Sr-Doping All-Inorganic CsPbBr3 Perovskite Thick Film for Self-Powered X-ray Detectors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
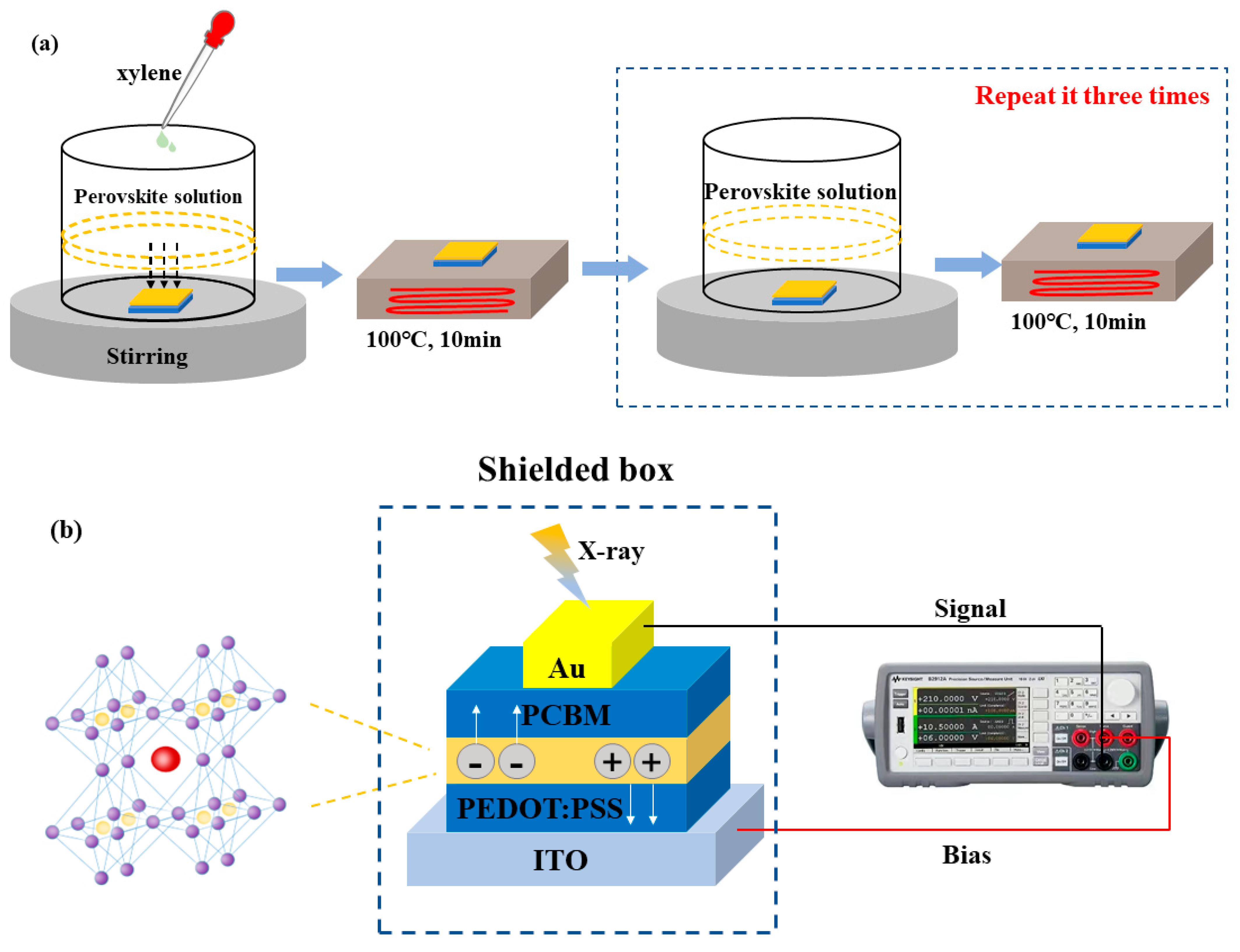
2.2. Device Fabrication
2.3. Characterizations
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kasap, S.; Frey, J.B.; Belev, G.; Tousignant, O.; Mani, H.; Greenspan, J.; Laperriere, L.; Bubon, O.; Reznik, A.; DeCrescenzo, G.; et al. Amorphous and Polycrystalline Photoconductors for Direct Conversion Flat Panel X-ray Image Sensors. Sensors 2011, 11, 5112–5157. [Google Scholar] [CrossRef] [Green Version]
- Yaffe, M.J.; Rowlands, J.A. X-ray detectors for digital radiography. Phys. Med. Biol. 1997, 42, 1. [Google Scholar] [CrossRef] [PubMed]
- Kasap, S.O.; Haugen, C.; Nesdoly, M.; Rowlands, J.A. Properties of a-Se for use in at panel X-ray image detectors. J. Non-Cryst. Solids 2000, 266, 1163–1167. [Google Scholar] [CrossRef]
- Chen, H.; Teale, S.; Chen, B.; Hou, Y.; Grater, L.; Zhu, T.; Bertens, K.; Park, S.M.; Atapattu, H.R.; Gao, Y.; et al. Quantum-size-tuned heterostructures enable efficient and stable inverted perovskite solar cells. Nat. Photonics 2022, 16, 352–358. [Google Scholar] [CrossRef]
- Hossain, M.I.; Shahiduzzaman, M.; Saleque, A.M.; Huqe, R.; Qarony, W.; Ahmed, S.; Akhtaruzzaman, M.; Knipp, D.; Tsang, Y.H.; Taima, T.; et al. Improved Nanophotonic Front Contact Design for High-Performance Perovskite Single-Junction and Perovskite/Perovskite Tandem Solar Cells. Sol. RRL 2021, 5, 2100509. [Google Scholar] [CrossRef]
- Yuce, H.; Perini, C.A.; Hidalgo, J.; Castro-Méndez, A.F.; Evans, C.; Betancur, P.F.; Vagott, J.N.; An, Y.; Bairley, K.; Demir, M.M.; et al. Understanding the impact of SrI2 additive on the properties of Sn-based halide perovskites. Opt. Mater. 2022, 123, 111806. [Google Scholar] [CrossRef]
- Shen, L.; Fang, Y.; Wang, D.; Bai, Y.; Deng, Y.; Wang, M.; Lu, Y.; Huang, J. A Self-Powered, Sub-nanosecond-Response Solution Processed Hybrid Perovskite Photodetector for Time Resolved Photoluminescence-Lifetime Detection. Adv. Mater. 2016, 28, 10794–10800. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, W.; Dong, H.; Li, G.; Xi, K.; Divitini, G.; Ran, C.; Yuan, F.; Zhang, M.; Jiao, B.; et al. A Strategy for Architecture Design of Crystalline Perovskite Light-Emitting Diodes with High Performance. Adv. Mater. 2018, 30, 1800251. [Google Scholar] [CrossRef]
- Thapa, S.; Adhikari, G.C.; Zhu, H.; Grigoriev, A.; Zhu, P. Zn-Alloyed All-Inorganic Halide Perovskite-Based White Light Emitting Diodes with Superior Color Quality. Sci. Rep. 2019, 9, 18636. [Google Scholar] [CrossRef] [Green Version]
- Dou, L.; Yang, Y.; You, J.; Hong, Z.; Chang, W.-H.; Li, G. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Hadar, I.; De Siena, M.C.; Klepov, V.V.; Pan, L.; Chung, D.Y.; Kanatzidis, M.G. Sensitivity and Detection Limit of Spectroscopic-Grade Perovskite CsPbBr3 Crystal for Hard X-ray Detection. Adv. Energy Mater. 2022, 32, 2112925. [Google Scholar]
- Zhou, L.; Lu, X.; Wu, J.; Jiang, H.; Chen, L.; Ouyang, X.; Lau, K.M. Self-Powered Fast-Response X-ray Detectors Based on Vertical GaN p-n Diodes. IEEE Electron. Device Lett. 2018, 5, 1044–1047. [Google Scholar] [CrossRef]
- Heo, J.H.; Park, J.K.; Yang, Y.M.; Lee, D.S.; Im, S.H. Self-powered flexible all-perovskite X-ray detectors with high sensitivity and fast response. ISCIENCE 2021, 24, 102927. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meng, C.; Huang, B.; Yang, D.; Xu, X.; Zeng, H. All-Perovskite Integrated X-ray Detector with Ultrahigh Sensitivity. Adv. Opt. Mater. 2020, 8, 2000273. [Google Scholar] [CrossRef]
- Jin, J.; Li, H.; Chen, C.; Zhang, B.; Xu, L.; Dong, B.; Song, H.; Dai, Q. Enhanced Performance of Perovskite Solar Cells with Zinc Chloride Additives. ACS Appl. Mater. Interfaces 2017, 9, 42875–42882. [Google Scholar] [CrossRef]
- Xu, L.; Yuan, S.; Zeng, H.; Song, J. A comprehensive review of doping in perovskite nanocrystals/quantum dots: Evolution of structure, electronics, optics, and light-emitting diodes. Mater. Today Nano 2019, 6, 100036. [Google Scholar] [CrossRef]
- Gao, Y.; Luo, C.; Yan, C.; Li, W.; Liu, C.; Yang, W. Copper-doping defect-lowered perovskite nanosheets for deep-blue light-emitting diodes. J. Colloid Interface Sci. 2022, 607, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, X.; Liu, Z.; Sun, B.; Li, J.; Xi, S.; Shi, T.; Tang, Z.; Liao, G. Enhancing the optical, morphological and electronic properties of the solution-processed CsPbIBr2 films by Li doping for efficient carbon-based perovskite solar cells. Appl. Surf. Sci. 2020, 499, 143990. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; Yang, X.; Wang, Y.; He, B.; Tang, Q. Lanthanide Ions Doped CsPbBr3 Halides for HTM-Free 10.14%-Efficiency Inorganic Perovskite Solar Cell with an Ultrahigh Open-Circuit Voltage of 1.594 V. Adv. Energy Mater. 2018, 8, 1802346. [Google Scholar] [CrossRef]
- Jacobsson, T.J.; Pazoki, M.; Hagfeldt, A.; Edvinsson, T. Goldschmidt’s Rules and Strontium Replacement in Lead Halogen Perovskite Solar Cells: Theory and Preliminary Experiments on CH3NH3SrI3. J. Phys. Chem. C 2015, 119, 25673–25683. [Google Scholar] [CrossRef]
- Chan, S.H.; Wu, M.C.; Lee, K.M.; Chen, W.C.; Lin, T.H.; Su, W.F. Enhancing perovskite solar cell performance and stability by doping barium in methylammonium lead halide. J. Mater. Chem. A 2017, 5, 18044–18052. [Google Scholar] [CrossRef]
- Liu, C.; Yang, D.; Tian, H.; Zhang, W.; Zhu, J. X-ray detectors based on CsPb1-xSrxBr3 thick films. Ceram. Int. 2023, 49, 5527–5531. [Google Scholar] [CrossRef]
- Gou, Z.; Huanglong, S.; Ke, W.; Sun, H.; Tian, H.; Gao, X.; Zhu, X.; Yang, D.; Wangyang, P. Self-Powered X-ray Detector Based on All-Inorganic Perovskite Thick Film with High Sensitivity Under Low Dose Rate. Phys. Status Solidi (RRL) Rapid Res. Lett. 2019, 13, 1900094. [Google Scholar] [CrossRef]
- Castro-Méndez, A.F.; Hidalgo, J.; Correa-Baena, J.P. The Role of Grain Boundaries in Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1901489. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Q.; Chen, D.; Zhang, Z.; Lin, Z.; Chang, J.; Zhang, J.; Zhang, C.; Hao, Y. Intermolecular Exchange Boosts Efficiency of Air-Stable, Carbon-Based All-Inorganic Planar CsPbIBr2 Perovskite Solar Cells to Over 9%. Adv. Energy Mater. 2018, 8, 1802080. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Zhang, C.; Ma, Y.; Fan, J.; Mai, Y. All-Inorganic CsPbI2Br Perovskite Solar Cells with High Efficiency Exceeding 13%. J. Am. Chem. Soc. 2018, 140, 3825–3828. [Google Scholar] [CrossRef]
- Gao, L.; Huang, S.; Chen, L.; Li, X.; Ding, B.; Huang, S.; Yang, G. Excellent Stability of Perovskite Solar Cells by Passivation Engineering. Solar RRL 2018, 2, 1800088. [Google Scholar] [CrossRef]
- Wu, J.; Xu, X.; Zhao, Y.; Shi, J.; Xu, Y.; Luo, Y.; Li, D.; Wu, H.; Meng, Q. DMF as an Additive in a Two-Step Spin-Coating Method for 20% Conversion Efficiency in Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 26937–26947. [Google Scholar] [CrossRef]
- Shai, X.; Zuo, L.; Sun, P.; Liao, P.; Huang, W.; Yao, E.-P.; Li, H.; Liu, S.; Shen, Y.; Yang, Y.; et al. Efficient planar perovskite solar cells using halide Sr-substituted Pb perovskite. Nano Energy 2017, 36, 213–222. [Google Scholar] [CrossRef]
- Nam, J.K.; Chai, S.U.; Cha, W.; Choi, Y.J.; Kim, W.; Jung, M.S.; Kwon, J.; Kim, D.; Park, J.H. Potassium Incorporation for Enhanced Performance and Stability of Fully Inorganic Cesium Lead Halide Perovskite Solar Cells. Nano Lett. 2017, 17, 2028–2033. [Google Scholar] [CrossRef]
- Yan, J.; Gao, F.; Tian, Y.; Li, Y.; Gong, W.; Wang, S.; Zhu, H.; Li, L. Controllable Perovskite Single Crystal Heterojunction for Stable Self-Powered Photo-Imaging and X-ray Detection. Adv. Opt. Mater. 2022, 10, 2200449. [Google Scholar] [CrossRef]
- Wei, H.; Fang, Y.; Mulligan, P.; Chuirazzi, W.; Fang, H.-H.; Wang, C.; Ecker, B.R.; Gao, Y.; Loi, M.A.; Cao, P.M.W.C.L.; et al. Sensitive X-ray detectors made of methylammonium lead tribromide perovskite single crystals. Nat. Photonics 2016, 10, 333–339. [Google Scholar] [CrossRef]
- Shrestha, S.; Fischer, R.; Matt, G.J.; Feldner, P.; Michel, T.; Osvet, A.; Levchuk, I.; Merle, B.; Golkar, S.; Chen, H.; et al. High-performance direct conversion X-ray detectors based on sintered hybrid lead triiodide perovskite wafers. Nat. Photonics 2017, 11, 436–440. [Google Scholar] [CrossRef]
- Yakunin, S.; Sytnyk, M.; Kriegner, D.; Shrestha, S.; Richter, M.; Matt, G.J.; Azimi, H.; Brabec, C.J.; Stangl, J.; Kovalenko, M.V.; et al. Detection of X-ray photons by solution-processed organic-inorganic perovskites. Nat. Photonics 2015, 9, 444–449. [Google Scholar] [CrossRef]
- Steele, J.A.; Pan, W.; Martin, C.; Keshavarz, M.; Debroye, E.; Yuan, H.; Banerjee, S.; Fron, E.; Jonckheere, D.; Kim, C.W.; et al. Photophysical Pathways in Highly Sensitive Cs2AgBiBr6Double-Perovskite Single-Crystal X-ray Detectors. Adv. Mater. 2018, 30, 1804450. [Google Scholar] [CrossRef]
- Wang, C.; Du, X.; Wang, S.; Deng, H.; Chen, C.; Niu, G.; Pang, J.; Li, K.; Lu, S.; Lin, X.; et al. Sb2Se3 film with grain size over 10 microm toward X-ray detection. Front. Optoelectron. 2021, 14, 341–351. [Google Scholar] [CrossRef]
- Daum, M.; Deumel, S.; Sytnyk, M.; Afify, H.A.; Hock, R.; Eigen, A.; Zhao, B.; Halik, M.; These, A.; Matt, G.J.; et al. Self-Healing Cs3Bi2Br3I6 Perovskite Wafers for X-ray Detection. Adv. Funct. Mater. 2021, 31, 2102713. [Google Scholar] [CrossRef]
- Sun, H.; Yang, D.; Liu, Y.; Zhu, X. Highly Flexible X-ray Detectors Based on Pure Inorganic Metal Iodide Polycrystalline Thin Films as Photon-to-Charge Conversion Layers. ACS Appl. Electron. Mater. 2019, 1, 2637–2645. [Google Scholar] [CrossRef]



| Material | Bias (V) | SC/Film | Absorbing Thickness | Dark Current | The Highest Detectable Dose Rate | Sensitivity (µC Gyair−1 cm−3 ) | SNR | Response Time | Ref |
|---|---|---|---|---|---|---|---|---|---|
| MAPbBr3 | 0.1 | SC | 2–3 mm | 29 nA cm−2 | / | 270 | / | 216 µs | [32] |
| MAPbI3 | 200 | PC | 1 mm | 6 µA cm−1 | / | 25,300 | / | / | [33] |
| CsPbBr3 | 0 | film | 80 µm | / | 0.082 μGy ms−1 | / | 39.2 | / | [23] |
| MAPbI3 | 80 | film | 60 µm | / | / | 25 | / | / | [34] |
| Cs2AgBiBr6 | 100 | SC | 2 mm | / | 61.12 μGy S−1 | 1600 | / | / | [35] |
| Sb2Se3 | −1 | film | 5 µm | 91.2 nA | 5.499 µGy S−1 | 21,000 | / | 2.5 ms | [36] |
| Cs3Bi2Br3I6 | 50 | PC | 0.7 mm | 12.5 nA | 2.2 mGy s−1 | 8.16 | / | / | [37] |
| BiI2 | 10 | film | 4–8 μm | 20 pA | / | / | / | 231–368 ms | [38] |
| CsPbBr3 | 0 | film | 160 µm | 5 pA | 0.955 μGy ms−1 | 288.11 | 58.8 | 0.056–0.233 s | this work |
| CsPbBr3:Sr | 0 | film | 160 µm | 12 pA | 0.955 μGy ms−1 | 517.02 | 154.9 | 0.053–0.148 s | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Zhang, W.; Yang, D.; Tian, H.; Zhu, J. Sr-Doping All-Inorganic CsPbBr3 Perovskite Thick Film for Self-Powered X-ray Detectors. Materials 2023, 16, 1783. https://doi.org/10.3390/ma16051783
Liu C, Zhang W, Yang D, Tian H, Zhu J. Sr-Doping All-Inorganic CsPbBr3 Perovskite Thick Film for Self-Powered X-ray Detectors. Materials. 2023; 16(5):1783. https://doi.org/10.3390/ma16051783
Chicago/Turabian StyleLiu, Chuanqi, Wen Zhang, Dingyu Yang, Haibo Tian, and Jun Zhu. 2023. "Sr-Doping All-Inorganic CsPbBr3 Perovskite Thick Film for Self-Powered X-ray Detectors" Materials 16, no. 5: 1783. https://doi.org/10.3390/ma16051783





