Can Differently Stabilized Silver Nanoparticles Modify Calcium Phosphate Precipitation?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Silver Nanoparticles
2.2.1. Citrate-Coated Silver Nanoparticles
2.2.2. AOT and PVP Coated AgNPs
2.3. Precipitation System
2.4. Characterization Methods
2.4.1. Powder X-ray Diffraction
2.4.2. Fourier Transform Infrared Spectroscopy
2.4.3. Atomic Absorption Spectroscopy
2.4.4. UV-Vis Spectroscopy
2.4.5. Dynamic and Electrophoretic Light Scattering
2.4.6. Thermogravimetric Analysis
2.4.7. Electron Paramagnetic Spectroscopy (EPR)
2.4.8. Transmission Electron Microscopy (TEM)
2.4.9. Scanning Electron Microscopy (SEM)
3. Results and Discussion
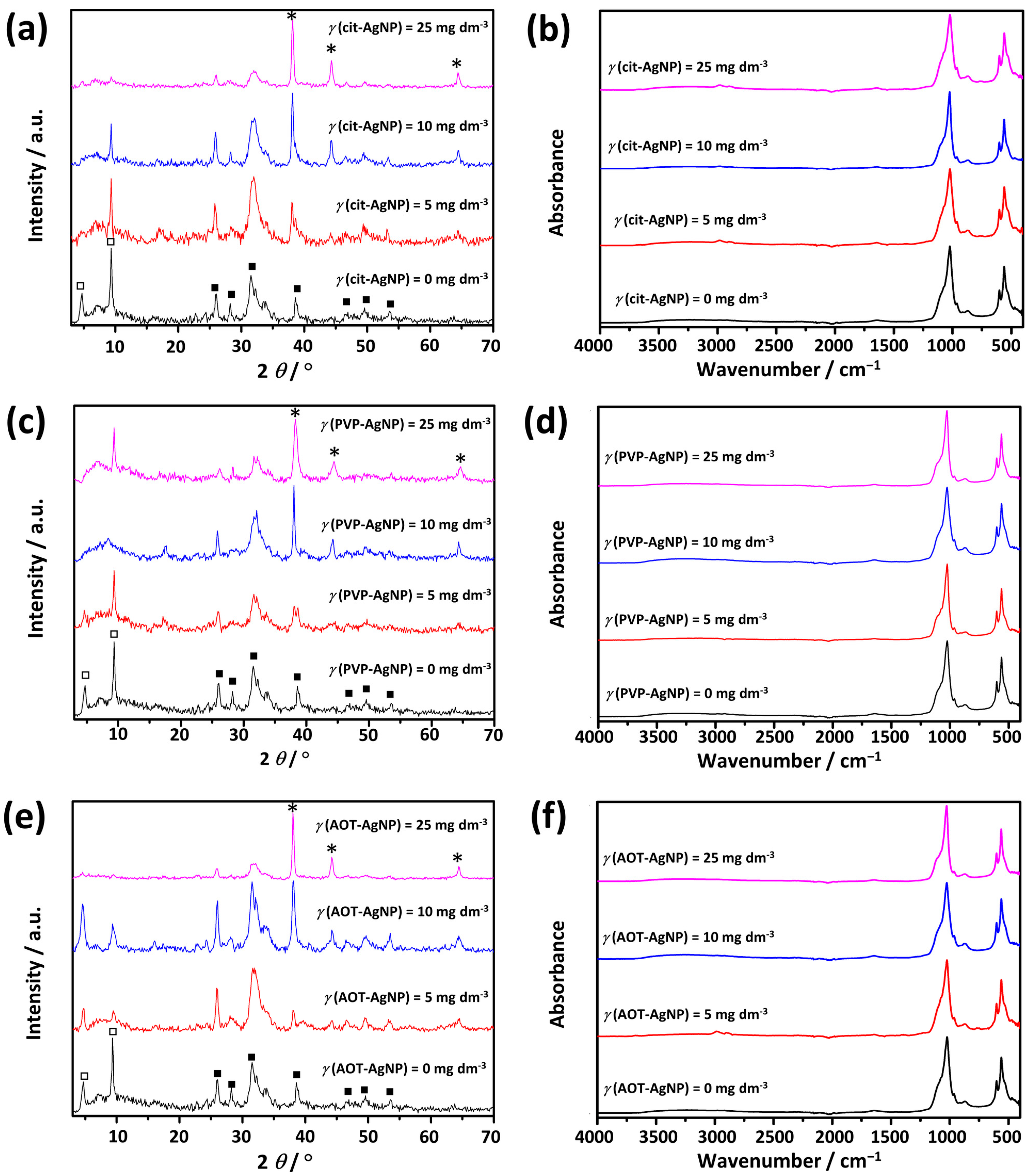
3.1. Characterization of AgNPs
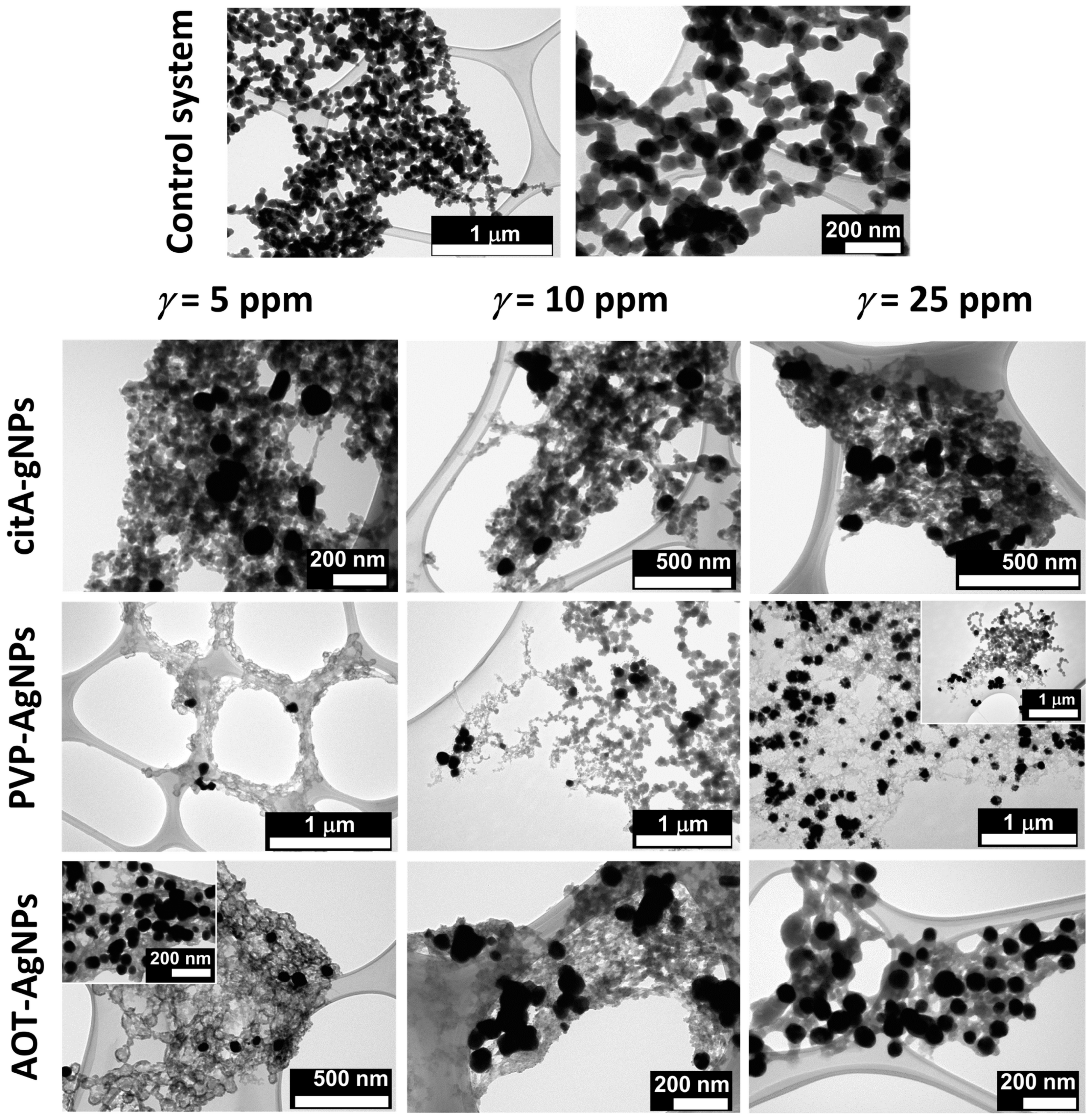
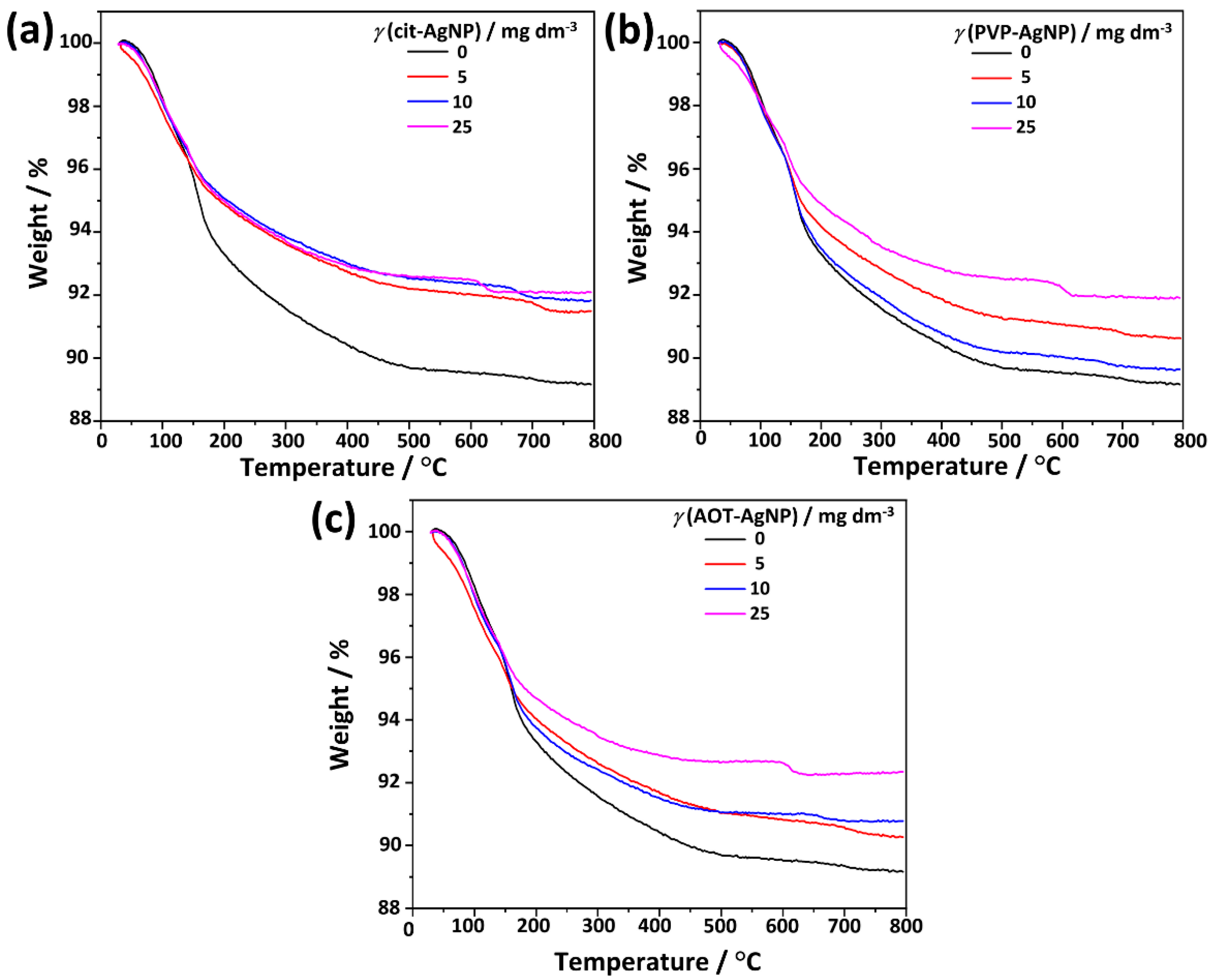
3.2. Influence of Silver Nanoparticles on Precipitation of Calcium Phosphates
3.2.1. Influence of AgNPs on Amorphous Calcium Phosphate
3.2.2. Influence of AgNPs on the Properties of Crystalline Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorozhkin, S.V. Calcium Orthophosphates (CaPO4): Occurrence and Properties. ProgBiomater 2016, 5, 9–70. [Google Scholar] [CrossRef] [Green Version]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates. In Application in Nature, Biology and Medicine; Pan Stanford Publishing: Singapore, 2012. [Google Scholar]
- Sikirić, M.D.; Füredi-Milhofer, H. The Influence of Surface Active Molecules on the Crystallization of Biominerals in Solution. Adv. Colloid Interfac. Sci. 2006, 128–130, 135–158. [Google Scholar] [CrossRef] [PubMed]
- Bleek, K.; Taubert, A. New Developments in Polymer-Controlled, Bioinspired Calcium Phosphate Mineralization from Aqueous Solution. Acta Biomater. 2013, 9, 6283–6321. [Google Scholar] [CrossRef] [PubMed]
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry; Oxford Chemistry Masters; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Nudelman, F.; Sommerdijk, N.A.J.M. Biomineralization as an Inspiration for Materials Chemistry. Angew. Chem. Int. Ed. 2012, 51, 6582–6596. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Wagner, H.D. The Material Bone: Structure-Mechanical Function Relations. Ann. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Crichton, R. Biomineralization. In Biological Inorganic Chemistry, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 517–544. [Google Scholar]
- Dorozhkin, S.V. Functionalized Calcium Orthophosphates (CaPO4) and Their Biomedical Applications. J. Mater. Chem. B 2019, 7, 7471–7489. [Google Scholar] [CrossRef]
- Canal, C.; Ginebra, M.P. Fibre-Reinforced Calcium Phosphate Cements: A Review. J. Mech. Behav. Biom. Mater. 2011, 4, 1658–1671. [Google Scholar] [CrossRef]
- Kargupta, R.; Bok, S.; Darr, C.M.; Crist, B.D.; Gangopadhyay, K.; Gangopadhyay, S.; Sengupta, S. Coatings and Surface Modifications Imparting Antimicrobial Activity to Orthopedic Implants. WIRES Nanomed. Nanobiotechnol. 2014, 6, 475–495. [Google Scholar] [CrossRef]
- Ben-Nissan, B.; Macha, I.; Cazalbou, S.; Choi, A.H. Calcium Phosphate Nanocoatings and Nanocomposites, Part 2: Thin Films for Slow Drug Delivery and Osteomyelitis. Nanomedicine 2016, 11, 531–544. [Google Scholar] [CrossRef] [Green Version]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver Nanoparticles Are Broad-Spectrum Bactericidal and Virucidal Compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percival, S.L.; Bowler, P.G.; Russell, D. Bacterial Resistance to Silver in Wound Care. J. Hospital Infect. 2005, 60, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Norton, R.; Finley, P.J. Clinically Isolated Bacteria Resistance to Silver-Based Wound Dressings. J. Wound Care 2021, 30, 238–247. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E.; Gazzano, M. Ion Substitution in Biological and Synthetic Apatites. In Biomineralization and Biomaterials; Aparicio, C., Ginebra, M.-P., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 235–266. [Google Scholar]
- Range, S.; Hagmeyer, D.; Rotan, O.; Sokolova, V.; Verheyen, J.; Siebers, B.; Epple, M. A Continuous Method to Prepare Poorly Crystalline Silver-Doped Calcium Phosphate Ceramics with Antibacterial Properties. RSC Adv. 2015, 5, 43172–43177. [Google Scholar] [CrossRef] [Green Version]
- Webster, T.J. Taylor Reducing Infections through Nanotechnology and Nanoparticles. Int. J. Nanomed. 2011, 6, 1463–1473. [Google Scholar] [CrossRef] [Green Version]
- Surmeneva, M.A.; Sharonova, A.A.; Chernousova, S.; Prymak, O.; Loza, K.; Tkachev, M.S.; Shulepov, I.A.; Epple, M.; Surmenev, R.A. Incorporation of Silver Nanoparticles into Magnetron-Sputtered Calcium Phosphate Layers on Titanium as an Antibacterial Coating. Colloid. Surface B 2017, 156, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Bartmanski, M.; Cieslik, B.; Glodowska, J.; Kalka, P.; Pawlowski, L.; Pieper, M.; Zielinski, A. Electrophoretic Deposition (EPD) of Nanohydroxyapatite-Nanosilver Coatings on Ti13Zr13Nb Alloy. Ceram. Int. 2017, 43, 11820–11829. [Google Scholar] [CrossRef]
- Hu, C.; Wu, L.; Zhou, C.; Sun, H.; Gao, P.; Xu, X.; Zhang, C.; Liang, J.; Fan, Y.; Sun, J.; et al. Berberine/Ag Nanoparticle Embedded Biomimetic Calcium Phosphate Scaffolds for Enhancing Antibacterial Function. Nanotechnol. Rev. 2020, 9, 568–579. [Google Scholar] [CrossRef]
- Correia, T.R.; Figueira, D.R.; de Sá, K.D.; Miguel, S.P.; Fradique, R.G.; Mendonça, A.G.; Correia, I.J. 3D Printed Scaffolds with Bactericidal Activity Aimed for Bone Tissue Regeneration. Int. J. Biol. Macromol. 2016, 93, 1432–1445. [Google Scholar] [CrossRef]
- Melo, M.A.; Orrego, S.; Weir, M.D.; Xu, H.H.K.; Arola, D.D. Designing Multiagent Dental Materials for Enhanced Resistance to Biofilm Damage at the Bonded Interface. ACS Appl. Mater. Interfaces 2016, 8, 11779–11787. [Google Scholar] [CrossRef] [PubMed]
- Clarin, A.; Ho, D.; Soong, J.; Looi, C.; Ipe, D.S.; Tadakamadla, S.K. The Antibacterial and Remineralizing Effects of Biomaterials Combined with DMAHDM Nanocomposite: A Systematic Review. Materials 2021, 14, 1688. [Google Scholar] [CrossRef]
- Keskar, M.; Sabatini, C.; Cheng, C.; Swihart, M.T. Synthesis and Characterization of Silver Nanoparticle-Loaded Amorphous Calcium Phosphate Microspheres for Dental Applications. Nanoscale Adv. 2019, 1, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Honda, M.; Kawanobe, Y.; Ishii, K.; Konishi, T.; Mizumoto, M.; Kanzawa, N.; Matsumoto, M.; Aizawa, M. In Vitro and in Vivo Antimicrobial Properties of Silver-Containing Hydroxyapatite Prepared via Ultrasonic Spray Pyrolysis Route. Mater. Sci. Eng. C 2013, 33, 5008–5018. [Google Scholar] [CrossRef]
- Nakamura, M.; Oyane, A.; Shimizu, Y.; Miyata, S.; Saeki, A.; Miyaji, H. Physicochemical Fabrication of Antibacterial Calcium Phosphate Submicrospheres with Dispersed Silver Nanoparticles via Coprecipitation and Photoreduction under Laser Irradiation. Acta Biomater. 2016, 46, 299–307. [Google Scholar] [CrossRef]
- López-Álvarez, M.; González-Rodríguez, L.; Gontad, F.; Teixeira-Santos, R.; Doiro, M.; Álvarez-Gómez, L.; Mergulhão, F.J.M.; González, P.; Serra, J. Dual Pulsed Laser Deposition of Ag Nanoparticles on Calcium Phosphate Coatings for Biomedical Applications. Biomed. Phys. Eng. Express 2022, 8, 065019. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Cassani, M.; Rubini, K.; Boga, C.; Bigi, A. (9R)-9-Hydroxystearate-Functionalized Anticancer Ceramics Promote Loading of Silver Nanoparticles. Nanomaterials 2018, 8, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forte, L.; Torricelli, P.; Bonvicini, F.; Boanini, E.; Gentilomi, G.A.; Lusvardi, G.; Della Bella, E.; Fini, M.; Vecchio Nepita, E.; Bigi, A. Biomimetic Fabrication of Antibacterial Calcium Phosphates Mediated by Polydopamine. J. Inorg. Biochem. 2018, 178, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Padmanabhan, V.P.; Sivashanmugam, P.; Kulandaivelu, R.; Sagadevan, S.; Sridevi, B.; Govindasamy, R.; Thiruvengadam, M. Biosynthesised Silver Nanoparticles Loading onto Biphasic Calcium Phosphate for Antibacterial and Bone Tissue Engineering Applications. Antibiotics 2022, 11, 1780. [Google Scholar] [CrossRef]
- Vasiliev, A.N.; Zlotnikov, E.; Riman, R.E. Calcium Phosphate-Encapsulated Silver Powder. Surf. Coat. Tec. 2009, 203, 1555–1558. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ergun, C.; Webster, T.J.; Bahadir, A.; Ueda, K.; Narushima, T.; Nakano, T. Effect of Precursor Deficiency Induced Ca/P Ratio on Antibacterial and Osteoblast Adhesion Properties of Ag-Incorporated Hydroxyapatite: Reducing Ag Toxicity. Materials 2021, 14, 3158. [Google Scholar] [CrossRef]
- Narendran, P.; Rajendran, A.; Garhnayak, M.; Garhnayak, L.; Nivedhitha, J.; Devi, K.C.; Pattanayak, D.K. Influence of PH on Wet-Synthesis of Silver Decorated Hydroxyapatite Nanopowder. Colloid. Surface B 2018, 169, 143–150. [Google Scholar] [CrossRef]
- Martínez-Sanmiguel, J.J.; G Zarate-Triviño, D.; Hernandez-Delgadillo, R.; Giraldo-Betancur, A.L.; Pineda-Aguilar, N.; Galindo-Rodríguez, S.A.; Franco-Molina, M.A.; Hernández-Martínez, S.P.; Rodríguez-Padilla, C. Anti-Inflammatory and Antimicrobial Activity of Bioactive Hydroxyapatite/Silver Nanocomposites. J. Biomater. Appl. 2019, 33, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Citradewi, P.W.; Hidayat, H.; Purwiandono, G.; Fatimah, I.; Sagadevan, S. Clitorea Ternatea-Mediated Silver Nanoparticle-Doped Hydroxyapatite Derived from Cockle Shell as Antibacterial Material. Chem. Phys. Lett. 2021, 769, 138412. [Google Scholar] [CrossRef]
- Sokolova, V.; Epple, M. Biological and Medical Applications of Calcium Phosphate Nanoparticles. Chem. Eur. J. 2021, 27, 7471–7488. [Google Scholar] [CrossRef]
- Falini, G.; Fermani, S. The Strategic Role of Adsorption Phenomena in Biomineralization: Biomineralization. Cryst. Res. Technol. 2013, 48, 864–876. [Google Scholar] [CrossRef]
- Munro, C.H.; Smith, W.E.; Garner, M.; Clarkson, J.; White, P.C. Characterization of the Surface of a Citrate-Reduced Colloid Optimized for Use as a Substrate for Surface-Enhanced Resonance Raman Scattering. Langmuir 1995, 11, 3712–3720. [Google Scholar] [CrossRef]
- Capjak, I.; Zebić Avdičević, M.; Sikirić, M.D.; Domazet Jurašin, D.; Hozić, A.; Pajić, D.; Dobrović, S.; Goessler, W.; Vinković Vrček, I. Behavior of Silver Nanoparticles in Wastewater: Systematic Investigation on the Combined Effects of Surfactants and Electrolytes in Model Systems. Envir. Sci. Water Res. Technol. 2018, 4, 2146–2159. [Google Scholar] [CrossRef]
- Vinković Vrček, I.; Pavičić, I.; Crnković, T.; Jurašin, D.; Babič, M.; Horák, D.; Lovrić, M.; Ferhatović, L.; Ćurlin, M.; Gajović, S. Does Surface Coating of Metallic Nanoparticles Modulate Their Interference with in Vitro Assays? RSC Adv. 2015, 5, 70787–70807. [Google Scholar] [CrossRef]
- Jurašin, D.D.; Ćurlin, M.; Capjak, I.; Crnković, T.; Lovrić, M.; Babič, M.; Horák, D.; Vinković Vrček, I.; Gajović, S. Surface Coating Affects Behavior of Metallic Nanoparticles in a Biological Environment. Beilstein J. Nanotechnol. 2016, 7, 246–262. [Google Scholar] [CrossRef] [Green Version]
- Uskoković, V. Visualizing Different Crystalline States during the Infrared Imaging of Calcium Phosphates. Vib. Spectrosc. 2020, 108, 103045. [Google Scholar] [CrossRef]
- Majer, M.; Roguljić, M.; Knežević, Ž.; Starodumov, A.; Ferenček, D.; Brigljević, V.; Mihaljević, B. Dose Mapping of the Panoramic 60Co Gamma Irradiation Facility at the Ruđer Bošković Institute–Geant4 Simulation and Measurements. Appl. Radiat. Isotopes 2019, 154, 108824. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for Industrial Radiation Sterilization of Disposable Medical Products (Cobalt-60 Gamma Irradiation); TECDOC Series; International Atomic Energy Agency: Vienna, Austria, 1989.
- Radiation Sterilization of Tissue Allografts: Requirements for Validation and Routine Control a Code of Practice; International Atomic Agency: Vienna, Austria, 2007.
- Wei, D.; Qian, W. Facile Synthesis of Ag and Au Nanoparticles Utilizing Chitosan as a Mediator Agent. Colloid. Surface B 2008, 62, 136–142. [Google Scholar] [CrossRef]
- Bar-Yosef Ofir, P.; Govrin-Lippman, R.; Garti, N.; Füredi-Milhofer, H. The Influence of Polyelectrolytes on the Formation and Phase Transformation of Amorphous Calcium Phosphate. Cryst. Growth Des. 2004, 4, 177–183. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Pathways to Biomineralization and Biodemineralization of Calcium Phosphates: The Thermodynamic and Kinetic Controls. Dalton Trans. 2009, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Selmani, A.; Coha, I.; Magdić, K.; Čolović, B.; Jokanović, V.; Šegota, S.; Gajović, S.; Gajović, A.; Jurašin, D.; Dutour Sikirić, M. Multiscale Study of the Influence of Cationic Surfactants on Amorphous Calcium Phosphate Precipitation. CrystEngComm 2015, 17, 8529–8548. [Google Scholar] [CrossRef] [Green Version]
- Füredi-Milhofer, H.; Purgarić, B.; Brečević, L.; Pavković, N. Precipitation of Calcium Phosphates from Electrolyte Solutions: I. A Study of the Precipitates in the Physiological PH Region. Calc. Tiss. Res. 1971, 8, 142–153. [Google Scholar] [CrossRef]
- Du, L.-W.; Bian, S.; Gou, B.-D.; Jiang, Y.; Huang, J.; Gao, Y.-X.; Zhao, Y.-D.; Wen, W.; Zhang, T.-L.; Wang, K. Structure of Clusters and Formation of Amorphous Calcium Phosphate and Hydroxyapatite: From the Perspective of Coordination Chemistry. Cryst. Growth Des. 2013, 13, 3103–3109. [Google Scholar] [CrossRef]
- Li, S.; Wang, L. Phosphorylated Osteopontin Peptides Inhibit Crystallization by Resisting the Aggregation of Calcium Phosphate Nanoparticles. CrystEngComm 2012, 14, 8037–8043. [Google Scholar] [CrossRef]
- Ding, H.; Pan, H.; Xu, X.; Tang, R. Toward a Detailed Understanding of Magnesium Ions on Hydroxyapatite Crystallization Inhibition. Cryst. Growth Des. 2014, 14, 763–769. [Google Scholar] [CrossRef]
- Wang, C.-G.; Liao, J.-W.; Gou, B.-D.; Huang, J.; Tang, R.-K.; Tao, J.-H.; Zhang, T.-L.; Wang, K. Crystallization at Multiple Sites inside Particles of Amorphous Calcium Phosphate. Cryst. Growth Des. 2009, 9, 2620–2626. [Google Scholar] [CrossRef]
- Yang, X.; Xie, B.; Wang, L.; Qin, Y.; Henneman, Z.J.; Nancollas, G.H. Influence of Magnesium Ions and Amino Acids on the Nucleation and Growth of Hydroxyapatite. CrystEngComm 2011, 13, 1153–1158. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, W.; Pan, H.; Jiang, S.; Tang, R. Stabilizing Amorphous Calcium Phosphate Phase by Citrate Adsorption. CrystEngComm 2014, 16, 1864–1867. [Google Scholar] [CrossRef]
- Erceg, I.; Kontrec, J.; Strasser, V.; Selmani, A.; Domazet Jurašin, D.; Ćurlin, M.; Džakula, B.N.; Matijaković Mlinarić, N.; Šegota, S.; Lyons, D.M.; et al. Precipitation of Calcium Phosphates and Calcium Carbonates in the Presence of Differently Charged Liposomes. Minerals 2022, 12, 208. [Google Scholar] [CrossRef]
- Erceg, I.; Selmani, A.; Gajović, A.; Panžić, I.; Iveković, D.; Faraguna, F.; Šegota, S.; Ćurlin, M.; Strasser, V.; Kontrec, J.; et al. Calcium Phosphate Formation on TiO2 Nanomaterials of Different Dimensionality. Colloid. Surface A 2020, 593, 124615. [Google Scholar] [CrossRef]
- Erceg, I.; Selmani, A.; Gajović, A.; Radatović, B.; Šegota, S.; Ćurlin, M.; Strasser, V.; Kontrec, J.; Kralj, D.; Maltar-Strmečki, N.; et al. Precipitation at Room Temperature as a Fast and Versatile Method for Calcium Phosphate/TiO2 Nanocomposites Synthesis. Nanomaterials 2021, 11, 1523. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Amorphous Calcium (Ortho)Phosphates. Acta Biomate. 2010, 6, 4457–4475. [Google Scholar] [CrossRef] [PubMed]
- Buljan Meić, I.; Kontrec, J.; Domazet Jurašin, D.; Selmani, A.; Njegić Džakula, B.; Maltar-Strmečki, N.; Lyons, D.M.; Plodinec, M.; Čeh, M.; Gajović, A.; et al. How Similar Are Amorphous Calcium Carbonate and Calcium Phosphate? A Comparative Study of Amorphous Phase Formation Conditions. CrystEngComm 2018, 20, 35–50. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Amorphous Calcium Phosphates: Synthesis, Properties and Uses in Biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.C. Fundamentals of Fourier Transform Infrared Spectroscopy, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Xie, B.; Halter, T.J.; Borah, B.M.; Nancollas, G.H. Tracking Amorphous Precursor Formation and Transformation during Induction Stages of Nucleation. Cryst. Growth Des. 2014, 14, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, L.; Qin, Y.; Sun, Z.; Henneman, Z.J.; Moradian-Oldak, J.; Nancollas, G.H. How Amelogenin Orchestrates the Organization of Hierarchical Elongated Microstructures of Apatite. J. Phys. Chem. B 2010, 114, 2293–2300. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.-M.; Lu, X.; Wang, K.-F.; Meng, F.-Z.; Jiang, O.; Zhang, H.-P.; Zhi, W.; Fang, L.-M. Silver Nanoparticles and Growth Factors Incorporated Hydroxyapatite Coatings on Metallic Implant Surfaces for Enhancement of Osteoinductivity and Antibacterial Properties. ACS App. Mater. Interfaces 2014, 6, 8580–8589. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.-C.; Chen, S.-Y.; Lee, H.-Y.; Bow, J.-S. Structural Characterization of Nano-Sized Calcium Deficient Apatite Powders. Biomaterials 2004, 25, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Koutsopoulos, S. Synthesis and Characterization of Hydroxyapatite Crystals: A Review Study on the Analytical Methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Mochales, C.; Wilson, R.M.; Dowker, S.E.P.; Ginebra, M.-P. Dry Mechanosynthesis of Nanocrystalline Calcium Deficient Hydroxyapatite: Structural Characterisation. J. Alloy. Compd. 2011, 509, 7389–7394. [Google Scholar] [CrossRef]
- Drouet, C. Apatite Formation: Why It May Not Work as Planned, and How to Conclusively Identify Apatite Compounds. BioMed. Res. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, B.O.; Markovic, M.; Brown, W.E. Octacalcium Phosphate. 3. Infrared and Raman Vibrational Spectra. Chem. Mater. 1993, 5, 1417–1423. [Google Scholar] [CrossRef]
- Füredi-Milhofer, H.; Sarig, S. Interactions between Polyelectrolytes and Sparingly Soluble Salts. Prog. Cryst. Growth Charact. Mater. 1996, 32, 45–74. [Google Scholar] [CrossRef]
- Buljan Meić, I.; Kontrec, J.; Domazet Jurašin, D.; Njegić Džakula, B.; Štajner, L.; Lyons, D.M.; Dutour Sikirić, M.; Kralj, D. Comparative Study of Calcium Carbonates and Calcium Phosphates Precipitation in Model Systems Mimicking the Inorganic Environment for Biomineralization. Cryst. Growth Des. 2017, 17, 1103–1117. [Google Scholar] [CrossRef]
- Furedi-Milhofer, H.; Moradian-Oldak, J.; Weiner, S.; Veis, A.; Mintz, K.P.; Addadf, L. Interactions of Matrix Proteins from Mineralized Tissues with Octacalcium Phosphate. Conn. Tissue Res. 1994, 30, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Siddiqi, S.A.; Hussain, T.; Chaudhry, A.A.; Rehman, I.U.; Khan, A.S.; Abbas, G. Synthesis and Characterization of Calcium Deficient Apatite Granules for Drug Eluting Bone Graft Applications. Ceram. Int. 2014, 40, 10719–10725. [Google Scholar] [CrossRef]
- Tõnsuaadu, K.; Gross, K.A.; Plūduma, L.; Veiderma, M. A Review on the Thermal Stability of Calcium Apatites. Therm. Ana. Calorim. 2012, 110, 647–659. [Google Scholar] [CrossRef]
- Lazić, S.; Zec, S.; Miljević, N.; Milonjić, S. The Effect of Temperature on the Properties of Hydroxyapatite Precipitated from Calcium Hydroxide and Phosphoric Acid. Thermochim. Acta 2001, 374, 13–22. [Google Scholar] [CrossRef]
- Shi, H.; Ye, X.; Zhang, J.; Wu, T.; Yu, T.; Zhou, C.; Ye, J. A Thermostability Perspective on Enhancing Physicochemical and Cytological Characteristics of Octacalcium Phosphate by Doping Iron and Strontium. Bioact. Mater. 2021, 6, 1267–1282. [Google Scholar] [CrossRef]
- Putlyaev, V.I.; Kukueva, E.V.; Safronova, T.V.; Ivanov, V.K.; Churagulov, B.R. Features of Octacalcium Phosphate Thermolysis. Refract. Ind. Ceram. 2014, 54, 420–424. [Google Scholar] [CrossRef]
- Murray, K.A.; Döbelin, N.; Albadarin, A.B.; Sadło, J.; Ren, G.; Collins, M.N.; O’Neill, C. The Influence of Sterilization on Octacalcium Phosphate for Clinical Applications. In Octacalcium Phosphate Biomaterials; Insley, G., Suzuki, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 55–84. [Google Scholar]
- Majeed Khan, M.A.; Kumar, S.; Ahamed, M.; Alrokayan, S.A.; AlSalhi, M.S. Structural and Thermal Studies of Silver Nanoparticles and Electrical Transport Study of Their Thin Films. Nanoscale. Res. Lett. 2011, 6, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adebayo-Tayo, B.; Salaam, A.; Ajibade, A. Green Synthesis of Silver Nanoparticle Using Oscillatoria Sp. Extract, Its Antibacterial, Antibiofilm Potential and Cytotoxicity Activity. Heliyon 2019, 5, e02502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebugle, A.; Zahidi, E.; Bonel, G. Effect of Structure and Composition on the Thermal Decomposition of Calcium Phosphates (Ca/P = 1.33). React. Solids 1986, 2, 151–161. [Google Scholar] [CrossRef]
- Guo, C.; Xue, J.; Tan, Y.; Dong, Y. Fabrication of Silver/Beta-tricalcium Phosphate Particle by a Simple Liquid Chemical Reduction Method. Micro. Nano Lett. 2016, 11, 391–394. [Google Scholar] [CrossRef]
- Callens, F.J.; Verbeeck, R.M.H.; Matthys, P.F.A.; Martens, L.C.; Boesman, E.R. The Contribution of CO33− and CO2− to the ESR Spectrum near G=2 of Powdered Human Tooth Enamel. Calc. Tiss. Int. 1987, 41, 124–129. [Google Scholar] [CrossRef]
- Erceg, I.; Maltar-Strmečki, N.; Jurašin, D.D.; Strasser, V.; Ćurlin, M.; Lyons, D.M.; Radatović, B.; Mlinarić, N.M.; Kralj, D.; Sikirić, M.D. Comparison of the Effect of the Amino Acids on Spontaneous Formation and Transformation of Calcium Phosphates. Crystals 2021, 11, 792. [Google Scholar] [CrossRef]
- Cevc, P.; Schara, M.; Ravnik, Č. Electron Paramagnetic Resonance Study of Irradiated Tooth Enamel. Radiat. Res. 1972, 51, 581–589. [Google Scholar] [CrossRef]
- Murzakhanov, F.F.; Grishin, P.O.; Goldberg, M.A.; Yavkin, B.V.; Mamin, G.V.; Orlinskii, S.B.; Fedotov, A.Y.; Petrakova, N.V.; Antuzevics, A.; Gafurov, M.R.; et al. Radiation-Induced Stable Radicals in Calcium Phosphates: Results of Multifrequency EPR, EDNMR, ESEEM, and ENDOR Studies. App. Sci. 2021, 11, 7727. [Google Scholar] [CrossRef]
- Gafurov, M.; Yavkin, B.; Mamin, G.; Orlinskii, S.; Smirnov, I.; Fedotov, A.; Komlev, V. Radiation Induced Paramagnetic Radicals in Synthetic Octacalcium Phosphate. IOP Conf. Ser. Earth and Environ. Sci. 2018, 155, 012018. [Google Scholar] [CrossRef]
- Shurtakova, D.V.; Yavkin, B.V.; Mamin, G.V.; Orlinskii, S.B.; Sirotinkin, V.P.; Fedotov, A.Y.; Shinkarev, A.; Antuzevics, A.; Smirnov, I.V.; Tovtin, V.I.; et al. X-Ray Diffraction and Multifrequency EPR Study of Radiation-Induced Room Temperature Stable Radicals in Octacalcium Phosphate. Radiat. Res. 2021, 195, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Murzakhanov, F.; Mamin, G.V.; Orlinskii, S.; Goldberg, M.; Petrakova, N.V.; Fedotov, A.Y.; Grishin, P.; Gafurov, M.R.; Komlev, V.S. Study of Electron-Nuclear Interactions in Doped Calcium Phosphates by Various Pulsed EPR Spectroscopy Techniques. ACS Omega 2021, 6, 25338–25349. [Google Scholar] [CrossRef]
- Callens, F.; Vanhaelewyn, G.; Matthys, P.; Boesman, E. EPR of Carbonate Derived Radicals: Applications in Dosimetry, Dating and Detection of Irradiated Food. Appl. Magn. Reson. 1998, 14, 235–254. [Google Scholar] [CrossRef]
- Grün, R.; Joannes-Boyau, R.; Stringer, C. Two Types of CO2− Radicals Threaten the Fundamentals of ESR Dating of Tooth Enamel. Q. Geochronol. 2008, 3, 150–172. [Google Scholar] [CrossRef]
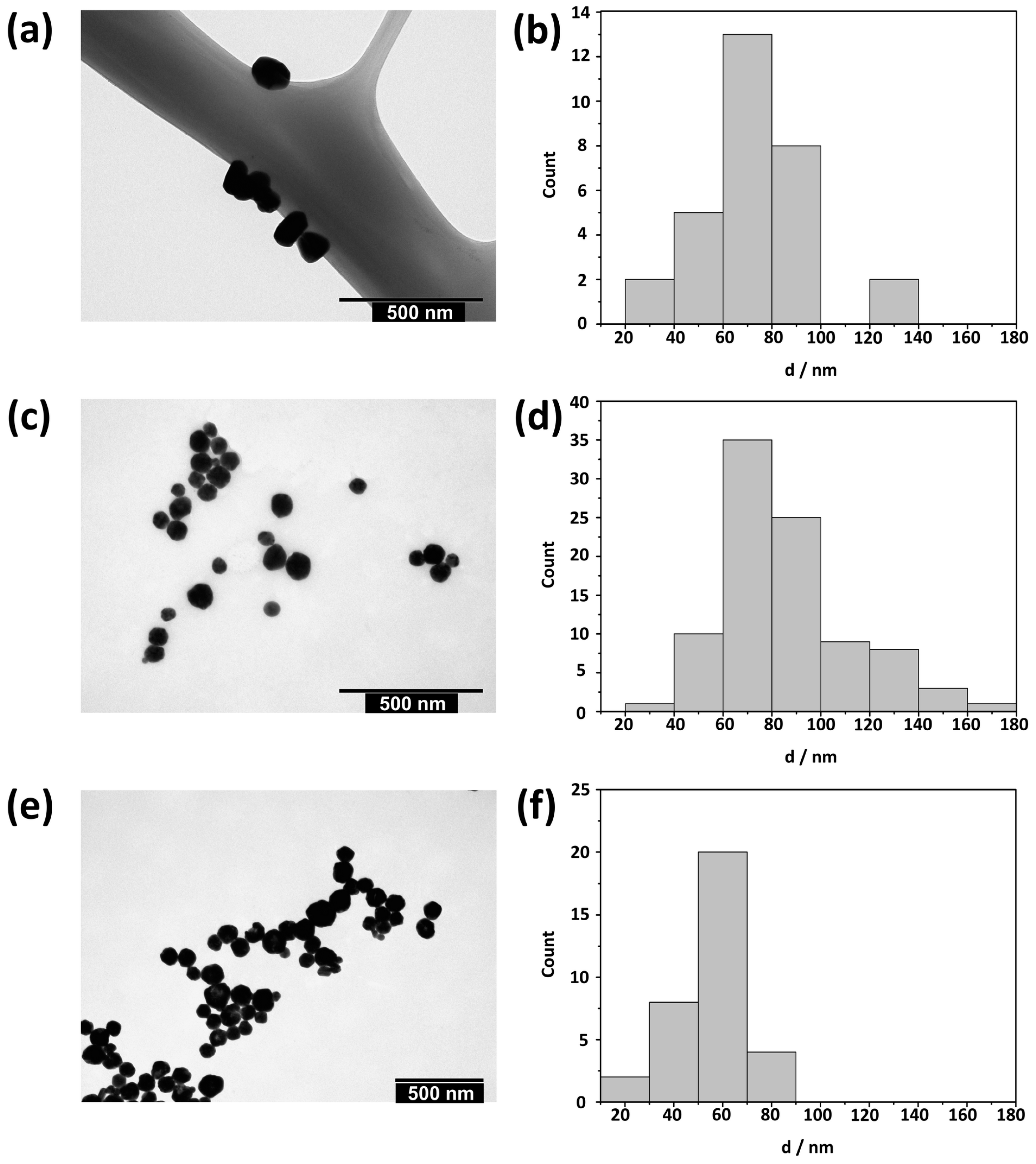
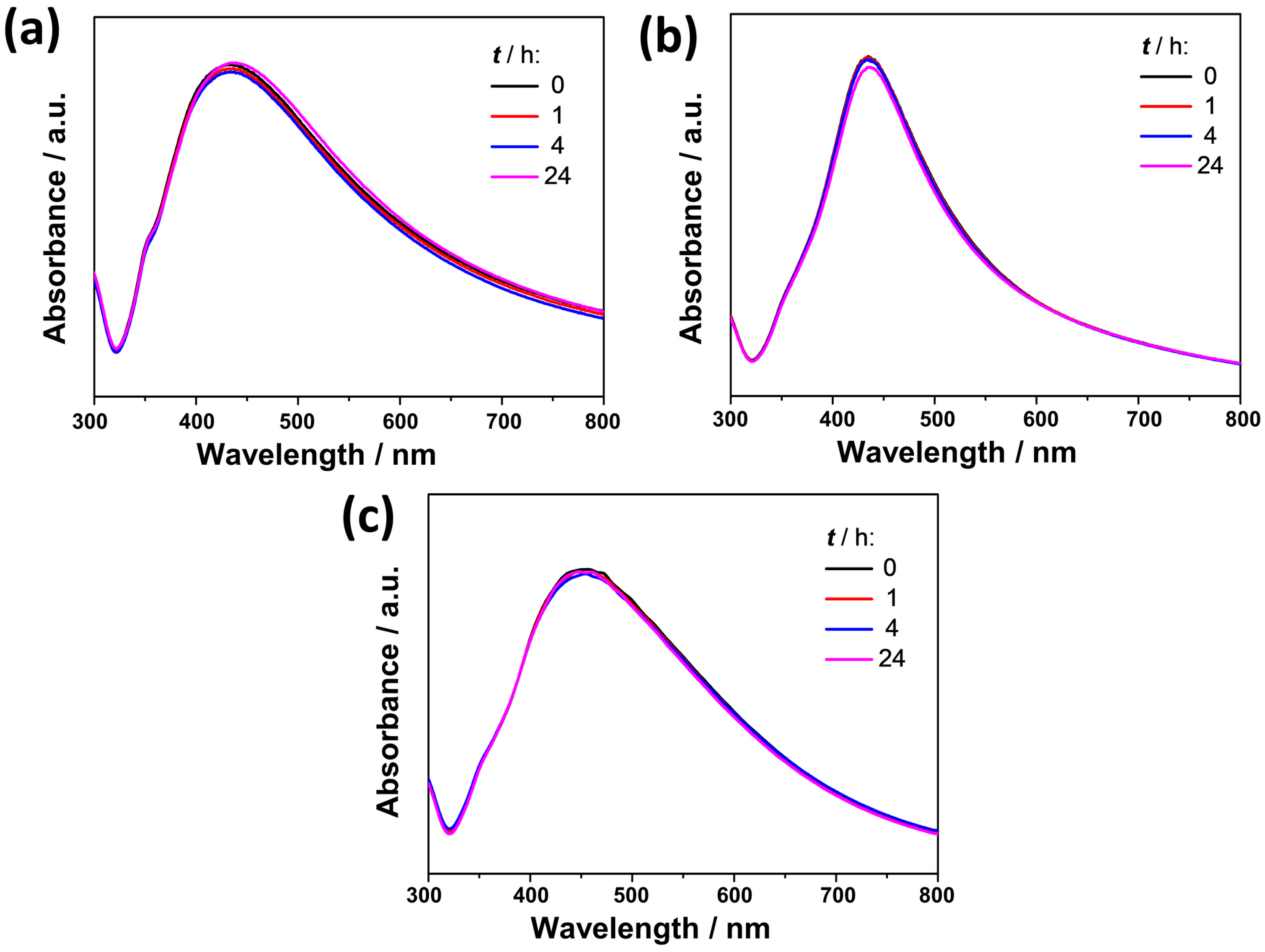
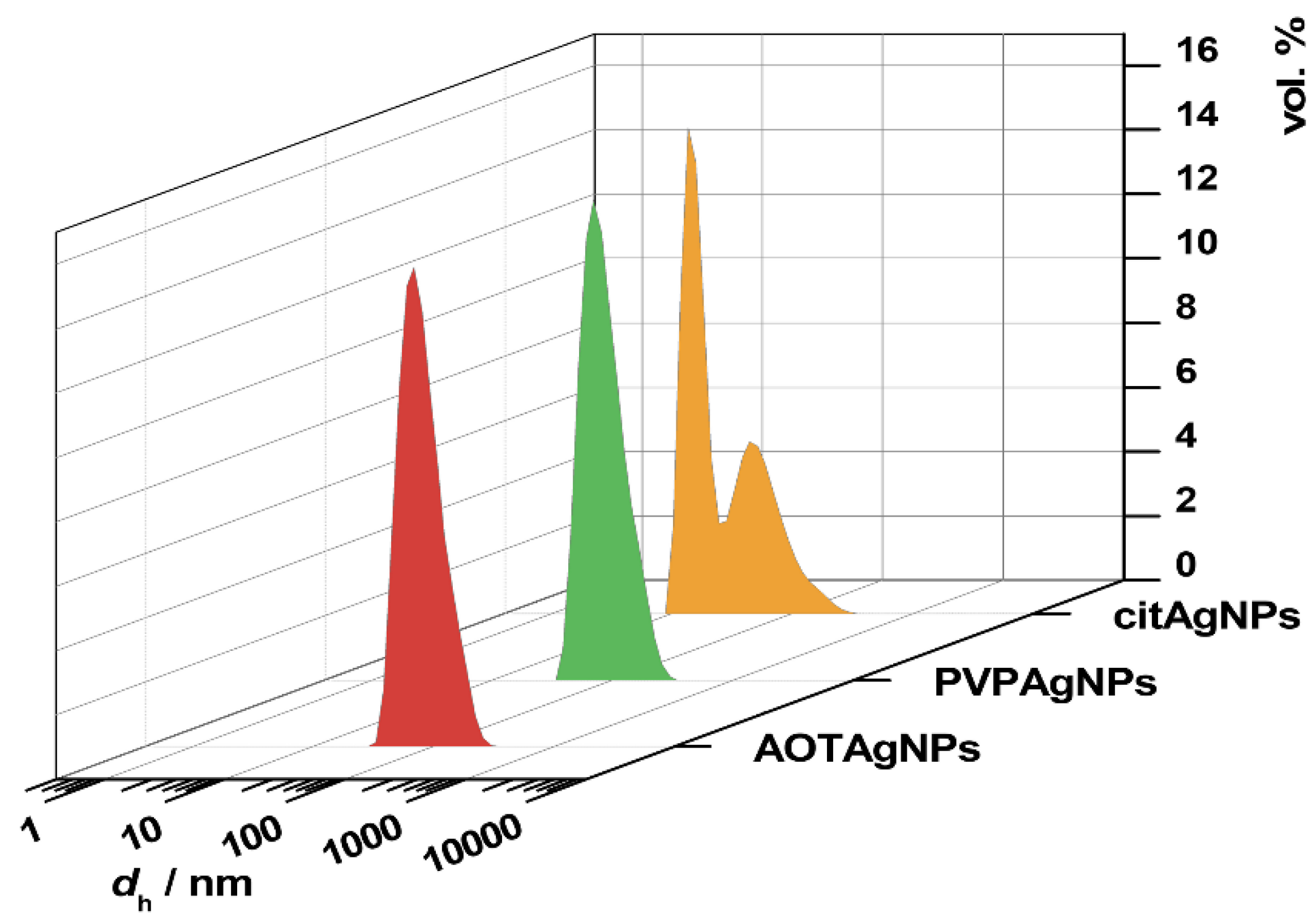

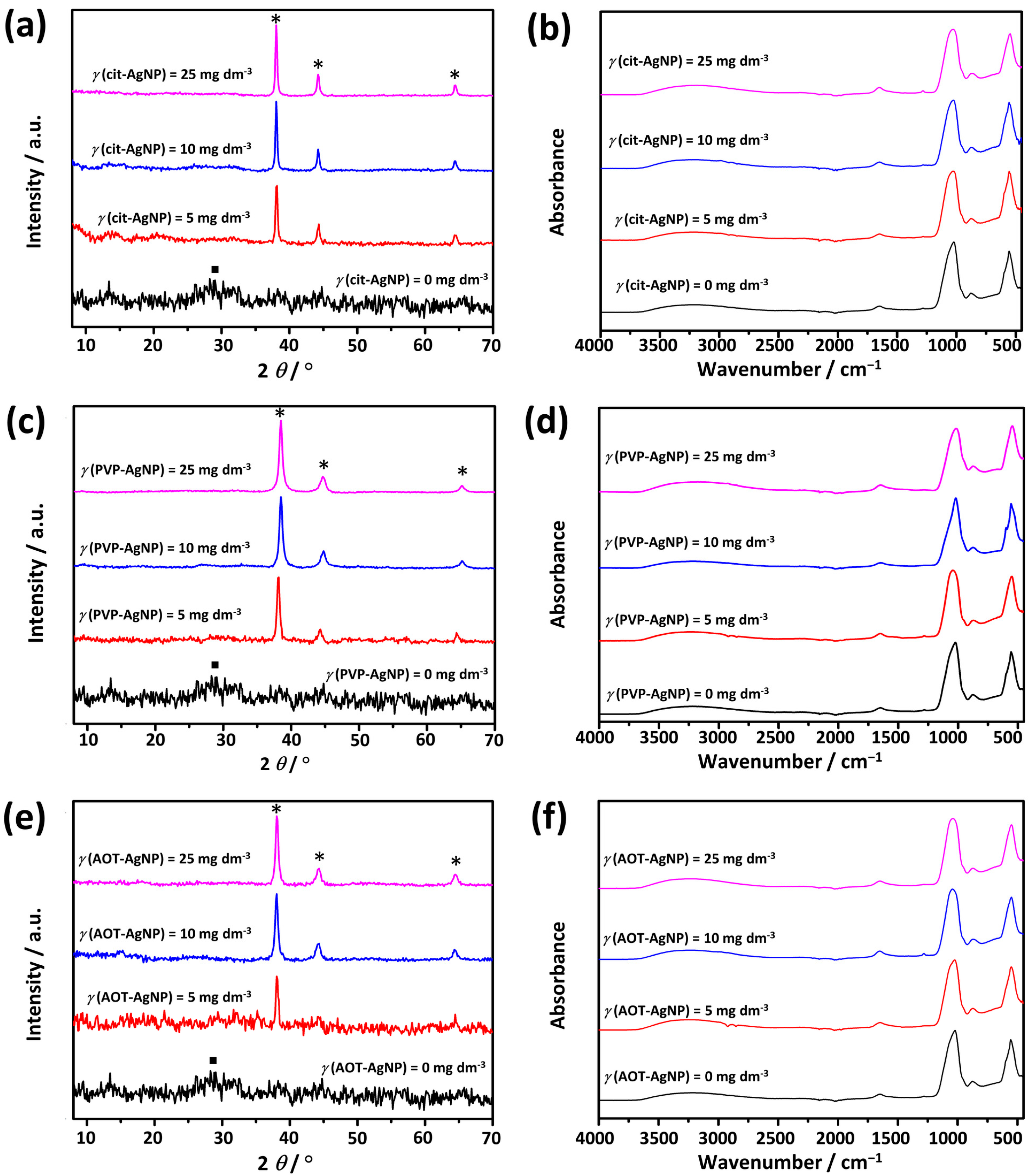


| Sample | Peak I | Peak II | ζ/mV | ||
|---|---|---|---|---|---|
| dh/nm | vol. % | dh/nm | vol. % | ||
| cit-AgNPs | 15.9 ± 2.0 | 59.7 ± 3.7 | 64.4 ± 2.6 | 40.3 ± 3.7 | −40.2 ± 1,4 |
| PVP-AgNPs | 89.9 ± 1.0 | 100 | −24.5 ± 1.6 | ||
| AOT-AgNPs | 84.8 ± 0.8 | 100 | −59.9 ± 2.5 | ||
| Sample | γ (AgNPs)/mg dm−3 | R | S |
|---|---|---|---|
| Control system | 0 | 0.536 | 0.443 |
| Cit-AgNPs | 5 | 0.719 | 0.729 |
| 10 | 0.611 | 0.542 | |
| 25 | 0.549 | 0.482 | |
| PVP-AgNPs | 5 | 0.692 | 0.720 |
| 10 | 0.577 | 0.508 | |
| 25 | 0.643 | 0.567 | |
| AOT-AgNPs | 5 | 0.698 | 0.719 |
| 10 | 0.643 | 0.589 | |
| 25 | 0.662 | 0.615 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inkret, S.; Ćurlin, M.; Smokrović, K.; Kalčec, N.; Peranić, N.; Maltar-Strmečki, N.; Domazet Jurašin, D.; Dutour Sikirić, M. Can Differently Stabilized Silver Nanoparticles Modify Calcium Phosphate Precipitation? Materials 2023, 16, 1764. https://doi.org/10.3390/ma16051764
Inkret S, Ćurlin M, Smokrović K, Kalčec N, Peranić N, Maltar-Strmečki N, Domazet Jurašin D, Dutour Sikirić M. Can Differently Stabilized Silver Nanoparticles Modify Calcium Phosphate Precipitation? Materials. 2023; 16(5):1764. https://doi.org/10.3390/ma16051764
Chicago/Turabian StyleInkret, Suzana, Marija Ćurlin, Kristina Smokrović, Nikolina Kalčec, Nikolina Peranić, Nadica Maltar-Strmečki, Darija Domazet Jurašin, and Maja Dutour Sikirić. 2023. "Can Differently Stabilized Silver Nanoparticles Modify Calcium Phosphate Precipitation?" Materials 16, no. 5: 1764. https://doi.org/10.3390/ma16051764







