Thermally Mendable Self-Healing Epoxy Coating for Corrosion Protection in Marine Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Self-Healing Epoxy Preparation and Coating Application
2.2. Experimental Characterization
3. Results and Discussion
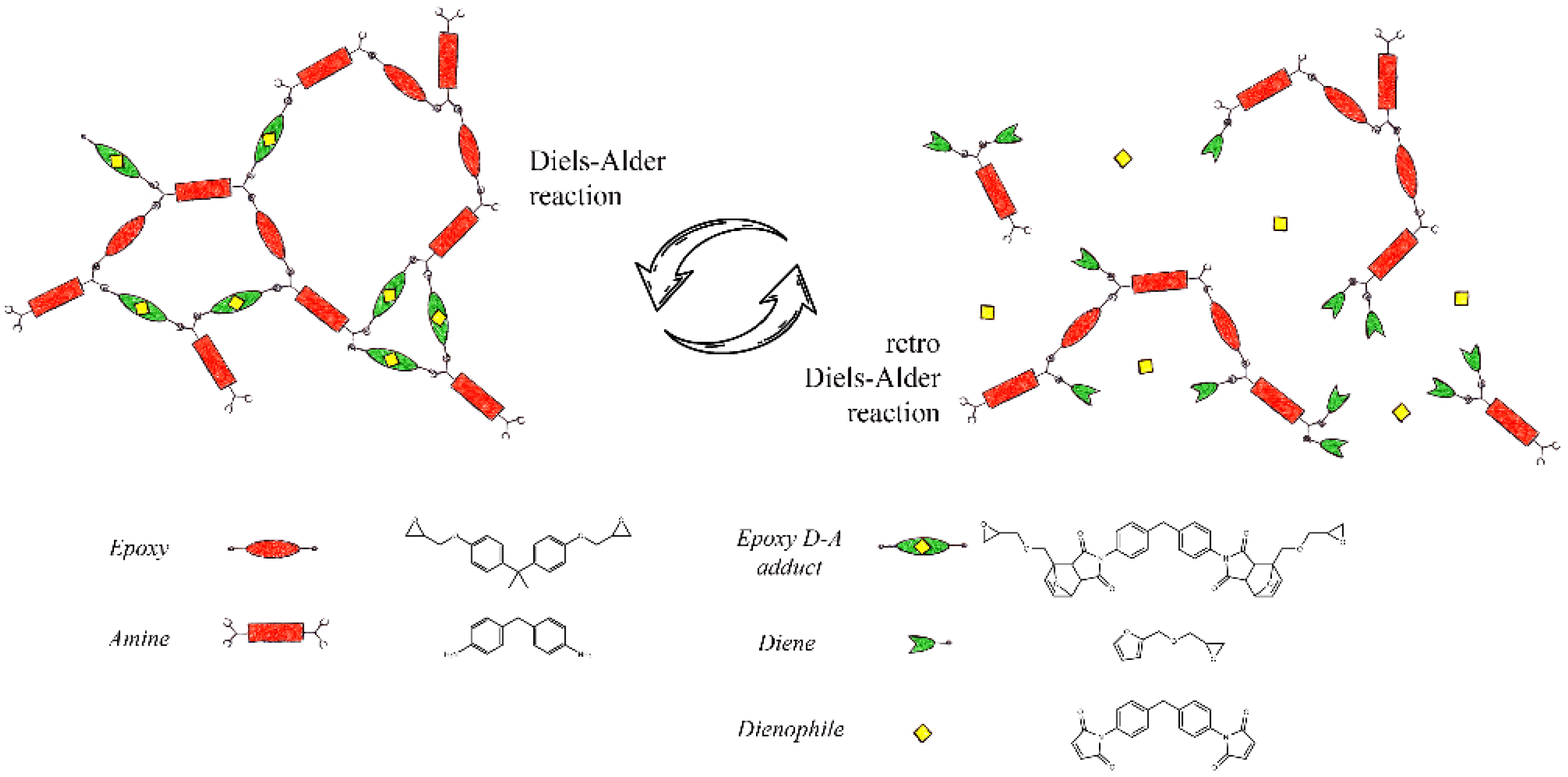
3.1. Diels–Alder Epoxy Resin’s Self-Healing Assessment
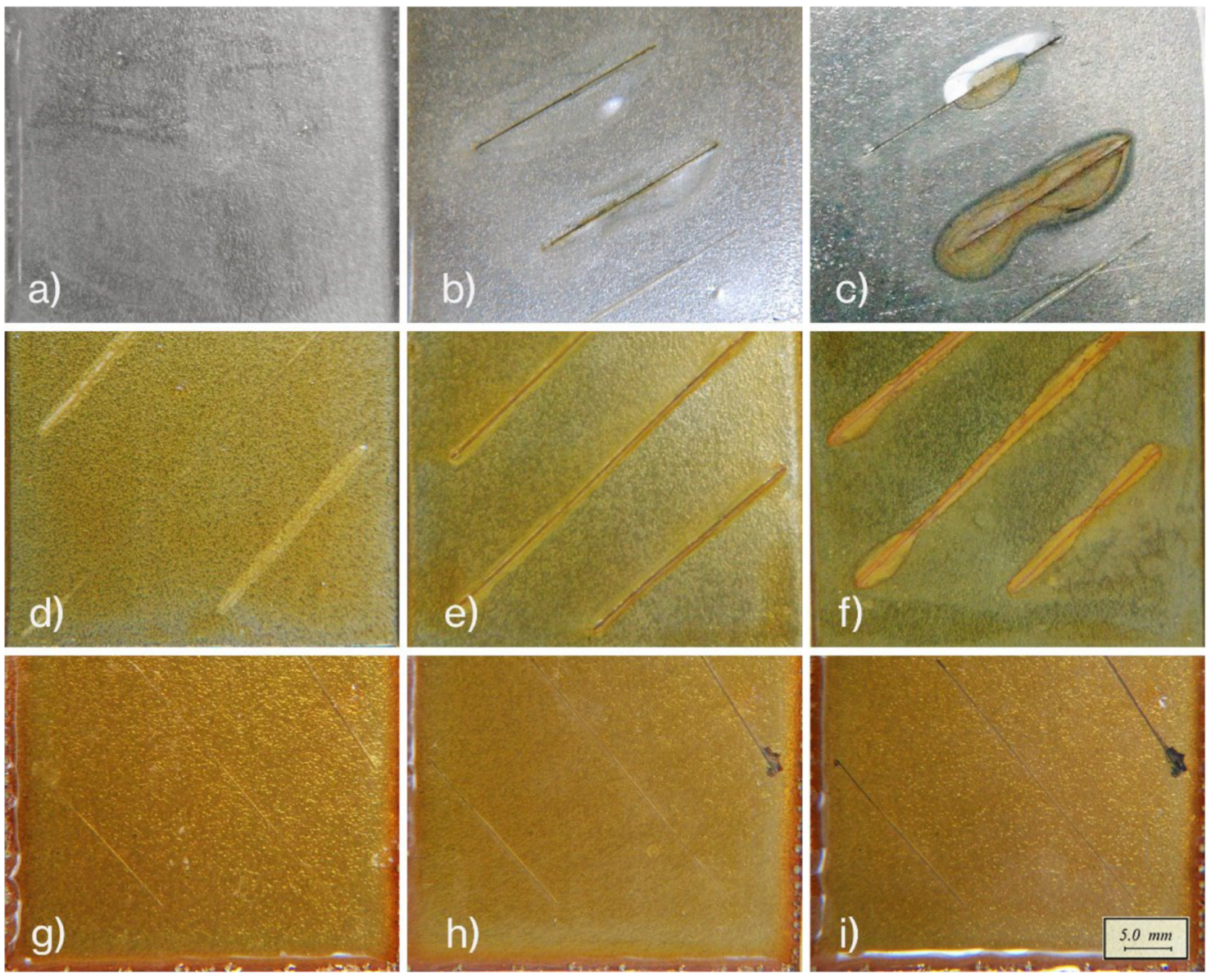
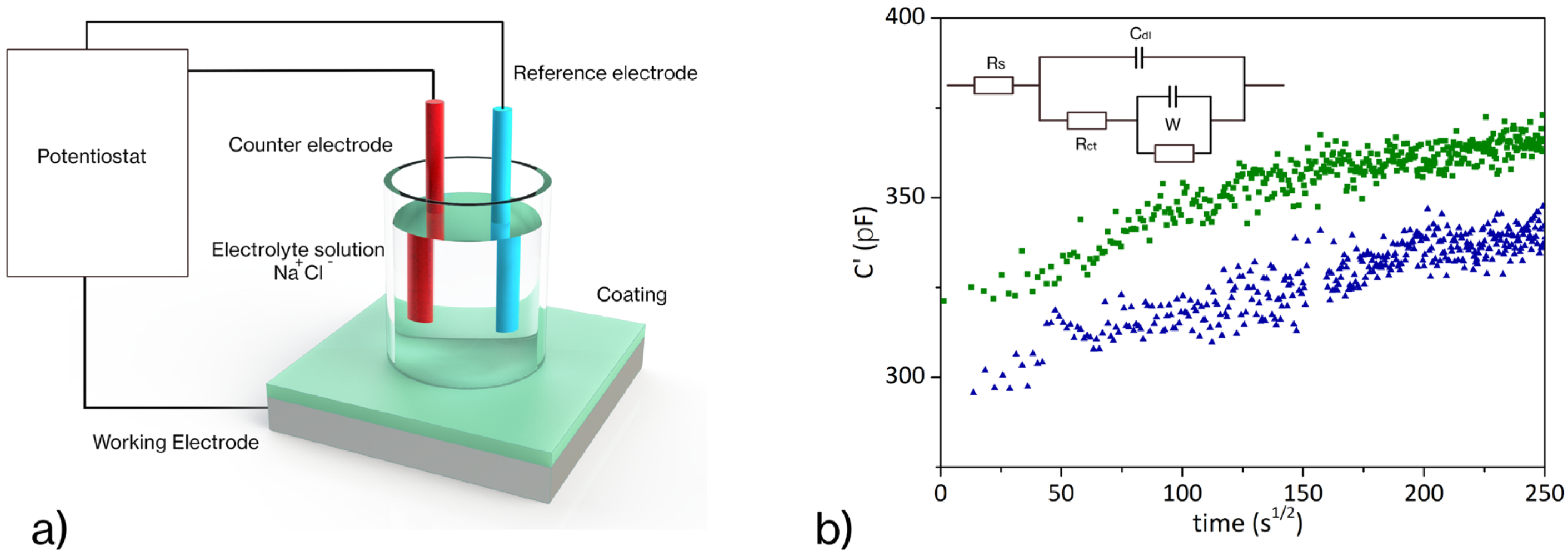
3.2. Corrosion Resistance Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- York, O.; Patel, S.; Young, M.-Y.; Zunino, J., III; Xanthos, M. Smart Polymeric Coatings-Recent Advances. Adv. Polym. Techn. 2007, 26, 1–13. [Google Scholar] [CrossRef]
- Iacono, S.; Martone, A.; Amendola, E. Corrosion-resistant self-healing coatings. AIP Conf. Proc. 2018, 1990, 020010. [Google Scholar] [CrossRef]
- Verma, A.; Jain, N.; Rastogi, S.; Dogra, V.; Sanjay, S.; Siengchin, S.; Mansour, R. Mechanism, Anti-Corrosion Protection and Components of Anti-Corrosion. In Polymer Coatings; CRC Press: Boca Raton, FL, USA, 2020; pp. 53–66. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, F.; Rong, M.; Zhang, M. Capsules-based self-healing polymers and polymer composites. In Recent Advances in Smart Self-Healing Polymers and Composites, 2nd ed.; Woodhead Publishing Limited: Cambridge, UK, 2022; pp. 113–140. [Google Scholar] [CrossRef]
- Madhusudhana, A.; Mohana, K. Synthesis of self-healing inhibitor releasing nanocapsules blended phenol novolac resin for high performance anticorrosion coating. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128762. [Google Scholar] [CrossRef]
- Udoh, I.; Shi, H.; Daniel, E.; Li, J.; Gu, S.; Liu, F.; Han, E. Active anticorrosion and self-healing coatings: A review with focus on multi-action smart coating strategies. J. Mater. Sci. Technol. 2022, 116, 224–237. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, H.; Yang, J. Synthesis of organic silane microcapsules for self-healing corrosion resistant polymer coatings. Corros. Sci. 2012, 65, 561–566. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, H.; Zhang, D.; Lou, Y.; Huang, L.; Ma, L.; Hao, X.; Dong, L.; Rosei, F.; Lau, W. Ultrafast and high-efficient self-healing epoxy coatings with active multiple hydrogen bonds for corrosion protection. Corros. Sci. 2021, 187, 109485. [Google Scholar] [CrossRef]
- Kim, H.; Yarin, A.; Lee, M. Self-healing corrosion protection film for marine environment. Compos. B Eng. 2020, 182, 107598. [Google Scholar] [CrossRef]
- Pepels, M.; Filot, I.; Klumperman, B.; Goossens, H. Self-healing systems based on disulfide—Thiol exchange reactions. Polym. Chem. 2013, 4, 4955–4965. [Google Scholar] [CrossRef]
- Dispinar, T.; Sanyal, R.; Sanyal, A. A Diels-Alder/retro Diels-Alder strategy to synthesize polymers bearing maleimide side chains. J. Polym. Sci. A Polym. Chem. 2007, 45, 4545–4551. [Google Scholar] [CrossRef]
- Iacono, S.; Martone, A.; Pastore, A.; Filippone, G.; Acierno, D.; Zarrelli, M.; Giordano, M.; Amendola, E. Thermally activated multiple self-healing diels-alder epoxy system. Polym. Eng. Sci. 2017, 57, 674–679. [Google Scholar] [CrossRef]
- Yasuda, K.; Sugane, K.; Shibata, M. Self-healing high-performance thermosets utilizing the furan/maleimide Diels-Alder and amine/maleimide Michael reactions. J. Polym. Res. 2020, 27, 18. [Google Scholar] [CrossRef]
- Iacono, S.; Martone, A.; Filippone, G.; Acierno, D.; Zarrelli, M.; Giordano, M.; Amendola, E. Insight on mendable resin made by combining Diels-Alder epoxy adducts with DGEBA. AIP Conf. Proc. 2016, 1736, 020075. [Google Scholar] [CrossRef]
- Mozhdehi, D.; Ayala, S.; Cromwell, O.; Guan, Z. Self-healing multiphase polymers via dynamic metal-ligand interactions. J. Am. Chem. Soc. 2014, 136, 16128–16131. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Broekhuis, A.; Picchioni, F. Reversible polymer networks containing covalent and hydrogen bonding interactions. Eur. Polym. J. 2014, 50, 127–134. [Google Scholar] [CrossRef]
- Cho, S.; White, S.; Braun, P.; Braun, P.; Institute, B.; Cho, S. Self-Healing Polymer Coatings. Adv. Mater. 2009, 21, 645–649. [Google Scholar] [CrossRef]
- Fierro, G.-P.; Pinto, F.; Iacono, S.; Martone, A.; Amendola, E.; Meo, M. Monitoring of self-healing composites: A nonlinear ultrasound approach. Smart Mater. Struct. 2017, 26, 115015. [Google Scholar] [CrossRef]
- del Olmo, R.; Tiringer, U.; Milošev, I.; Visser, P.; Arrabal, R.; Matykina, E.; Mol, J. Hybrid sol-gel coatings applied on anodized AA2024-T3 for active corrosion protection. Surf. Coat. Technol. 2021, 419, 127251. [Google Scholar] [CrossRef]
- Carlton, G. Hexavalent Chromium Exposures During Full-Aircraft Corrosion Control. Am. Ind. Hyg. Assoc. J. 2003, 64, 668–672. [Google Scholar] [CrossRef]
- Onyeachu, I.; Solomon, M. Benzotriazole derivative as an effective corrosion inhibitor for low carbon steel in 1 M HCl and 1 M HCl + 3.5 wt% NaCl solutions. J. Mol. Liq. 2020, 313, 113536. [Google Scholar] [CrossRef]
- Castaldo, R.; de Luna, M.; Siviello, C.; Gentile, G.; Lavorgna, M.; Amendola, E.; Cocca, M. On the acid-responsive release of benzotriazole from engineered mesoporous silica nanoparticles for corrosion protection of metal surfaces. J. Cult. Herit. 2020, 44, 317–324. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Polyaniline-cerium oxide (PAni-CeO2) coated graphene oxide for enhancement of epoxy coating corrosion protection performance on mild steel. Corros. Sci. 2018, 137, 111–126. [Google Scholar] [CrossRef]
- Kausar, A. Polymer coating technology for high performance applications: Fundamentals and advances. J. Macromol. Sci. Part A 2018, 55, 440–448. [Google Scholar] [CrossRef]
- Monetta, T.; Bellucci, F.; Nicodemo, L.; Nicolais, L. Protective properties of epoxy-based organic coatings on mild steel. Prog. Org. Coat. 1993, 21, 353–369. [Google Scholar] [CrossRef]
- Martone, A.; Iacono, S.; Xiao, S.; Xu, F.; Amendola, E. An integrated strategy to promote self-healing at interface in composites based on Diels-Alder epoxy. AIP Conf. Proc. 2018, 1981, 020046. [Google Scholar] [CrossRef]
- Oliver, W.; Pharr, G. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Strandman-Long, L.; Murto, J. Furfuryl alcohol—III. Infrared, matrix infrared and Raman spectra and ab initio and normal coordinate calculations. Spectrochim Acta A 1981, 37, 643–653. [Google Scholar] [CrossRef]
- Geitner, R.; Kötteritzsch, J.; Siegmann, M.; Bocklitz, T.; Hager, M.; Schubert, U.; Gräfe, S.; Dietzek, B.; Schmitt, M.; Popp, J. Two-dimensional Raman correlation spectroscopy reveals molecular structural changes during temperature-induced self-healing in polymers based on the Diels–Alder reaction. Phys. Chem. Chem. Phys. 2015, 17, 22587–22595. [Google Scholar] [CrossRef]
- Zheng, S.; Ashcroft, I. A depth sensing indentation study of the hardness and modulus of adhesives. Int. J. Adhes. Adhes. 2005, 25, 67–76. [Google Scholar] [CrossRef]
- Mansfeld, F. Use of electrochemical impedance spectroscopy for the study of corrosion protection by polymer coatings. J. Appl. Electrochem. 1995, 25, 187–202. [Google Scholar] [CrossRef]
- Wang, W.; Xu, L.; Li, X.; Lin, Z.; Yang, Y.; An, E. Self-healing mechanisms of water triggered smart coating in seawater. J. Mater. Chem. A Mater. 2014, 2, 1914–1921. [Google Scholar] [CrossRef]
- Brasher, D.; Kingsbury, A. Electrical measurements in the study of immersed paint coatings on metal. I. Comparison between capacitance and gravimetric methods of estimating water-uptake. J. Appl. Chem. 1954, 4, 62–72. [Google Scholar] [CrossRef]
- Margarit-Mattos, I. EIS and organic coatings performance: Revisiting some key points. Electrochim. Acta 2020, 354, 136725. [Google Scholar] [CrossRef]
- van der Wel, G.; Adan, O. Moisture in Organic Coatings—A review. Prog. Org. Coat. 1999, 37, 1–14. [Google Scholar] [CrossRef]
- de Rosa, L.; Monetta, T.; Bellucci, F. Moisture Uptake in Organic Coatings Monitored with EIS. Mater. Sci. Forum 1998, 289–292, 315–326. [Google Scholar] [CrossRef]
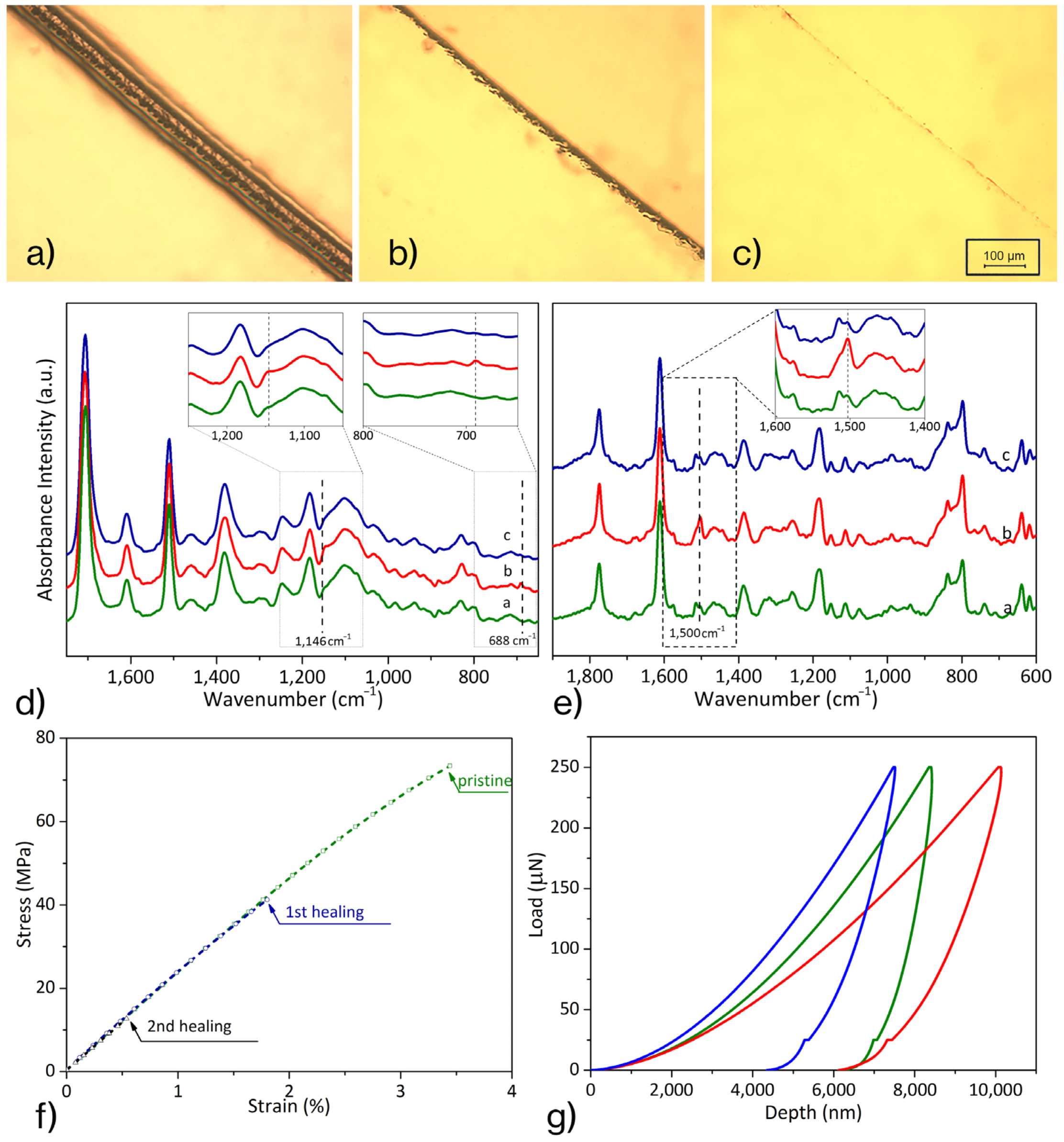
| Description | Elastic Modulus, GPa | Hardness, GPa |
|---|---|---|
| Pristine 2Ph2Epo65 | 4.89 ± 0.35 | 0.19 ± 0.02 |
| After 20 min @ 120 °C | 2.60 ± 0.29 | 0.16 ± 0.02 |
| Additional 6 h @ 90 °C | 4.10 ± 0.63 | 0.21 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amendola, E.; Palmieri, B.; Dello Iacono, S.; Martone, A. Thermally Mendable Self-Healing Epoxy Coating for Corrosion Protection in Marine Environments. Materials 2023, 16, 1775. https://doi.org/10.3390/ma16051775
Amendola E, Palmieri B, Dello Iacono S, Martone A. Thermally Mendable Self-Healing Epoxy Coating for Corrosion Protection in Marine Environments. Materials. 2023; 16(5):1775. https://doi.org/10.3390/ma16051775
Chicago/Turabian StyleAmendola, Eugenio, Barbara Palmieri, Stefania Dello Iacono, and Alfonso Martone. 2023. "Thermally Mendable Self-Healing Epoxy Coating for Corrosion Protection in Marine Environments" Materials 16, no. 5: 1775. https://doi.org/10.3390/ma16051775








