Impact of 1,8-Diiodooctane (DIO) Additive on the Active Layer Properties of Cu2ZnSnS4 Kesterite Thin Films Prepared by Electrochemical Deposition for Photovoltaic Applications
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharmin, A.; Bashar, M.; Sultana, M.; Al Mamun, S.M. Sputtered single-phase kesterite Cu2ZnSnS4 (CZTS) thin film for photovoltaic applications: Post annealing parameter optimization and property analysis. AIP Adv. 2020, 10, 015230. [Google Scholar] [CrossRef] [Green Version]
- Zhuk, S.; Kushwaha, A.; Wong, T.K.; Masudy-Panah, S.; Smirnov, A.; Dalapati, G.K. Critical review on sputter-deposited Cu2ZnSnS4 (CZTS) based thin film photovoltaic technology focusing on device architecture and absorber quality on the solar cells performance. Sol. Energy Mater. Sol. Cells 2017, 171, 239–252. [Google Scholar] [CrossRef]
- Ravindiran, M.; Praveenkumar, C. Status review and the future prospects of CZTS based solar cell–A novel approach on the device structure and material modeling for CZTS based photovoltaic device. Renew. Sustain. Energy Rev. 2018, 94, 317–329. [Google Scholar] [CrossRef]
- Busari, R.; Taleatu, B.; Adewinbi, S.; Adewumi, O.; Fasasi, A.Y. Surface Characterization of Spin Coated Quaternary Chalcogenide CZT (S, O) Thin Film for Optoelectronic Applications. J. Photonic Mater. Technol. 2019, 5, 38–45. [Google Scholar] [CrossRef]
- Deokate, R.; Chavan, H.; Im, H.; Inamdar, A. Spray-deposited kesterite Cu2ZnSnS4 (CZTS): Optical, structural, and electrical investigations for solar cell applications. Ceram. Int. 2022, 48, 795–802. [Google Scholar] [CrossRef]
- Ramanujam, J.; Singh, U. Copper indium gallium selenide based solar cells—A review. Energy Environ. Sci. 2017, 10, 1306–1319. [Google Scholar] [CrossRef]
- Schorr, S.; Gurieva, G.; Guc, M.; Dimitrievska, M.; Pérez-Rodríguez, A.; Izquierdo-Roca, V.; Schnohr, C.; Kim, J.; Jo, W.; Merino, J. Point defects, compositional fluctuations, and secondary phases in non-stoichiometric kesterites. J. Phys. Energy 2019, 2, 012002. [Google Scholar] [CrossRef] [Green Version]
- Akcay, N.; Ataser, T.; Ozen, Y.; Ozcelik, S. Influence of sulfurization time on the properties of Cu2ZnSnS4 thin films deposited on Mo-coated soda lime glass substrates by Co-sputtering technique. Thin Solid Film. 2020, 704, 138028. [Google Scholar] [CrossRef]
- Peksu, E.; Karaagac, H. Characterization of Cu2ZnSnS4 thin films deposited by one-step thermal evaporation for a third generation solar cell. J. Alloy. Compd. 2021, 862, 158503. [Google Scholar] [CrossRef]
- Ashfaq, A.; Jacob, J.; Bano, N.; Nabi, M.; Ali, A.; Ahmad, W.; Mahmood, K.; Arshad, M.; Ikram, S.; Rehman, U. A two step technique to remove the secondary phases in CZTS thin films grown by sol-gel method. Ceram. Int. 2019, 45, 10876–10881. [Google Scholar] [CrossRef]
- Özdal, T.; Chtouki, T.; Kavak, H.; Figa, V.; Guichaoua, D.; Erguig, H.; Mysliwiec, J.; Sahraoui, B. Effect of annealing temperature on morphology and optoelectronics properties of spin-coated CZTS thin films. J. Inorg. Organomet. Polym. Mater. 2021, 31, 89–99. [Google Scholar] [CrossRef]
- Pandiyan, R.; Elhmaidi, Z.; Sekkat, Z.; Abd-lefdil, M.; El Khakani, M. Reconstructing the energy band electronic structure of pulsed laser deposited CZTS thin films intended for solar cell absorber applications. Appl. Surf. Sci. 2017, 396, 1562–1570. [Google Scholar] [CrossRef]
- Borate, H.; Bhorde, A.; Waghmare, A.; Nair, S.; Subhash, P.; Punde, A.; Shinde, P.; Vairale, P.; Jadkar, V.; Waykar, R. Single-step electrochemical deposition of CZTS thin films with enhanced photoactivity. ES Mater. Manuf. 2020, 11, 30–39. [Google Scholar] [CrossRef]
- Bermudez, V. An overview on electrodeposited Cu(In, Ga) (Se, S)2 thin films for photovoltaic devices. Sol. Energy 2018, 175, 2–8. [Google Scholar] [CrossRef]
- Jiang, F.; Li, S.; Ozaki, C.; Harada, T.; Ikeda, S. Co-Electrodeposited Cu2ZnSnS4 Thin Film Solar Cell and Cu2ZnSnS4 Solar Cell–BiVO4 Tandem Device for Unbiased Solar Water Splitting. Sol. RRL 2018, 2, 1700205. [Google Scholar] [CrossRef]
- Zhang, Y.; Parnell, A.; Pontecchiani, F.; Cooper, J.; Thompson, R.; Jones, R.; King, S.; Lidzey, D.; Bernardo, G. Understanding and controlling morphology evolution via DIO plasticization in PffBT4T-2OD/PC71BM devices. Sci. Rep. 2017, 7, 44269. [Google Scholar] [CrossRef] [Green Version]
- Mkawi, E.M.; Ibrahim, M.K.M.; Ali, M.; Farrukh, M.; Mohamed, A. Influence of triangle wave pulse on the properties of Cu2ZnSnS4 thin films prepared by single step electrodeposition. Sol. Energy Mater. Sol. Cells 2014, 130, 91–98. [Google Scholar] [CrossRef]
- Maddipatla, D.; Saeed, T.; Narakathu, B.; Obare, S.; Atashbar, M. Incorporating a novel hexaazatriphenylene derivative to a flexible screen-printed electrochemical sensor for copper ion detection in water samples. IEEE Sens. J. 2020, 20, 12582–12591. [Google Scholar] [CrossRef]
- Li, Q.; Fu, J.; Zhu, W.; Chen, Z.; Shen, B.; Wu, L.; Xi, Z.; Wang, T.; Lu, G.; Zhu, J.-J.; et al. Tuning Sn-catalysis for electrochemical reduction of CO2 to CO via the core/shell Cu/SnO2 structure. J. Am. Chem. Soc. 2017, 139, 4290–4293. [Google Scholar] [CrossRef]
- Guha, B.; Islam, F.; Khan, K. Studies on redox equilibrium and electrode potentials. IJARIIE 2018, 4, 1092–1102. [Google Scholar]
- Ansari, M.Z.; Khare, N. Effect of intrinsic strain on the optical band gap of single phase nanostructured Cu2ZnSnS4. Mater. Sci. Semicond. Process. 2017, 63, 220–226. [Google Scholar] [CrossRef]
- Nefzi, C.; Askri, B.; Dabaki, Y.; Kamoun-Turki, N. Synthesis of new promising quaternary Cu2InSnS4 absorber layer: Physical behaviors, wettability and photocatalysis applications. J. Alloy. Compd. 2022, 898, 162771. [Google Scholar] [CrossRef]
- Buldu, D.; Cantas, A.; Turkoglu, F.; Akca, F.; Meric, E.; Ozdemir, M.; Tarhan, E.; Ozyuzer, L.; Aygun, G. Influence of sulfurization temperature on Cu2ZnSnS4 absorber layer on flexible titanium substrates for thin film solar cells. Phys. Scr. 2018, 93, 024002. [Google Scholar] [CrossRef]
- Sawant, J.; Pathan, H.; Kale, R. Photoelectrochemical properties of spray deposited Cu2ZnSnS4 photoelectrode: Enhancement in photoconversion efficiency with film thickness. ES Energy Environ. 2020, 10, 73–79. [Google Scholar] [CrossRef]
- Kaza, J.; Pasumarthi, M.; Avadhani, P. Superstrate and substrate thin film configuration of CdS/CZTS solar cell fabricated using SILAR method. Opt. Laser Technol. 2020, 131, 106413. [Google Scholar] [CrossRef]
- Gadallah, A.-S.; Salim, M.; Atwee, T.; Ghander, A. Effect of Al doping on structural, morphological, optical, and electrical properties of Cu2ZnSnS4 thin films prepared by sol-gel spin coating. Optik 2018, 159, 275–282. [Google Scholar] [CrossRef]
- Babichuk, I.; Semenenko, M.; Caballero, R.; Datsenko, O.; Golovynskyi, S.; Qiu, R.; Huang, C.; Hu, R.; Babichuk, I.; Ziniuk, R. Raman mapping of MoS2 at Cu2ZnSnS4/Mo interface in thin film. Sol. Energy 2020, 205, 154–160. [Google Scholar] [CrossRef]
- Gür, E.; Sarıtaş, S.; Demir, E.; Demir, K.; Coşkun, C. CZTS growth for solar cell application by electrochemical deposition, pH effect. In Proceedings of the 2019 IEEE Regional Symposium on Micro and Nanoelectronics (RSM), IEEE, Putrajaya, Malaysia, 21–23 August 2019; pp. 123–125. [Google Scholar]
- Olgar, M. Optimization of sulfurization time and temperature for fabrication of Cu2ZnSnS4 (CZTS) thin films. Superlattices Microstruct. 2019, 126, 32–41. [Google Scholar] [CrossRef]
- Baskaran, P.; Nisha, K.; Harish, S.; Ikeda, H.; Archana, J.; Navaneethan, M. Enhanced catalytic performance of Cu2ZnSnS4/MoS2 nanocomposites based counter electrode for Pt-free dye-sensitized solar cells. J. Alloy. Compd. 2022, 894, 162166. [Google Scholar] [CrossRef]
- Vishwakarma, M.; Agrawal, K.; Hadermann, J.; Mehta, B. Investigating the effect of sulphurization on volatility of compositions in Cu-poor and Sn-rich CZTS thin films. Appl. Surf. Sci. 2020, 507, 145043. [Google Scholar] [CrossRef]
- Rondiya, S.; Rokade, A.; Jadhavar, A.; Nair, S.; Chaudhari, M.; Kulkarni, R.; Mayabadi, A.; Funde, A.; Pathan, H.; Jadkar, S. Effect of calcination temperature on the properties of CZTS absorber layer prepared by RF sputtering for solar cell applications. Mater. Renew. Sustain. Energy 2017, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Avendaño, C.; Courel, M.; Enríquez, J.; Valderrama, R.; Ayala, A.; De Los Santos, I.; Pérez, J. Formation of SnSSe thin films by heat treatment of SnS thin films in S/Se atmosphere. Mater. Res. Express 2019, 6, 076413. [Google Scholar] [CrossRef]
- Feng, W.; Han, J.; Ge, J.; Peng, X.; Liu, Y.; Jian, Y.; Yuan, L.; Xiong, X.; Cha, L.; Liao, C. Influence of annealing temperature on CZTS thin film surface properties. J. Electron. Mater. 2017, 46, 288–295. [Google Scholar] [CrossRef]
- Fouad, S.; El Radaf, I.; Sharma, P.; El-Bana, M. Multifunctional CZTS thin films: Structural, optoelectrical, electrical and photovoltaic properties. J. Alloy. Compd. 2018, 757, 124–133. [Google Scholar] [CrossRef]
- Thiruvenkadam, S.; Jovina, D.; Rajesh, A. The influence of deposition temperature in the photovoltaic properties of spray deposited CZTS thin films. Sol. Energy 2014, 106, 166–170. [Google Scholar] [CrossRef]
- Vanalakar, S.; Agwane, G.; Gang, M.; Patil, P.; Kim, J.; Kim, J. A mild hydrothermal route to synthesis of CZTS nanoparticle inks for solar cell applications. Phys. Status Solidi C 2015, 12, 500–503. [Google Scholar] [CrossRef]
- Bencherif, H.; Dehimi, L.; Mahsar, N.; Kouriche, E.; Pezzimenti, F. Modeling and optimization of CZTS kesterite solar cells using TiO2 as efficient electron transport layer. Mater. Sci. Eng. B 2022, 276, 115574. [Google Scholar] [CrossRef]
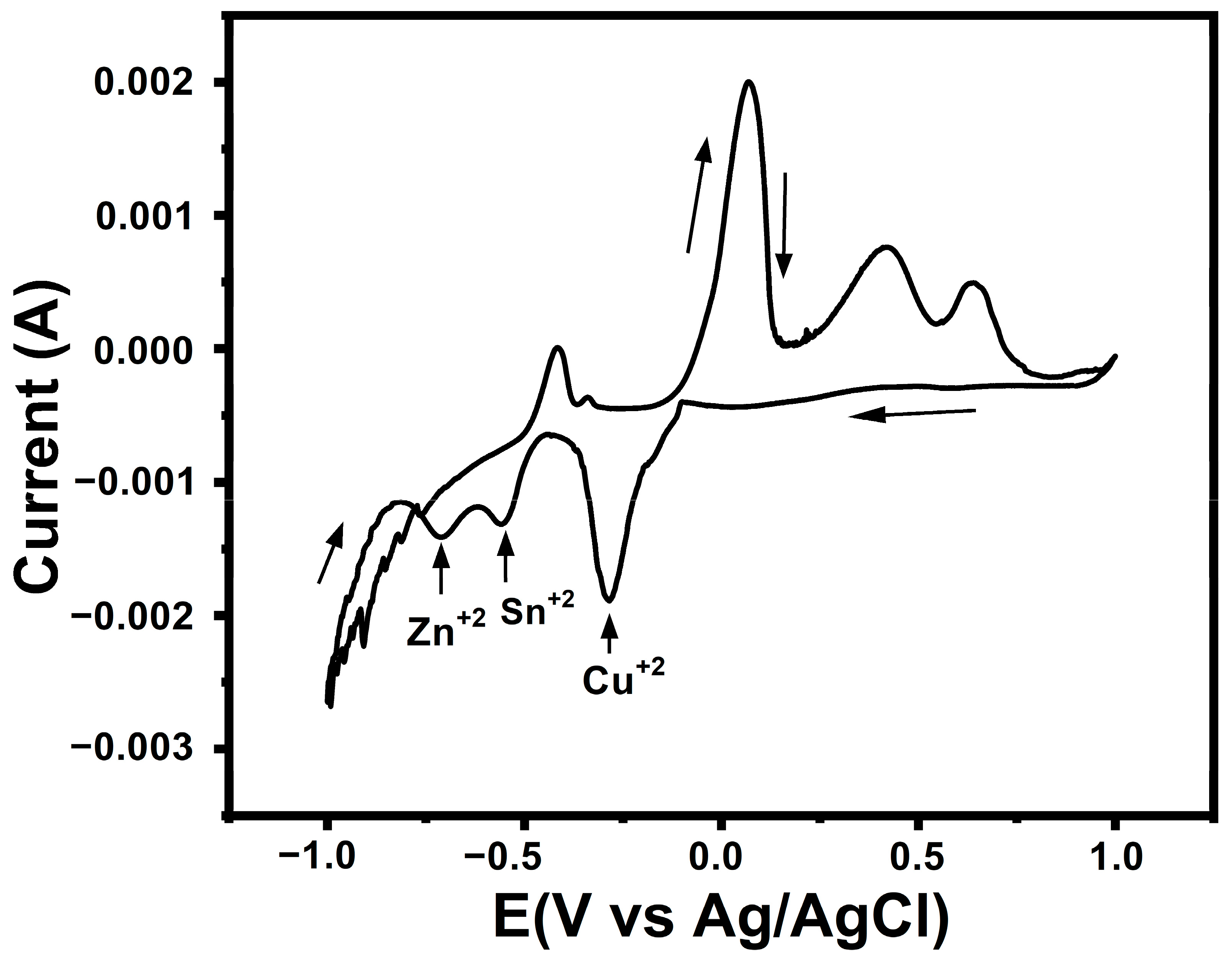

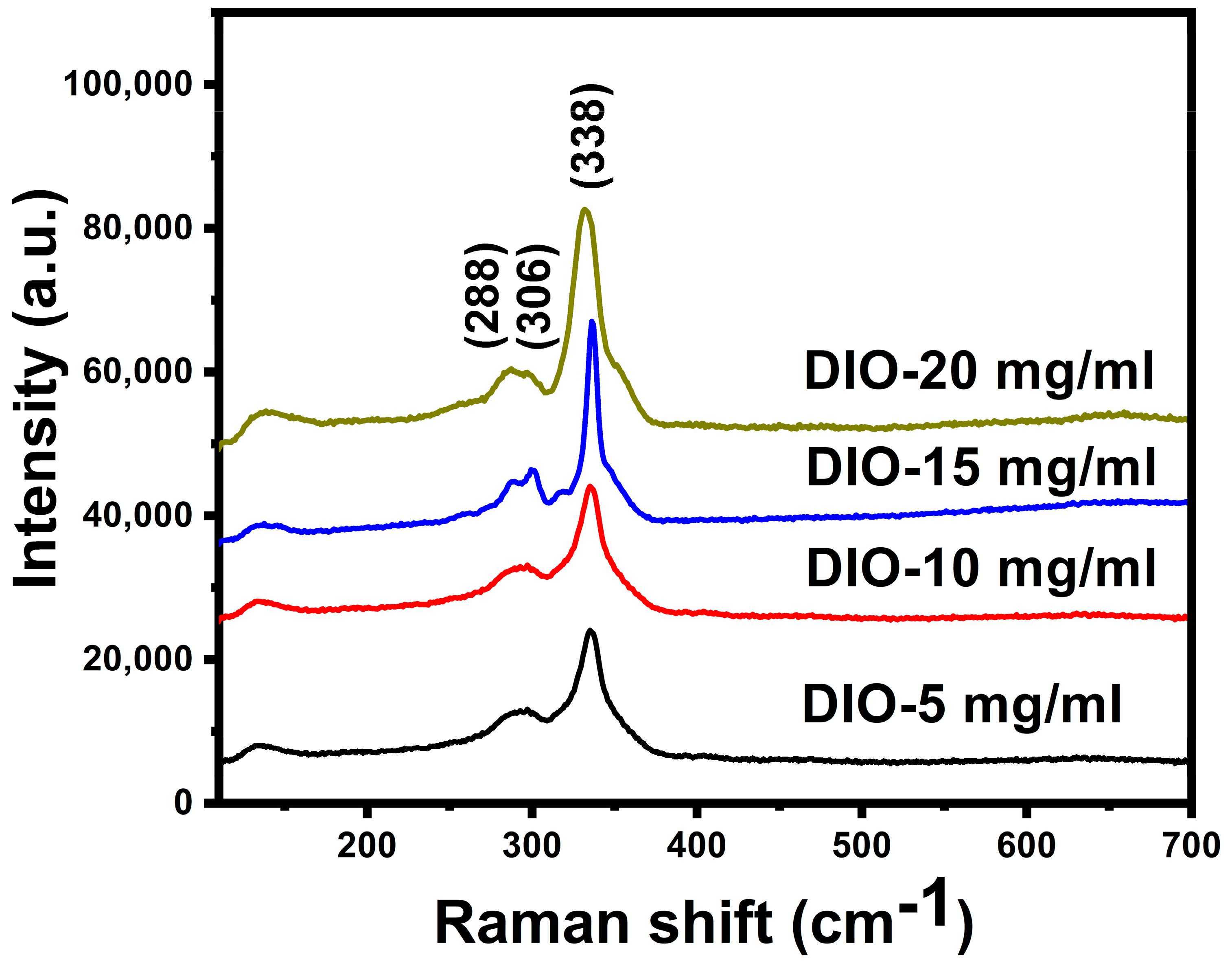
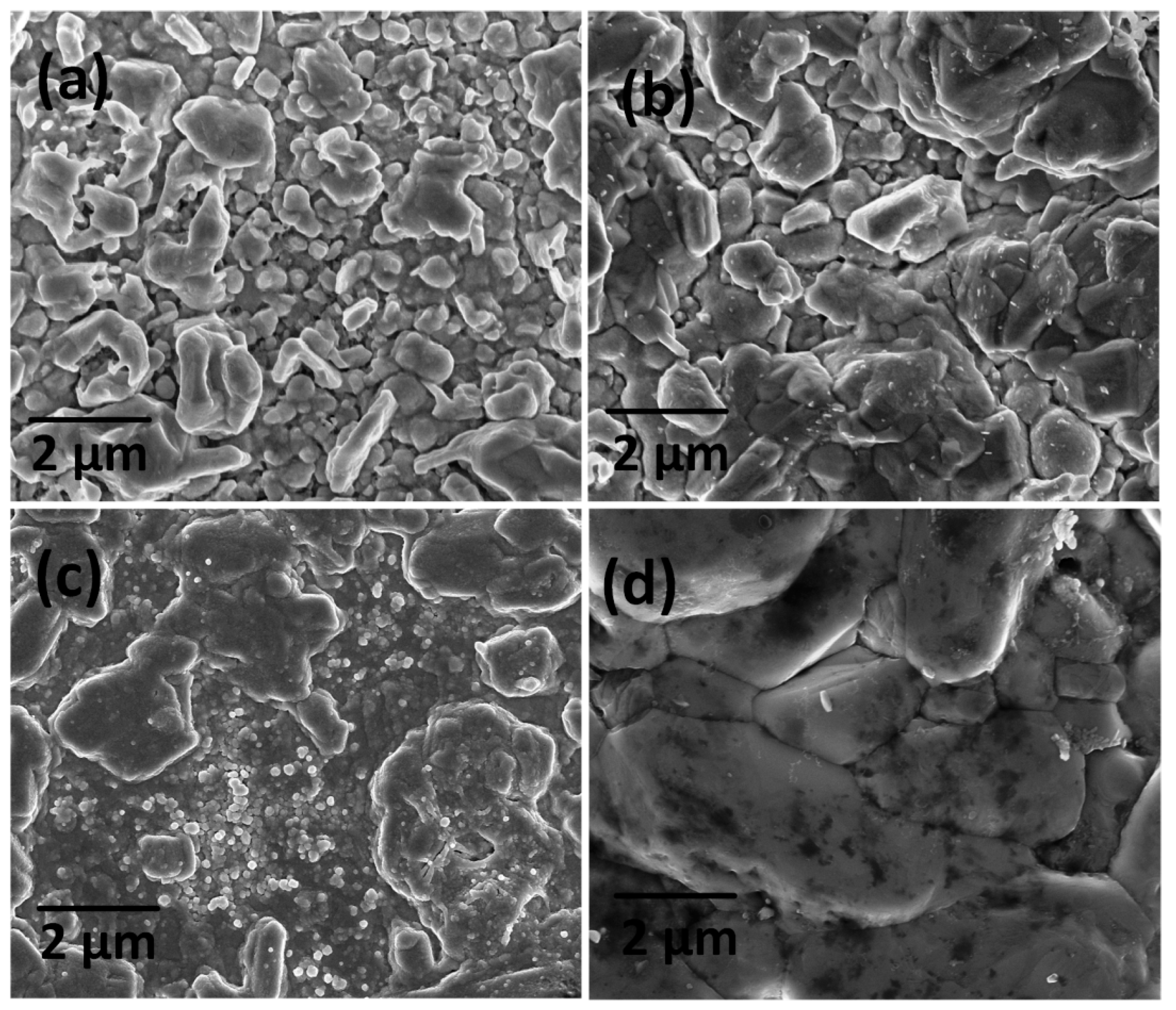
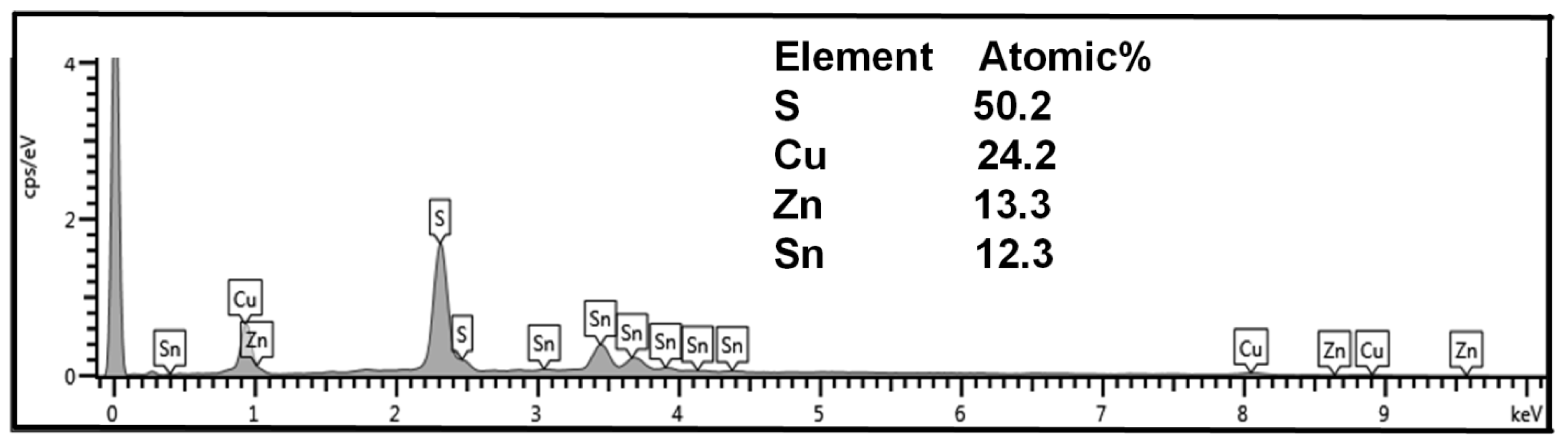
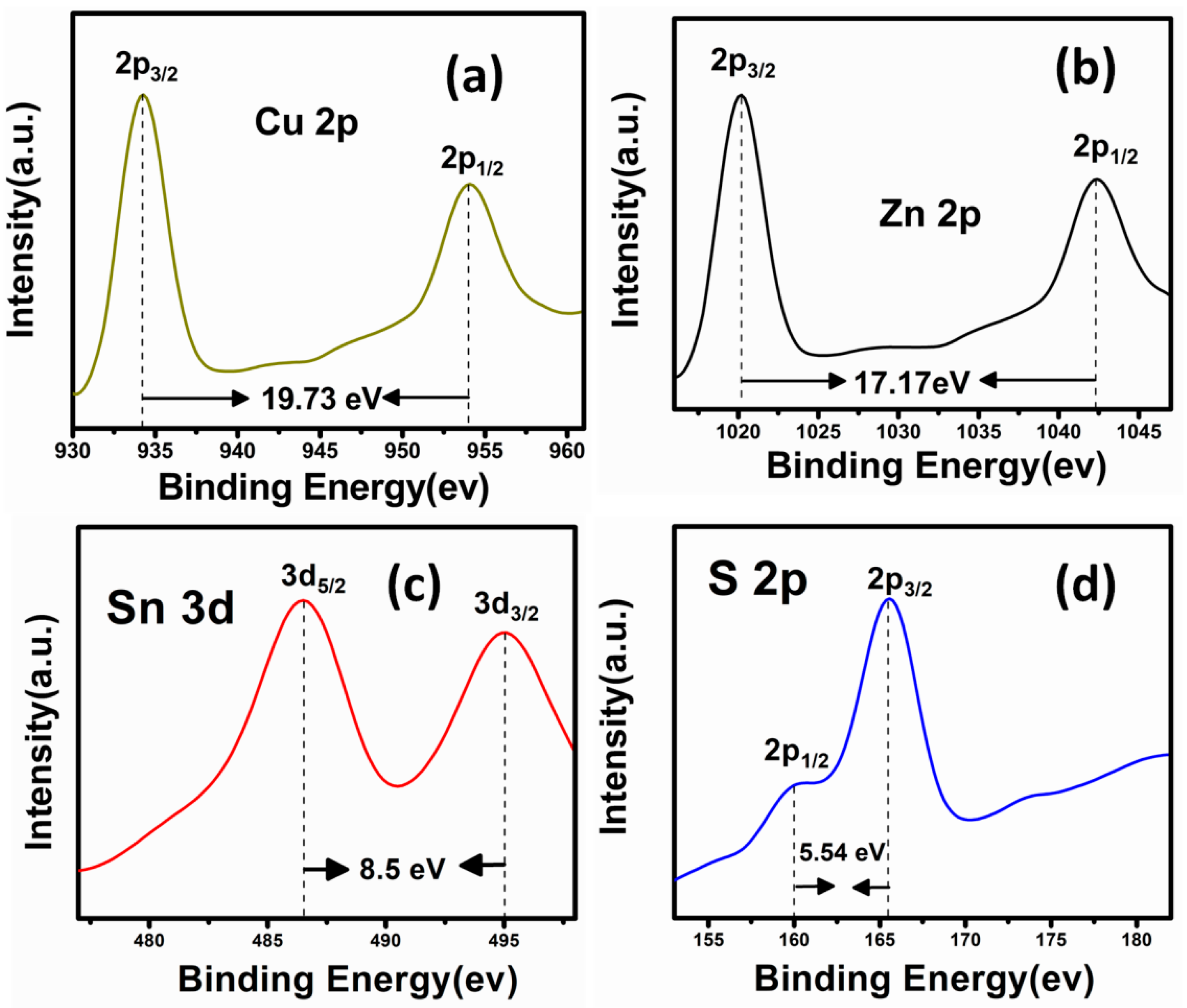
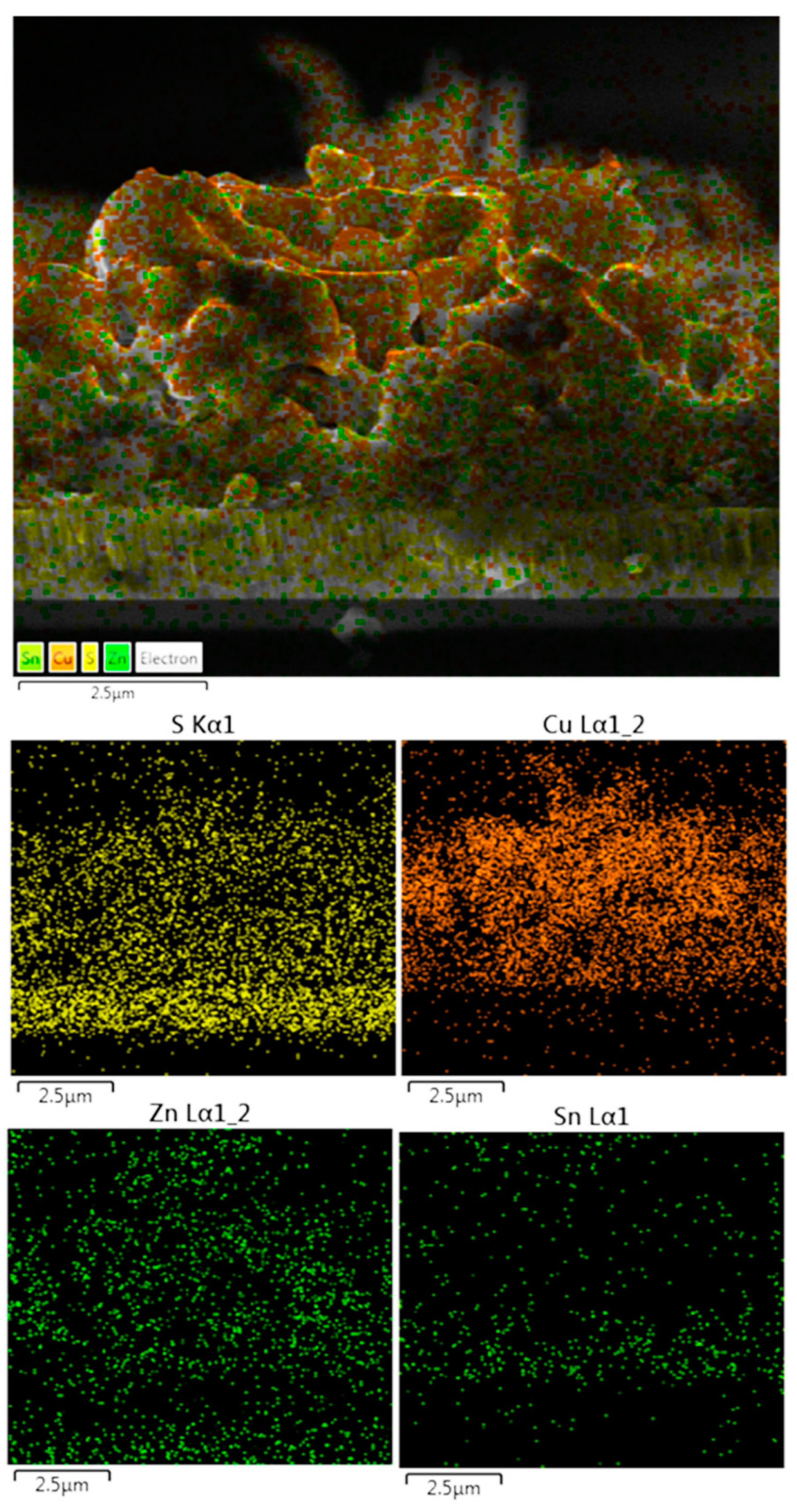
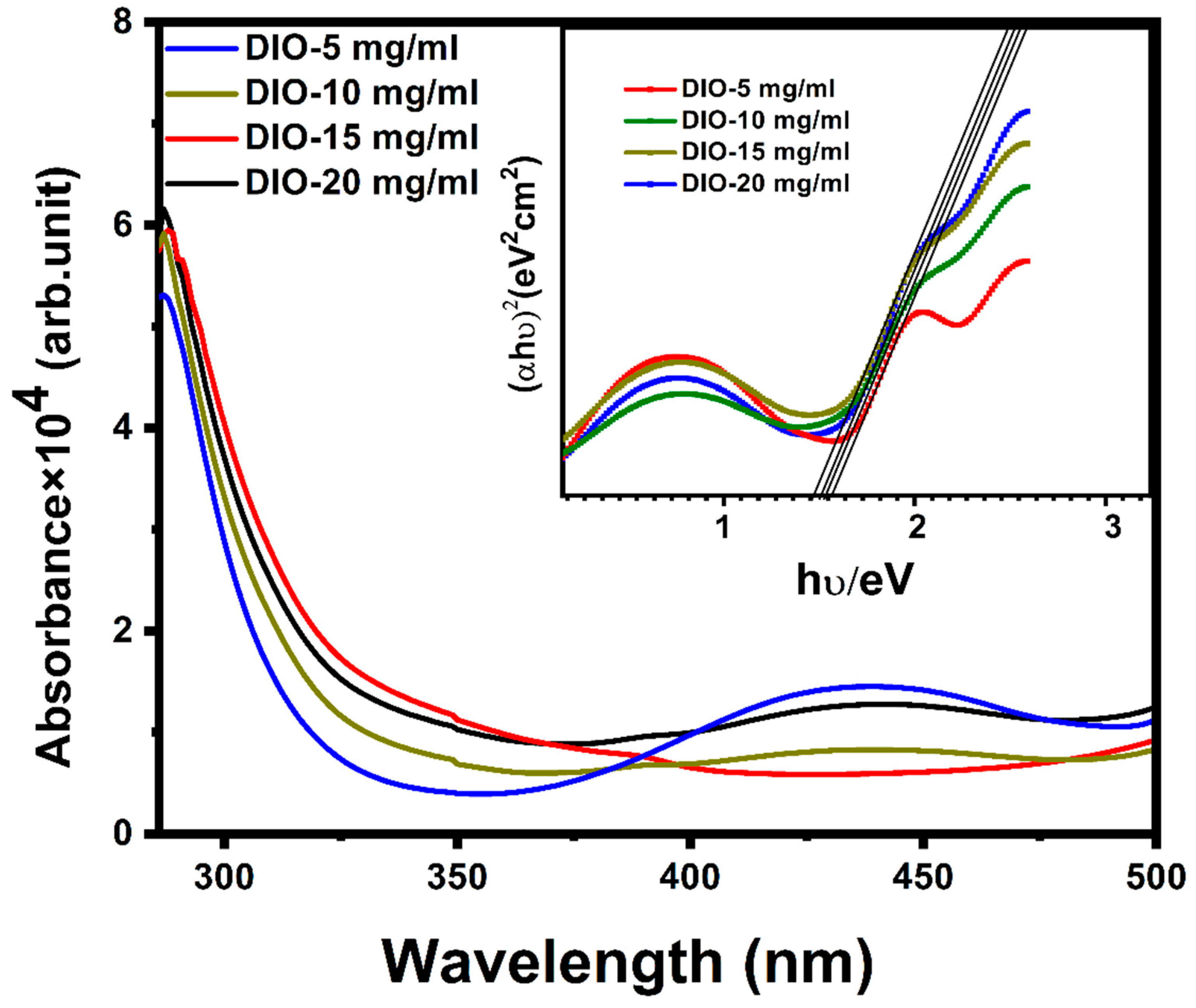
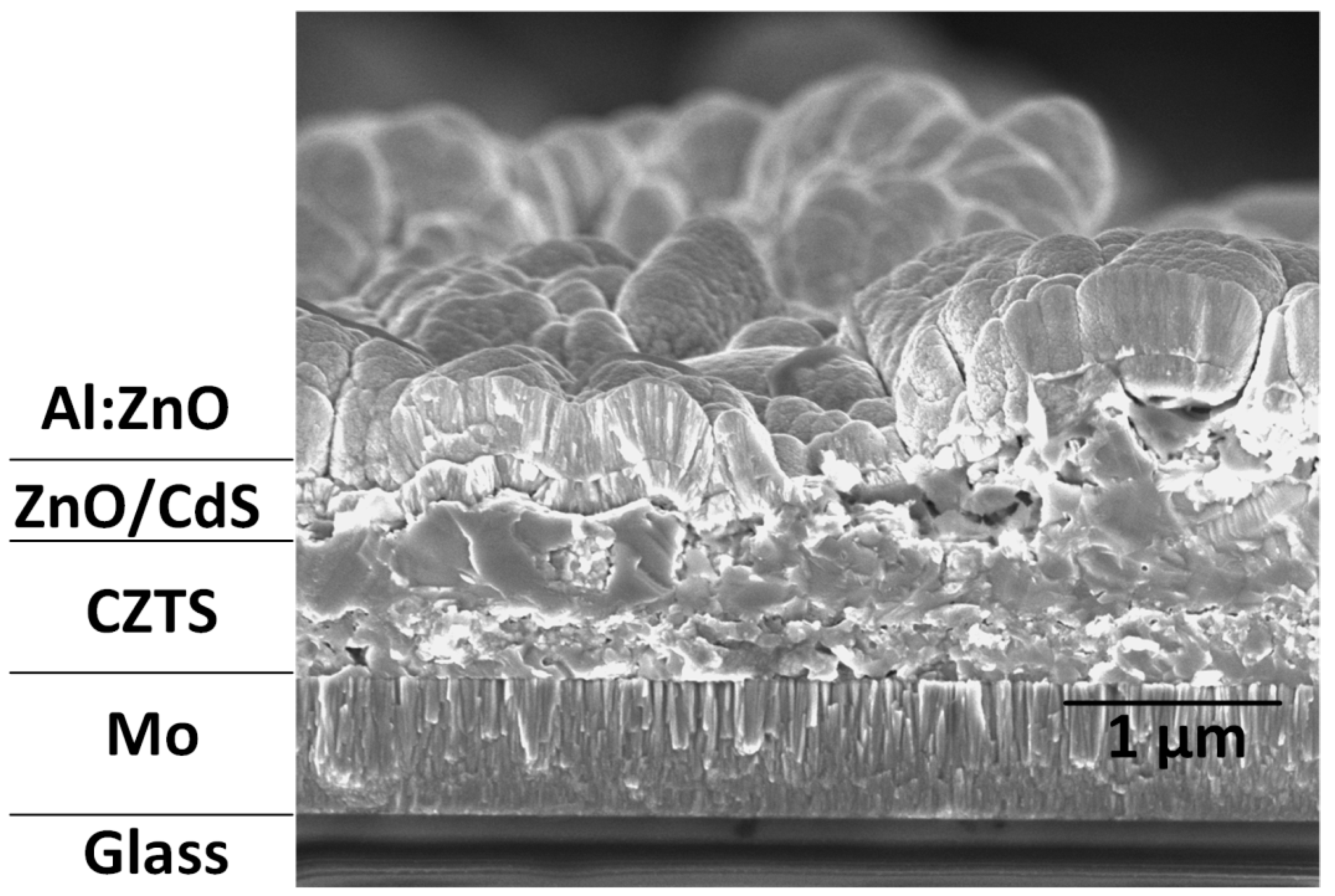

| Sample | Cu (at%) | Zn (at%) | Sn (at%) | S (at%) | Cu/(Zn + Sn) | Zn/Sn |
|---|---|---|---|---|---|---|
| DIO-5 mg/mL | 26.5 | 12.8 | 13.2 | 48.3 | 1.03 | 0.97 |
| DIO-10 mg/mL | 25.6 | 12.9 | 12.9 | 48.5 | 0.99 | 1.00 |
| DIO-15 mg/ml | 24.7 | 13.1 | 12.5 | 49.6 | 0.96 | 1.04 |
| DIO-20 mg/mL | 24.2 | 13.3 | 12.3 | 50.2 | 0.94 | 1.08 |
| Sample Voc (mV) | Jsc (mA/cm2) FF (%) | η (%) Rs (Ω cm2) | Rsh (Ω cm2) |
|---|---|---|---|
| DIO-5 mg/mL 432 | 11.74 61.3 | 2.36 23.37 | 443.2 |
| DIO-10 mg/mL 433 | 14.35 63.3 | 3.84 21.44 | 635.6 |
| DIO-15 mg/mL 445 | 15.88 64.1 | 4.52 16.25 | 774.4 |
| DIO-20 mg/mL 456 | 16.44 64.3 | 4.82 14.27 | 958.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkawi, E.M.; Al-Hadeethi, Y.; Arkook, B.; Bekyarova, E. Impact of 1,8-Diiodooctane (DIO) Additive on the Active Layer Properties of Cu2ZnSnS4 Kesterite Thin Films Prepared by Electrochemical Deposition for Photovoltaic Applications. Materials 2023, 16, 1659. https://doi.org/10.3390/ma16041659
Mkawi EM, Al-Hadeethi Y, Arkook B, Bekyarova E. Impact of 1,8-Diiodooctane (DIO) Additive on the Active Layer Properties of Cu2ZnSnS4 Kesterite Thin Films Prepared by Electrochemical Deposition for Photovoltaic Applications. Materials. 2023; 16(4):1659. https://doi.org/10.3390/ma16041659
Chicago/Turabian StyleMkawi, Elmoiz Merghni, Yas Al-Hadeethi, Bassim Arkook, and Elena Bekyarova. 2023. "Impact of 1,8-Diiodooctane (DIO) Additive on the Active Layer Properties of Cu2ZnSnS4 Kesterite Thin Films Prepared by Electrochemical Deposition for Photovoltaic Applications" Materials 16, no. 4: 1659. https://doi.org/10.3390/ma16041659





