Mechanical Performance and Cytotoxicity of an Alginate/Polyacrylamide Bipolymer Network Developed for Medical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Double Polymer Network Alginate/Polyacrylamide
2.3. Mechanical Properties—Compression Test
2.4. Preliminary Study
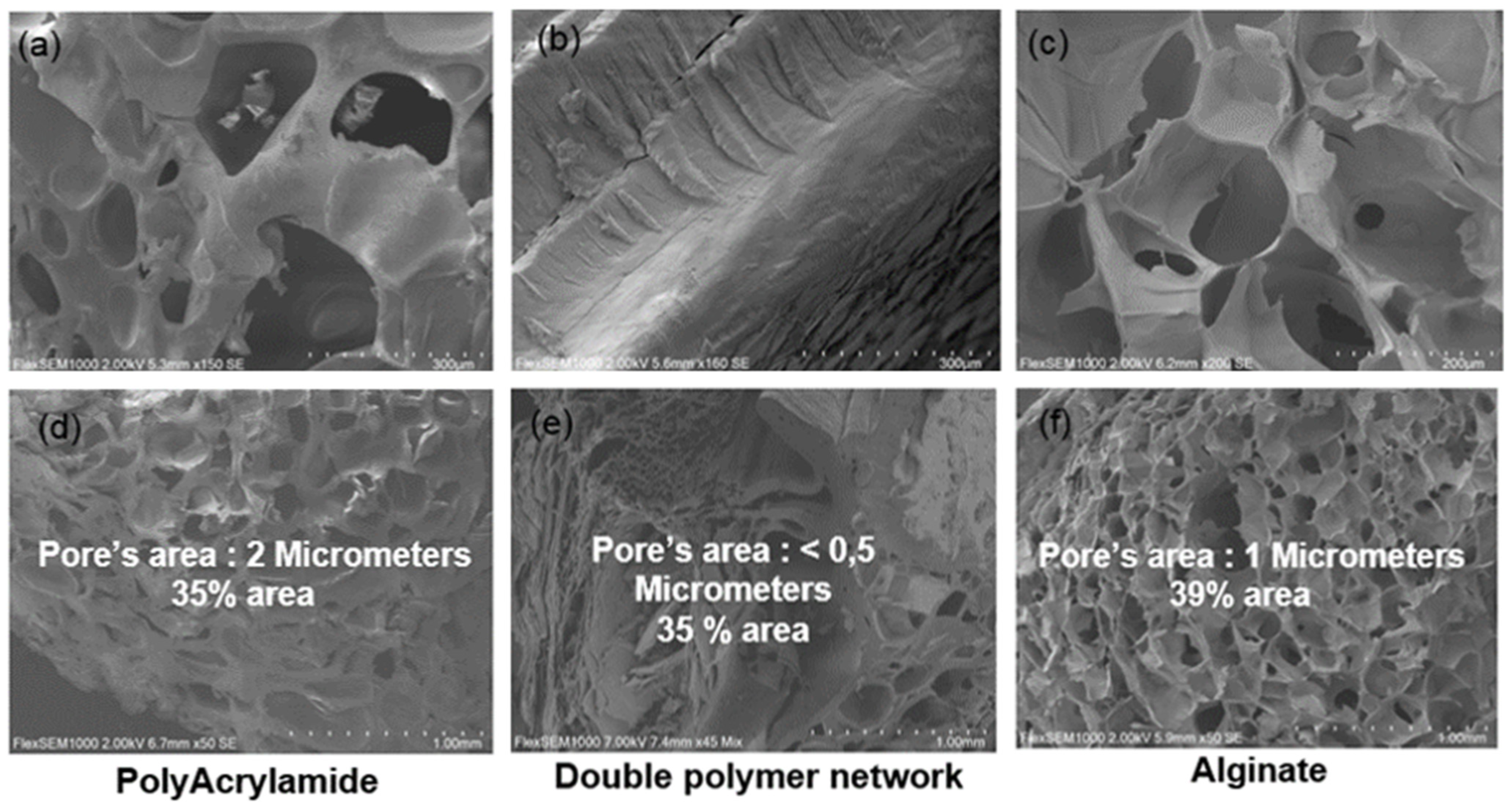
2.4.1. Microstructure Evaluation
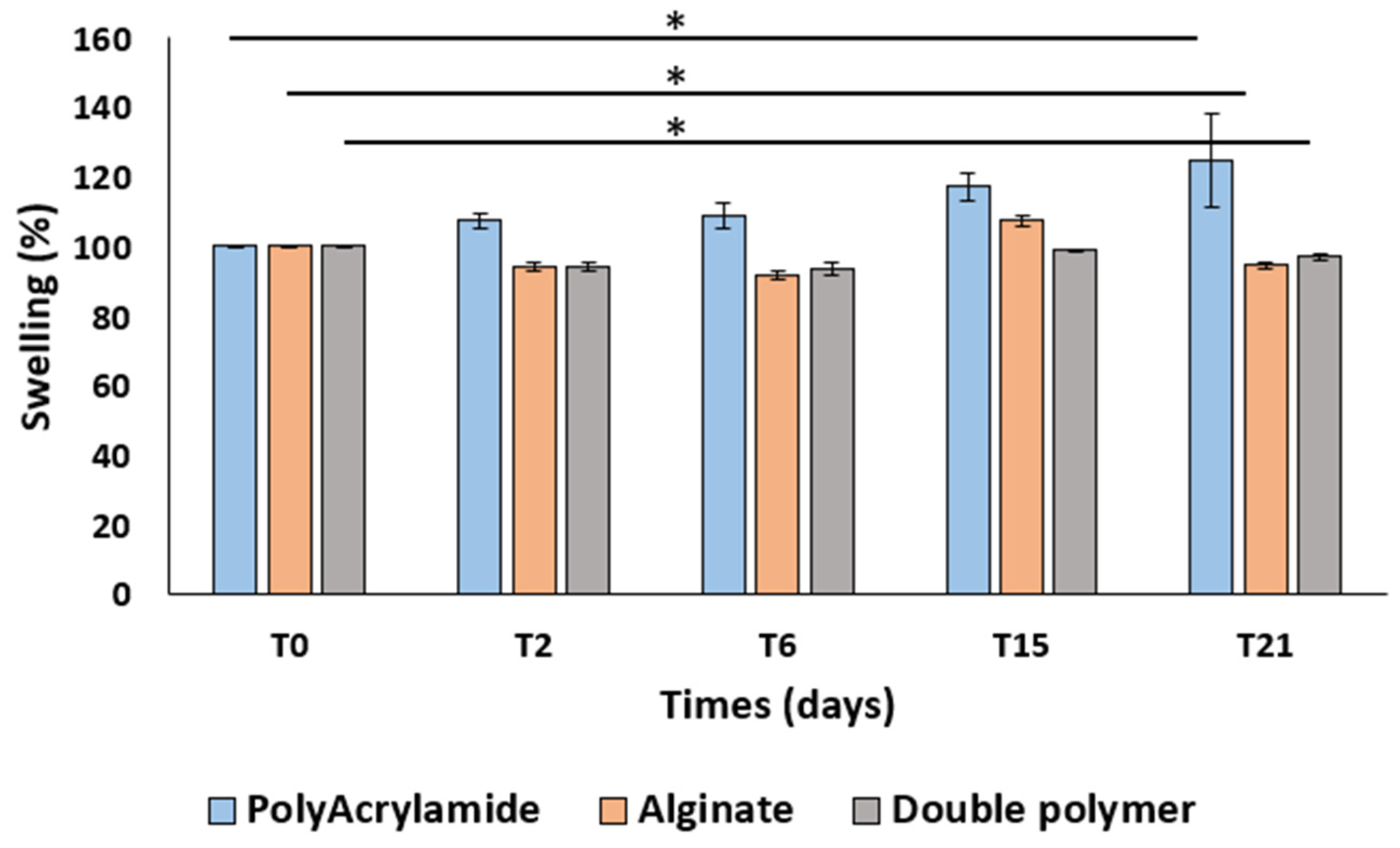
2.4.2. Swelling Test
2.4.3. Extractable and Leachable Test
2.4.4. In Vitro Cytotoxicity Assessment
2.4.5. Cell Culture
2.4.6. MTT Assay
3. Results and Discussion
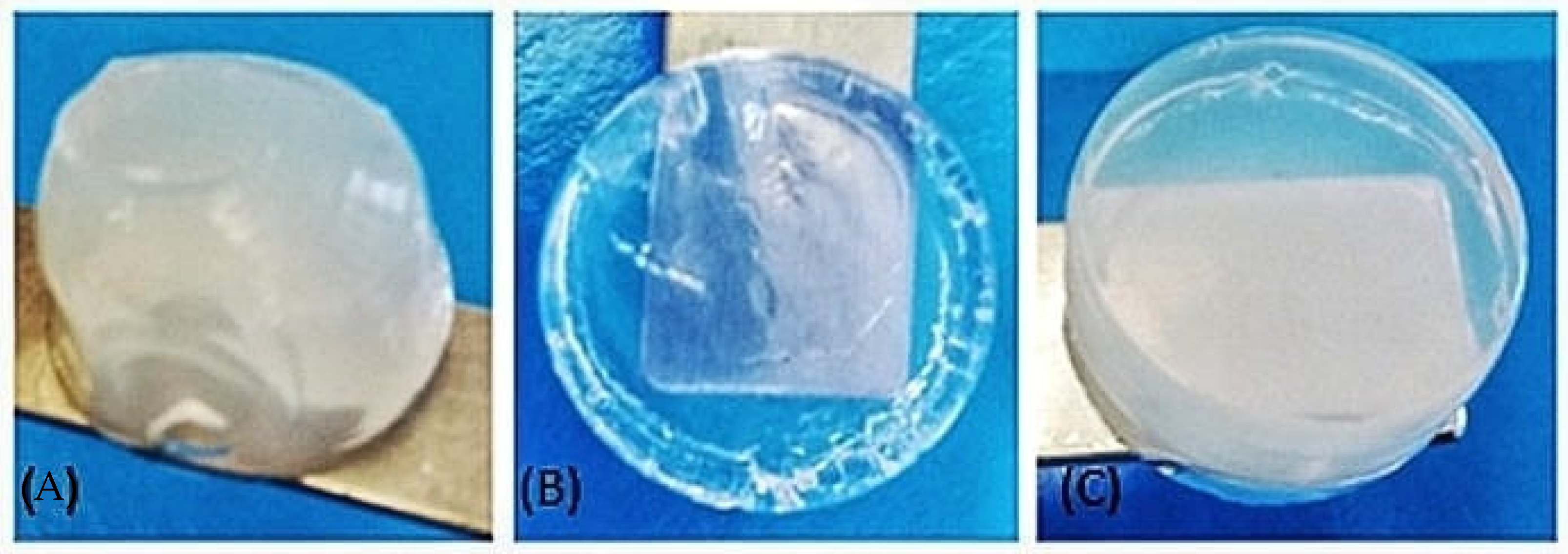
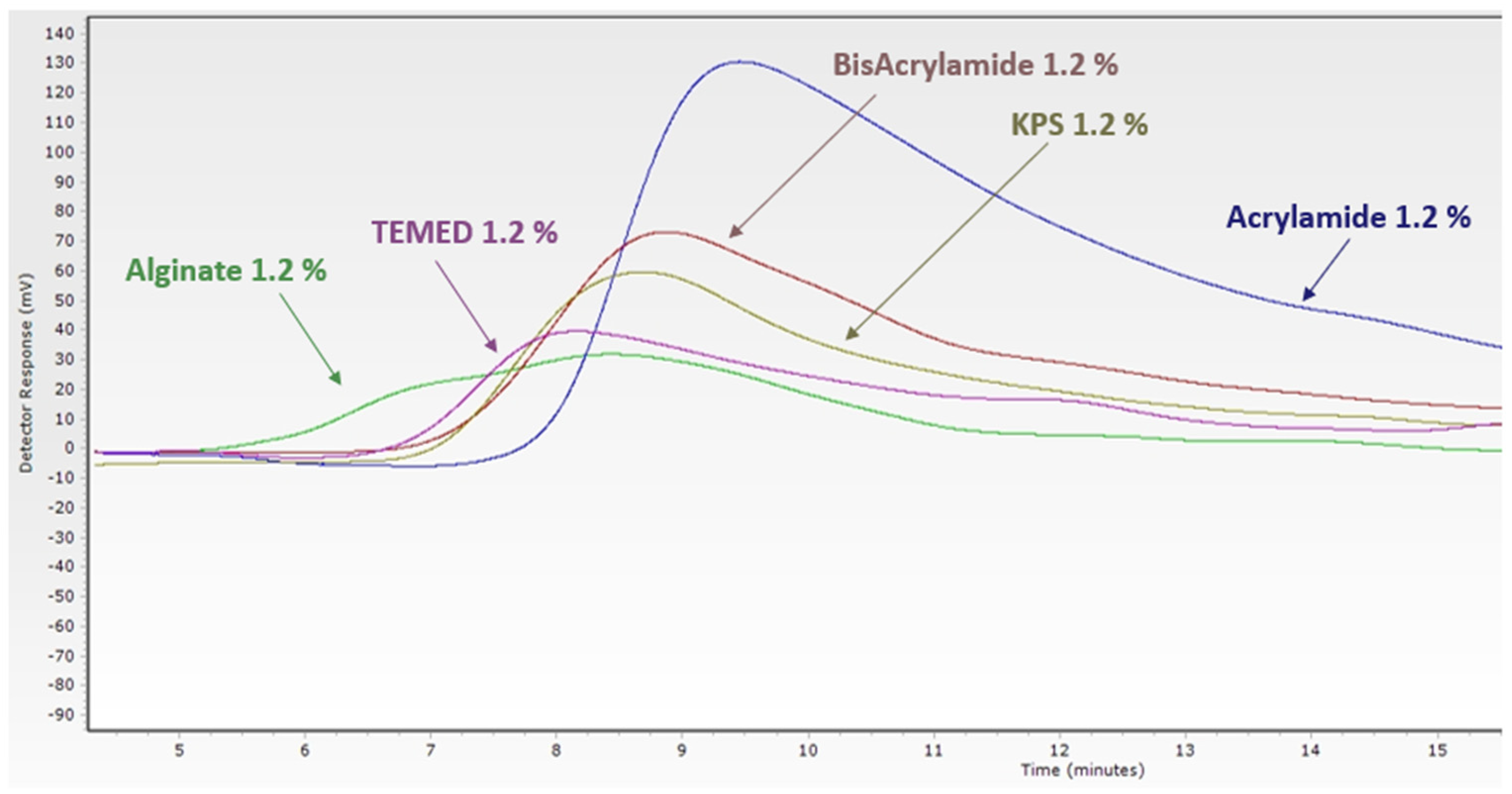
3.1. Characterization of Biomaterial Scaffolds
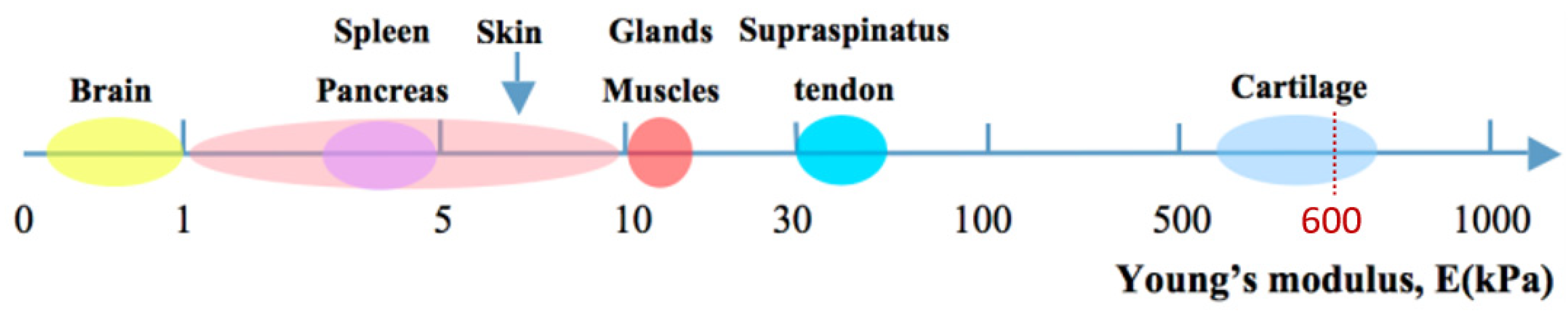
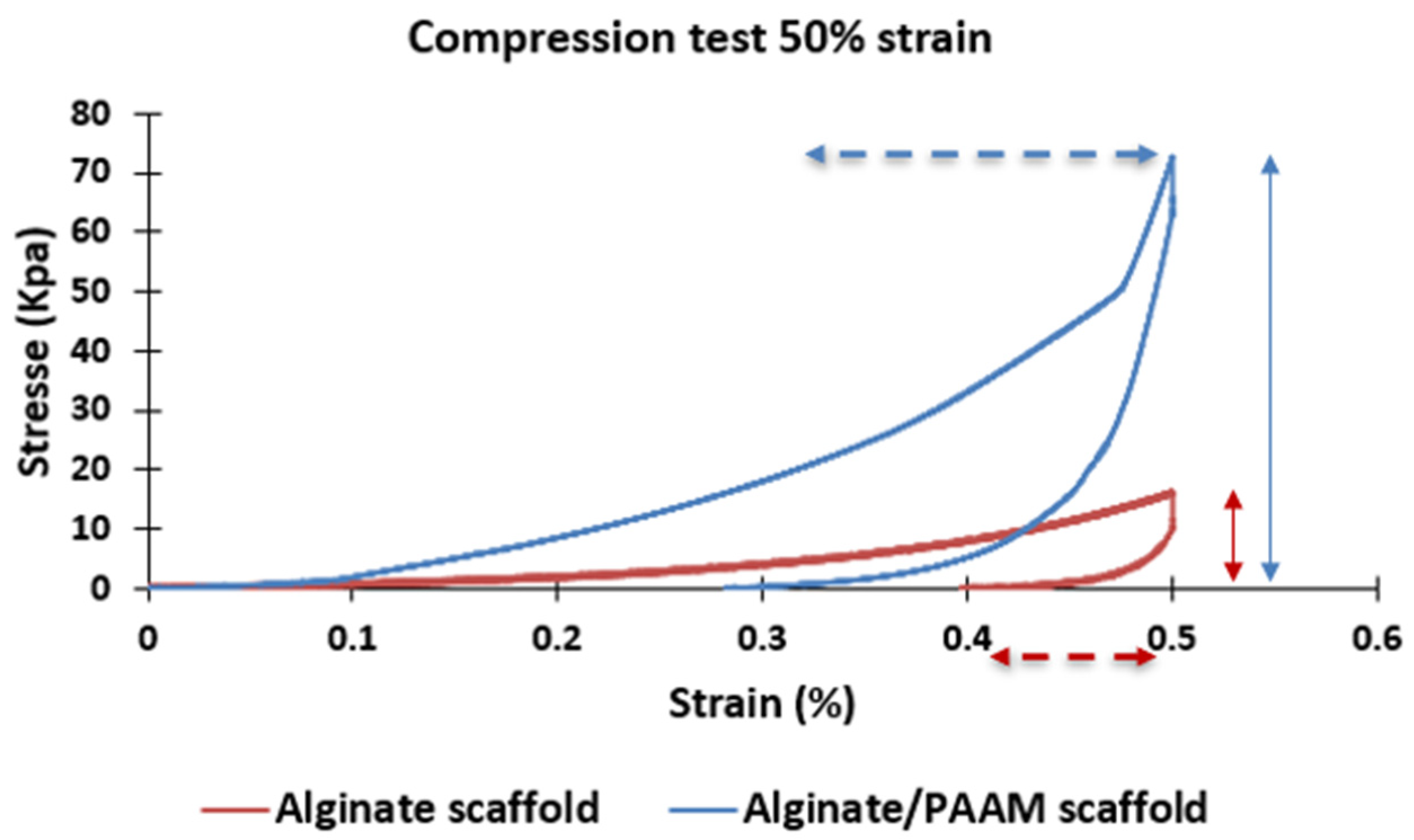
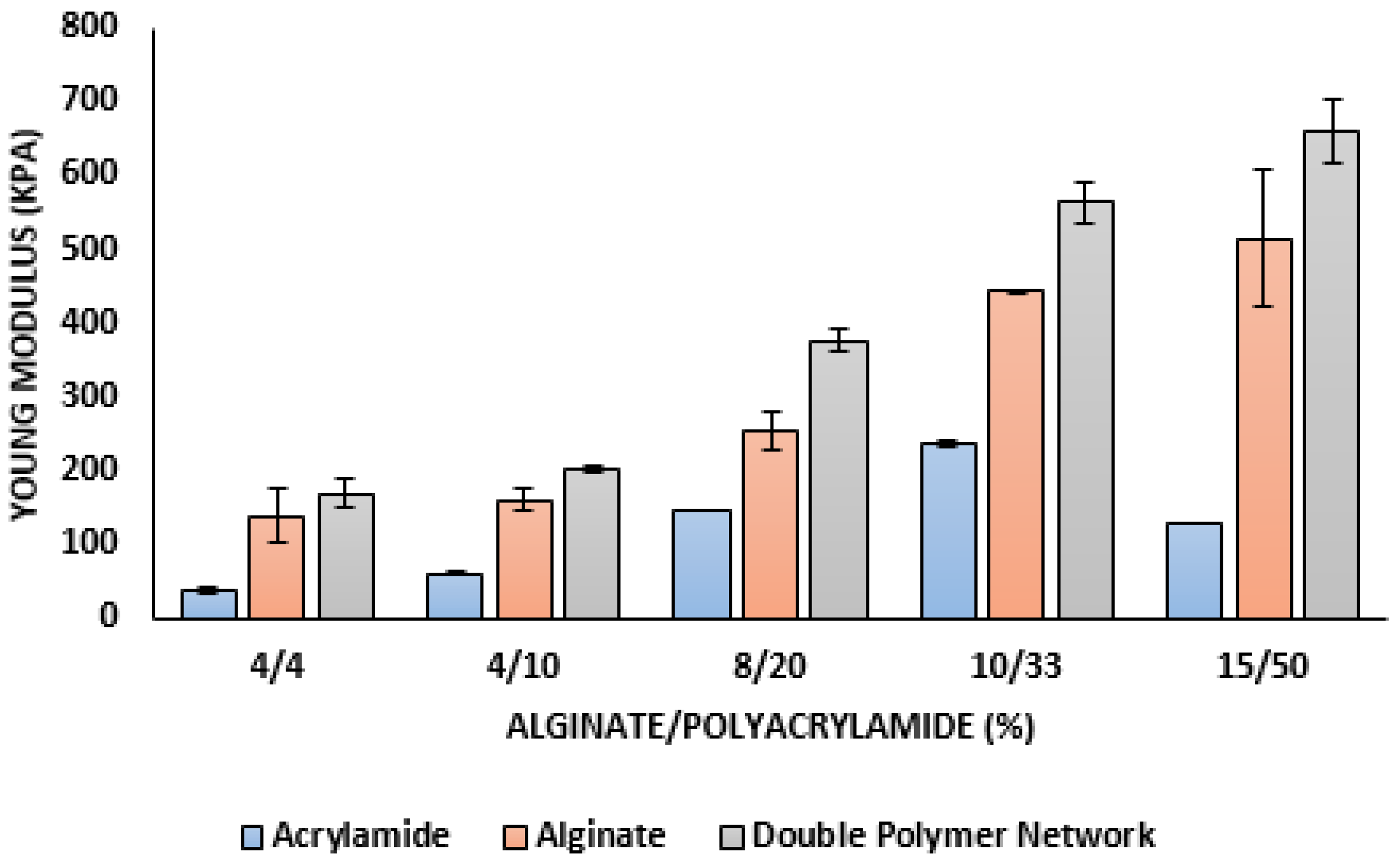
3.2. Mechanical Properties
3.3. Preliminary Study
3.3.1. Extractable and Leachable Test
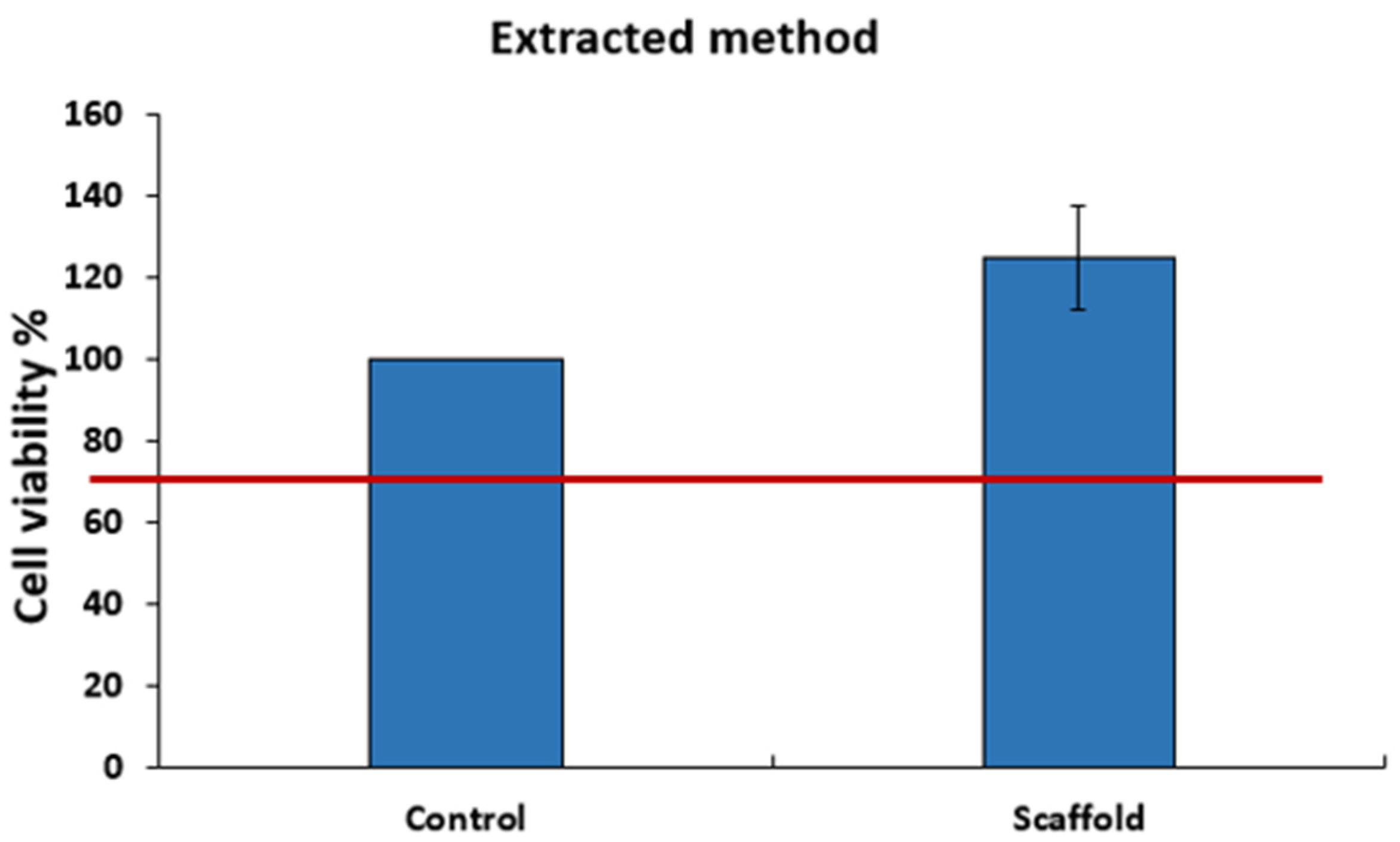
3.3.2. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutolf, M.P. Spotlight on hydrogels. Nat. Mater. 2009, 8, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Tay, R.; Khan, M.; Ee, P.L.R.; Hedrick, J.L.; Yang, Y.Y. Synthetic hydrogels for controlled stem cell differentiation. Soft Matter 2009, 6, 67–81. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, H.; Poh, P.S.P.; Machens, H.-G.; Schilling, A.F. Hydrogels for Engineering of Perfusable Vascular Networks. Int. J. Mol. Sci. 2015, 16, 15997–16016. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Yuan, Y.; Chi, F. Biomimetic alginate/polyacrylamide porous scaffold supports human mesenchymal stem cell proliferation and chondrogenesis. Mater. Sci. Eng. C 2014, 42, 622–628. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The Role of Tissue Engineering in Articular Cartilage Repair and Regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Keeney, M.; Lai, J.H.; Yang, F. Recent progress in cartilage tissue engineering. Curr. Opin. Biotechnol. 2011, 22, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A. Biomaterials in Tissue Engineering. Nat. Biotechnol. 1995, 13, 565–576. [Google Scholar] [CrossRef]
- Peter, S.J.; Miller, M.; Yasko, A.; Yaszemski, M.; Mikos, A.G. Polymer concepts in tissue engineering. J. Biomed. Mater. Res. 1998, 43, 422–427. [Google Scholar] [CrossRef]
- Isogai, N.; Landis, W.; Kim, T.H.; Gerstenfeld, L.C.; Upton, J.; Vacanti, J.P. Formation of Phalanges and Small Joints by Tissue-Engineering*†. J. Bone Jt. Surg. 1999, 81, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sato, T.; Ushida, T.; Ochiai, N.; Tateishi, T. Tissue Engineering of Cartilage Using a Hybrid Scaffold of Synthetic Polymer and Collagen. Tissue Eng. 2004, 10, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Darnell, M.C.; Sun, J.-Y.; Mehta, M.; Johnson, C.; Arany, P.R.; Suo, Z.; Mooney, D.J. Performance and biocompatibility of extremely tough alginate/polyacrylamide hydrogels. Biomaterials 2013, 34, 8042–8048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- García-Peñas, A.; Wang, Y.; Mena-Palomo, I.; López-Collazo, E.; Díaz-García, D.; Gómez-Ruiz, S.; Stadler, F.J. Preparation, thermoresponsive behavior, and preliminary biological study of functionalized poly(N-isopropylacrylamide-co-dopamine methacrylamide) copolymers with an organotin(IV) compound. Polym. Test. 2021, 94, 107046. [Google Scholar] [CrossRef]
- Moral-Zamorano, M.; Quijada-Garrido, I.; San-Miguel, V.; Serrano, B.; Baselga, J.; Hashmi, S.; Stadler, F.J.; García-Peñas, A. Concentration Effect over Thermoresponse Derived from Organometallic Compounds of Functionalized Poly(N-isopropylacrylamide-co-dopamine Methacrylamide). Polymers 2021, 13, 3921. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [Green Version]
- Stevens, M.M.; Qanadilo, H.F.; Langer, R.; Shastri, V.P. A rapid-curing alginate gel system: Utility in periosteum-derived cartilage tissue engineering. Biomaterials 2004, 25, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H. Recent Applications of Polyacrylamide as Biomaterials. Recent Pat. Mater. Sci. 2008, 1, 19–40. [Google Scholar] [CrossRef]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of Partially Oxidized Alginate and Its Potential Application for Tissue Engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Alsberg, E.; Kaigler, D.; Lee, K.Y.; Mooney, D. Controlling Degradation of Hydrogels via the Size of Crosslinked Junctions. Adv. Mater. 2004, 16, 1917–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakrishnan, B.; Jayakrishnan, A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials 2005, 26, 3941–3951. [Google Scholar] [CrossRef]
- Gåserød, O.; Smidsrød, O.; Skjåk-Bræk, G. Microcapsules of alginate-chitosan—I: A quantitative study of the interaction between alginate and chitosan. Biomaterials 1998, 19, 1815–1825. [Google Scholar] [CrossRef]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Bello, G.; Jackson, I.T.; Keskin, M.; Kelly, C.; Dajani, K.; Studinger, R.; Kim, E.M.H.; Lincoln, D.; Silberberg, B.; Lee, A. The Use of Polyacrylamide Gel in Soft-Tissue Augmentation: An Experimental Assessment. Plast. Reconstr. Surg. 2007, 119, 1326–1336. [Google Scholar] [CrossRef]
- Raymond, S.; Weintraub, L. Acrylamide Gel as a Supporting Medium for Zone Electrophoresis. Science 1959, 130, 711. [Google Scholar] [CrossRef]
- Christensen, L.H.; Breiting, V.B.; Aasted, A.; Jørgensen, A.; Kebuladze, I. Long-Term Effects of Polyacrylamide Hydrogel on Human Breast Tissue. Plast. Reconstr. Surg. 2003, 111, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 844:2021; Rigid Cellular Plastics—Determination of Compression Properties (ISO 844:2021). iTeh Standards Store: Etobicoke, ON, Canada. Available online: https://standards.iteh.ai/catalog/standards/cen/cda438ec-0c6d-4b6a-b27f-2e3798bddaae/en-iso-844-2021 (accessed on 13 December 2022).
- Integrated Safety and Risk Assessment for Medical Devices and Combination Products|SHAYNE GAD|Springer. Available online: https://www.springer.com/gp/book/9783030352400 (accessed on 19 July 2021).
- Feilden, A. Update on Undertaking Extractable and Leachable Testing; ISmithers: Akron, OH, USA, 2011. [Google Scholar]
- A General Overview of the Suitability for Intended Use Requirements for Materials Used in Pharmaceutical Systems—Leachables and Extractables Handbook—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781118147672.ch2 (accessed on 19 July 2021).
- Polymers|Free Full-Text|A Systematic Study on the Degradation Products Generated from Artificially Aged Microplastics. Available online: https://www.mdpi.com/2073-4360/13/12/1997 (accessed on 19 July 2021).
- Rosenbluth, S.; Weddington, G.; Guess, W.; Autian, J. Tissue Culture Method for Screening Toxicity of Plastic Materials to be Used in Medical Practice. J. Pharm. Sci. 1965, 54, 156–159. [Google Scholar] [CrossRef]
- Bernard, M.; Jubeli, E.; Bakar, J.; Saunier, J.; Yagoubi, N. Impact of simulated biological aging on physicochemical and biocompatibility properties of cyclic olefin copolymers. Mater. Sci. Eng. C 2018, 97, 377–387. [Google Scholar] [CrossRef]
- Arda, K.; Ciledag, N.; Aktas, E.; Arıbas, B.K.; Köse, S.K. Quantitative Assessment of Normal Soft-Tissue Elasticity Using Shear-Wave Ultrasound Elastography. Am. J. Roentgenol. 2011, 197, 532–536. [Google Scholar] [CrossRef]
- Hoenig, E.; Leicht, U.; Winkler, T.; Mielke, G.; Beck, K.; Peters, F.; Schilling, A.F.; Morlock, M.M. Mechanical Properties of Native and Tissue-Engineered Cartilage Depend on Carrier Permeability: A Bioreactor Study. Tissue Eng. Part A 2013, 19, 1534–1542. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Fernandez, P.; Bausch, A.R. The compaction of gels by cells: A case of collective mechanical activity. Integr. Biol. 2009, 1, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Bender, R.J.; Durand, K.L.; Anseth, K.S. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: Engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol. Bioeng. 2004, 86, 747–755. [Google Scholar] [CrossRef] [PubMed]



| ALG/PAAM | Alginate Percentage (%) | Polyacrilamide Percentage (%) |
|---|---|---|
| 4/4 | 4 | 4 |
| 4/10 | 4 | 10 |
| 8/20 | 8 | 20 |
| 10/33 | 10 | 33 |
| 15/50 | 15 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouchati, A.; Yagoubi, N. Mechanical Performance and Cytotoxicity of an Alginate/Polyacrylamide Bipolymer Network Developed for Medical Applications. Materials 2023, 16, 1789. https://doi.org/10.3390/ma16051789
Mouchati A, Yagoubi N. Mechanical Performance and Cytotoxicity of an Alginate/Polyacrylamide Bipolymer Network Developed for Medical Applications. Materials. 2023; 16(5):1789. https://doi.org/10.3390/ma16051789
Chicago/Turabian StyleMouchati, Abdullah, and Najet Yagoubi. 2023. "Mechanical Performance and Cytotoxicity of an Alginate/Polyacrylamide Bipolymer Network Developed for Medical Applications" Materials 16, no. 5: 1789. https://doi.org/10.3390/ma16051789
APA StyleMouchati, A., & Yagoubi, N. (2023). Mechanical Performance and Cytotoxicity of an Alginate/Polyacrylamide Bipolymer Network Developed for Medical Applications. Materials, 16(5), 1789. https://doi.org/10.3390/ma16051789






