Crystallization of Slag Films of CaO-Al2O3-BaO-CaF2-Li2O-Based Mold Fluxes for High-Aluminum Steels’ Continuous Casting
Abstract
1. Introduction
2. Materials and Methods
Measurements and Analysis
3. Results and Discussion
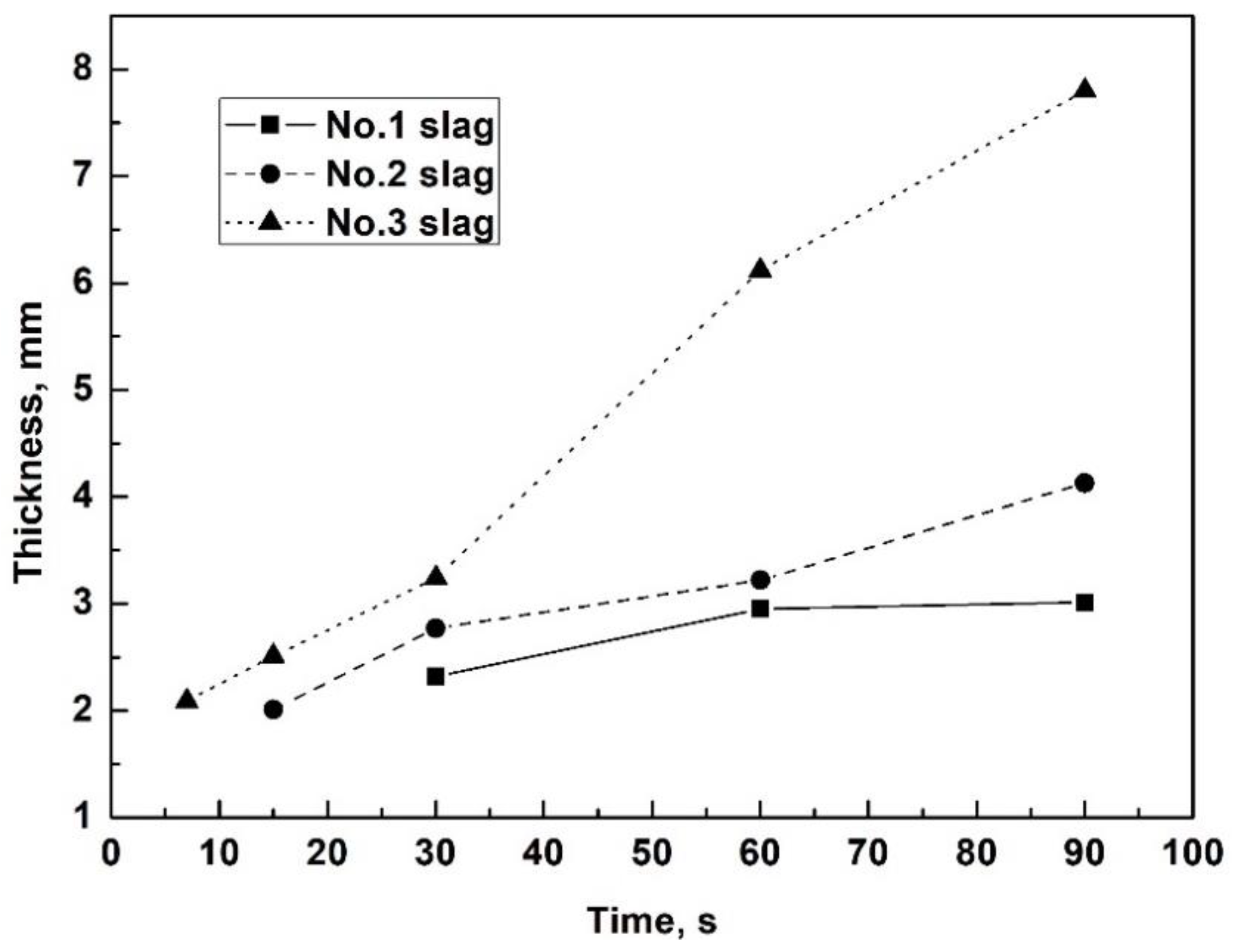
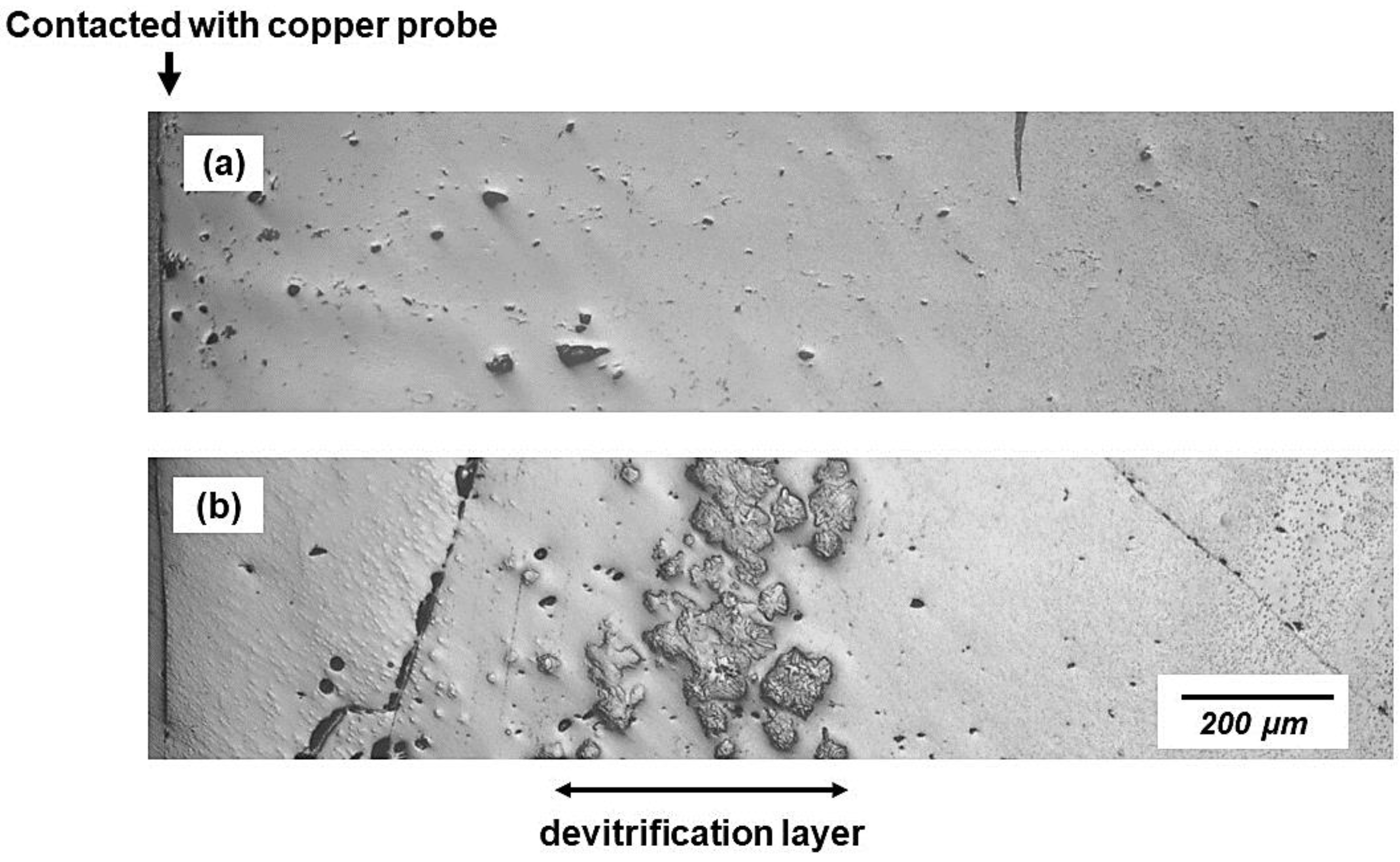
3.1. Evolution of Thickness and Devitrification of Slag Films
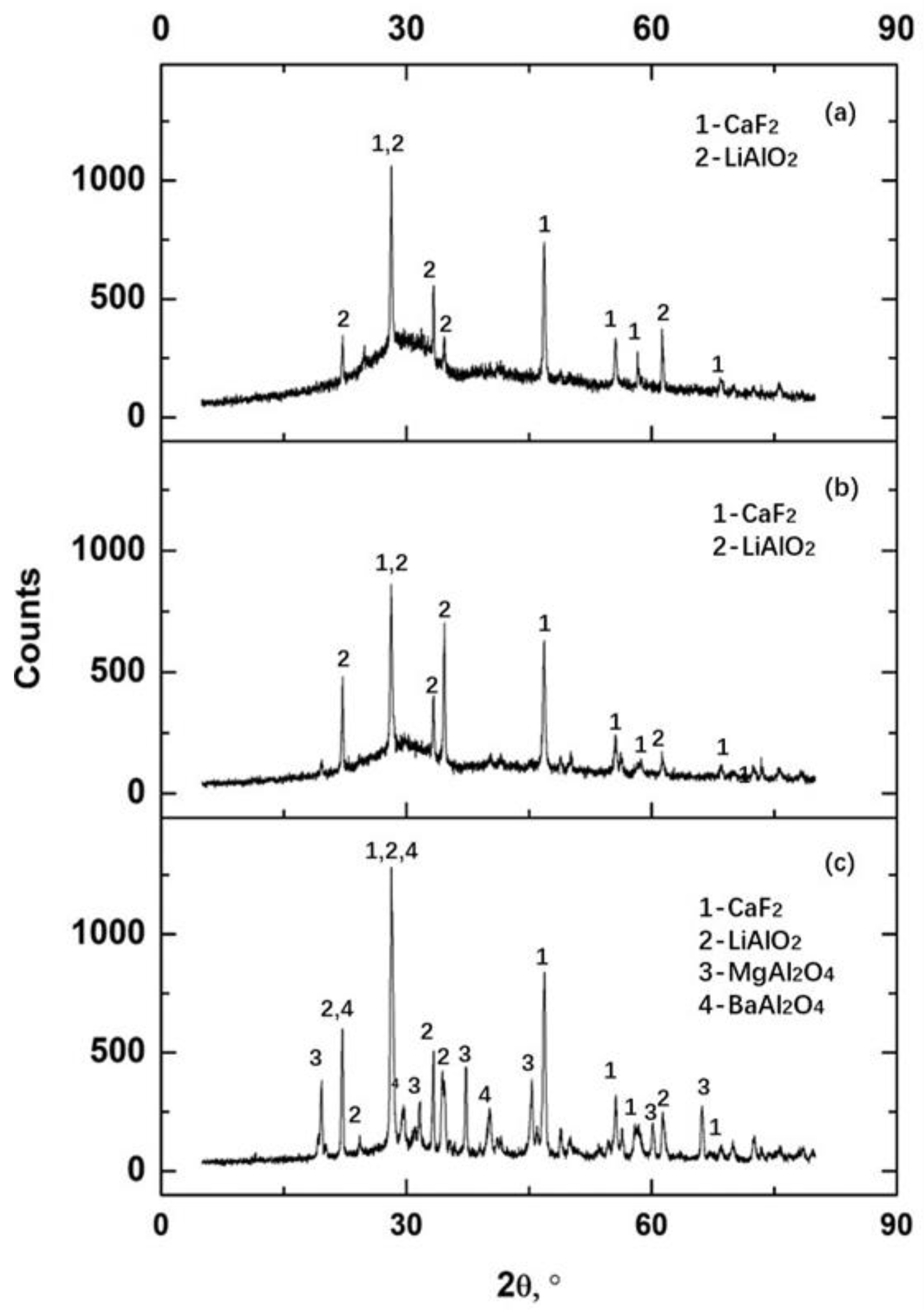
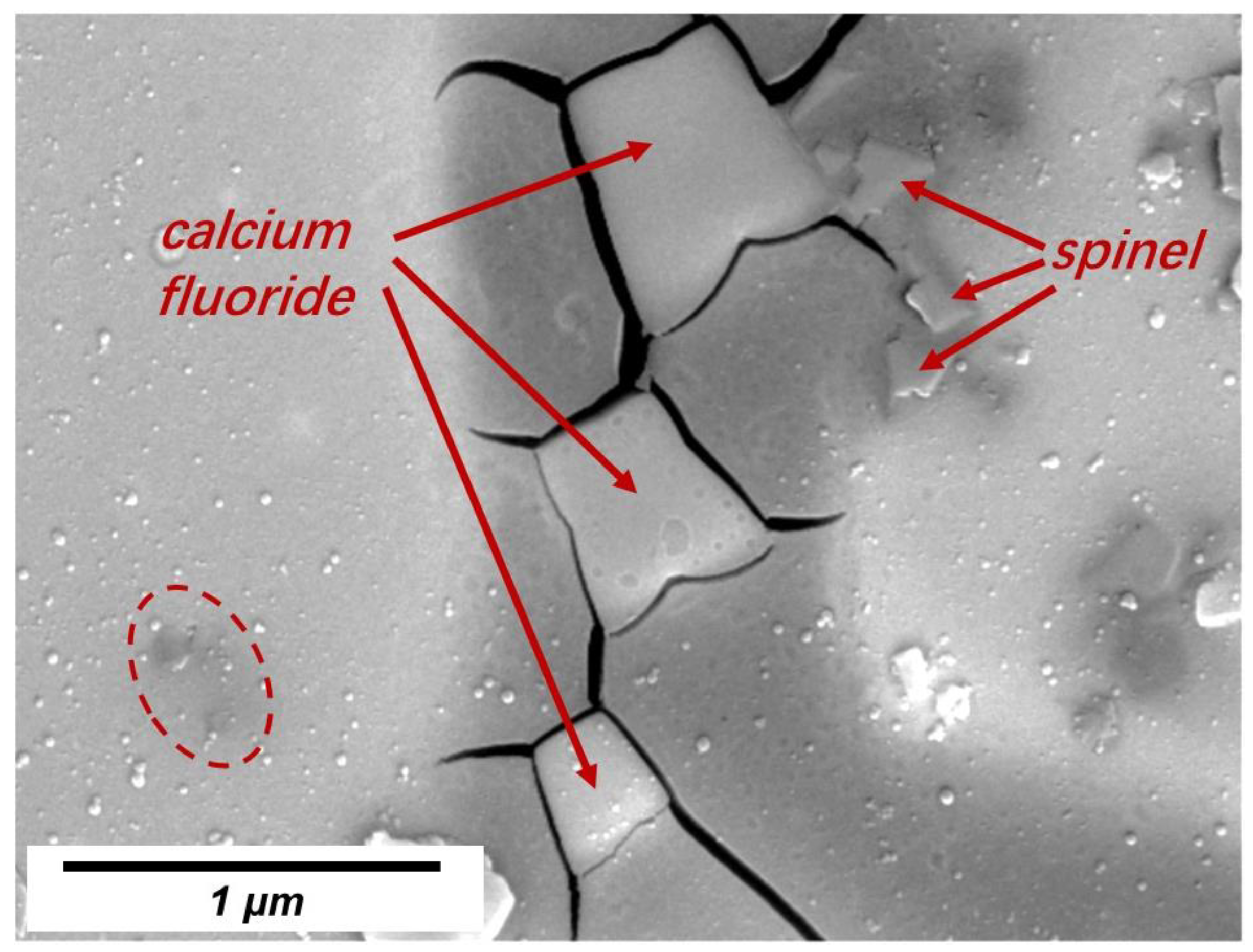
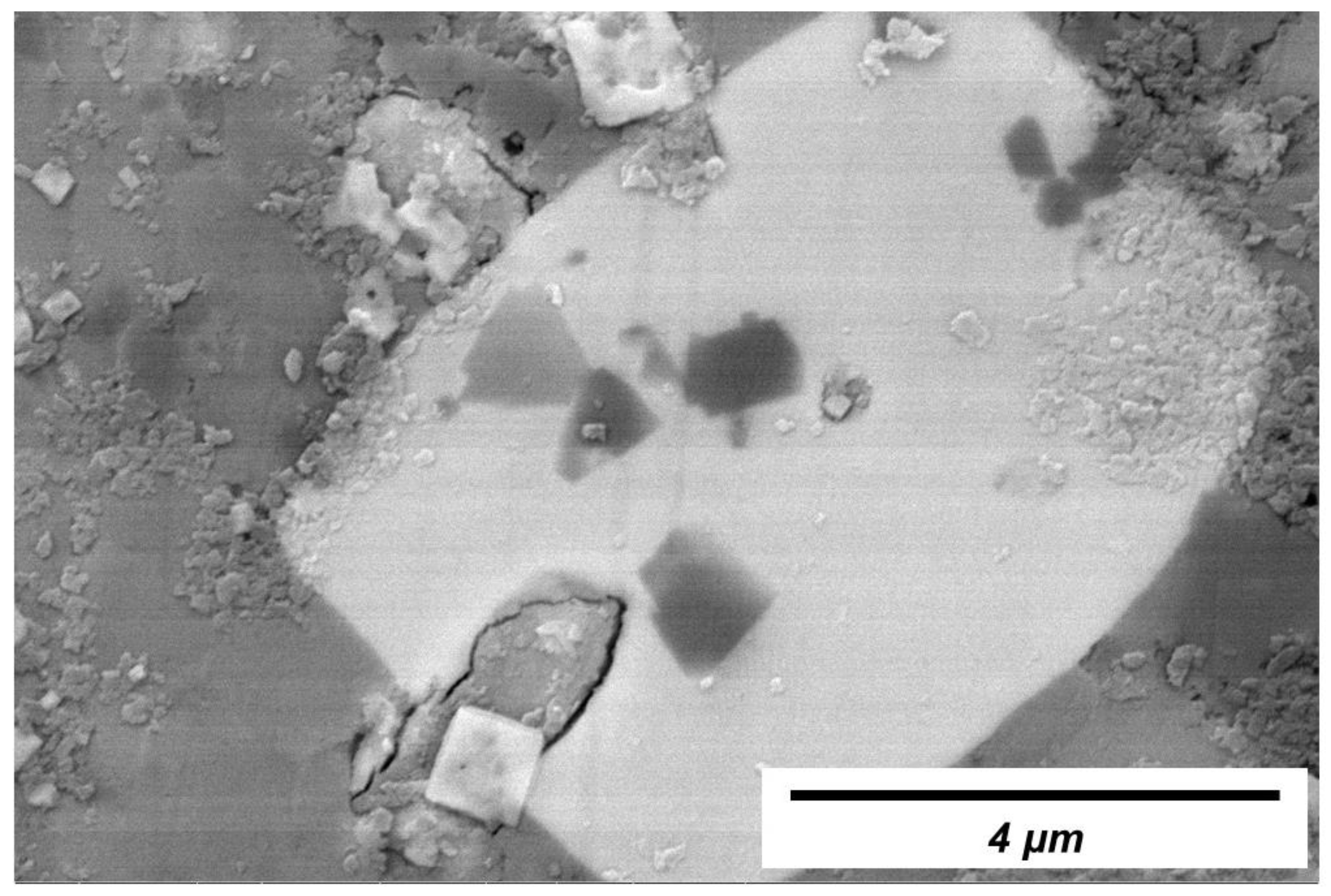
3.2. Crystals in Slag Films
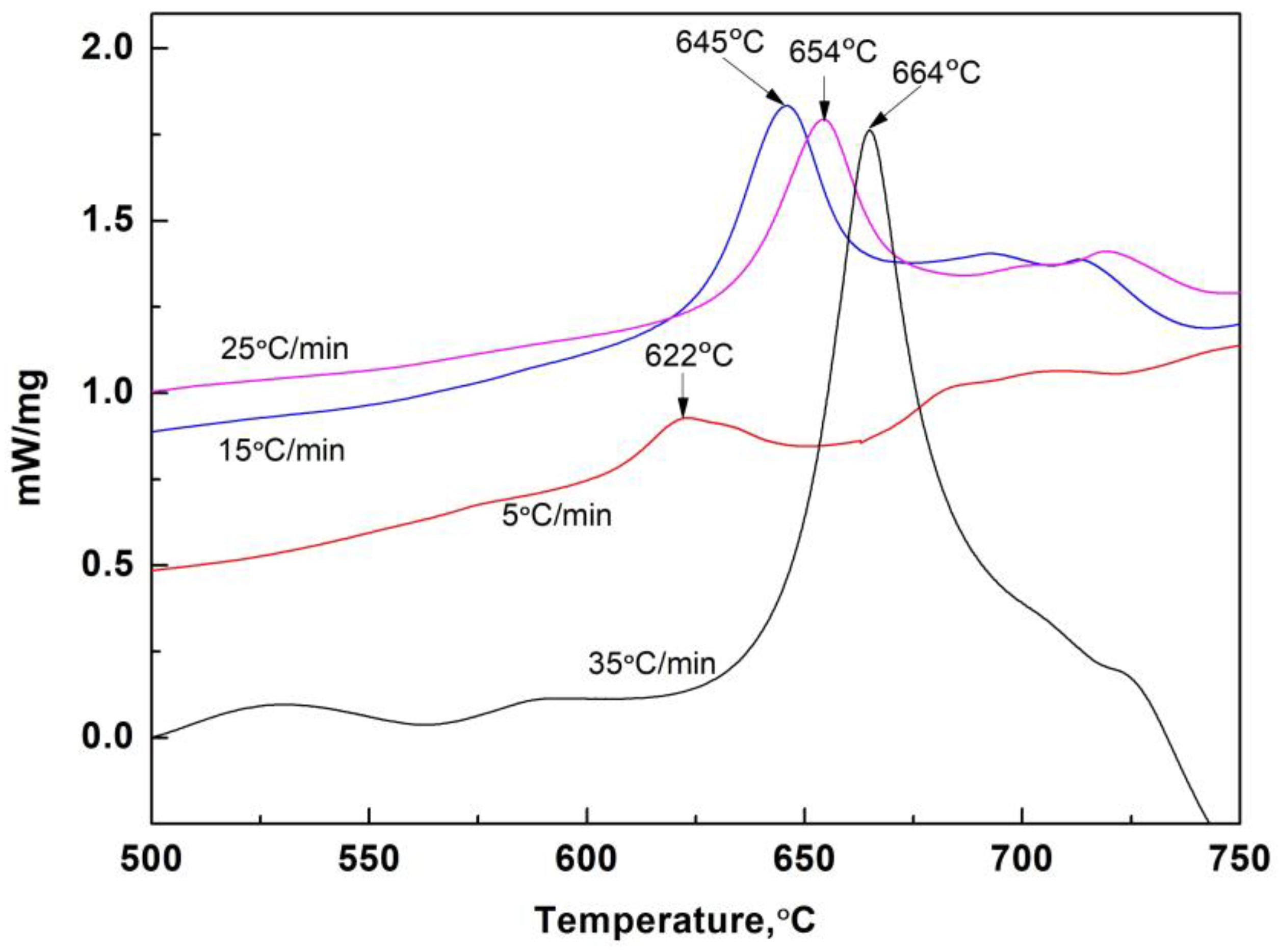
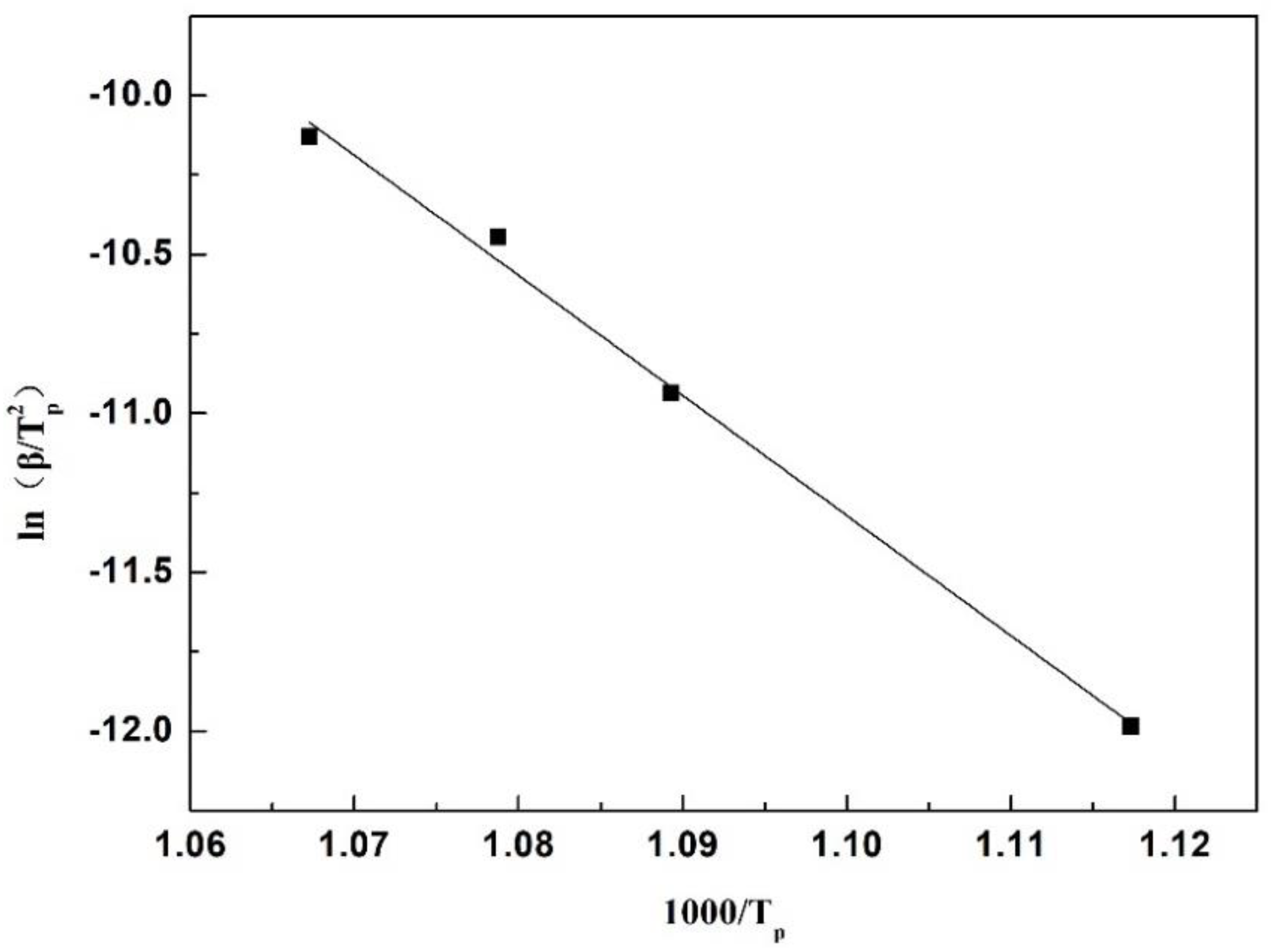
3.3. Kinetic Conditions of Crystallization
4. Conclusions
- (1)
- After adding extra Al2O3, the growing speed and thickness of the slag films increased significantly, especially for films with 10 wt% Al2O3 addition, and more time was required to reach a steady state of film thickness.
- (2)
- LiAlO2, CaF2, and BaAl2O4 were the major crystals precipitated in slag films of No. 1 and No. 2. BaAl2O4 usually precipitated and used initial crystal particles as their nuclei. Spinel (MgAl2O4) precipitated in the films when an extra 10 wt% of Al2O3 was added. The results suggest that the MgO in the slag or the absorption of Al2O3 from liquid steels should be controlled.
- (3)
- For the glassy state of No. 1 slag, when the heating rate reached 35 °C/min during devitrification, the softening temperature decreased to approximately 700 °C. Lower heating rates were associated with higher softening temperatures.
- (4)
- The apparent activation energy of initial devitrification crystallization for the original slag (No. 1) was 314.16 KJ/mol. After adding Al2O3, this activation energy decreased to 297.32 KJ/mol and 269.46 KJ/mol for 5 wt% and 10 wt% Al2O3 addition, respectively. The crystallization ratio of the slag films also increased after adding extra Al2O3.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mills, K.C.; Fox, A.B. The role of mould fluxes in continuous casting-so simple and yet so complex. ISIJ Int. 2003, 43, 1479–1486. [Google Scholar] [CrossRef]
- Cho, J.W.; Emi, T.; Shibata, H.; Suzuki, M. Heat transfer across mold flux film in mold during initial solidification in continuous casting of steel. ISIJ Int. 1998, 38, 834–842. [Google Scholar] [CrossRef]
- Yoon, D.W.; Cho, J.W.; Kim, S.H. Controlling radiative heat transfer across the mold flux layer by the scattering effect of the borosilicate mold flux system with metallic iron. Metall. Mater. Trans. B 2017, 48, 1951–1961. [Google Scholar] [CrossRef]
- Susa, M.; Mills, K.C.; Richardson, M.J.; Taylor, R. Thermal properties of slag films taken from continuous casting mould. Ironmak. Steelmak. 1994, 21, 279–286. [Google Scholar]
- Mills, K.C. Structure and Properties of slags used in the continuous casting of steel: Part 1 conventional mould powders. ISIJ Int. 2016, 56, 1–13. [Google Scholar] [CrossRef]
- Bhagurkar, A.G.; Qin, R. The Microstructure formation in slag solidification at continuous casting mold. Metals 2022, 12, 617. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, M.S.; Kang, Y.B. A reaction between high Mn–high Al steel and CaO-SiO2-type molten mold flux: Reduction of additive oxide components in mold flux by Al in steel. Metall. Mater. Trans. B 2020, 50, 2077–2082. [Google Scholar] [CrossRef]
- Wang, W.; Lu, B.; Xiao, D. A review of mold flux development for the casting of high-Al steels. Metall. Mater. Trans. B 2016, 47, 384–389. [Google Scholar] [CrossRef]
- XYan, X.; Pan, W.; Wang, X.; Zhang, X.; He, S.; Wang, Q. Electrical conductivity, viscosity and structure of CaO–Al2O3-based mold slags for continuous casting of high-Al steels. Metall. Mater. Trans. B 2021, 52, 2526–2535. [Google Scholar]
- Ou, G.L.; Liu, Y.C.; Yen, S.Y.; Wang, H.Y.; Su, Y.H.; Lu, M.J.; Lin, S.K. Reactivity and thermo-physical properties of MnO-modified CaO-Al2O3-based mold fluxes for advanced high-strength steels. J. Mater. Res. Technol. 2020, 9, 12091–12101. [Google Scholar] [CrossRef]
- Gao, E.; Wang, W.; Zhang, L. Effect of alkaline earth metal oxides on the viscosity and structure of the CaO-Al2O3 based mold flux for casting high-al steels. J. Non-Cryst. Solids 2017, 473, 79–86. [Google Scholar] [CrossRef]
- SShirayama, S.; Aoki, H.; Yanaba, Y.; Kim, Y.; Morita, K. Relationship between thermal conductivity and structure of the CaO–BO1.5–AlO1.5 System. ISIJ Int. 2020, 60, 392–399. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, J.; Cui, H. Simulation for mass transfer kinetics at slag-steel interface during high Al steel continuous casting. ISIJ Int. 2019, 59, 2256–2263. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, J.; Yan, B. Interfacial phenomena and reaction kinetics between high Al molten steel and CaO-SiO2-type flux. Metals 2022, 12, 391. [Google Scholar] [CrossRef]
- Gu, S.; Wen, G.; Ding, Z.; Tang, P.; Liu, Q. Effect of shear stress on isothermal crystallization behavior of CaO-Al2O3-SiO2-Na2O-CaF2 slags. Materials 2018, 11, 1085. [Google Scholar] [CrossRef]
- Leng, M.; Lai, F.; Li, J. Effect of cooling rate on phase and crystal morphology transitions of CaO–SiO2-based systems and CaO–Al2O3-based systems. Materials 2019, 12, 62. [Google Scholar] [CrossRef]
- Perrot, C.; Pontoire, J.N.; Marchionni, C.; Ridolfi, M.R.; Sancho, L.F. Several slag rims and lubrication behaviours in slab casting. Metall. Res. Technol. 2005, 102, 887–896. [Google Scholar] [CrossRef]
- He, S.; Li, Z.; Chen, Z.; Wu, T.; Wang, Q. Review of mold fluxes for continuous casting of high-alloy (Al, Mn, Ti) steels. Steel Res. Int. 2019, 90, 180042. [Google Scholar] [CrossRef]
- Long, X.; Wang, Q.; He, S.; Pistorius, P.C. Structure evolution of slag films of ultrahigh-basicity mold flux during solidification. Metall. Mater. Trans. B 2017, 48, 1938–1942. [Google Scholar] [CrossRef]
- Long, X.; He, S.; Wang, Q.; Pistorius, P.C. Structure of solidified films of mold flux for peritectic steel. Metall. Mater. Trans. B 2017, 48, 1652–1658. [Google Scholar] [CrossRef]
- Assis, K.L.S.; Pistorius, P.C. Improved cold-finger measurement of heat flux through solidified mould flux. Ironmak. Steelmak. 2018, 45, 502–508. [Google Scholar] [CrossRef]
- Assis, K.L.S. Heat Transfer through Mold Fluxes: A New Approach to Measure Thermal Properties of Slags; Carnegie Mellon University: Pittsburgh, PA, USA, 2016. [Google Scholar]
- Nakada, H.; Susa, M.; Seko, Y.; Hayashi, M.; Nagata, K. Mechanism of heat transfer reduction by crystallization of mold flux for continuous casting. ISIJ Int. 2008, 48, 446–453. [Google Scholar] [CrossRef]
- Xiao, L.O.N.G.; He, S.P.; Xu, J.F.; Huo, X.L.; Qian, W.A.N.G. Properties of high basicity mold fluxes for peritectic steel slab casting. J. Iron Steel Res. Int. 2012, 19, 39–45. [Google Scholar]
- Zeng, J.; Long, X.; You, X.; Li, M.; Wang, Q.; He, S. Structure of solidified films of CaO-SiO2-Na2O based low-fluorine mold flux. Metals 2019, 9, 93. [Google Scholar] [CrossRef]

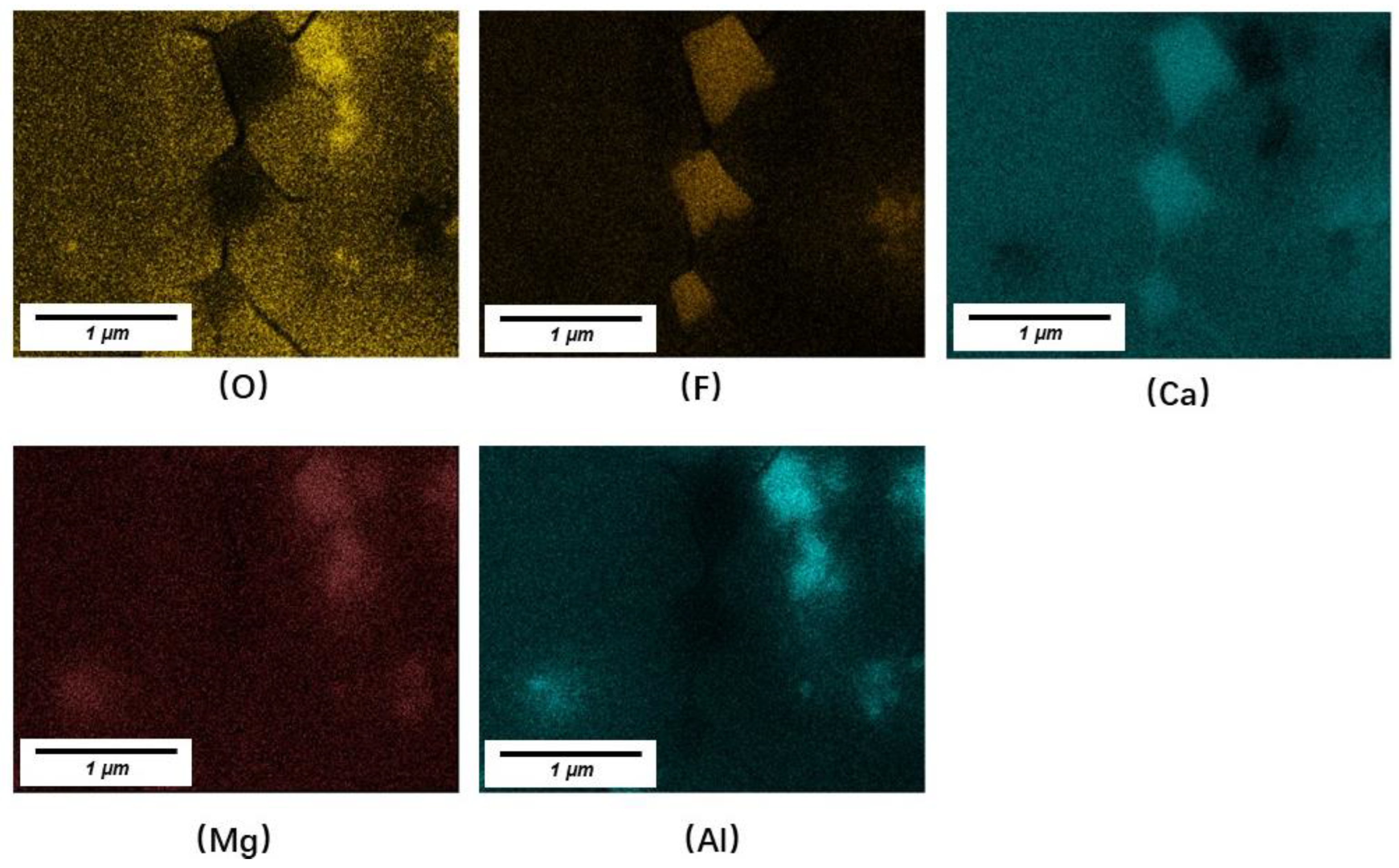
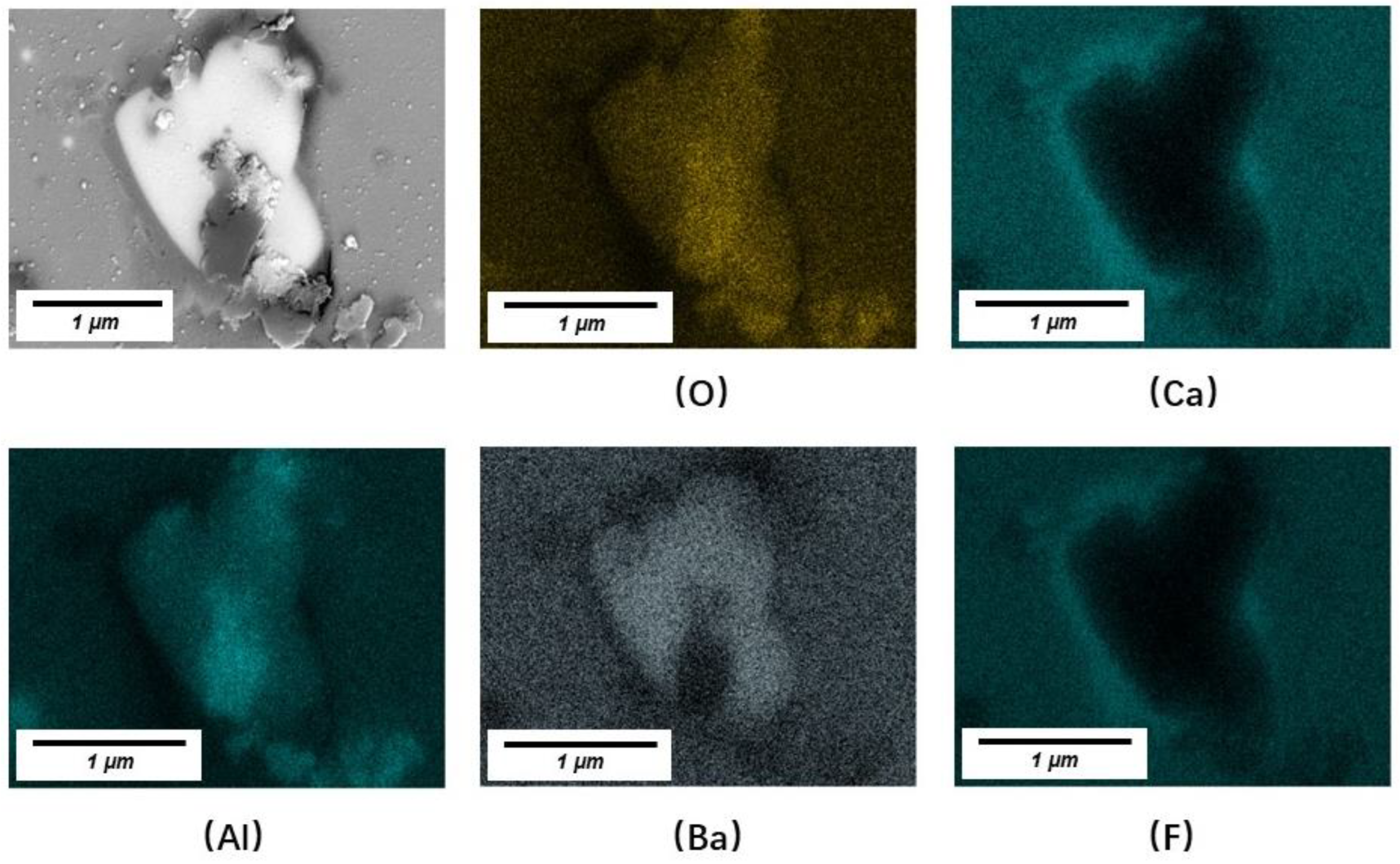
| No. | CaO | Al2O3 | BaO | F | Li2O | B2O3 | MgO |
|---|---|---|---|---|---|---|---|
| 1 | 24 | 32 | 15 | 10 | 6 | 4.5 | 2 |
| 2 | 24 | 37 | 15 | 10 | 6 | 4.5 | 2 |
| 3 | 24 | 42 | 15 | 10 | 6 | 4.5 | 2 |
| Solidification Time, s | No. 1 Slag | No. 2 Slag | No. 3 Slag | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bulk Temperature of Molten Slag, °C | |||||||||
| 1400 | 1350 | 1300 | 1400 | 1350 | 1300 | 1400 | 1350 | 1300 | |
| Average Film Thickness, mm | |||||||||
| 7 | - | - | - | - | - | - | 1.61 | 2.09 | 3.35 |
| 15 | - | - | 2.54 | 1.67 | 2.01 | 3.29 | 1.88 | 2.51 | 4.37 |
| 30 | 1.73 | 2.32 | 2.99 | 1.89 | 2.77 | 3.97 | 1.98 | 3.24 | 5.60 |
| 60 | 2.23 | 2.95 | 4.45 | 2.68 | 3.22 | 4.33 | 2.62 | 6.12 | - |
| 90 | 2.45 | 3.01 | 5.56 | 2.71 | 4.13 | 6.21 | 2.97 | 7.80 | 9.17 |
| Solidification Time, s | No. 1 Slag | No. 2 Slag | No. 3 Slag | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bulk Temperature of Molten Slag, °C | |||||||||
| 1400 | 1350 | 1300 | 1400 | 1350 | 1300 | 1400 | 1350 | 1300 | |
| Crystal Phase | |||||||||
| 7 | - | - | - | - | - | - | G | G | AC |
| 15 | - | - | B | G | AB | AB | BC | ABC | ABC |
| 30 | AB | AB | AB | AB | AB | ABD | ABC | ABCD | ABCD |
| 60 | ABD | ABD | ABD | ABD | ABD | ABD | ABCD | ABCD | - |
| 90 | ABD | ABD | ABD | ABD | ABCD | ABD | ABCD | ABCD | ABCD |
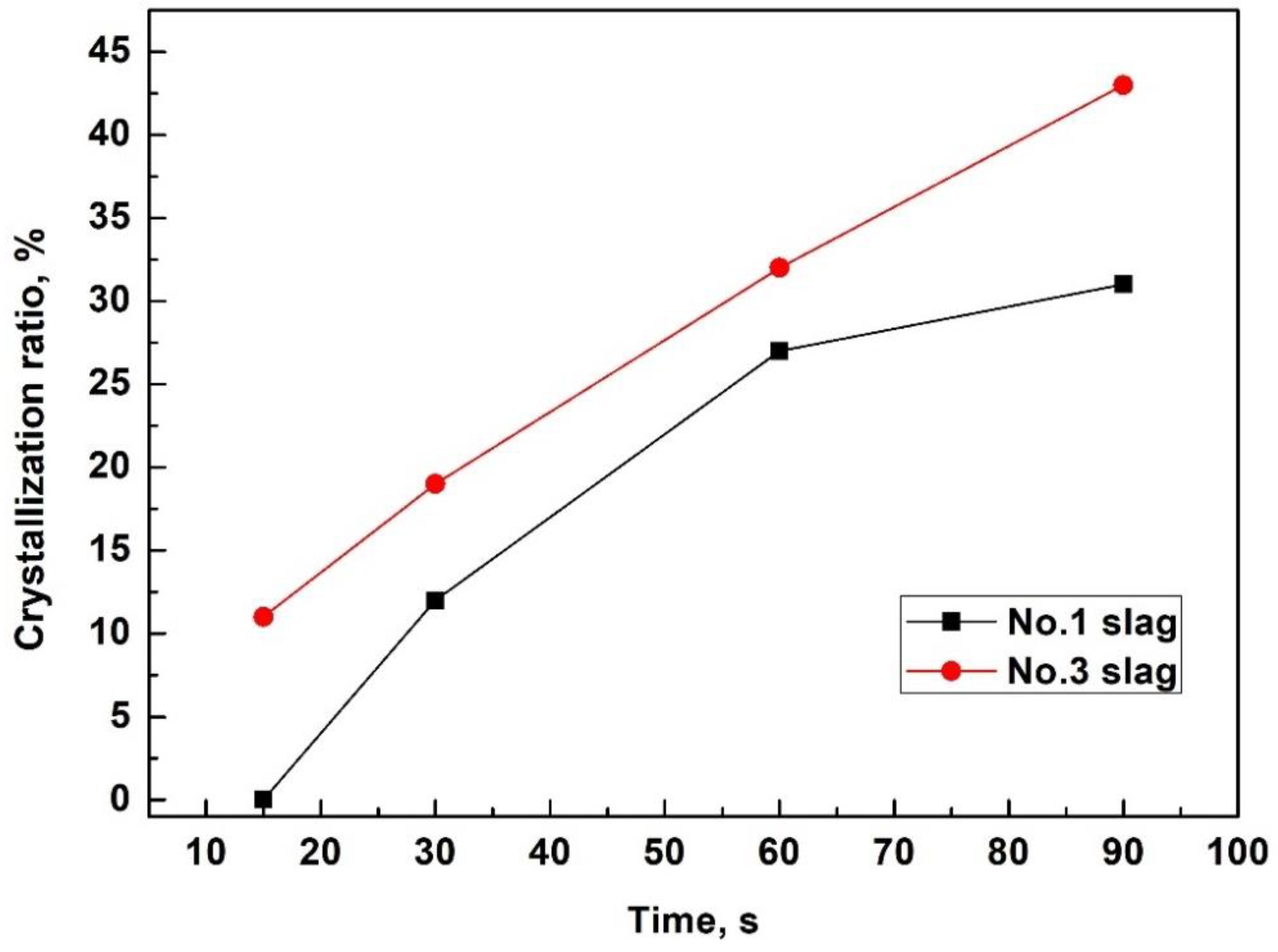
| Slag No. | Solidified Time, s | CaF2 | Spinel (MgAl2O4) | LiAlO2 | BaAl2O4 | Crystal Ratio of Film |
|---|---|---|---|---|---|---|
| No. 1 | 30 | 2 | 0 | 10 | 0 | 12 |
| 60 | 8 | 0 | 13 | 6 | 27 | |
| 90 | 9 | 0 | 15 | 7 | 31 | |
| No. 3 | 7 | 0 | 0 | 0 | 0 | 0 |
| 15 | 0 | 3 | 8 | 0 | 11 | |
| 30 | 5 | 3 | 11 | 0 | 19 | |
| 60 | 7 | 4 | 14 | 7 | 32 | |
| 90 | 12 | 4 | 16 | 11 | 43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, X.; Long, S.; Luo, W.; Li, X.; Tu, C.; Na, Y.; Xu, J. Crystallization of Slag Films of CaO-Al2O3-BaO-CaF2-Li2O-Based Mold Fluxes for High-Aluminum Steels’ Continuous Casting. Materials 2023, 16, 1903. https://doi.org/10.3390/ma16051903
Long X, Long S, Luo W, Li X, Tu C, Na Y, Xu J. Crystallization of Slag Films of CaO-Al2O3-BaO-CaF2-Li2O-Based Mold Fluxes for High-Aluminum Steels’ Continuous Casting. Materials. 2023; 16(5):1903. https://doi.org/10.3390/ma16051903
Chicago/Turabian StyleLong, Xiao, Shaolei Long, Wenbo Luo, Xiang Li, Changping Tu, Yunhao Na, and Jinxin Xu. 2023. "Crystallization of Slag Films of CaO-Al2O3-BaO-CaF2-Li2O-Based Mold Fluxes for High-Aluminum Steels’ Continuous Casting" Materials 16, no. 5: 1903. https://doi.org/10.3390/ma16051903
APA StyleLong, X., Long, S., Luo, W., Li, X., Tu, C., Na, Y., & Xu, J. (2023). Crystallization of Slag Films of CaO-Al2O3-BaO-CaF2-Li2O-Based Mold Fluxes for High-Aluminum Steels’ Continuous Casting. Materials, 16(5), 1903. https://doi.org/10.3390/ma16051903






