zhou, X.J.; Yu, L.P.; Lu, X.Z.; Zhou, D.W. Catalytic effect and mechanism of NiCu solid solutions on hydrogen storage properties of MgH2. Renew. Energy 2020, 154, 1229–1239. [Google Scholar] [CrossRef]
- Zhang, J.; He, L.; Yao, Y.; Zhou, X.-j.; Jiang, L.-k.; Peng, P. Hydrogen storage properties of magnesium hydride catalyzed by Ni-based solid solutions. Trans. Nonferrous Met. Soc. China 2022, 32, 604–617. [Google Scholar] [CrossRef]
- Liu, G.; Qiu, F.; Li, J.; Wang, Y.; Li, L.; Yan, C.; Jiao, L.; Yuan, H. NiB nanoparticles: A new nickel-based catalyst for hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2012, 37, 17111–17117. [Google Scholar] [CrossRef]
- Zhang, Q.; Zang, L.; Huang, Y.; Gao, P.; Jiao, L.; Yuan, H.; Wang, Y. Improved hydrogen storage properties of MgH2 with Ni-based compounds. Int. J. Hydrogen Energy 2017, 42, 24247–24255. [Google Scholar] [CrossRef]
- Xie, X.; Ma, X.; Liu, P.; Shang, J.; Li, X.; Liu, T. Formation of Multiple-Phase Catalysts for the Hydrogen Storage of Mg Nanoparticles by Adding Flowerlike NiS. ACS Appl. Mater. Interfaces 2017, 9, 5937–5946. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Zhang, J.; Guo, X.; Yang, X. Improved MgH2 kinetics and cyclic stability by fibrous spherical NiMoO4 and rGO. J. Taiwan Inst. Chem. E. 2022, 134, 104311. [Google Scholar] [CrossRef]
- Meng, Q.; Huang, Y.; Ye, J.; Xia, G.; Wang, G.; Dong, L.; Yang, Z.; Yu, X. Electrospun carbon nanofibers with in-situ encapsulated Ni nanoparticles as catalyst for enhanced hydrogen storage of MgH2. J. Alloys Compd. 2021, 851, 156874. [Google Scholar] [CrossRef]
- Duan, C.; Tian, Y.; Wang, X.; Wu, M.; Fu, D.; Zhang, Y.; Lv, W.; Su, Z.; Xue, Z.; Wu, Y. Ni-CNTs as an efficient confining framework and catalyst for improving dehydriding/rehydriding properties of MgH2. Renew. Energy 2022, 187, 417–427. [Google Scholar] [CrossRef]
- Hou, Q.; Zhang, J.; XinTao, G.; Xu, G.; Yang, X. Synthesis of low-cost biomass charcoal-based Ni nanocatalyst and evaluation of their kinetic enhancement of MgH2. Int. J. Hydrogen Energy 2022, 47, 15209–15223. [Google Scholar] [CrossRef]
- Zeng, L.; Lan, Z.; Li, B.; Liang, H.; Wen, X.; Huang, X.; Tan, J.; Liu, H.; Zhou, W.; Guo, J. Facile synthesis of a Ni3S2@C composite using cation exchange resin as an efficient catalyst to improve the kinetic properties of MgH2. J. Magnes. Alloys 2021, in press. [CrossRef]
- El-Eskandarany, M.S.; Shaban, E.; Ali, N.; Aldakheel, F.; Alkandary, A. In-situ catalyzation approach for enhancing the hydrogenation/dehydrogenation kinetics of MgH2 powders with Ni particles. Sci. Rep. 2016, 6, 37335. [Google Scholar] [CrossRef]
- Dan, L.; Wang, H.; Liu, J.; Ouyang, L.; Zhu, M. H2 Plasma Reducing Ni Nanoparticles for Superior Catalysis on Hydrogen Sorption of MgH2. ACS Appl. Energy Mater. 2022, 5, 4976–4984. [Google Scholar] [CrossRef]
- Motavalli, A.; Rajabi, M. Catalytic effect of melt-spun Ni3FeMn alloy on hydrogen desorption properties of nanocrystalline MgH2 synthesized by mechanical alloying. Int. J. Hydrogen Energy 2014, 39, 17047–17053. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Matrouk, H.; Behbehani, M.; Shaban, E.; Alkandary, A.; Aldakheel, F.; Al-Saidi, M. Amorphous-versus big cube- Zr2Ni for improving the kinetics of hydrogenation/dehydrogenation behaviors for MgH2 powders. Mater. Chem. Phys. 2018, 203, 17–26. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, L.; Yao, Z.; Yan, N.; Sun, Z.; Yang, X.; Zhu, X.; Hu, S.; Chen, L. Facile synthesized Fe nanosheets as superior active catalyst for hydrogen storage in MgH2. Int. J. Hydrogen Energy 2019, 44, 21955–21964. [Google Scholar] [CrossRef]
- Santos, S.F.; Ishikawa, T.T.; Botta, W.J.; Huot, J. MgH2 + FeNb nanocomposites for hydrogen storage. Mater. Chem. Phys. 2014, 147, 557–562. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Z.; Liu, Z.; Tang, Q.; Zhu, Y.; Lin, H.; Zhang, Y.; Zhang, J.; Liu, Y.; Li, L. Synergistic effect of rGO supported Ni3Fe on hydrogen storage performance of MgH2. Int. J. Hydrogen Energy 2020, 45, 16622–16633. [Google Scholar] [CrossRef]
- Singh, S.; Bhatnagar, A.; Shukla, V.; Vishwakarma, A.K.; Soni, P.K.; Verma, S.K.; Shaz, M.A.; Sinha, A.S.K.; Srivastava, O.N. Ternary transition metal alloy FeCoNi nanoparticles on graphene as new catalyst for hydrogen sorption in MgH2. Int. J. Hydrogen Energy 2020, 45, 774–786. [Google Scholar] [CrossRef]
- Gao, S.; Wang, X.; Liu, H.; He, T.; Wang, Y.; Li, S.; Yan, M. CNTs decorated with CoFeB as a dopant to remarkably improve the dehydrogenation/rehydrogenation performance and cyclic stability of MgH2. Int. J. Hydrogen Energy 2020, 45, 28964–28973. [Google Scholar] [CrossRef]
- Ismail, M. Influence of different amounts of FeCl3 on decomposition and hydrogen sorption kinetics of MgH2. Int. J. Hydrogen Energy 2014, 39, 2567–2574. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, L.; Li, Y.; Guo, S.; Yu, H.; Wang, W.; Ren, K.; Zhang, W.; Han, S. Effect of ternary transition metal sulfide FeNi2S4 on hydrogen storage performance of MgH2. J. Magnes. Alloys 2022, in press. [CrossRef]
- Song, M.; Zhang, L.; Zheng, J.; Yu, Z.; Wang, S. Constructing graphene nanosheet-supported FeOOH nanodots for hydrogen storage of MgH2. Int. J. Miner. Metall. Mater. 2022, 29, 1464–1473. [Google Scholar] [CrossRef]
- Zhou, D.; Cui, K.; Zhou, Z.; Liu, C.; Zhao, W.; Li, P.; Qu, X. Enhanced hydrogen-storage properties of MgH2 by Fe–Ni catalyst modified three-dimensional graphene. Int. J. Hydrogen Energy 2021, 46, 34369–34380. [Google Scholar] [CrossRef]
- Hou, Q.; Zhang, J.; Zheng, Z.A.; Yang, X.; Ding, Z. Ni3Fe/BC nanocatalysts based on biomass charcoal self-reduction achieves excellent hydrogen storage performance of MgH2. Dalton Trans. 2022, 51, 14960–14969. [Google Scholar] [CrossRef]
- Ren, S.; Fu, Y.; Zhang, L.; Cong, L.; Xie, Y.; Yu, H.; Wang, W.; Li, Y.; Jian, L.; Wang, Y.; et al. An improved hydrogen storage performance of MgH2 enabled by core-shell structure Ni/Fe3O4@MIL. J. Alloys Compd. 2022, 892, 162048. [Google Scholar] [CrossRef]
- Song, M.; Zhang, L.; Yao, Z.; Zheng, J.; Shang, D.; Chen, L.; Li, H. Unraveling the degradation mechanism for the hydrogen storage property of Fe nanocatalyst-modified MgH2. Inorg. Chem. Front. 2022, 9, 3874–3884. [Google Scholar] [CrossRef]
- Shan, J.; Li, P.; Wan, Q.; Zhai, F.; Zhang, J.; Li, Z.; Liu, Z.; Volinsky, A.A.; Qu, X. Significantly improved dehydrogenation of ball-milled MgH2 doped with CoFe2O4 nanoparticles. J. Power Sources 2014, 268, 778–786. [Google Scholar] [CrossRef]
- Gattia, D.M.; Jangir, M.; Jain, I.P. Study on nanostructured MgH2 with Fe and its oxides for hydrogen storage applications. J. Alloys Compd. 2019, 801, 188–191. [Google Scholar] [CrossRef]
- Shahi, R.R.; Bhatnagar, A.; Pandey, S.K.; Dixit, V.; Srivastava, O.N. Effects of Ti-based catalysts and synergistic effect of SWCNTs-TiF3 on hydrogen uptake and release from MgH2. Int. J. Hydrogen Energy 2014, 39, 14255–14261. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Yu, H.; Lu, Z.; He, J.; Zheng, J.; Wu, F.; Chen, L. Achieving superior hydrogen storage properties of MgH2 by the effect of TiFe and carbon nanotubes. Chem. Eng. J. 2021, 422, 130101. [Google Scholar] [CrossRef]
- Hu, C.; Zheng, Z.; Si, T.; Zhang, Q. Enhanced hydrogen desorption kinetics and cycle durability of amorphous TiMgVNi3-doped MgH2. Int. J. Hydrogen Energy 2022, 47, 3918–3926. [Google Scholar] [CrossRef]
- Pandey, S.K.; Bhatnagar, A.; Shukla, V.; Kesarwani, R.; Deshpandey, U.; Yadav, T.P. Catalytic mechanism of TiO2 quantum dots on the de/re-hydrogenation characteristics of magnesium hydride. Int. J. Hydrogen Energy 2021, 46, 37340–37350. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, Z.; Zhang, M.; Gao, M.; Hu, J.; Du, F.; Liu, Y.; Pan, H. A novel solid-solution MXene (Ti0.5V0.5)3C2 with high catalytic activity for hydrogen storage in MgH2. Materialia 2018, 1, 114–120. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Z.; Jian, N.; Hu, J.; Du, F.; Yao, J.; Gao, M.; Liu, Y.; Pan, H. A novel complex oxide TiVO3.5 as a highly active catalytic precursor for improving the hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2018, 43, 23327–23335. [Google Scholar] [CrossRef]
- Gao, H.; Shi, R.; Liu, Y.; Zhu, Y.; Zhang, J.; Li, L.; Hu, X. Facet-dependent catalytic activity of two-dimensional Ti3C2Tx MXene on hydrogen storage performance of MgH2. J. Magnes. Alloys 2022, in press. [CrossRef]
- Soni, P.K.; Bhatnagar, A.; Shukla, V.; Shaz, M.A. Improved de/re-hydrogenation properties of MgH2 catalyzed by graphene templated Ti–Ni–Fe nanoparticles. Int. J. Hydrogen Energy 2022, 47, 21391–21402. [Google Scholar] [CrossRef]
- Verma, S.K.; Bhatnagar, A.; Shukla, V.; Soni, P.K.; Pandey, A.P.; Yadav, T.P.; Srivastava, O.N. Multiple improvements of hydrogen sorption and their mechanism for MgH2 catalyzed through TiH2@Gr. Int. J. Hydrogen Energy 2020, 45, 19516–19530. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, X.; Luo, B.; Liu, M.; Chen, M.; Chen, L. Superior de/hydrogenation performances of MgH2 catalyzed by 3D flower-like TiO2@C nanostructures. J. Energy Chem. 2020, 46, 191–198. [Google Scholar] [CrossRef]
- Gao, H.; Shi, R.; Zhu, J.; Liu, Y.; Shao, Y.; Zhu, Y.; Zhang, J.; Li, L.; Hu, X. Interface effect in sandwich like Ni/Ti3C2 catalysts on hydrogen storage performance of MgH2. Appl. Surf. Sci. 2021, 564, 150302. [Google Scholar] [CrossRef]
- Gao, H.; Shi, R.; Shao, Y.; Liu, Y.; Zhu, Y.; Zhang, J.; Hu, X.; Li, L.; Ba, Z. One-step self-assembly of TiO2/MXene heterostructures for improving the hydrogen storage performance of magnesium hydride. J. Alloys Compd. 2022, 895, 162635. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Wang, Y.; Jiao, L.; Yuan, H. Catalytic effects of different Ti-based materials on dehydrogenation performances of MgH2. J. Alloys Compd. 2015, 645, S509–S512. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Sandhya, K.S.; Ramasamy, D.; Shaula, A.; Fagg, D.P. Transformation of Metallic Ti to TiH2 Phase in the Ti/MgH2 Composite and Its Influence on the Hydrogen Storage Behavior of MgH2. ChemPhysChem 2020, 21, 1195–1201. [Google Scholar] [CrossRef]
- Ren, C.; Fang, Z.Z.; Zhou, C.; Lu, J.; Ren, Y.; Zhang, X.; Luo, X. In situ X-ray diffraction study of dehydrogenation of MgH2 with Ti-based additives. Int. J. Hydrogen Energy 2014, 39, 5868–5873. [Google Scholar] [CrossRef]
- Khodaparast, V.; Rajabi, M. Hydrogen Desorption Properties of MgH2-5 wt% Ti-Mn-Cr Composite via Combined Melt Spinning and Mechanical Alloying. Procedia Mater. Sci. 2015, 11, 611–615. [Google Scholar] [CrossRef]
- Haghparast, M.R.; Rajabi, M. Hydrogen Desorption Properties of MgH2-5 at% Ti-Cr-Mn-Fe-V Composite Via Combined Vacuum Arc Remelting and Mechanical Alloying. Procedia Mater. Sci. 2015, 11, 605–610. [Google Scholar] [CrossRef]
- Zou, R.; Adedeji Bolarin, J.; Lei, G.; Gao, W.; Li, Z.; Cao, H.; Chen, P. Microwave-assisted reduction of Ti species in MgH2-TiO2 composite and its effect on hydrogen storage. Chem. Eng. J. 2022, 450, 138072. [Google Scholar] [CrossRef]
- Ren, L.; Zhu, W.; Li, Y.; Lin, X.; Xu, H.; Sun, F.; Lu, C.; Zou, J. Oxygen Vacancy-Rich 2D TiO2 Nanosheets: A Bridge Toward High Stability and Rapid Hydrogen Storage Kinetics of Nano-Confined MgH2. Nano-Micro Lett. 2022, 14, 144. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Du, Y.; Liao, W. Catalytic effect of Ti2C MXene on the dehydrogenation of MgH2. Int. J. Hydrogen Energy 2019, 44, 6787–6794. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Y.; Zhang, M. Excellent catalytic activity of two-dimensional Ti2C and Ti2CT2 (T = O, F, OH) monolayers on hydrogen storage of MgH2: First-principles calculations. Int. J. Hydrogen Energy 2021, 46, 33176–33185. [Google Scholar] [CrossRef]
- Wu, Z.; Fang, J.; Liu, N.; Wu, J.; Kong, L. The Improvement in Hydrogen Storage Performance of MgH2 Enabled by Multilayer Ti3C2. Micromachines 2021, 12, 1190. [Google Scholar] [CrossRef]
- Gao, H.; Shi, R.; Liu, Y.; Zhu, Y.; Zhang, J.; Hu, X.; Li, L. Enhanced hydrogen storage performance of magnesium hydride with incompletely etched Ti3C2Tx: The nonnegligible role of Al. Appl. Surf. Sci. 2022, 600, 154140. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Z.; Cai, Z.; Yan, N.; Lu, X.; Zhu, X.; Chen, L. Enhanced hydrogen storage properties of MgH2 by the synergetic catalysis of Zr0.4Ti0.6Co nanosheets and carbon nanotubes. Appl. Surf. Sci. 2020, 504, 144465. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Hu, Y.; Sun, C.; Leng, H.; Li, Q.; Wu, C. Enhanced catalytic effect of TiO2@rGO synthesized by one-pot ethylene glycol-assisted solvothermal method for MgH2. J. Alloys Compd. 2021, 881, 160644. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, G.; Zhang, D.; Fan, Y.; Liu, B. Striking enhanced effect of PrF3 particles on Ti3C2 MXene for hydrogen storage properties of MgH2. J. Alloys Compd. 2022, 914, 165291. [Google Scholar] [CrossRef]
- Peng, C.; Yang, C.; Zhang, Q. Few-layer MXene Ti3C2Tx supported Ni@C nanoflakes as a catalyst for hydrogen desorption of MgH2. J. Mater. Chem. A 2022, 10, 12409–12417. [Google Scholar] [CrossRef]
- da Conceição, M.O.T.; Brum, M.C.; dos Santos, D.S. The effect of V, VCl3 and VC catalysts on the MgH2 hydrogen sorption properties. J. Alloys Compd. 2014, 586, S101–S104. [Google Scholar] [CrossRef]
- Milošević, S.; Kurko, S.; Pasquini, L.; Matović, L.; Vujasin, R.; Novaković, N.; Novaković, J.G. Fast hydrogen sorption from MgH2–VO2(B) composite materials. J. Power Sources 2016, 307, 481–488. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Ahmed, S.A.; Shaban, E. Metallic Glassy V45Zr20Ni20Cu10Al3Pd2 Alloy Powders for Superior Hydrogenation/Dehydrogenation Kinetics of MgH2. Mater. Today: Proc. 2018, 5, 13718–13725. [Google Scholar]
- Pang, Y.; Wang, Y.; Yang, J.; Zheng, S. Engineering dual-functional VB2 nanoparticles in MgH2 for highly efficient hydrogen storage. Compos. Commun. 2021, 26, 100781. [Google Scholar] [CrossRef]
- Meng, Y.; Ju, S.; Chen, W.; Chen, X.; Xia, G.; Sun, D.; Yu, X. Design of Bifunctional Nb/V Interfaces for Improving Reversible Hydrogen Storage Performance of MgH2. Small Struct. 2022, 3, 2200119. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, Z.; Jian, N.; Gao, M.; Hu, J.; Du, F.; Pan, H.; Liu, Y. Vanadium oxide nanoparticles supported on cubic carbon nanoboxes as highly active catalyst precursors for hydrogen storage in MgH2. J. Mater. Chem. A 2018, 6, 16177–16185. [Google Scholar] [CrossRef]
- Zang, J.; Wang, S.; Hu, R.; Man, H.; Zhang, J.; Wang, F.; Sun, D.; Song, Y.; Fang, F. Ni, beyond thermodynamic tuning, maintains the catalytic activity of V species in Ni3(VO4)2 doped MgH2. J. Mater. Chem. A 2021, 9, 8341–8349. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, L.; Yan, N.; Zheng, J.; Bian, T.; Yang, Z.; Su, S. Realizing Hydrogen De/Absorption Under Low Temperature for MgH2 by Doping Mn-Based Catalysts. Nanomaterials 2020, 10, 1745. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.-y.; Wu, F.-y.; Sun, Z.; Zheng, J.-g.; Zhang, L.-t.; Chen, L.-x. Mn nanoparticles enhanced dehydrogenation and hydrogenation kinetics of MgH2 for hydrogen storage. Trans. Nonferrous Met. Soc. China 2021, 31, 3469–3477. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, X.; Sun, Z.; Yan, N.; Yu, H.; Lu, Z.; Zhu, X. Superior catalytic effect of facile synthesized LaNi4.5Mn0.5 submicro-particles on the hydrogen storage properties of MgH2. J. Alloys Compd. 2020, 844, 156069. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Z.; Yao, Z.; Yang, L.; Yan, N.; Lu, X.; Xiao, B.; Zhu, X.; Chen, L. Excellent catalysis of Mn3O4 nanoparticles on the hydrogen storage properties of MgH2: An experimental and theoretical study. Nanoscale Adv. 2020, 2, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Nong, J.; Wen, X.; Liang, H.; Guo, J.; Zhou, W.; Huang, X.; Liu, H.; Ning, H.; Lan, Z. Facile and low-cost synthesis of carbon-supported manganese monoxide nanocomposites and evaluation of their superior catalytic effect toward magnesium hydride. J. Alloys Compd. 2021, 887, 161380. [Google Scholar] [CrossRef]
- Wang, P.; Tian, Z.; Wang, Z.; Xia, C.; Yang, T.; Ou, X. Improved hydrogen storage properties of MgH2 using transition metal sulfides as catalyst. Int. J. Hydrogen Energy 2021, 46, 27107–27118. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, Q.; Chang, J.; Zhang, D.; Peng, Y.; Yang, X. Improvement of hydrogen storage performance of MgH2 by MnMoO4 rod composite catalyst. Solid State Sci. 2021, 121, 106750. [Google Scholar] [CrossRef]
- Meena, P.; Singh, R.; Sharma, V.K.; Jain, I.P. Role of NiMn9.3Al4.0Co14.1Fe3.6 alloy on dehydrogenation kinetics of MgH2. J. Magnes. Alloys 2018, 6, 318–325. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).