Effects of K2TiF6 and Electrolyte Temperatures on Energy Consumption and Properties of MAO Coatings on 6063 Aluminum Alloy
Abstract
:1. Introduction
2. Experiment
2.1. Experimental Materials and Film Fabrication
2.2. Coating Characterizations
2.3. Energy Consumption
2.4. Corrosion Behavior of the Films
3. Results
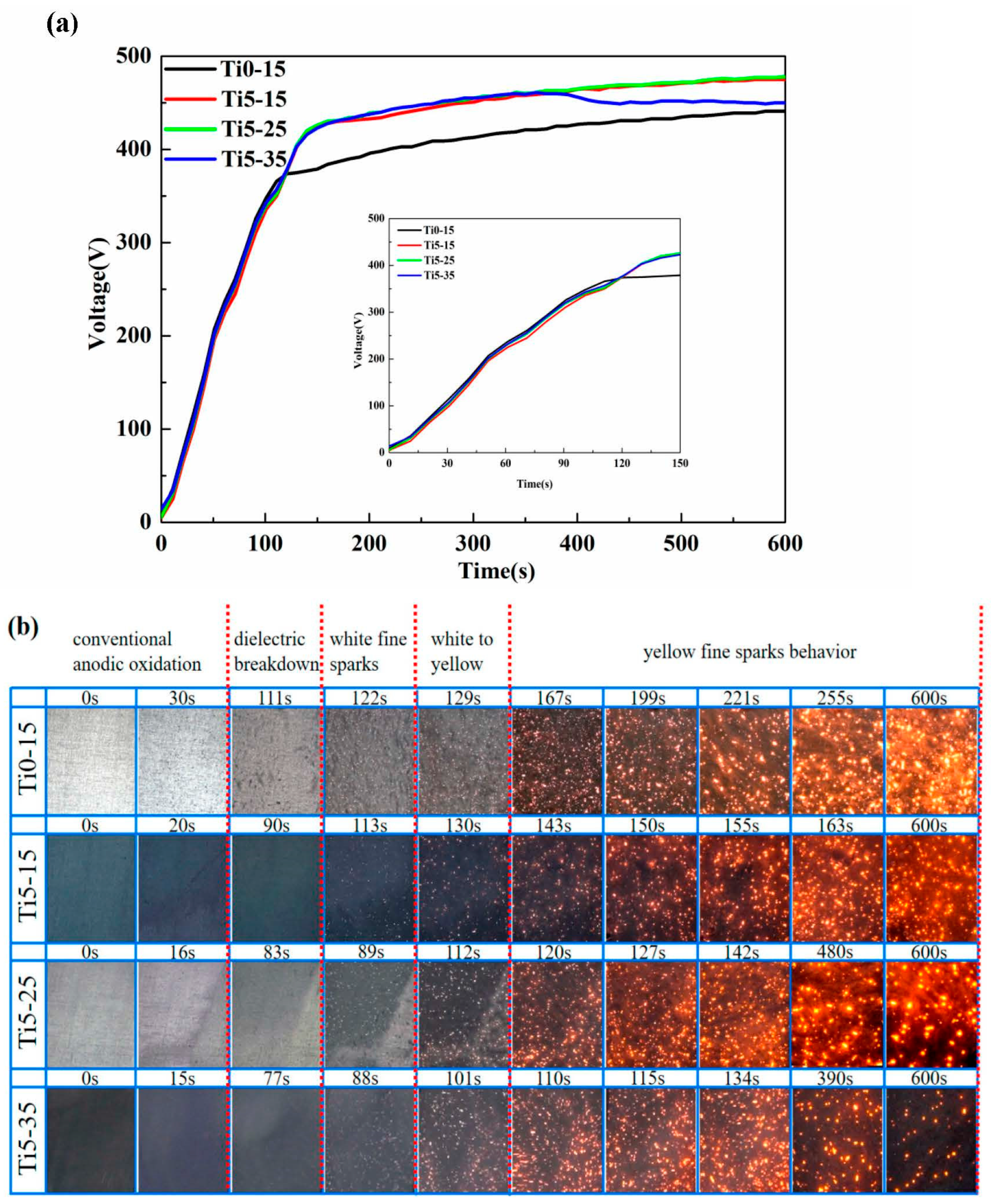
3.1. Evolution of Voltage and Discharge
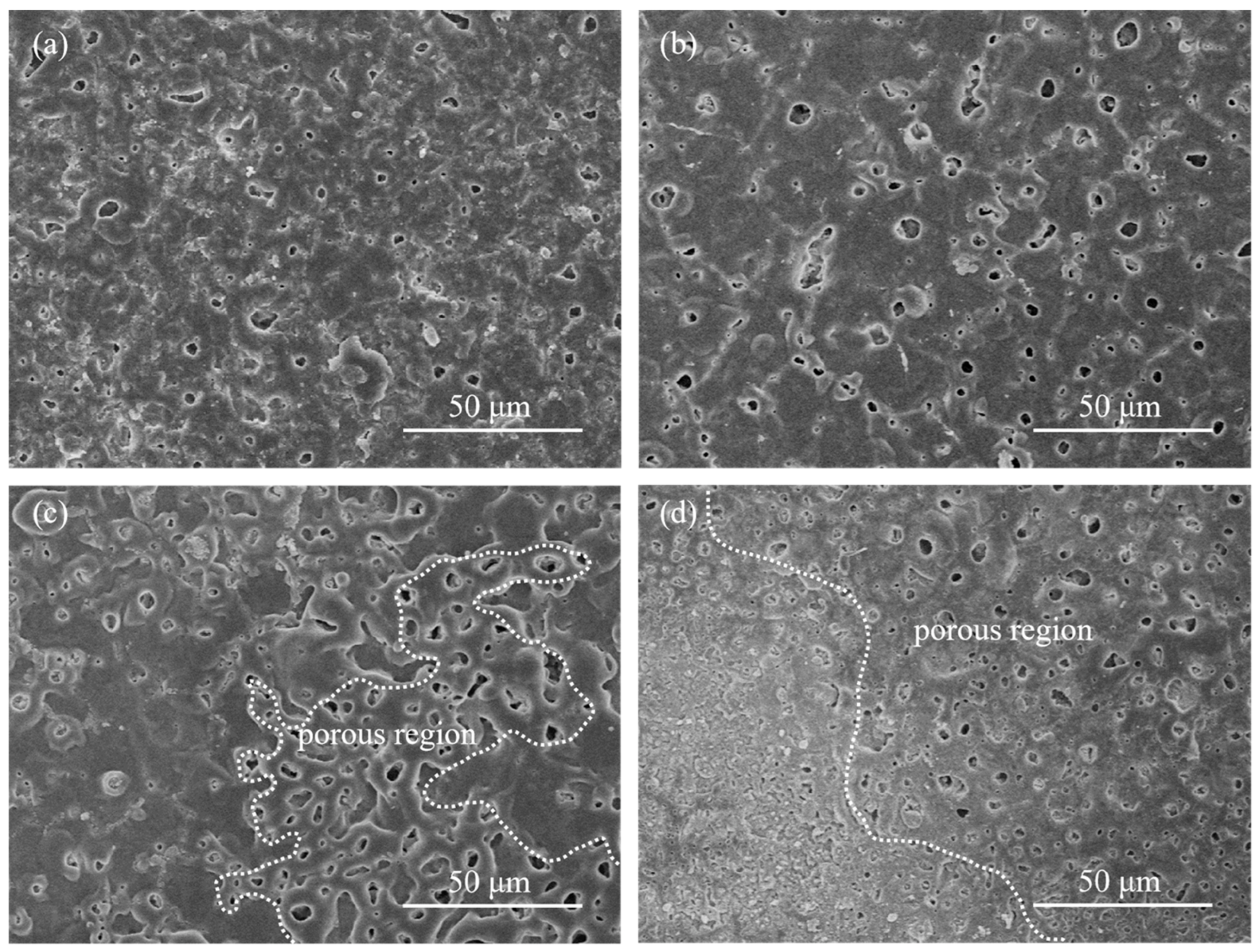
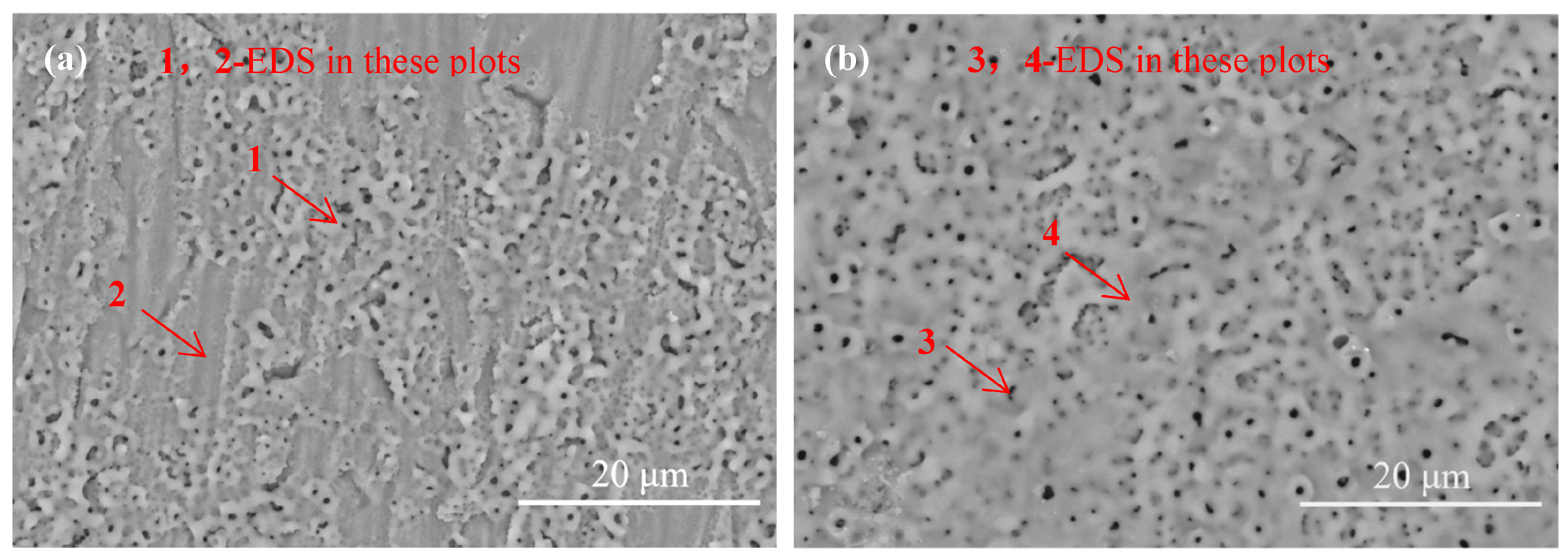
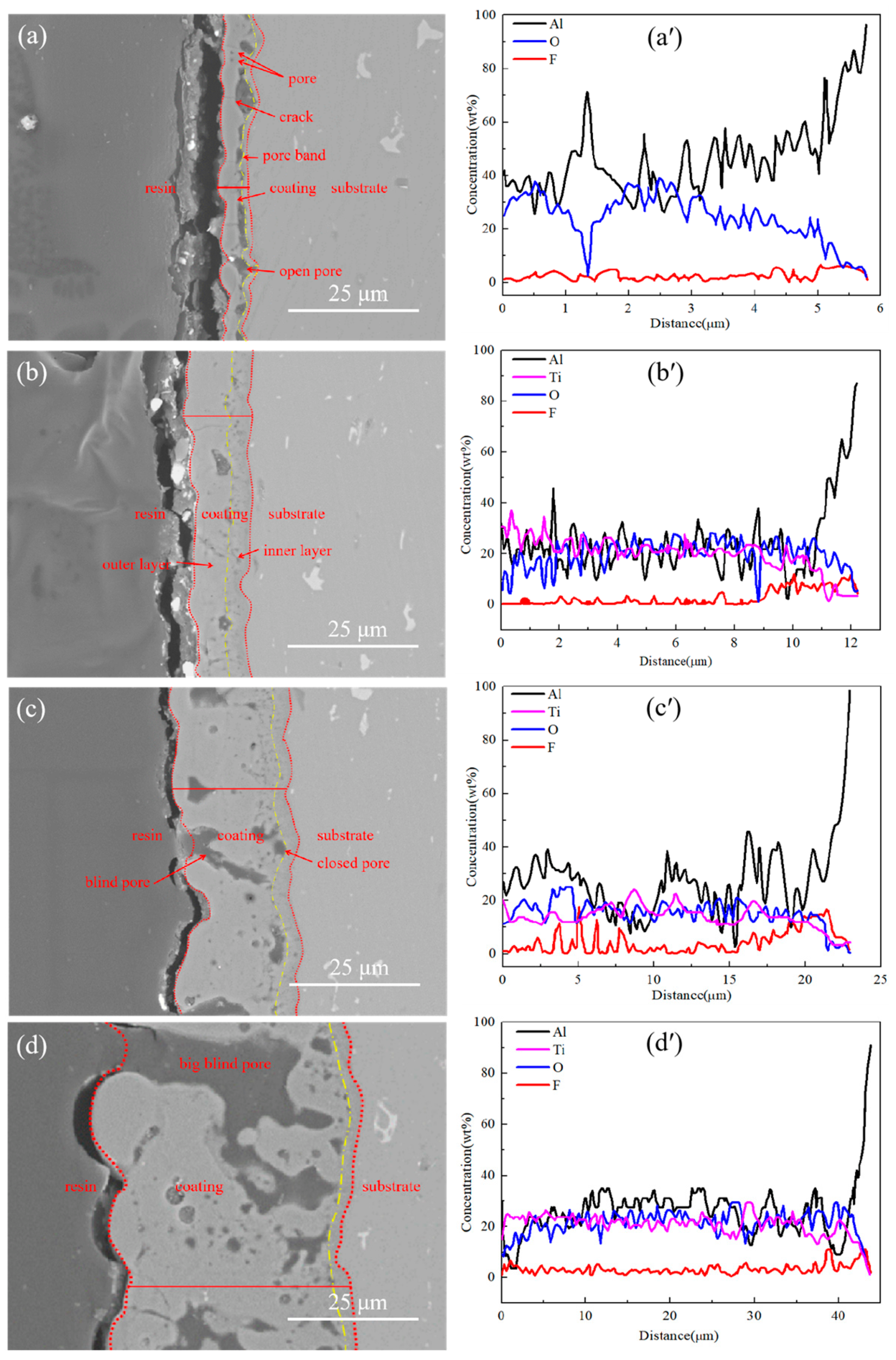
3.2. Microstructure of the Films
3.3. Phase Composition
3.4. Corrosion Resistance of the Films
3.5. Influence of the Electrolyte Temperature on the Coating Growth
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yerokhin, A.; Shatrov, A.; Samsonov, V.; Shashkov, P.; Pilkington, A.; Leyland, A.; Matthews, A. Oxide ceramic coatings on aluminium alloys produced by a pulsed bipolar plasma electrolytic oxidation process. Surf. Coat. Technol. 2005, 199, 150–157. [Google Scholar] [CrossRef]
- Peng, Z.; Xu, H.; Liu, S.; Qi, Y.; Liang, J. Wear and Corrosion Resistance of Plasma Electrolytic Oxidation Coatings on 6061 Al Alloy in Electrolytes with Aluminate and Phosphate. Materials 2021, 14, 4037. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.; Qin, G.; Ling, K.; Lv, X.; Wang, N.; Li, W. Layer-by-layer self-assembled polyurea layers onto MAO surface for enhancing corrosion protection to aluminum alloy 6063. Surf. Coat. Technol. 2021, 405, 126653. [Google Scholar] [CrossRef]
- Lv, X.; Zou, G.; Ling, K.; Yang, W.; Mo, Q.; Li, W. Tribological properties of MAO/MoS2 self-lubricating composite coating by microarc oxidation and hydrothermal reaction. Surf. Coat. Technol. 2021, 406, 126630. [Google Scholar] [CrossRef]
- Cheng, Y.-L.; Xue, Z.-G.; Wang, Q.; Wu, X.-Q.; Matykina, E.; Skeldon, P.; Thompson, G. New findings on properties of plasma electrolytic oxidation coatings from study of an Al–Cu–Li alloy. Electrochim. Acta 2013, 107, 358–378. [Google Scholar] [CrossRef]
- Troughton, S.; Nominé, A.; Henrion, G.; Clyne, T. Synchronised electrical monitoring and high speed video of bubble growth associated with individual discharges during plasma electrolytic oxidation. Appl. Surf. Sci. 2015, 359, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Qin, G.; Zhang, Y.; Yang, E.; Peng, Z.; Wei, W.; Xie, X.; Li, W. Effect of post-sealing treatment with different concentrations of NaH2PO4 on corrosion resistance of MAO coating on 6063 aluminum alloy. Surf. Coat. Technol. 2022, 443, 128604. [Google Scholar] [CrossRef]
- Sopchenski, L.; Robert, J.; Touzin, M.; Tricoteaux, A.; Olivier, M.-G. Improvement of wear and corrosion protection of PEO on AA2024 via sol-gel sealing. Surf. Coat. Technol. 2021, 417, 127195. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, Z.; Bai, S.; Wang, J. Influence of Sealing Treatment on the Corrosion Resistance of PEO Coated Al-Zn-Mg-Cu Alloy in Various Environments. Coatings 2019, 9, 867. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Liu, C.; Xu, T.; Jiang, B.; Li, H.; Yang, B. Effects of current output modes on the growth kinetics and corrosion resistance of micro-arc oxidation coatings on magnesium alloy. Mater. Res. Express 2021, 8, 066407. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, Y.; Hao, X.; Zhang, H.; Wu, W.; Han, G.; Dou, Z.; Wei, Y.; Zhang, Y.; Chen, F. Preparation and characterization of the low-energy plasma electrolysis oxide coatings on Mg Li alloy. Surf. Coat. Technol. 2022, 440, 128445. [Google Scholar] [CrossRef]
- Gębarowski, W.; Pietrzyk, S. Growth Characteristics of the Oxide Layer on Aluminium in the Process of Plasma Electrolytic Oxidation. Arch. Met. Mater. 2014, 59, 407–411. [Google Scholar] [CrossRef]
- Yao, J.; Wang, S.; Zhou, Y.; Dong, H. Effects of the power supply mode and loading parameters on the characteristics of micro-arc oxidation coatings on magnesium alloy. Metals 2020, 10, 1452. [Google Scholar] [CrossRef]
- Egorkin, V.; Gnedenkov, S.; Sinebryukhov, S.; Vyaliy, I.; Gnedenkov, A.; Chizhikov, R. Increasing thickness and protective properties of PEO-coatings on aluminum alloy. Surf. Coat. Technol. 2018, 334, 29–42. [Google Scholar] [CrossRef]
- Mohedano, M.; Mingo, B.; Mora-Sánchez, H.; Matykina, E.; Arrabal, R. Effects of pre-anodizing and phosphates on energy consumption and corrosion performance of PEO coatings on AA6082. Surf. Coat. Technol. 2021, 409, 126892. [Google Scholar] [CrossRef]
- Mohedano, M.; Lopez, E.; Mingo, B.; Moon, S.; Matykina, E.; Arrabal, R. Energy consumption, wear and corrosion of PEO coatings on preanodized Al alloy: The influence of current and frequency. J. Mater. Res. Technol. 2022, 21, 2061–2075. [Google Scholar] [CrossRef]
- Cheng, Y.; Cao, J.; Mao, M.; Peng, Z.; Skeldon, P.; Thompson, G. High growth rate, wear resistant coatings on an Al–Cu–Li alloy by plasma electrolytic oxidation in concentrated aluminate electrolytes. Surf. Coat. Technol. 2015, 269, 74–82. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Yin, X.; Du, N. Influence of electrolyte components on the microstructure and growth mechanism of plasma electrolytic oxidation coatings on 1060 aluminum alloy. Surf. Coat. Technol. 2020, 381, 125214. [Google Scholar] [CrossRef]
- Rahmati, M.; Raeissi, K.; Toroghinejad, M.R.; Hakimizad, A.; Santamaria, M. The multi-effects of K2TiF6 additive on the properties of PEO coatings on AZ31 Mg alloy. Surf. Coat. Technol. 2020, 402, 126296. [Google Scholar] [CrossRef]
- Xiong, Y.; Hu, Q.; Hu, X.; Song, R. Microstructure and corrosion resistance of Ti3O5-HA bio-ceramic coating fabricated on AZ80 magnesium alloy. Surf. Coat. Technol. 2017, 325, 239–247. [Google Scholar] [CrossRef]
- Sridhar, S.; Arunnellaiappan, T.; Rameshbabu, N.; Mika, S.; Viswanathan, A. Solar photocatalytic activity of nitrogen doped TiO2 coating by micro-arc oxidation. Surf. Eng. 2016, 33, 779–786. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Zhang, X.-Y.; Huang, X.; Gong, X.-P.; Zhang, J.-J.; Hu, X.-J. Microstructure and properties of microarc oxidation coating formed on aluminum alloy with compound additives nano-TiO2 and nano-ZnO. Rare Met. 2017, 37, 976–982. [Google Scholar] [CrossRef]
- Raj, V.; Ali, M.M. Formation of ceramic alumina nanocomposite coatings on aluminium for enhanced corrosion resistance. J. Mater. Process. Technol. 2009, 209, 5341–5352. [Google Scholar] [CrossRef]
- Habazaki, H.; Tsunekawa, S.; Tsuji, E.; Nakayama, T. Formation and characterization of wear-resistant PEO coatings formed on β-titanium alloy at different electrolyte temperatures. Appl. Surf. Sci. 2012, 259, 711–718. [Google Scholar] [CrossRef]
- Al Bosta, M.M.S.; Ma, K.-J. Influence of electrolyte temperature on properties and infrared emissivity of MAO ceramic coating on 6061 aluminum alloy. Infrared Phys. Technol. 2014, 67, 63–72. [Google Scholar] [CrossRef]
- Sreekanth, D.; Rameshbabu, N.; Venkateswarlu, K. Effect of various additives on morphology and corrosion behavior of ceramic coatings developed on AZ31 magnesium alloy by plasma electrolytic oxidation. Ceram. Int. 2012, 38, 4607–4615. [Google Scholar] [CrossRef]
- Sreekanth, D.; Rameshbabu, N.; Venkateswarlu, K.; Subrahmanyam, C.; Krishna, L.R.; Rao, K.P. Effect of K2TiF6 and Na2B4O7 as electrolyte additives on pore morphology and corrosion properties of plasma electrolytic oxidation coatings on ZM21 magnesium alloy. Surf. Coat. Technol. 2013, 222, 31–37. [Google Scholar] [CrossRef]
- Tang, M.; Li, W.; Liu, H.; Zhu, L. Influence of K2TiF6 in electrolyte on characteristics of the microarc oxidation coating on aluminum alloy. Curr. Appl. Phys. 2012, 12, 1259–1265. [Google Scholar] [CrossRef]
- Tang, M.; Feng, Z.; Li, G.; Zhang, Z.; Zhang, R. High-corrosion resistance of the microarc oxidation coatings on magnesium alloy obtained in potassium fluotitanate electrolytes. Surf. Coat. Technol. 2015, 264, 105–113. [Google Scholar] [CrossRef]
- Fernández-López, P.; Alves, S.; López-Ortega, A.; José-Lombera, J.S.; Bayón, R. High performance tribological coatings on a secondary cast Al–Si alloy generated by Plasma Electrolytic Oxidation. Ceram. Int. 2021, 47, 31238–31250. [Google Scholar] [CrossRef]
- Li, L.; Yang, E.; Yan, Z.; Xie, X.; Wei, W.; Li, W. Effect of pre-anodized film on micro-arc oxidation process of 6063 aluminum alloy. Materials 2022, 15, 5221. [Google Scholar] [CrossRef]
- Zhao, Z.; Pan, Q.; Yan, J.; Ye, J.; Liu, Y. Direct current micro-arc oxidation coatings on Al-Zn-Mg-Mn-Zr extruded alloy with tunable structures and properties templated by discharge stages. Vacuum 2018, 150, 155–165. [Google Scholar] [CrossRef]
- Arunnellaiappan, T.; Krishna, L.; Anoop, S.; Rani, R.; Rameshbabu, N. Fabrication of multifunctional black PEO coatings on AA7075 for spacecraft applications. Surf. Coat. Technol. 2016, 307, 735–746. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Cai, W.; Shan, A.; Jiang, Z. Effects of fluoride on the structure and properties of microarc oxidation coating on aluminium alloy. J. Alloys Compd. 2010, 505, 188–193. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Santamaria, M.; Asghari, M. Effects of pulse current mode on plasma electrolytic oxidation of 7075 Al in Na2WO4 containing solution: From unipolar to soft-sparking regime. Electrochim. Acta 2018, 284, 618–629. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; O Northwood, D.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 43, 105203. [Google Scholar] [CrossRef]
- Ling, K.; Mo, Q.; Lv, X.; Qin, G.; Yang, W.; Li, L.; Li, W. Growth characteristics and corrosion resistance of micro-arc oxidation coating on Al–Mg composite plate. Vacuum 2022, 195, 110640. [Google Scholar] [CrossRef]
- Habazaki, H.; Fushimi, K.; Shimizu, K.; Skeldon, P.; Thompson, G. Fast migration of fluoride ions in growing anodic titanium oxide. Electrochem. Commun. 2007, 9, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-X.; Li, F.-B.; Li, X.-Z. Effects of peptizing conditions on nanometer properties and photocatalytic activity of TiO2 hydrosols prepared by H2TiO3. J. Hazard. Mater. 2008, 155, 90–99. [Google Scholar] [CrossRef] [Green Version]
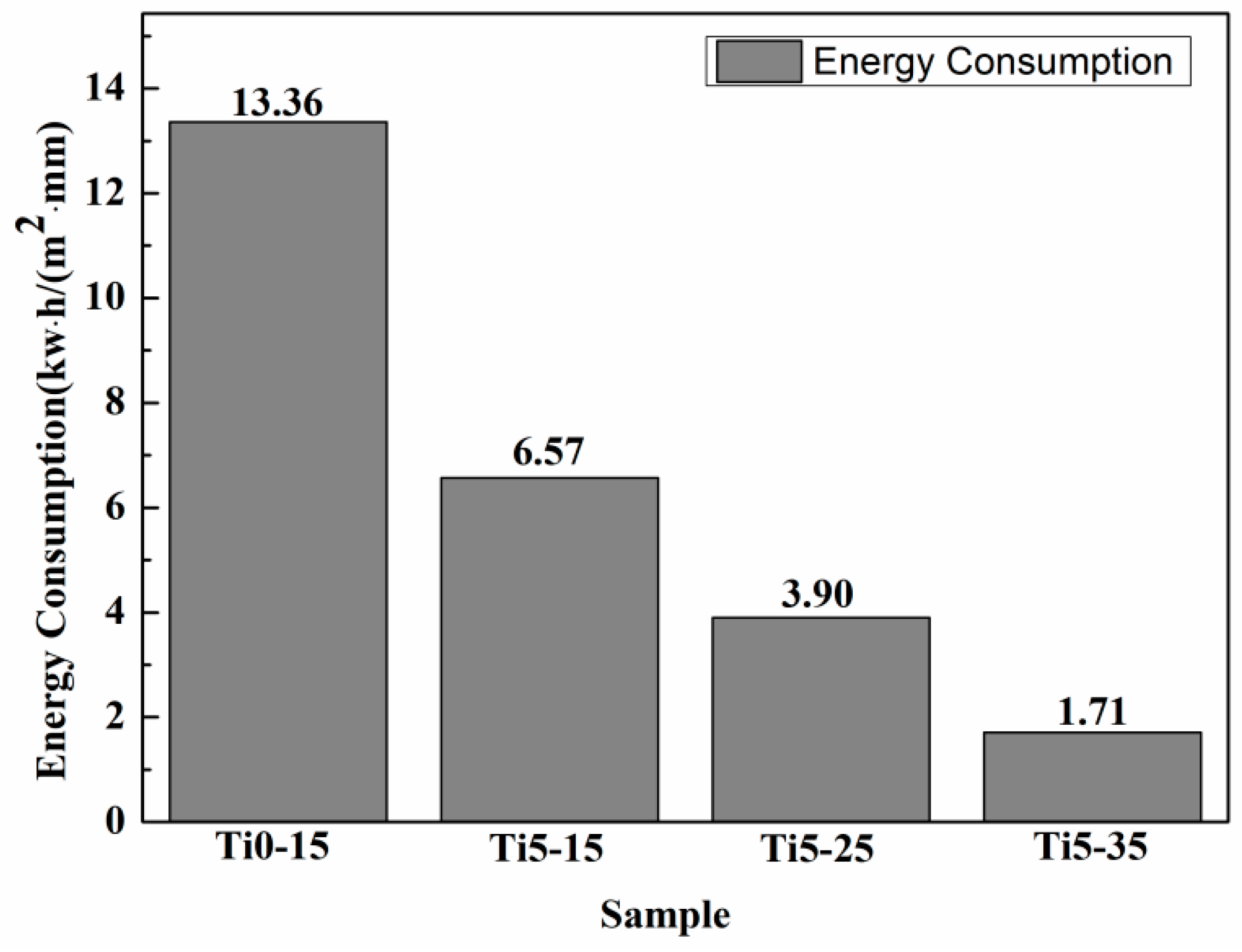
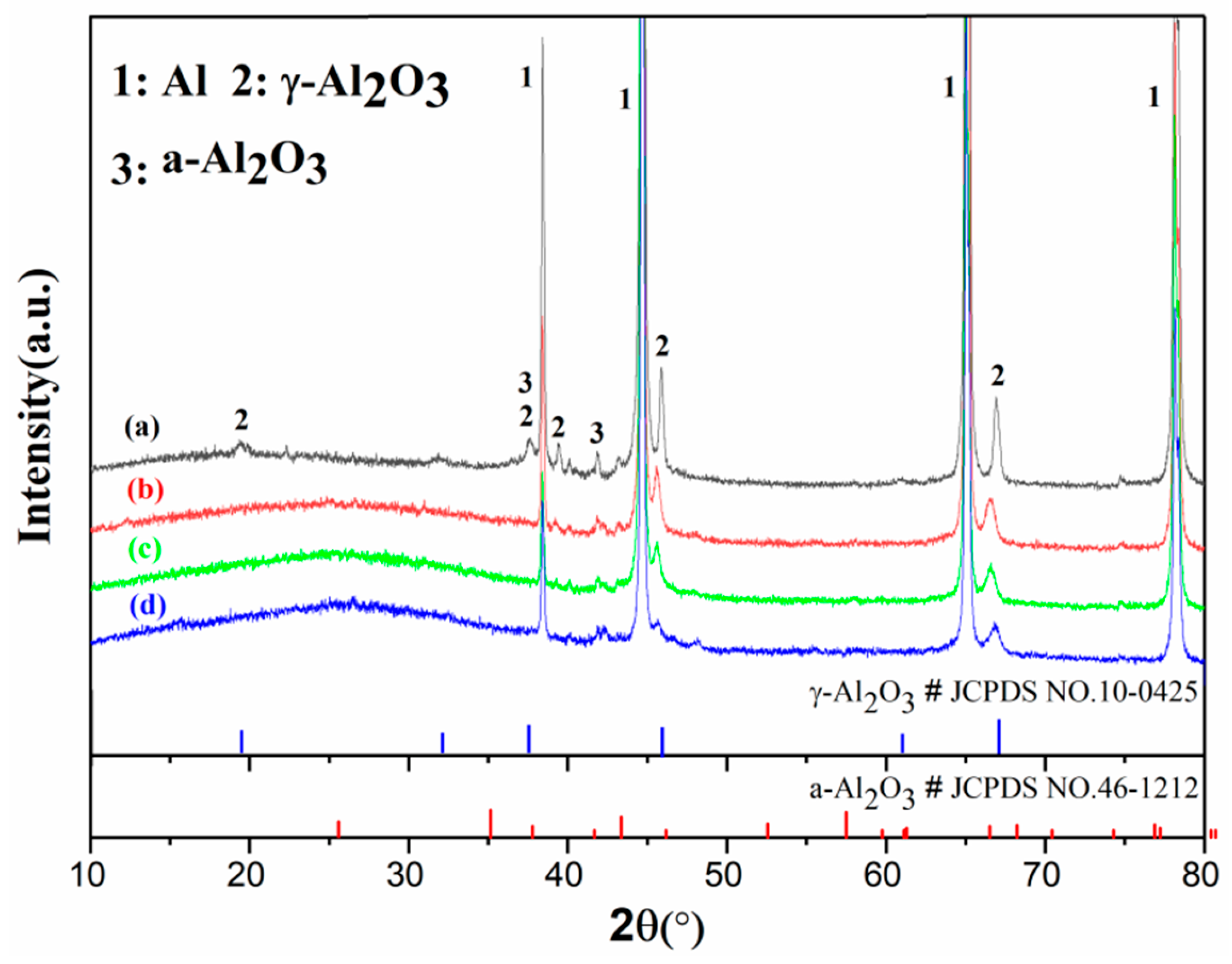



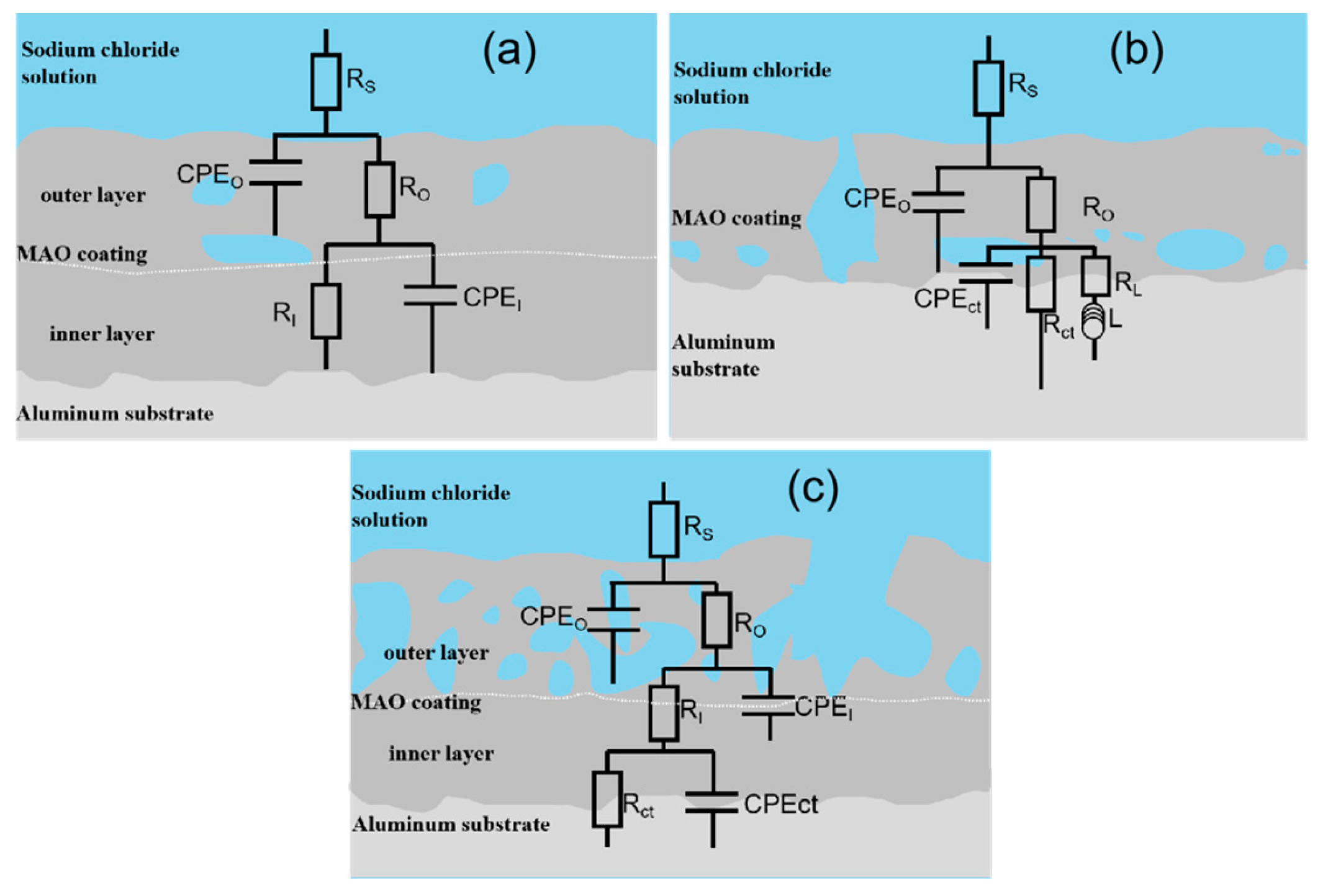

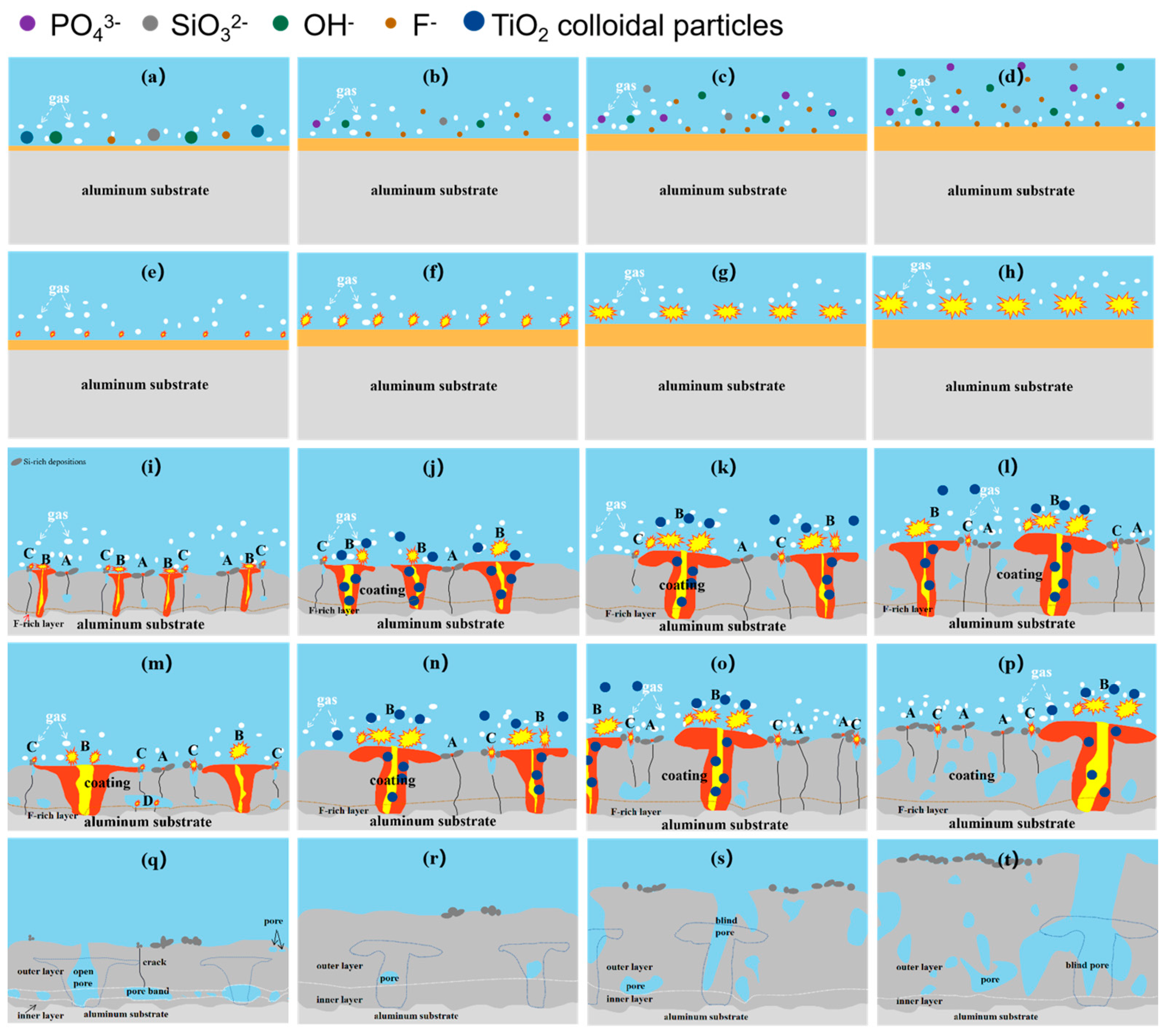
| Sample/ Coating Code | Electrolyte Composition | Cooling Temperature | Conductivity (κ, mS ± 0.1) | pH |
|---|---|---|---|---|
| Ti0-15 | 10 g/L Na2SiO3, 10 g/L Na3PO4, 2 g/L NaF | 15 °C | 17.92 | 12.17 |
| Ti5-15 | 10 g/L Na2SiO3, 10 g/L Na3PO4, 2 g/L NaF, 5 g/L K2TiF6 | 15 °C | 16.79 | 8.18 |
| Ti5-25 | 10 g/L Na2SiO3, 10 g/L Na3PO4, 2 g/L NaF, 5 g/L K2TiF6 | 25 °C | 17.49 | 8.11 |
| Ti5-35 | 10 g/L Na2SiO3, 10 g/L Na3PO4, 2 g/L NaF, 5 g/L K2TiF6 | 35 °C | 17.65 | 8.03 |
| Element/wt% | Al | O | Si | P | Na | F | Ti | K |
|---|---|---|---|---|---|---|---|---|
| Ti0-15 | 61.99 | 28.216 | 6.129 | 2.694 | 0.971 | 0 | - | - |
| Ti5-15 | 35.733 | 29.581 | 4.523 | 4.181 | 1.245 | 0.516 | 23.535 | 0.686 |
| Ti5-25 | 32.763 | 28.081 | 7.042 | 4.019 | 4.06 | 3.824 | 18.587 | 1.624 |
| Ti5-35 | 26.54 | 27.728 | 8.904 | 4.982 | 8.17 | 4.086 | 17.034 | 2.557 |
| Element/wt% | Al | O | Si | P | Na | F | Ti | K |
|---|---|---|---|---|---|---|---|---|
| 90″-surface | 58.073 | 23.413 | 1.926 | 2.198 | 0.148 | 9.383 | 4.606 | 0.242 |
| 90″-point1 | 51.224 | 31.886 | 3.147 | 2.695 | 0.358 | 4.965 | 5.255 | 0.166 |
| 90″-point2 | 69.767 | 16.374 | 0.936 | 1.014 | 0.124 | 10.194 | 1.536 | 0.055 |
| 130″-surface | 39.577 | 30.77 | 4.723 | 3.162 | 0.622 | 1.032 | 19.924 | 0.19 |
| 130″-point3 | 41.043 | 26.731 | 3.694 | 3.112 | 0.611 | 3.896 | 20.855 | 0.058 |
| 130″-point4 | 34.810 | 37.370 | 6.841 | 3.075 | 0.668 | 0.278 | 16.814 | 0.124 |
| Coating | Inner Layer | Outer Layer | All Coating | |||
|---|---|---|---|---|---|---|
| Sample Thickness (μm) | Average | Standard Deviation | Average | Standard Deviation | Average | Standard Deviation |
| Ti0-15 | 1.2 | 0.2 | 4.6 | 0.5 | 5.8 | 0.7 |
| Ti5-15 | 3.9 | 0.3 | 8.5 | 0.8 | 12.3 | 1.0 |
| Ti5-25 | 2.5 | 0.3 | 18.7 | 1.3 | 21.3 | 1.5 |
| Ti5-35 | 2.0 | 0.2 | 44.0 | 4.0 | 47.9 | 4.1 |
| Sample | βa | βc | Ecorr | icorr | Rcorr |
|---|---|---|---|---|---|
| (mV·dec−1) | (mV·dec−1) | (V) | (A·cm−2) | (mm·a−1) | |
| Ti0-15 | 3096.2 | 147.95 | −1.424 | 6.297 × 10−4 | 0.617 |
| Ti5-15 | 11,609 | 223.62 | −1.183 | 7.238 × 10−5 | 0.071 |
| Ti5-25 | 3260.3 | 222.67 | −1.224 | 1.354 × 10−4 | 0.133 |
| Ti5-35 | 3930.2 | 173.9 | −1.359 | 3.545 × 10−4 | 0.348 |
| Sample | RS | RO | RI | RCT | CPEO | CPEI | CPEct | L | Chi Square | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Ω·cm2) | (Ω·cm2) | (Ω·cm2) | (Ω·cm2) | YO | nO | YI | nI | Yct | nct | (H·cm−2) | ||
| (Ω−1·sn·cm−2) | (Ω−1·sn·cm−2) | (Ω−1·sn·cm−2) | ||||||||||
| Ti0-15 | 10 | 120 | - | 226 | 1.61 × 10−5 | 0.92 | 3.71 × 10−5 | 0.91 | 950 | 9.74 × 10−4 | ||
| Ti5-15 | 28 | 1.01 × 106 | 5.94 × 106 | - | 9.49 × 10−7 | 0.75 | 1.22 × 10−6 | 0.86 | - | - | - | 1.01 × 10−3 |
| Ti5-25 | 47 | 1.3 × 105 | 9.52 × 105 | - | 6.26 × 10−6 | 0.74 | 4.37 × 10−6 | 0.87 | - | - | - | 1.51 × 10−3 |
| Ti5-35 | 16 | 319 | 2196 | 7714 | 6.2 × 10−6 | 0.75 | 1.14 × 10−5 | 0.80 | 1.23 × 10−6 | 0.96 | - | 1.25 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Yang, E.; Zhang, Z.; Wei, W.; Li, D.; Zhao, X.; Yang, R.; Li, W. Effects of K2TiF6 and Electrolyte Temperatures on Energy Consumption and Properties of MAO Coatings on 6063 Aluminum Alloy. Materials 2023, 16, 1830. https://doi.org/10.3390/ma16051830
Xie X, Yang E, Zhang Z, Wei W, Li D, Zhao X, Yang R, Li W. Effects of K2TiF6 and Electrolyte Temperatures on Energy Consumption and Properties of MAO Coatings on 6063 Aluminum Alloy. Materials. 2023; 16(5):1830. https://doi.org/10.3390/ma16051830
Chicago/Turabian StyleXie, Xiaomeng, Erhui Yang, Ziying Zhang, Wu Wei, Dong Li, Xiaolian Zhao, Ruixia Yang, and Weizhou Li. 2023. "Effects of K2TiF6 and Electrolyte Temperatures on Energy Consumption and Properties of MAO Coatings on 6063 Aluminum Alloy" Materials 16, no. 5: 1830. https://doi.org/10.3390/ma16051830






