Long-Term Stability in Electronic Properties of Textile Organic Electrochemical Transistors for Integrated Applications
Abstract
1. Introduction
2. Experimental Section
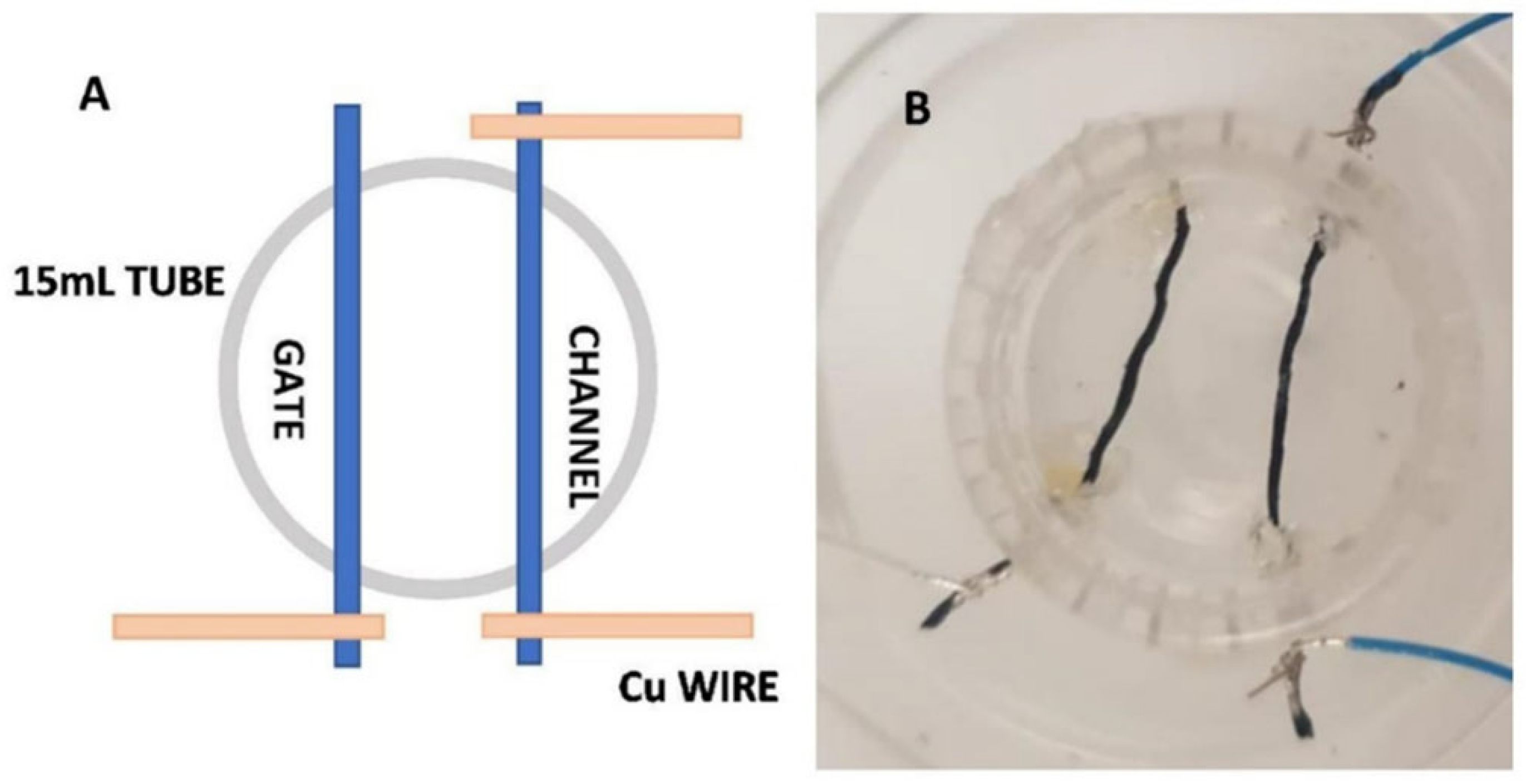
2.1. Sensor Design
2.2. Functionalization with Ethylene Glycol in Solution
2.3. Functionalization with Sulfuric Acid Post-Treatment
2.4. Device Fabrication and Characterization
2.5. Study Design
- (i)
- The channel current at the beginning of each cycle (I0), which depends on the hole mobility of the PEDOT:PSS channel [19].
- (ii)
- The sensor response (R), calculated as the relative variation of the current at the channel measured for the on/off Vg cycle: (I − I0)/I0. In the existing literature regarding organic electrochemical sensors’ (OECTs) response, modulation and sensitivity are synonym and are often used interchangeably. Thus, in the following sections of this paper, the sensor response is occasionally called modulation or sensitivity.
- (iii)
- The gate–source current (Igs), measured as the maximum current flowing from the gate to the source when Vg is applied.
- (iv)
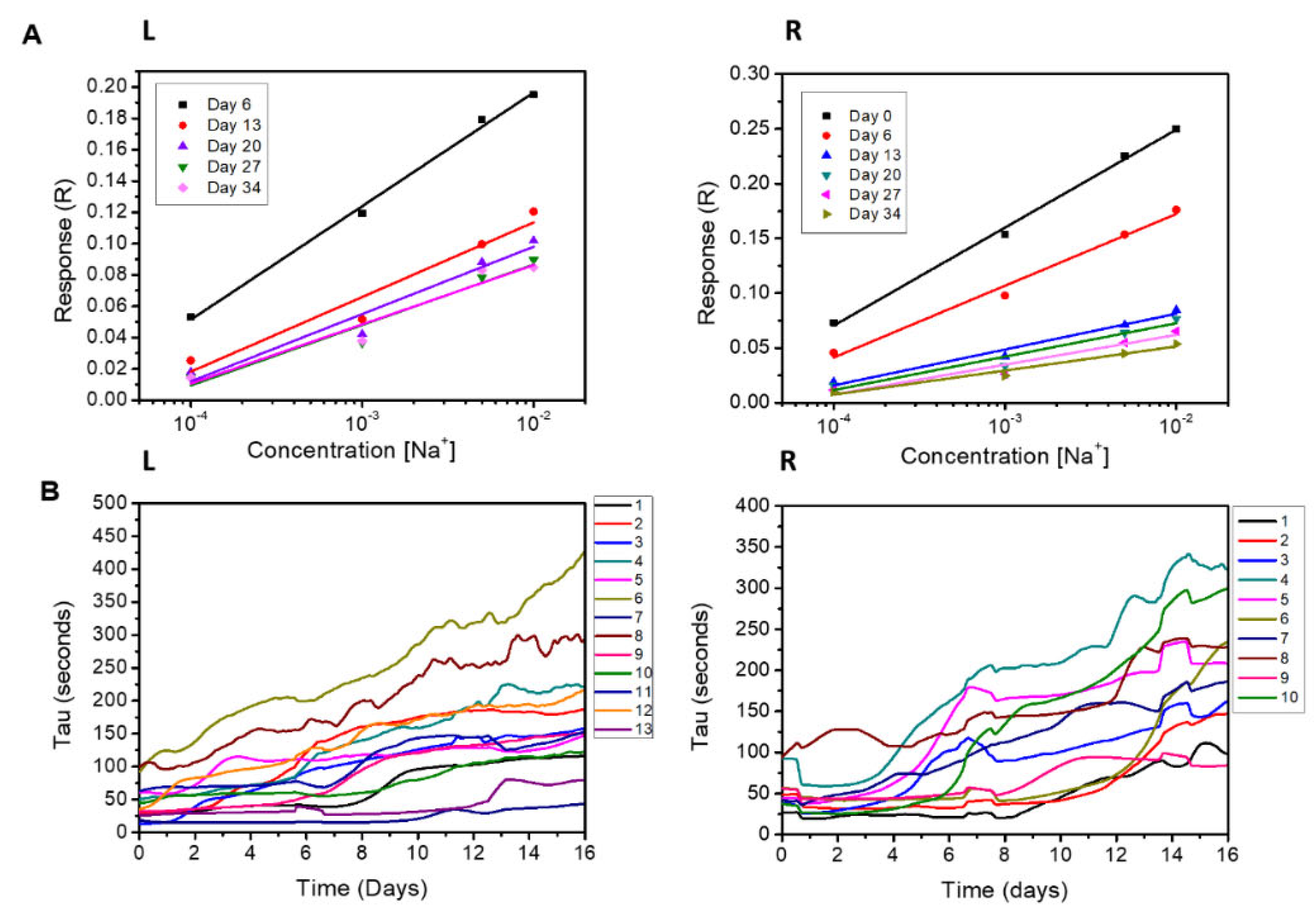
- The sensitivity (S), defined as the slope of the response R against the concentration of the solute, as measured for a constant Vg.
- (v)
- The time constant τ, i.e., the time required for the system’s response to reach the 66% of its steady-state value [27], which is found by non-linear fitting of data with an exponential curve of the form , where A is a model parameter and t is the time. τ is related to the reaction kinetics of de-doping [28]: it depends on the diffusion of cations into the channel and represents an important parameter to measure the effectiveness of the channel de-doping rate.
2.6. Image Analysis of PEDOT:PSS Functionalized Cotton Fibers
3. Results
3.1. Exploitation of EG as the Plasticizer
3.2. Post-Treatment with Sulfuric Acid
3.3. Image Analysis of the PEDOT:PSS Polymer Fiber
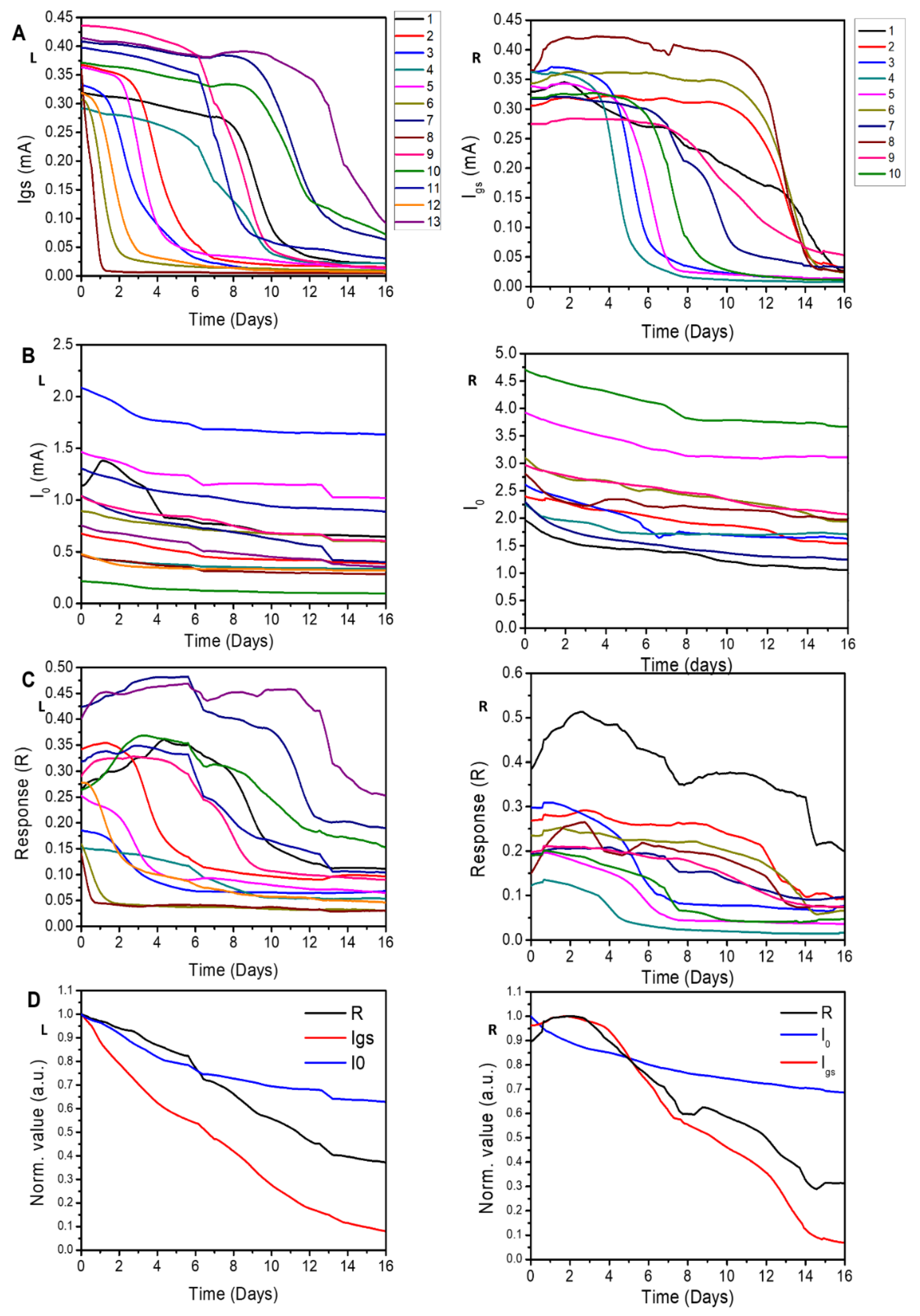
3.4. Effect of the Applied Voltage on Device Lifetime for EG Samples
3.5. Effect of the Applied Voltage on Device Lifetime for SA Samples
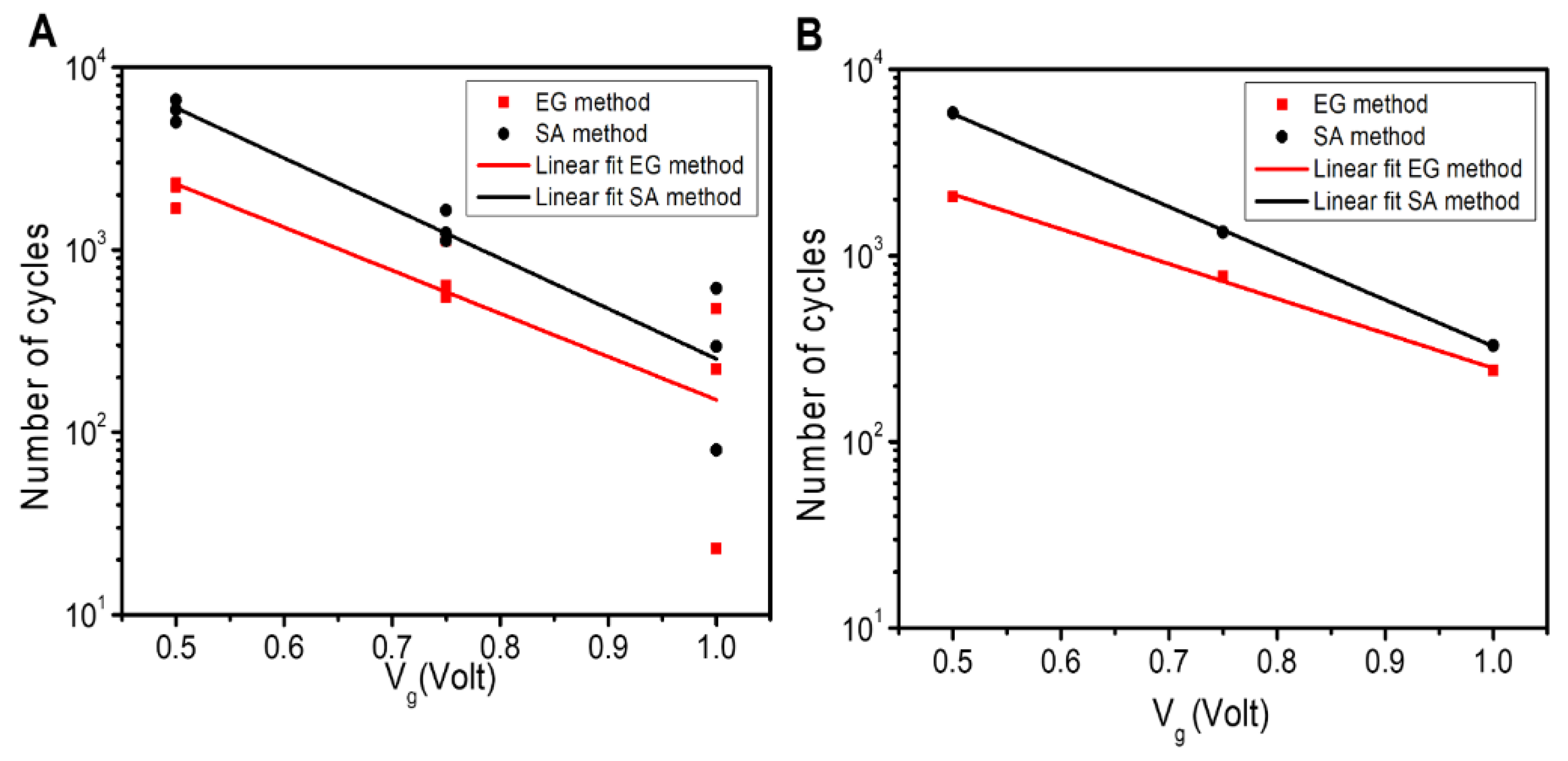
3.6. Correlation between the Lifetime of Device and Vg
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donahue, M.J.; Sanchez-Sanchez, A.; Inal, S.; Qu, J.; Owens, R.M.; Mecerreyes, D.; Malliaras, G.G.; Martin, D.C. Tailoring PEDOT properties for applications in bioelectronics. Mater. Sci. Eng. R Rep. 2020, 140, 100546. [Google Scholar] [CrossRef]
- Tarabella, G.; Mahvash Mohammadi, F.; Coppedè, N.; Barbero, F.; Iannotta, S.; Santato, C.; Cicoira, F. New opportunities for organic electronics and bioelectronics: Ions in action. Chem. Sci. 2013, 4, 1395–1409. [Google Scholar] [CrossRef]
- Lee, I.; Lee, K. The Internet of Things (IoT): Applications, investments, and challenges for enterprises. Bus. Horiz. 2015, 58, 431–440. [Google Scholar] [CrossRef]
- Madakam, S.; Ramaswamy, R.; Tripathi, S. Internet of Things (IoT): A Literature Review. J. Comput. Commun. 2015, 3, 164–173. [Google Scholar] [CrossRef]
- Rogers, B.; Zonfrilli, C.; Kreher, T.; Della, R.T. The Internet of Things: From theory to reality. Forbes Insight 2017, 2017, 1–24. [Google Scholar]
- Volkov, A.V.; Wijeratne, K.; Mitraka, E.; Ail, U.; Zhao, D.; Tybrandt, K.; Andreasen, J.W.; Berggren, M.; Crispin, X.; Zozoulenko, I.V. Understanding the Capacitance of PEDOT:PSS. Adv. Funct. Mater. 2017, 27, 1–10. [Google Scholar] [CrossRef]
- Gentile, F.; Coppedè, N.; Tarabella, G.; Villani, M.; Calestani, D.; Candeloro, P.; Iannotta, S.; Di Fabrizio, E. Microtexturing of the conductive PEDOT:PSS Polymer for superhydrophobic organic electrochemical transistors. BioMed Res. Int. 2014, 2014, 302694. [Google Scholar] [CrossRef]
- Verma, D.; Singh, K.R.; Yadav, A.K.; Nayak, V.; Singh, J.; Solanki, P.R.; Singh, R.P. Internet of things (IoT) in nano-integrated wearable biosensor devices for healthcare applications. Biosens. Bioelectron. X 2022, 11, 100153. [Google Scholar] [CrossRef]
- Strakosas, X.; Bongo, M.; Owens, R.M. The organic electrochemical transistor for biological applications. J. Appl. Polym. Sci. 2015, 132, 1–14. [Google Scholar] [CrossRef]
- Battista, E.; Lettera, V.; Villani, M.; Calestani, D.; Gentile, F.; Antonio, P.; Iannotta, S.; Zappettini, A.; Netti, P.A.; Iannotta, S.; et al. Enzymatic sensing with laccase-functionalized textile organic biosensors. Org. Electron. 2017, 40, 51–57. [Google Scholar] [CrossRef]
- Coppedè, N.; Tarabella, G.; Villani, M.; Calestani, D.; Iannotta, S.; Zappettini, A. Human stress monitoring through an organic cotton-fiber biosensor. J. Mater. Chem. B 2014, 2, 5620–5626. [Google Scholar] [CrossRef] [PubMed]
- Vurro, F.; Janni, M.; Coppedè, N.; Gentile, F.; Manfredi, R.; Bettelli, M.; Zappettini, A. Development of an in vivo sensor to monitor the effects of vapour pressure deficit (VPD) changes to improve water productivity in agriculture. Sensors 2019, 19, 4667. [Google Scholar] [CrossRef] [PubMed]
- Tarabella, G.; Villani, M.; Calestani, D.; Mosca, R.; Iannotta, S.; Zappettini, A.; Coppedè, N. A single cotton fiber organic electrochemical transistor for liquid electrolyte saline sensing. J. Mater. Chem. 2012, 22, 23830–23834. [Google Scholar] [CrossRef]
- Coppedè, N.; Giannetto, M.; Villani, M.; Lucchini, V.; Battista, E.; Careri, M.; Zappettini, A. Ion selective textile organic electrochemical transistor for wearable sweat monitoring. Org. Electron. 2020, 78, 105579. [Google Scholar] [CrossRef]
- Coppedè, N.; Janni, M.; Bettelli, M.; Maida, C.L.; Gentile, F.; Villani, M.; Ruotolo, R.; Iannotta, S.; Marmiroli, N.; Marmiroli, M.; et al. An in vivo biosensing, biomimetic electrochemical transistor with applications in plant science and precision farming. Sci. Rep. 2017, 7, 16195. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef]
- Amato, D.; Montanaro, G.; Vurro, F.; Coppedé, N.; Briglia, N.; Petrozza, A.; Janni, M.; Zappettini, A.; Cellini, F.; Nuzzo, V. Towards In Vivo Monitoring of Ions Accumulation in Trees: Response of an in Planta Organic Electrochemical Transistor Based Sensor to Water Flux Density, Light, Vapor Pressure Deficit Variation. Appl. Sci. 2021, 11, 4729. [Google Scholar] [CrossRef]
- Janni, M.; Coppede, N.; Bettelli, M.; Briglia, N.; Petrozza, A.; Summerer, S.; Vurro, F.; Danzi, D.; Cellini, F.; Marmiroli, N.; et al. In Vivo Phenotyping for the Early Detection of Drought Stress in Tomato. Plant Phenomics 2019, 2019, 6168209. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Fan, X.; Nie, W.; Tsai, H.; Wang, N.; Huang, H.; Cheng, Y.; Wen, R.; Ma, L.; Yan, F.; Xia, Y. PEDOT:PSS for Flexible and Stretchable Electronics: Modifications, Strategies, and Applications. Adv. Sci. 2019, 6, 1900813. [Google Scholar] [CrossRef] [PubMed]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, 1806133. [Google Scholar] [CrossRef] [PubMed]
- Al-Oqla, F.M.; Sapuan, S.M.; Anwer, T.; Jawaid, M.; Hoque, M.E. Natural fiber reinforced conductive polymer composites as functional materials: A review. Synth. Met. 2015, 206, 42–54. [Google Scholar] [CrossRef]
- Zeglio, E.; Inganäs, O. Active Materials for Organic Electrochemical Transistors. Adv. Mater. 2018, 30, 1800941. [Google Scholar] [CrossRef]
- D’Angelo, P.; Marasso, S.L.; Verna, A.; Ballesio, A.; Parmeggiani, M.; Sanginario, A.; Tarabella, G.; Demarchi, D.; Pirri, C.F.; Cocuzza, M.; et al. Scaling Organic Electrochemical Transistors Down to Nanosized Channels. Small 2019, 15, e1902332. [Google Scholar] [CrossRef] [PubMed]
- ElMahmoudy, M.; Inal, S.; Charrier, A.; Uguz, I.; Malliaras, G.G.; Sanaur, S. Tailoring the Electrochemical and Mechanical Properties of PEDOT:PSS Films for Bioelectronics. Macromol. Mater. Eng. 2017, 302, 1–8. [Google Scholar] [CrossRef]
- Coppede, N.; Vurro, F.; Manfredi, R.; Janni, M.; Zappettini, A.; Gentile, F. Introducing State Variables in Organic Electrochemical Transistors With Application to Biophysical Systems. IEEE Sens. J. 2019, 19, 11753–11758. [Google Scholar] [CrossRef]
- Malara, N.; Gentile, F.; Coppedè, N.; Coluccio, M.L.; Candeloro, P.; Perozziello, G.; Ferrara, L.; Giannetto, M.; Careri, M.; Castellini, A.; et al. Superhydrophobic lab-on-chip measures secretome protonation state and provides a personalized risk assessment of sporadic tumour. npj Precis. Oncol. 2018, 2, 1–13. [Google Scholar] [CrossRef]
- Güne, A.; Kalkan, H.; Efkan, K. Optimizing the Color-to-Grayscale Conversion for Image Classification; Springer: Berlin/Heidelberg, Germany, 2016; Volume 10, pp. 853–860. [Google Scholar]
- Prats-Montalbán, J.M.; de Juan, A.; Ferrer, A. Multivariate image analysis: A review with applications. Chemom. Intell. Lab. Syst. 2011, 107, 1–23. [Google Scholar] [CrossRef]
- Coppede, N.; Villani, M.; Gentile, F. Diffusion Driven Selectivity in Organic Electrochemical Transistors. Sci. Rep. 2014, 4, 4297. [Google Scholar] [CrossRef]
- Ouyang, J.; Xu, Q.; Chu, C.W.; Yang, Y.; Li, G.; Shinar, J. On the mechanism of conductivity enhancement in poly(3,4- ethylenedioxythiophene):poly(styrene sulfonate) film through solvent treatment. Polymer (Guildf) 2004, 45, 8443–8450. [Google Scholar] [CrossRef]
- Kim, S.-M.; Kim, C.-H.; Kim, Y.; Kim, N.; Lee, W.-J.; Lee, E.-H.; Kim, D.; Park, S.; Lee, K.; Rivnay, J.; et al. Influence of PEDOT:PSS crystallinity and composition on electrochemical transistor performance and long-term stability. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]

| y = a + b ∗ x | Value | Standard Error | Adj. R-Square | |
|---|---|---|---|---|
| Ethylene Glycol | a | 4.542 | 0.260 | 0.961 |
| b | −2.364 | 0.335 | ||
| Sulfuric Acid | a | 5.154 | 0.080 | 0.997 |
| b | −2.752 | 0.103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manfredi, R.; Vurro, F.; Janni, M.; Bettelli, M.; Gentile, F.; Zappettini, A.; Coppedè, N. Long-Term Stability in Electronic Properties of Textile Organic Electrochemical Transistors for Integrated Applications. Materials 2023, 16, 1861. https://doi.org/10.3390/ma16051861
Manfredi R, Vurro F, Janni M, Bettelli M, Gentile F, Zappettini A, Coppedè N. Long-Term Stability in Electronic Properties of Textile Organic Electrochemical Transistors for Integrated Applications. Materials. 2023; 16(5):1861. https://doi.org/10.3390/ma16051861
Chicago/Turabian StyleManfredi, Riccardo, Filippo Vurro, Michela Janni, Manuele Bettelli, Francesco Gentile, Andrea Zappettini, and Nicola Coppedè. 2023. "Long-Term Stability in Electronic Properties of Textile Organic Electrochemical Transistors for Integrated Applications" Materials 16, no. 5: 1861. https://doi.org/10.3390/ma16051861
APA StyleManfredi, R., Vurro, F., Janni, M., Bettelli, M., Gentile, F., Zappettini, A., & Coppedè, N. (2023). Long-Term Stability in Electronic Properties of Textile Organic Electrochemical Transistors for Integrated Applications. Materials, 16(5), 1861. https://doi.org/10.3390/ma16051861









