Preparation of Anorthite/Mullite In Situ and Phase Transformation in Porcelain
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Methods
2.3. Calculation
3. Results and Discussion
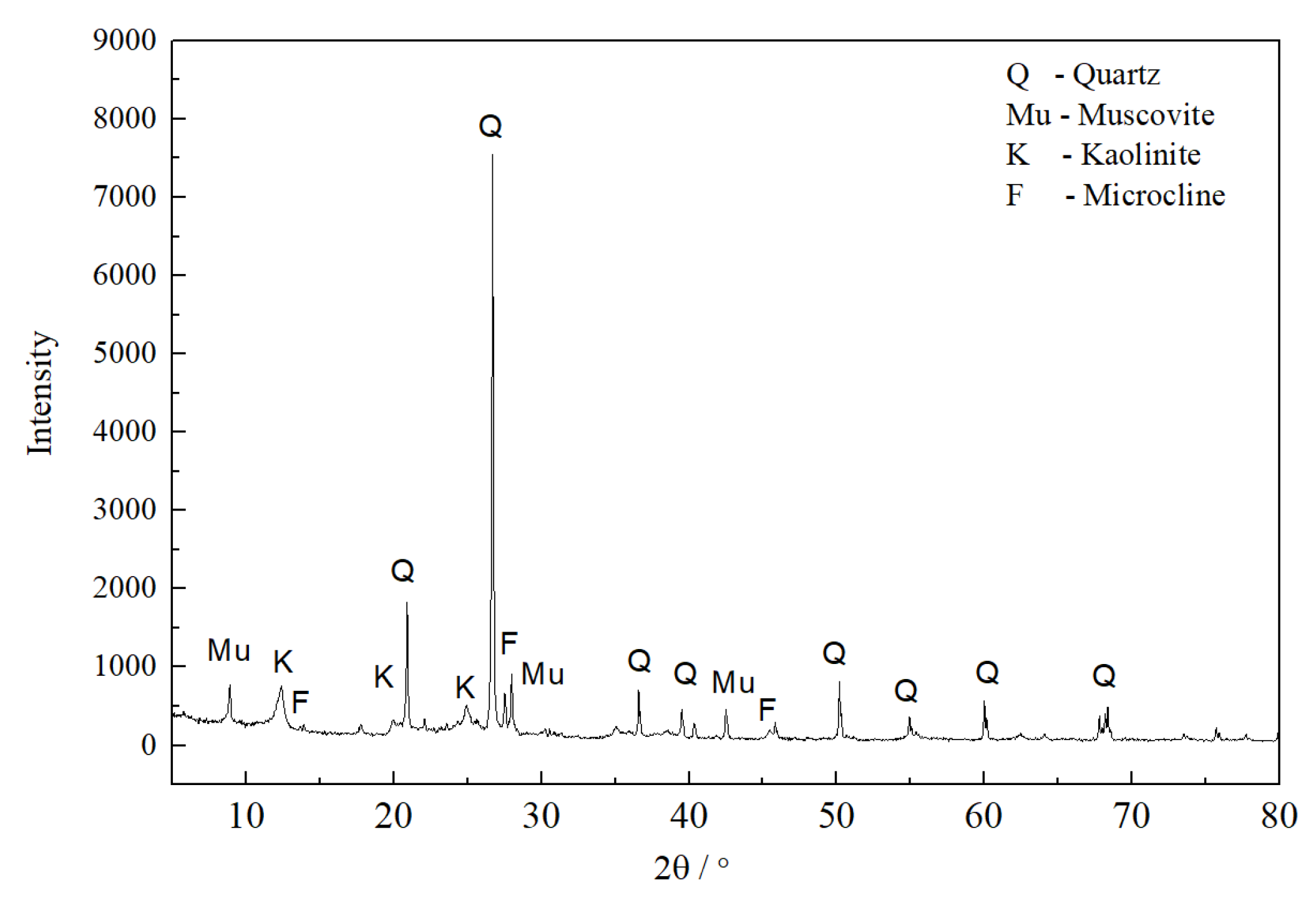
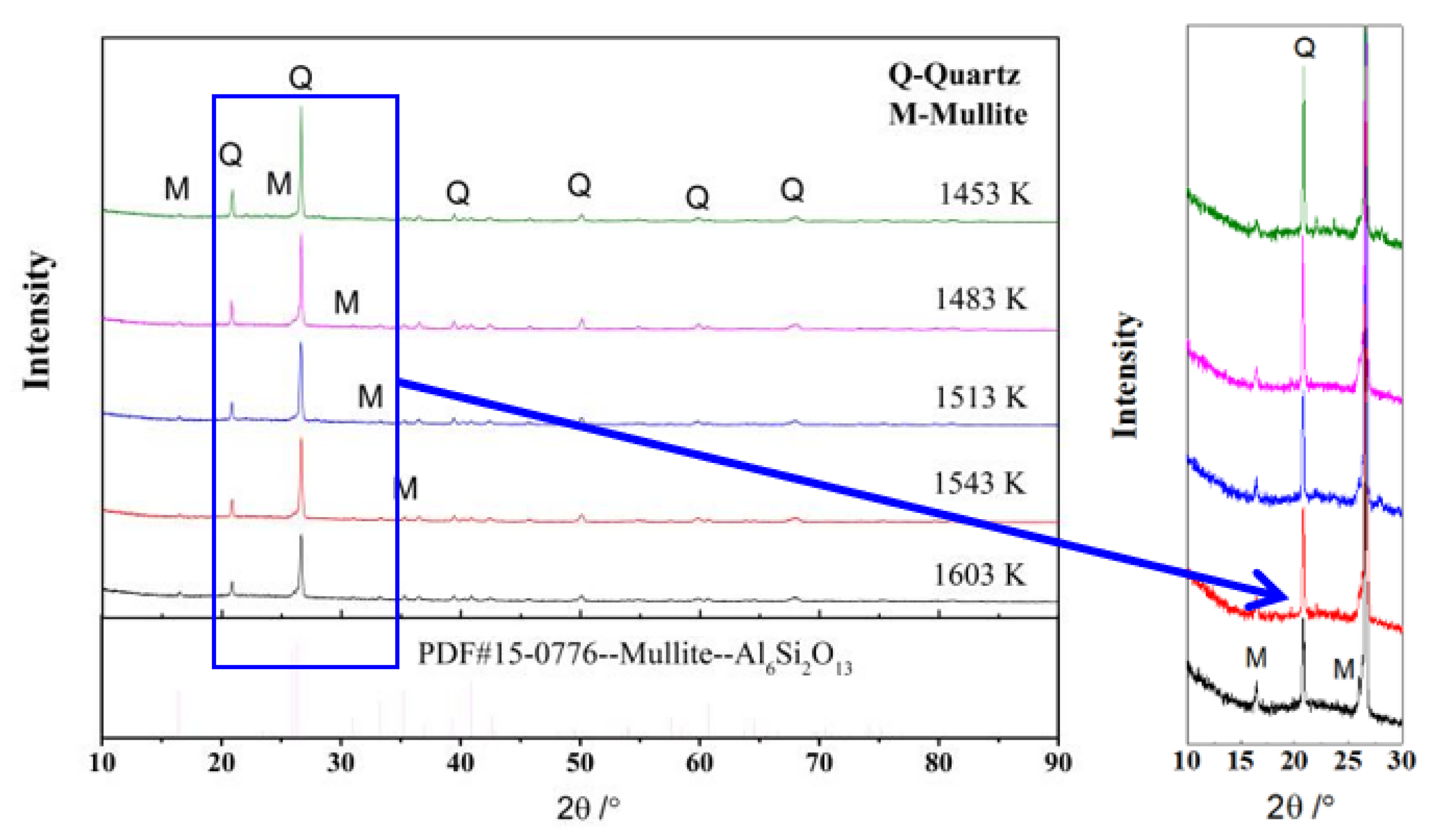
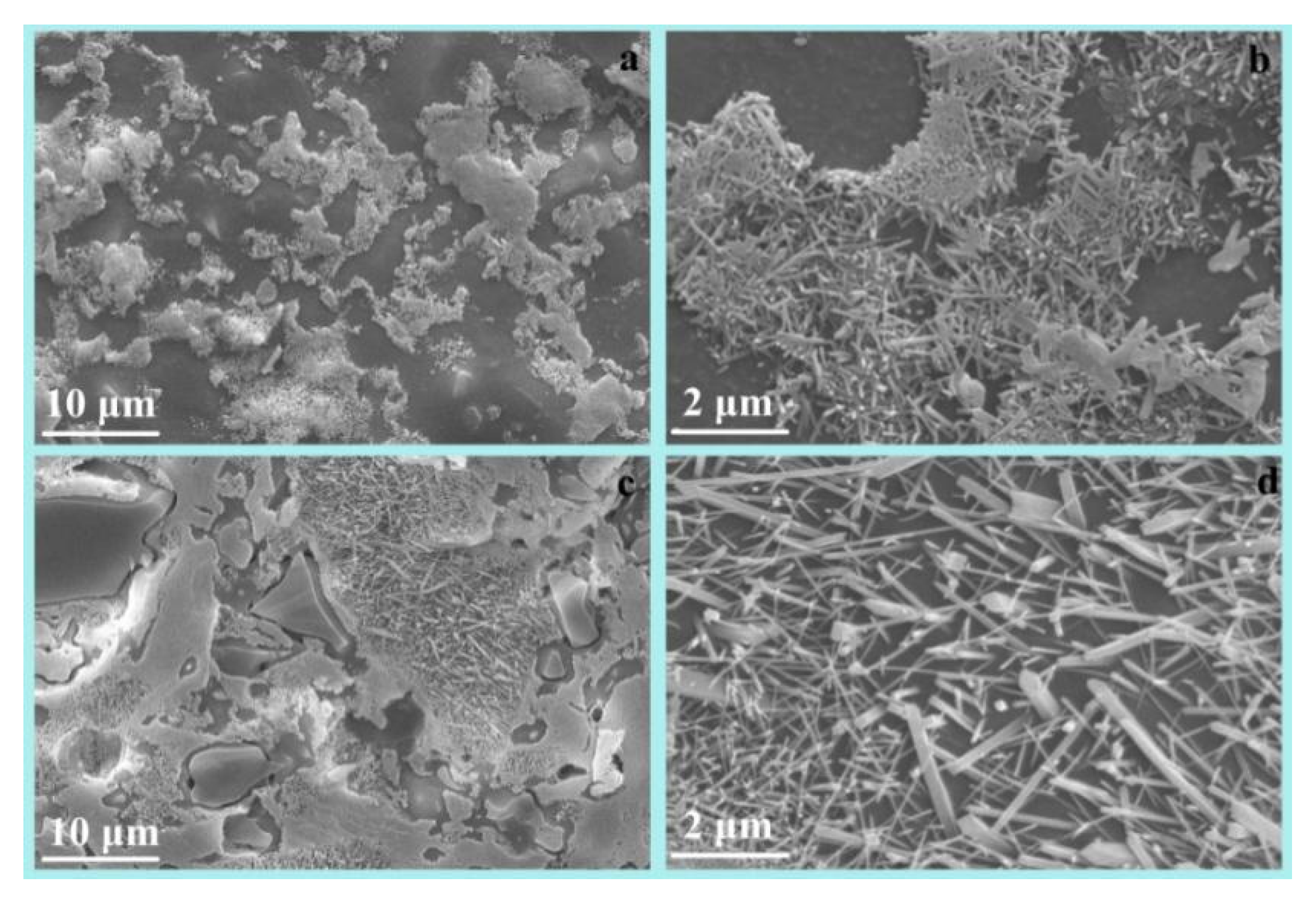
3.1. Properties of the Sintered Samples
3.2. Thermodynamic Analysis of the Conversion of Anorthite and Mullite
| CaO + Al2O3 + 2SiO2 → CaO•Al2O3•2SiO2 | Reaction 1 |
| 3Al2O3 + 2SiO2 → 3Al2O3•2SiO2 | Reaction 2 |
| 3(CaO•Al2O3•2SiO2) → 3Al2O3•2SiO2 + 3CaO + 4SiO2 | Reaction 3 |
| CaO•Al2O3•2SiO2 + 2(Al2O3•2SiO2) → 3Al2O3•2SiO2 + CaO + 4SiO2 | Reaction 4 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitouni, S.; Harabi, A. Sintering and mechanical properties of porcelains prepared from algerian raw materials. Cerâmica 2011, 57, 453–460. [Google Scholar] [CrossRef]
- Contreras, J.E.; Taha-Tijerina, J.; López-Perales, J.F.; Banda-Muñoz, F.; Díaz-Tato, L.; Rodríguez, E.A. Enhancing the quartz-clay-feldspar system by nano-Al2O3 addition for electrical insulators: From laboratory to prototype scale. Mater. Chem. Phys. 2021, 263, 124389. [Google Scholar] [CrossRef]
- Su, C.; Zhang, Y.; Wang, L.; Zhang, S.; Wang, Q. Mechanical properties of reinforced porcelain slabs with mullite whiskers introduced by aluminum silicate fiber. Ceram. Int. 2022, 48, 18909–18917. [Google Scholar] [CrossRef]
- Fuertes, V.; Reinosa, J.J.; Fernández, J.F.; Enríquez, E. Engineered feldspar-based ceramics: A review of their potential in ceramic industry. J. Eur. Ceram. Soc. 2022, 42, 307–326. [Google Scholar] [CrossRef]
- Lin, S.; Yu, Y.; Tan, Y.; Yang, H.; Zhong, M.; Zhang, C.; Zhang, Z.; Wu, Y. The Mechanism of Porcelain Toughened by Activated Kaolinite in a Lower Sintering Temperature. Materials 2022, 15, 3867. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Fu, R.; Lv, J.; Zhang, X.; Chen, X.; Li, G.; Liu, X. Enhanced bonding strength of Al2O3/AlN ceramics joined via glass frit with gradient thermal expansion coefficient. Ceram. Int. 2020, 46, 12806–12811. [Google Scholar] [CrossRef]
- Hallmann, L.; Ulmer, P.; Wille, S.; Kern, M. Effect of differences in coefficient of thermal expansion of veneer and Y-TZP ceramics on interface phase transformation. J. Pros. Dent. 2014, 112, 591–599. [Google Scholar] [CrossRef]
- Hirata, Y. Theoretical analyses of thermal shock and thermal expansion coefficients of metals and ceramics. Ceram. Int. 2015, 41, 1145–1153. [Google Scholar] [CrossRef]
- Wang, B.; Yang, X.; Zeng, D.; Yang, J.; Ishizaki, K.; Niihara, K. Effect of glassy bonding materials on the properties of LAS/SiC porous ceramics with near zero thermal expansion. J. Eur. Ceram. Soc. 2014, 34, 91–95. [Google Scholar] [CrossRef]
- Fedorova, A.; Michelsen, L.; Scheffler, M. Polymer-derived ceramic tapes with small and negative thermal expansion coefficients. J. Eur. Ceram. Soc. 2018, 38, 719–725. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Zhou, N.; Nian, S.; Li, J.; Wu, Z.; Cao, W. Research on the characterization of a porcelainised material fabricated by adding a small amount of anorthite chamotte. Ceram. Int. 2017, 43, 12402–12407. [Google Scholar] [CrossRef]
- Wu, J.; Yu, J.; Xu, X.; Liu, Y.; Zhang, Z.; Wei, P. Preparation and thermal shock resistance of anorthite solar thermal energy storage ceramics from magnesium slag. Ceram. Int. 2022, 48, 33604–33614. [Google Scholar] [CrossRef]
- Li, C.; Bian, C.; Han, Y.; Wang, C.; An, L. Mullite whisker reinforced porous anorthite ceramics with low thermal conductivity and high strength. J. Eur. Ceram. Soc. 2016, 36, 761–765. [Google Scholar] [CrossRef]
- Wu, L.; Li, C.; Chen, Y.; Wang, C. Seed assisted in-situ synthesis of porous anorthite/mullite whisker ceramics by foam-freeze casting. Ceram. Int. 2021, 47, 11193–11201. [Google Scholar] [CrossRef]
- Yahya, N.A.; Rashid, T.N.I.T.A.; Hasmaliza, M. Effect of sintering temperature on physical properties of Trong clay and prefired material in low clay whiteware ceramic fabrication. Mater. Tod. Proce. 2022, 66, 2716–2719. [Google Scholar] [CrossRef]
- Lin, Y. Preparation and Properties of Porous Anorthite/Mullite Composite Ceramics. Master’s Thesis, Beijing Jiaotong University, Beijing, China, 2011. [Google Scholar]
- Dong, W.; Bao, Q.; Gu, X.; Hu, K.; Jiang, J. Preparation of the anorthite/mullite composites by the in-situ growth method. Elect. Comp. Mater. 2010, 20, 12–14. [Google Scholar]
- Ilic, M.; Cheeseman, C.; Sollars, C.; Knightb, J. Mineralogy and microstructure of sintered lignite coal fly ash. Fuel 2003, 82, 331–336. [Google Scholar] [CrossRef]
- Lin, S.; Yu, Y.; Zhang, Z.; Zhang, C.; Zhong, M.; Wang, L.; Lu, S.; Xu, W.; Li, N.; Huang, X. The synergistic mechanisms of citric acid and oxalic acid on the rapid dissolution of kaolinite. Appl. Clay Sci. 2020, 196, 105756. [Google Scholar] [CrossRef]
- Yuan, K.; Liao, L.; Wan, H.; Wang, C.; Wang, C. Quantitative Analysis of Cristobalite and α-Quartz in Bentonite by X-Ray Powder Diffraction—Comparison Between External Standard and K-Value Method. J. Chin. Ceram. Soc. 2011, 39, 377–382. [Google Scholar]
- Yu, X.; Yu, C.; Ran, Q.; Liu, J. Quantitative Analysis of Cement and Its Hydration Product by Rietveld External Standard Method. Mater. Repor. 2019, 33, 2337–2342. [Google Scholar]
- Vasić, M.V.; Mijatović, N.; Radojević, Z. Aplitic granite waste as raw material for the production of outdoor ceramic floor tiles. Materials 2022, 15, 3145. [Google Scholar] [CrossRef] [PubMed]
- Ye, D. Handbook of Practical Inorganic Thermodynamics Data, 2nd ed.; Metallurgical Industry Press: Beijing, China, 2002; pp. 72–924. [Google Scholar]
- Zhou, L.; Wang, H.; Yu, Q. Energy consumption and conservation for sintering daily-use ceramics. China Ceram. 2010, 46, 57–59. [Google Scholar]
- Conconi, M.S.; Rendtorff, N.M.; Aglietti, E.F. Evaluation of non crystalline phase in AZS refractories by xrd methods. N. J. Glas. Ceram. 2011, 1, 28–33. [Google Scholar] [CrossRef]
- Liu, C.; Tang, G.; Luo, L.; Chen, W. Phase separation inducing controlled crystallization of GeSe2-Ga2Se3-CsI glasses for fabricating infrared transmitting glass–ceramics. J. Am. Ceram. Soc. 2009, 92, 245–248. [Google Scholar] [CrossRef]
- Nenadović, S.; Gulicovski, J.; Mirković, M.; Kljajević, L.; Bošković, I.; Vukčević, M.; Nenadović, M. Structural, mechanical and chemical properties of low content carbon geopolymer. Sustainability 2022, 14, 4885. [Google Scholar] [CrossRef]


| The Substance | Al2O3 | SiO2 | CaO | CaO•Al2O3•2SiO2 | 3Al2O3•2SiO2 | Al2O3•2SiO2 |
|---|---|---|---|---|---|---|
| /J·mol−1 | −1,675,274 | −908,346 | −634,294 | −4,222,493 | −6,819,209 | −3,211,220 |
| T/K | Al2O3 | SiO2 | CaO | CaO•Al2O3•2SiO2 | 3Al2O3•2SiO2 | Al2O3•2SiO2 |
|---|---|---|---|---|---|---|
| 1300 | 124.10 | 85.54 | 74.02 | 382.57 | 574.53 | 306.95 |
| 1400 | 130.82 | 89.36 | 77.00 | 399.84 | 602.12 | 321.74 |
| 1500 | 137.27 | 93.01 | 79.85 | 416.48 | 628.59 | 335.90 |
| 1600 | 143.46 | 96.50 | 82.57 | 432.52 | 653.99 | 349.47 |
| 1700 | 149.42 | 99.86 | 85.18 | 448.00 | 678.42 | 362.50 |
| 1800 | 155.15 | 103.08 | 87.69 | 462.96 | 701.91 | 375.04 |
| Samples | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | TiO2 | MnO | P2O5 | Others | LOI 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | 68.38 | 26.18 | 1.15 | 0.09 | 0.05 | 0.26 | 3.55 | 0.08 | 0.12 | 0.02 | 0.12 | 8.95 |
| M | 66.01 | 25.74 | 1.12 | 0.09 | 1.67 | 0.25 | 3.41 | 0.08 | 0.12 | 1.39 | 0.12 | 8.91 |
| Sample | R 1 | M 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1453 K | 1483 K | 1513 K | 1543 K | 1603 K | 1453 K | 1483 K | 1513 K | 1543 K | |
| Water absorption/% | 8.63 ± 0.11 | 3.17 ± 0.06 | 1.63 ± 0.09 | 1.17 ± 0.03 | 0.36 ± 0.02 | 1.84 ± 0.07 | 0.39 ± 0.02 | 0.22 ± 0.01 | 0.21 ± 0.04 |
| Bulk density/(g·cm−3) | 1.88 ± 0.03 | 2.05 ± 0.05 | 2.17 ± 0.09 | 2.26 ± 0.13 | 2.39 ± 0.43 | 2.11 ± 0.06 | 2.43 ± 0.08 | 2.44 ± 0.12 | 2.42 ± 0.10 |
| Bending strength/MPa | 39.15 ± 1.07 | 48.14 ± 0.84 | 56.32 ± 0.65 | 69.16 ± 0.73 | 78.23 ± 0.76 | 63.43 ± 0.98 | 74.38 ± 0.55 | 95.31 ± 0.63 | 90.17 ± 0.47 |
| Temperature/K | Anorthite | Mullite | Quartz | Amorphous Phase | The Sum of Anorthite and Mullite |
|---|---|---|---|---|---|
| 1453 | 4.27 | 19.21 | 31.28 | 45.24 | 23.48 |
| 1483 | 7.03 | 20.43 | 24.23 | 48.31 | 27.46 |
| 1513 | 4.16 | 27.23 | 15.17 | 53.44 | 31.39 |
| 1543 | - | 32.53 | 11.86 | 55.61 | 32.53 |
| T/K | Reaction 1 | Reaction 2 | ||||
|---|---|---|---|---|---|---|
(J·mol−1) | ΔΦ (J·mol−1·K−1) | (J·mol−1) | (J·mol−1) | ΔΦ (J·mol−1·K−1) | (J·mol−1) | |
| 1300 | −96,233 | 13.37 | −113,614 | 23,305 | 31.15 | −17,190 |
| 1400 | 13.3 | −114,853 | 30.94 | −20,011 | ||
| 1500 | 13.34 | −116,243 | 30.76 | −22,835 | ||
| 1600 | 13.49 | −117,817 | 30.61 | −25,671 | ||
| 1700 | 13.68 | −119,489 | 30.44 | −28,443 | ||
| 1800 | 13.96 | −121,361 | 30.30 | −31,235 | ||
| T/K | Reaction 3 | Reaction 4 | ||||
|---|---|---|---|---|---|---|
(J·mol−1) | ΔΦ (J·mol−1·K−1) | (J·mol−1) | (J·mol−1) | ΔΦ (J·mol−1·K−1) | (J·mol−1) | |
| 1300 | 301,960 | −8.96 | 313,609 | −441,954 | −5.76 | −434,466 |
| 1400 | −8.98 | 314,538 | −6.76 | −432,490 | ||
| 1500 | −9.29 | 315,891 | −7.80 | −430,254 | ||
| 1600 | −9.85 | 317,714 | −8.90 | −427,714 | ||
| 1700 | −10.62 | 320,011 | −9.96 | −425,022 | ||
| 1800 | −11.58 | 322,811 | −11.12 | −421,938 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-M.; Yu, Y.-L.; Zhong, M.-F.; Yang, H.; Liu, Y.; Li, H.; Zhang, C.-Y.; Zhang, Z.-J. Preparation of Anorthite/Mullite In Situ and Phase Transformation in Porcelain. Materials 2023, 16, 1616. https://doi.org/10.3390/ma16041616
Lin S-M, Yu Y-L, Zhong M-F, Yang H, Liu Y, Li H, Zhang C-Y, Zhang Z-J. Preparation of Anorthite/Mullite In Situ and Phase Transformation in Porcelain. Materials. 2023; 16(4):1616. https://doi.org/10.3390/ma16041616
Chicago/Turabian StyleLin, Shao-Min, Ya-Ling Yu, Ming-Feng Zhong, Huan Yang, Yang Liu, Hang Li, Chen-Yang Zhang, and Zhi-Jie Zhang. 2023. "Preparation of Anorthite/Mullite In Situ and Phase Transformation in Porcelain" Materials 16, no. 4: 1616. https://doi.org/10.3390/ma16041616
APA StyleLin, S.-M., Yu, Y.-L., Zhong, M.-F., Yang, H., Liu, Y., Li, H., Zhang, C.-Y., & Zhang, Z.-J. (2023). Preparation of Anorthite/Mullite In Situ and Phase Transformation in Porcelain. Materials, 16(4), 1616. https://doi.org/10.3390/ma16041616





