The Influence of Titanium Oxide Nanoparticles and UV Radiation on the Electrical Properties of PEDOT:PSS-Coated Cotton Fabrics
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Fabrication of Conductive Cotton
2.3. Characterization Method
3. Results and Discussion
3.1. Effect of TiO2 on the Electrical Properties
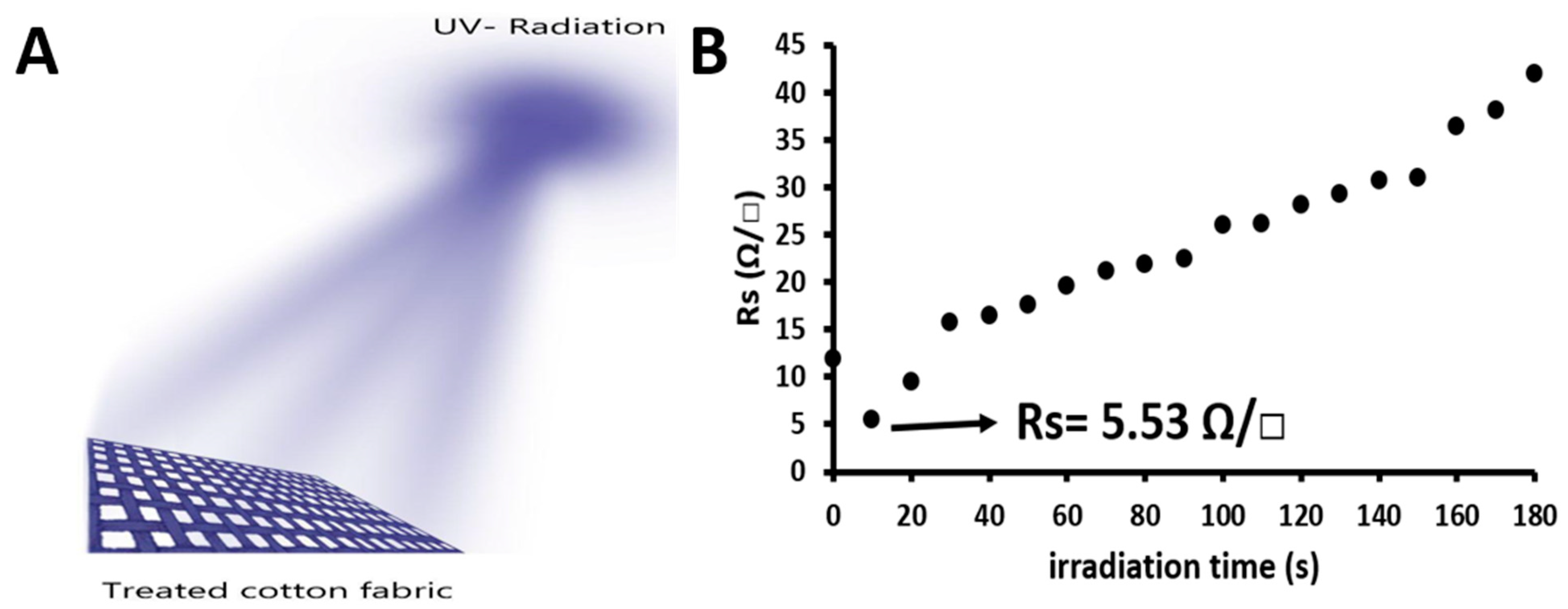
3.2. Effect of UV Irradiation on the Electrical Properties
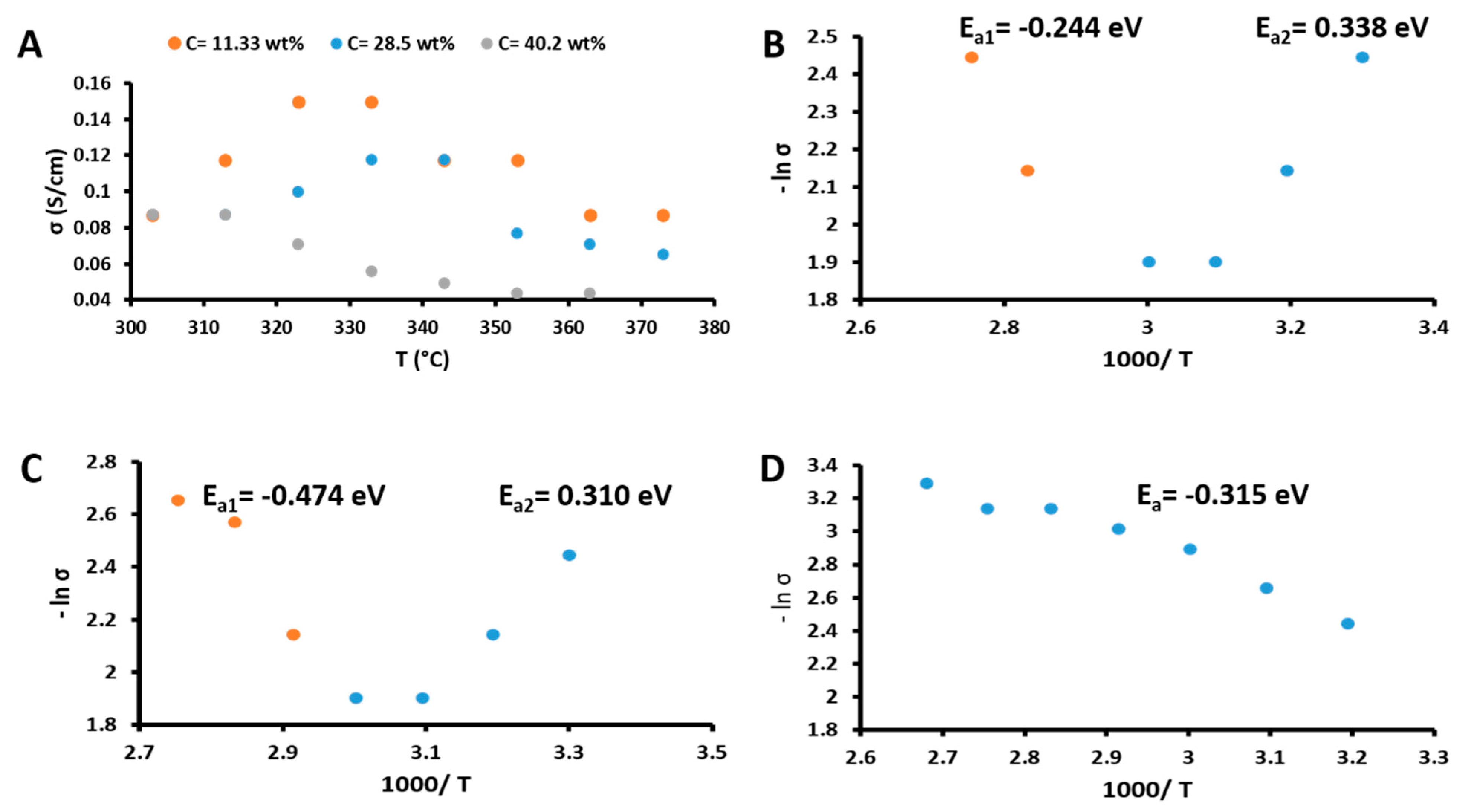
3.3. Electrical Conductivity as a Function of Temperature
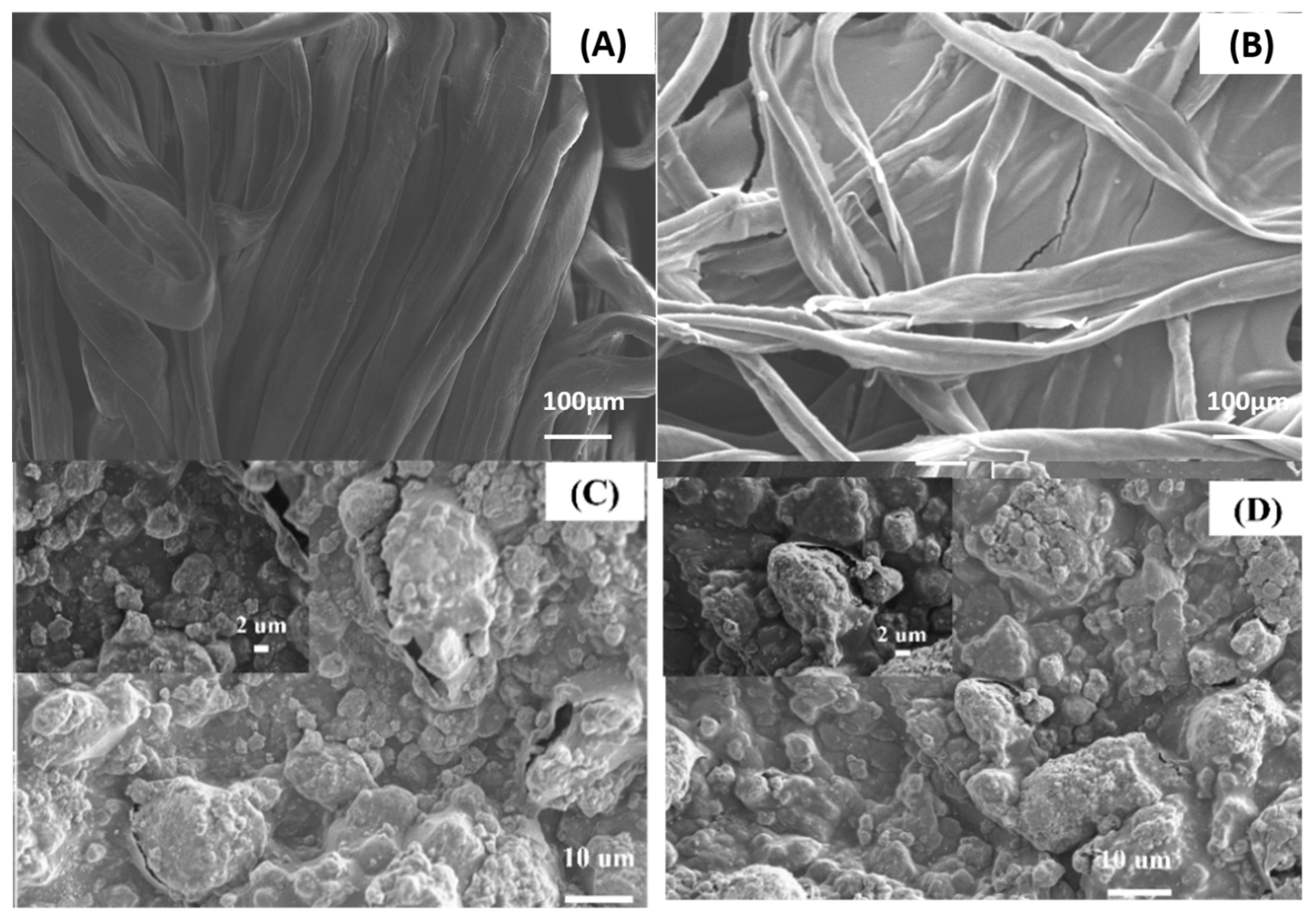
3.4. Morphological Characterization
3.5. Elemental Analysis
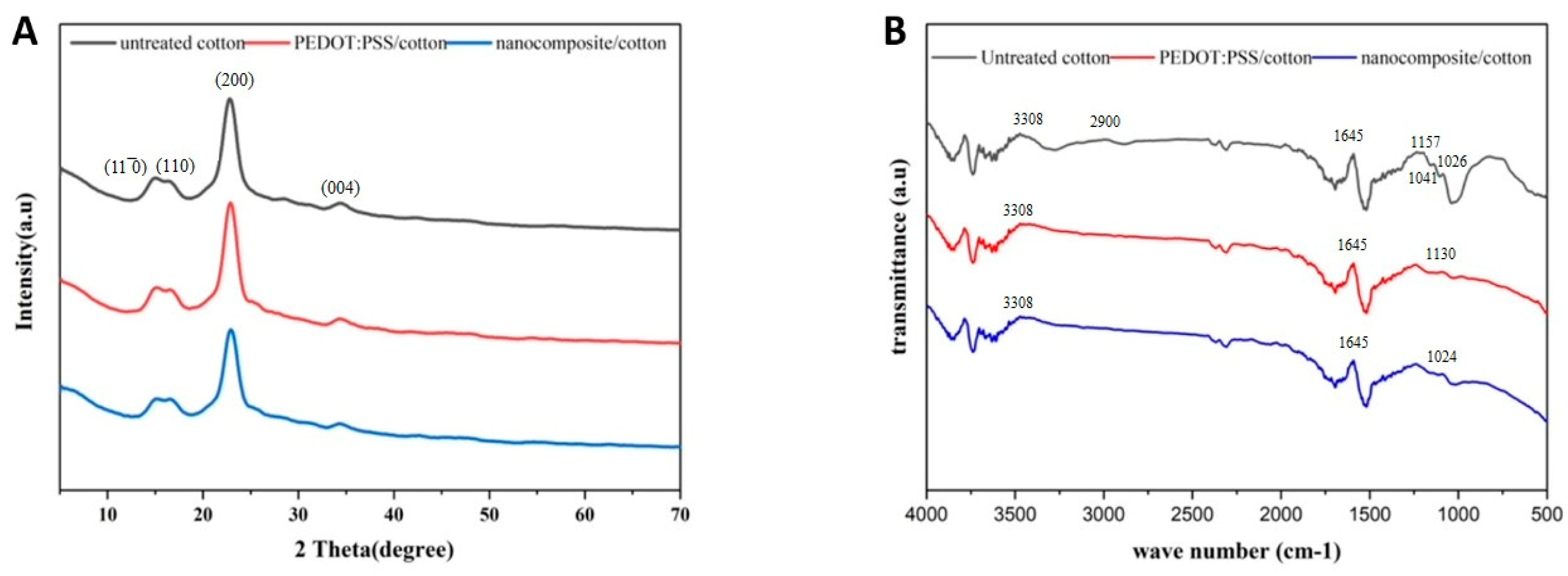
3.6. XRD Analysis
3.7. FTIR Analysis
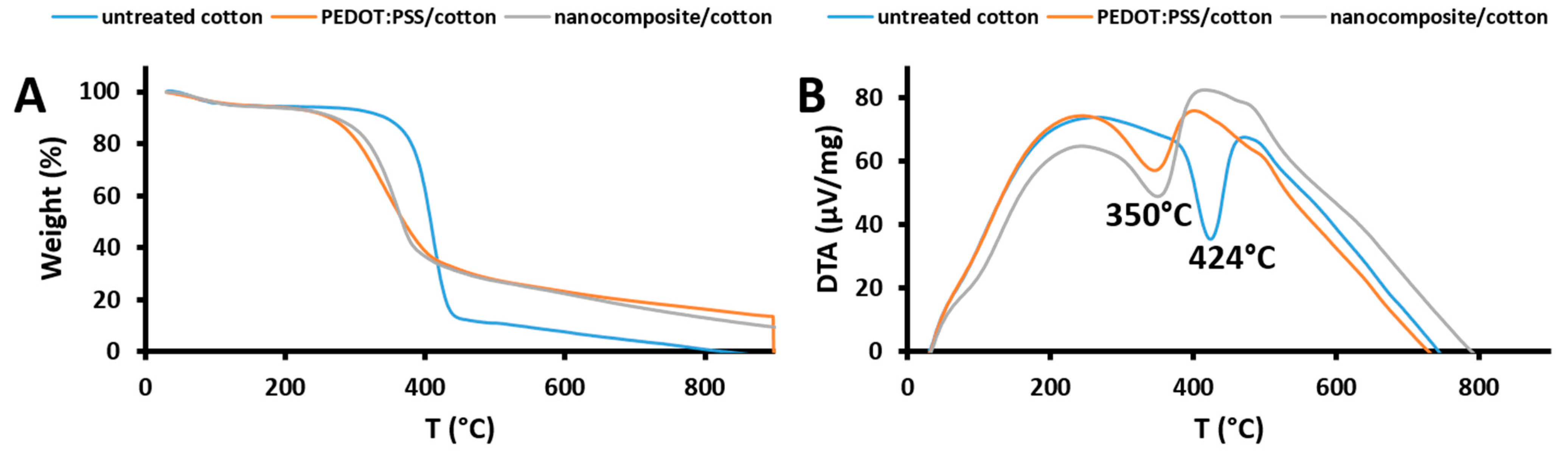
3.8. Thermal Analysis
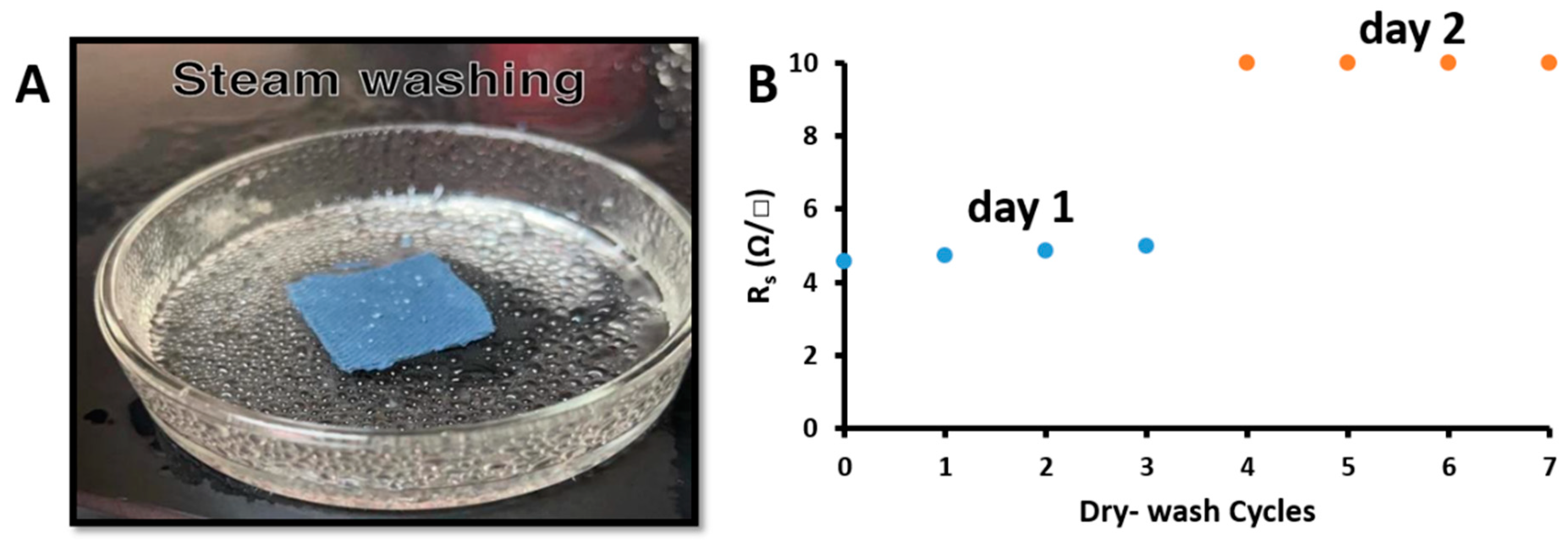
3.9. Washability
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef] [PubMed]
- Barman, J.; Tirkey, A.; Batra, S.; Paul, A.A.; Panda, K.; Deka, R.; Babu, P.J. The Role of Nanotechnology Based Wearable Electronic Textiles in Biomedical and Healthcare Applications. Mater. Today Commun. 2022, 32, 104055. [Google Scholar] [CrossRef]
- Mondal, S. Nanomaterials for UV protective textiles. J. Ind. Text. 2022, 51, 5592S–5621S. [Google Scholar] [CrossRef]
- Kiran, S.; Meda, U.S.; Ranga, S.; Basavaraja, R.J. Advances in Incorporation of Nanomaterials Onto Fabrics. ECS Trans. 2022, 107, 4853–4862. [Google Scholar] [CrossRef]
- Visakh, P.M.; Semkin, A.; Balakrishnan, R.; Lazovic, S. Nanotechnology in Electronics: Materials, Properties, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Zhu, Q.; Chua, M.H.; Ong, P.J.; Lee, J.J.C.; Chin, K.L.O.; Wang, S.; Kai, D.; Ji, R.; Kong, J.; Dong, Z.; et al. Recent advances in nanotechnology-based functional coatings for the built environment. Mater. Today Adv. 2022, 15, 100270. [Google Scholar] [CrossRef]
- Gautam, S.; Mishra, A.; Koundal, P. Nanotechnology for Functional/High-Performance/Smart Textiles. In Synthesis and Applications of Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2022; pp. 525–534. [Google Scholar]
- Goharshadi, E.K.; Goharshadi, K.; Moghayedi, M. The use of nanotechnology in the fight against viruses: A critical review. Coord. Chem. Rev. 2022, 464, 214559. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Dable-Tupas, G.; De Guzman, M.B. Applications of nanotechnology in pharmaceutical products. In Applications of Nanotechnology in Drug Discovery and Delivery; Elsevier: Berlin/Heidelberg, Germany, 2022; pp. 119–156. [Google Scholar]
- Dar, T.B.; Bhat, A.R.; Biteghe, F.A.N.; Bhat, A.R.; Malindi, Z. Nanotechnology and Nanomedicine. In Fundamentals and Advances in Medical Biotechnology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 325–361. [Google Scholar]
- Honarvar, M.G.; Latifi, M. Overview of wearable electronics and smart textiles. J. Text. Inst. 2017, 108, 631–652. [Google Scholar] [CrossRef]
- Chen, X.; An, J.; Cai, G.; Zhang, J.; Chen, W.; Dong, X.; Zhu, L.; Tang, B.; Wang, J.; Wang, X. Environmentally friendly flexible strain sensor from waste cotton fabrics and natural rubber latex. Polymers 2019, 11, 404. [Google Scholar] [CrossRef] [Green Version]
- Alamer, F.A. Structural and electrical properties of conductive cotton fabrics coated with the composite polyaniline/carbon black. Cellulose 2018, 25, 2075–2082. [Google Scholar] [CrossRef]
- Maráková, N.; Humpolíček, P.; Kašpárková, V.; Capáková, Z.; Martinková, L.; Bober, P.; Trchová, M.; Stejskal, J. Antimicrobial activity and cytotoxicity of cotton fabric coated with conducting polymers, polyaniline or polypyrrole, and with deposited silver nanoparticles. Appl. Surf. Sci. 2017, 396, 169–176. [Google Scholar] [CrossRef]
- Güneşoğlu, C.; Güneşoğlu, S.; Wei, S.; Guo, Z. The enhanced electrical conductivity of cotton fabrics via polymeric nanocomposites. Fibers Polym. 2016, 17, 402–407. [Google Scholar] [CrossRef]
- Alamer, F.A.; Beyari, R.F. Overview of the Influence of Silver, Gold, and Titanium Nanoparticles on the Physical Properties of PEDOT:PSS-Coated Cotton Fabrics. Nanomaterials 2022, 12, 1609. [Google Scholar] [CrossRef]
- Perelshtein, I.; Applerot, G.; Perkas, N.; Grinblat, J.; Gedanken, A. A one-step process for the antimicrobial finishing of textiles with crystalline TiO2 nanoparticles. Chem. Eur. J. 2012, 18, 4575–4582. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Amparán, M.A.; Martínez-Cornejo, V.; Cedeño-Caero, L.; Hernandez-Hernandez, K.A.; Cadena-Nava, R.D.; Alonso-Núñez, G.; Moyado, S.F. Characterization and photocatalytic activity of TiO2 nanoparticles on cotton fabrics, for antibacterial masks. Appl. Nanosci. 2022, 12, 4019–4032. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, M.; Kazerouni, Y.; Mohammadi, A.; Hashemi, M.; Hejazi, I.; Seyfi, J.; Khonakdar, H.A.; Davachi, S.M. Superhydrophobic cotton fabrics coated by chitosan and titanium dioxide nanoparticles with enhanced antibacterial and UV-protecting properties. Int. J. Biol. Macromol. 2021, 171, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Yu, S.; Amirfazli, A.; Siddiqui, A.R.; Li, W. Recent advances in antibacterial superhydrophobic coatings. Adv. Eng. Mater. 2022, 24, 2101053. [Google Scholar] [CrossRef]
- Stan, M.S.; Nica, I.C.; Popa, M.; Chifiriuc, M.C.; Iordache, O.; Dumitrescu, I.; Diamandescu, L.; Dinischiotu, A. Reduced graphene oxide/TiO2 nanocomposites coating of cotton fabrics with antibacterial and self-cleaning properties. J. Ind. Text. 2019, 49, 277–293. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Shaarawy, S.; Abdel-Aziz, M.S.; Katry, H.A.E.M.; Youssef, A.M. Functionalization of cotton fabrics with titanium oxide doped silver nanoparticles: Antimicrobial and UV protection activities. Luminescence 2022, 37, 854–864. [Google Scholar] [CrossRef]
- Zahid, M.; Papadopoulou, E.L.; Suarato, G.; Binas, V.D.; Kiriakidis, G.; Gounaki, I.; Moira, O.; Venieri, D.; Bayer, I.S.; Athanassiou, A. Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review. Text. Res. J. 2004, 1, 1154–1164. [Google Scholar] [CrossRef]
- Alamer, F.A.; Althagafy, K.; Alsalmi, O.; Aldeih, A.; Alotaiby, H.; Althebaiti, M.; Alghamdi, H.; Alotibi, N.; Saeedi, A.; Zabarmawi, Y.; et al. Review on PEDOT:PSS-Based Conductive Fabric. ACS Omega 2022, 7, 35371–35386. [Google Scholar] [CrossRef]
- Elschner, A.; Kirchmeyer, S.; Lovenich, W.; Merker, U.; Reuter, K. PEDOT: Principles and Applications of an Intrinsically Conductive Polymer; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Alamer, F.A.; Badawi, N.M.; Alodhayb, A.; Okasha, R.M.; Kattan, N.A. Effect of dopant on the conductivity and stability of three different cotton fabrics impregnated with PEDOT:PSS. Cellulose 2020, 27, 531–543. [Google Scholar] [CrossRef]
- Eslamian, M.; Soltani-Kordshuli, F. Development of multiple-droplet drop-casting method for the fabrication of coatings and thin solid films. J. Coatings Technol. Res. 2018, 15, 271–280. [Google Scholar] [CrossRef]
- Ghosh, S.; Ganguly, S.; Remanan, S.; Das, N.C. Fabrication and investigation of 3D tuned PEG/PEDOT: PSS treated conductive and durable cotton fabric for superior electrical conductivity and flexible electromagnetic interference shielding. Compos. Sci. Technol. 2019, 181, 107682. [Google Scholar] [CrossRef]
- Maithani, Y.; Singh, A.; Mehta, B.R.; Singh, J.P. PEDOT: PSS treated cotton-based textile dry electrode for ECG sensing. Mater. Today Proc. 2022, 62, 4052–4057. [Google Scholar] [CrossRef]
- Alvarado, A.E.S. A Flexible Demonstrator Composed of a Printed Integrated Circuit Based on OFETs, a Flexible Battery and an Electrochromic Display, as a Precursor of a Fully-Printed Smart Label for Metered-Dose Inhalers. Ph.D. Thesis, Politecnico di Torino, Turin, Italy, 2019. [Google Scholar]
- Tseghai, G.B.; Mengistie, D.A.; Malengier, B.; Fante, K.A.; Van Langenhove, L. PEDOT: PSS-based conductive textiles and their applications. Sensors 2020, 20, 1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuksel, R.; Unalan, H.E. Textile supercapacitors-based on MnO2/SWNT/conducting polymer ternary composites. Int. J. Energy Res. 2015, 39, 2042–2052. [Google Scholar] [CrossRef]
- Alamer, F.A.; Badawi, N.M. Fully Flexible, Highly Conductive Threads Based on single walled carbon nanotube (SWCNTs) and poly (3, 4 ethylenedioxy thiophene) poly (styrenesulfonate)(PEDOT: PSS). Adv. Eng. Mater. 2021, 23, 2100448. [Google Scholar] [CrossRef]
- Darvishzadeh, A.; Nasouri, K. Manufacturing, modeling, and optimization of nickel-coated carbon fabric for highly efficient EMI shielding. Surf. Coatings Technol. 2021, 409, 126957. [Google Scholar] [CrossRef]
- Alamer, F.A.; Alnefaie, M.A.; Salam, M.A. Preparation and characterization of multi-walled carbon nanotubes-filled cotton fabrics. Results Phys. 2022, 33, 105205. [Google Scholar] [CrossRef]
- Alamer, F.A. Highly Conductive Flexible Conductor Based on PEDOT: PSS/MWCNTs Nano Composite. Crystals 2023, 13, 192. [Google Scholar] [CrossRef]
- Alamer, F.A.; Otley, M.T.; Ding, Y.; Sotzing, G.A. Solid-State High-Throughput Screening for Color Tuning of Electrochromic Polymers. Adv. Mater. 2013, 25, 6256–6260. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Y.; Xu, Q.; Chen, Q.; Zhang, Y.; Xu, Z. Fabrication of durable and conductive cotton fabric using silver nanoparticles and PEDOT: PSS through mist polymerization. Appl. Surf. Sci. 2022, 592, 153314. [Google Scholar] [CrossRef]
- Kumar Anbalagan, A.; Gupta, S.; Chaudhary, M.; Kumar, R.R.; Chueh, Y.L.; Tai, N.H.; Lee, C.H. Consequences of gamma-ray irradiation on structural and electronic properties of PEDOT: PSS polymer in air and vacuum environments. RSC Adv. 2021, 11, 20752–20759. [Google Scholar] [CrossRef]
- Tang, F.C.; Chang, J.; Wu, F.C.; Cheng, H.L.; Hsu, S.L.C.; Chen, J.S.; Chou, W.Y. Alignment of poly(3,4-ethylenedioxythiophene) polymer chains in photovoltaic cells by ultraviolet irradiation. J. Mater. Chem. 2012, 22, 22409–22417. [Google Scholar] [CrossRef]
- Mechiakh, R.; Sedrine, N.B.; Chtourou, R.; Bensaha, R. Correlation between microstructure and optical properties of nano-crystalline TiO2 thin films prepared by sol–gel dip coating. Appl. Surf. Sci. 2010, 257, 670–676. [Google Scholar] [CrossRef]
- Hiremath, R.K.; Rabinal, M.K.; Mulimani, B.G. Simple setup to measure electrical properties of polymeric films. Rev. Sci. Instrum. 2006, 77, 126106. [Google Scholar] [CrossRef]
- Cho, E.-C.; Li, C.-P.; Huang, J.-H.; Lee, K.-C.; Huang, J.-H. Three-dimensional conductive nanocomposites based on multiwalled carbon nanotube networks and PEDOT: PSS as a flexible transparent electrode for optoelectronics. ACS Appl. Mater. Interfaces 2015, 7, 11668–11676. [Google Scholar] [CrossRef]
- Tanaka, S.; Zakhidov, A.; Ovalle-Robles, R.; Yoshida, Y.; Hiromitsu, I.; Fujita, Y.; Yoshino, K. Semitransparent organic photovoltaic cell with carbon nanotube-sheet anodes and Ga-doped ZnO cathodes. Synth. Met. 2009, 159, 2326–2328. [Google Scholar] [CrossRef]
- Otley, M.T.; Alamer, F.A.; Guo, Y.; Santana, J.; Eren, E.; Li, M.; Lombardi, J.; Sotzing, G.A. Phase segregation of PEDOT: PSS on textile to produce materials of >10 A mm—2 current carrying capacity. Macromol. Mater. Eng. 2017, 302, 1600348. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Feng, G.; Ge, M. On the mechanism of conductivity enhancement in PEDOT: PSS/PVA blend fiber induced by UV-light irradiation. Appl. Phys. A 2020, 126, 1–7. [Google Scholar]
- Acharya, S.; Hu, Y.; Abidi, N. Cellulose dissolution in ionic liquid under mild conditions: Effect of hydrolysis and temperature. Fibers 2021, 9, 5. [Google Scholar] [CrossRef]
- Nindiyasari, F.; Griesshaber, E.; Zimmermann, T.; Manian, A.; Randow, C.; Zehbe, R.; Fernández-Díaz, L.; Ziegler, A.; Fleck, C.; Schmahl, W.W. Characterization and mechanical properties investigation of the cellulose/gypsum composite. J. Compos. Mater. 2016, 50, 657–672. [Google Scholar] [CrossRef] [Green Version]
- Garside, P.; Wyeth, P. Identification of cellulosic fibres by FTIR spectroscopy-thread and single fibre analysis by attenuated total reflectance. Stud. Conserv. 2003, 48, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Khasim, S.; Pasha, A.; Badi, N.; Lakshmi, M.; Al-Ghamdi, S.A.; AL-Aoh, H.A. PVA Treated PEDOT-PSS: TiO2 Nanocomposite Based High-Performance Sensors towards Detection of Relative Humidity and Soil Moisture Content for Agricultural Applications. J. Polym. Environ. 2021, 29, 612–623. [Google Scholar] [CrossRef]


| Sample | C(wt%) of the Dope PEDOT:PSS | C(wt%) of TiO2 | Rs (Ω/□) |
|---|---|---|---|
| 1 | 9.11 | 0 | 20.71 |
| 2 | 9.11 | 2.22 | 3.85 |
| 3 | 9.11 | 2.92 | 2.68 |
| 4 | 9.11 | 3.39 | 3.86 |
| 5 | 9.11 | 5.29 | 5.46 |
| 6 | 9.11 | 13.29 | 13.57 |
| Substrate | Nanocomposite Materials | Rs (Ω/□) | Ref. |
|---|---|---|---|
| Cotton | PEDOT:PSS without nanoparticles | 20.70 | This work |
| films | PEDOT:PSS/MWCNTs | 51 | [43] |
| Quartz | PEDOT:PSS/MWCNTs | ~500–700 | [44] |
| PET | PEDOT:PSS/SiO2 | 3.20 | [45] |
| Cotton | PEDOT:PSS/TiO2 | 13.57 | This work |
| Chemical Bond | Bond Energy (kJ/mol) |
|---|---|
| C–C | 347.7 |
| C–S | 370 |
| C–O | 351.5 |
| TiO2 Concentration (wt%) | Activation Energy (eV) | |
|---|---|---|
| Ea1 (303–333 K) | Ea2 (343–373 K) | |
| 2.22 | 0.3378 | −0.2438 |
| 19.39 | 0.3100 | −0.4738 |
| 31.09 | −0.315 | |
| Untreated Cotton | Cotton Treated with PEDOT:PSS | |||||||
|---|---|---|---|---|---|---|---|---|
| Element Line | E (keV) | M (%) | At (%) | Error | E (keV) | M (%) | At (%) | Error |
| C-K | 0.277 | 37.73 | 44.66 | 0.277 | 50.74 | 58.79 | ||
| O-K | 0.525 | 62.27 | 55.34 | 0.525 | 45.51 | 39.58 | ||
| S-K | - | - | - | 2.307 | 3.75 | 1.63 | ||
| Cotton Treated with Nanocomposite with 2.22 wt% TiO2 | Cotton Treated with Nanocomposite with 13.29 wt% TiO2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Element Line | E (keV) | M (%) | At (%) | Error | E (keV) | M (%) | At (%) | Error |
| C-K | 0.277 | 9.34 | 16.44 | 0.277 | 15.49 | 24.78 | ||
| O-K | 0.525 | 48.77 | 64.46 | 4 | 0.525 | 50.81 | 61.01 | |
| S-K | 2.307 | 2.77 | 1.83 | 2.307 | 3.52 | 2.11 | ||
| Ti-K | 5 | 39.12 | 17.27 | 5 | 30.18 | 12.10 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhashmi Alamer, F.; Beyari, R.F. The Influence of Titanium Oxide Nanoparticles and UV Radiation on the Electrical Properties of PEDOT:PSS-Coated Cotton Fabrics. Materials 2023, 16, 1738. https://doi.org/10.3390/ma16041738
Alhashmi Alamer F, Beyari RF. The Influence of Titanium Oxide Nanoparticles and UV Radiation on the Electrical Properties of PEDOT:PSS-Coated Cotton Fabrics. Materials. 2023; 16(4):1738. https://doi.org/10.3390/ma16041738
Chicago/Turabian StyleAlhashmi Alamer, Fahad, and Rawan F. Beyari. 2023. "The Influence of Titanium Oxide Nanoparticles and UV Radiation on the Electrical Properties of PEDOT:PSS-Coated Cotton Fabrics" Materials 16, no. 4: 1738. https://doi.org/10.3390/ma16041738





