Correlating Hydration of Alkali-Activated Slag Modified by Organic Additives to the Evolution of Its Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Methods
2.2.1. Isothermal Heat Conduction Calorimetry
2.2.2. Physical-Mechanical Testing
2.2.3. Pore Solution Composition
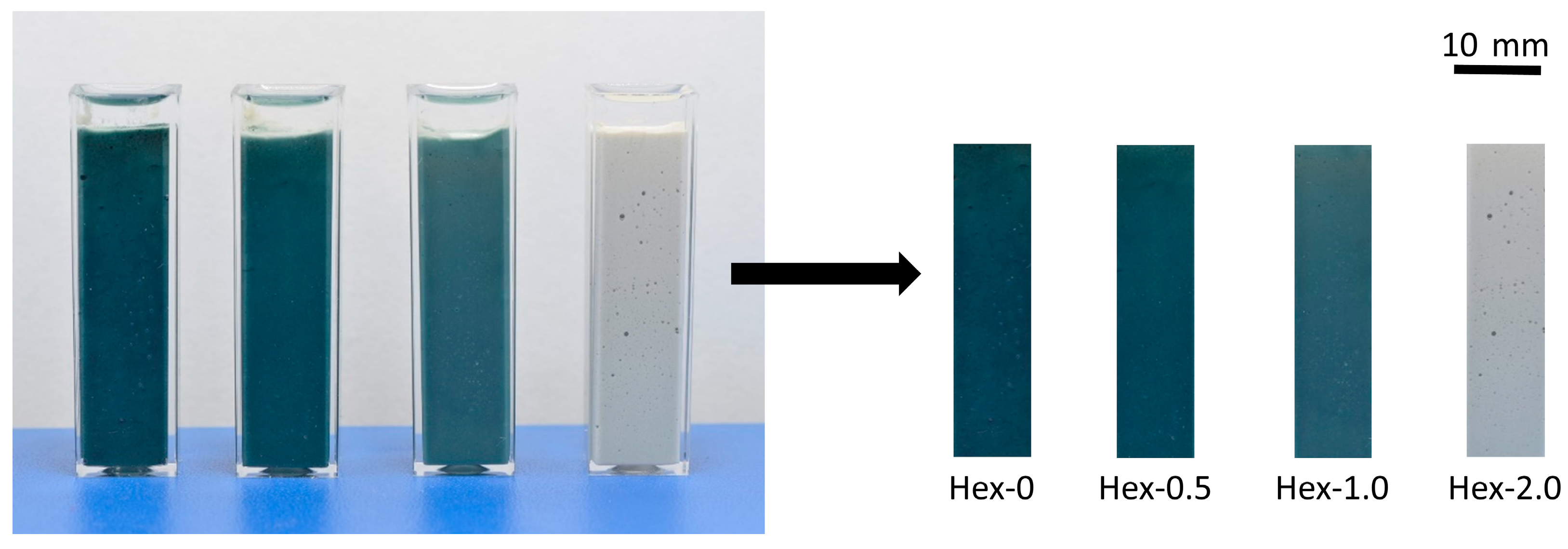
2.2.4. Assessment of Color Change
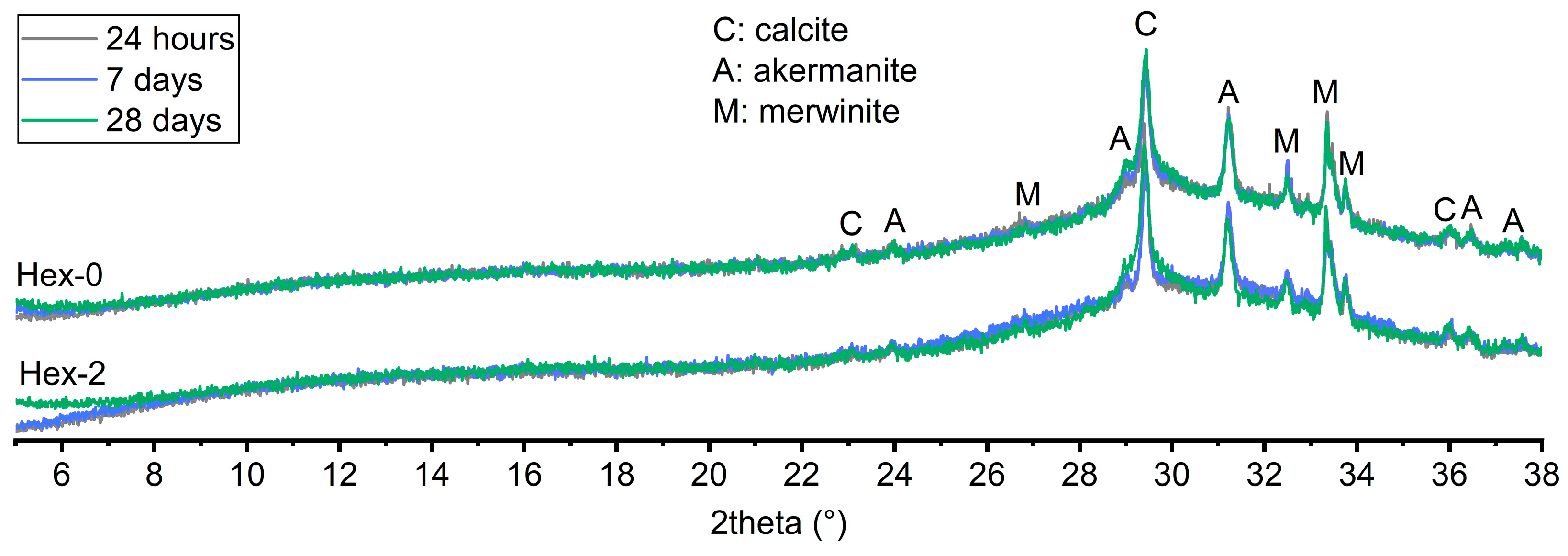
2.2.5. Phase Assemblage
3. Results and Their Discussion
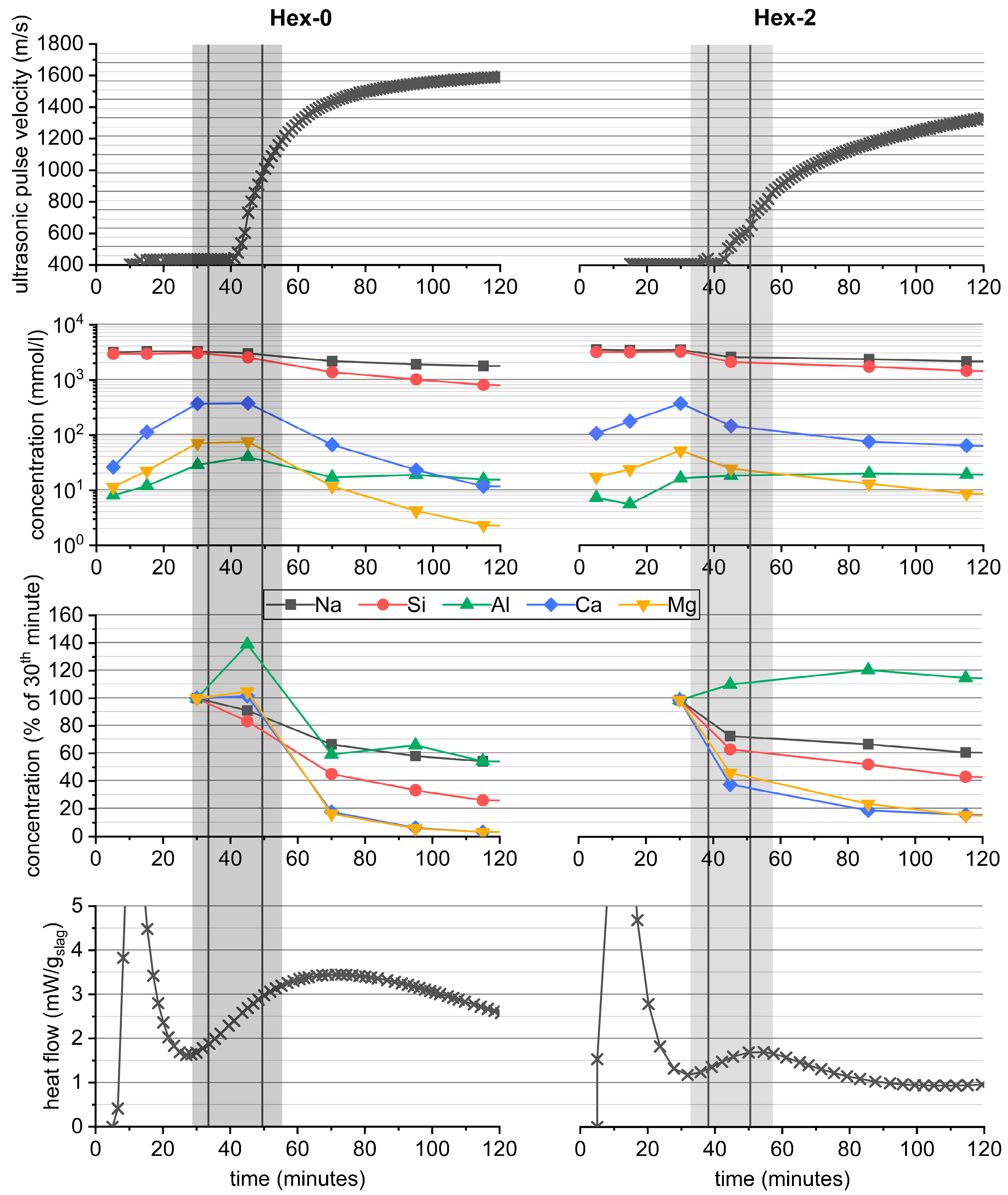
3.1. Overall Course of Hydration and Hydration Products
3.2. Correlations during the Gelation Stages of AAS Hydration
3.3. Correlations during the Bulk Hydration Stages of AAS Hydration
4. Conclusions
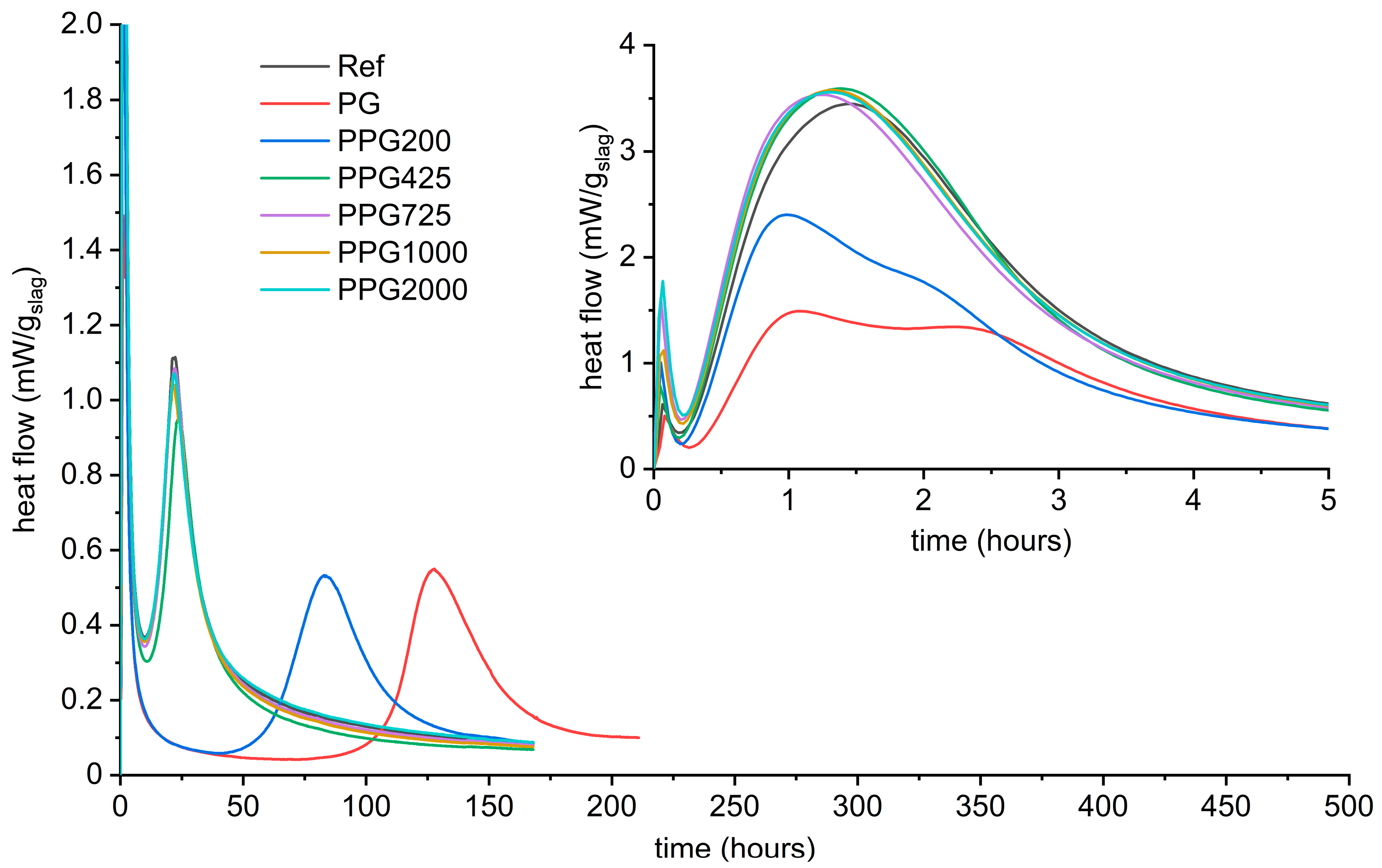
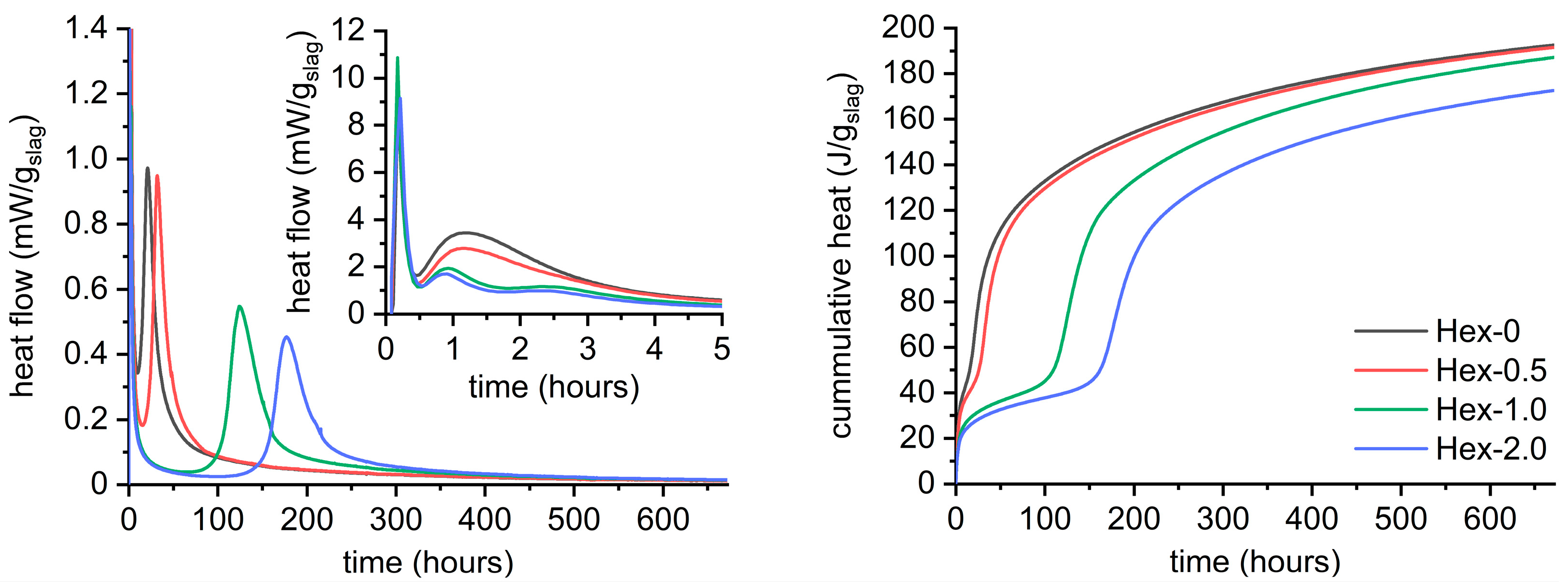
- Hexylene glycol and many other organic admixtures significantly affected the hydration process of AAS by means of decreased intensity of the gelation peak and simultaneously prolonged induction period, i.e., retardation of the bulk hydration peak.
- The position of the second peak is crucial for the timing of setting or loss of workability, while the intensity of this peak is related to the initial compressive strengths that reach a few to several MPa. After that, the hydration goes into the induction period of minimized heat flow and can last several days or even weeks depending on the type and dose of the organic admixture.
- The bulk hydration peak perfectly correlates with a great increase in strength, autogenous shrinkage, and ultrasonic pulse velocity, as well as with the onset of a fascinating color change from off-white to green/blue, characteristic for AAS. Therefore, the color change is a very simple and practical indicator of the bulk hydration of the slag and thus tells something about the maturity of the material, particularly under anoxic conditions.
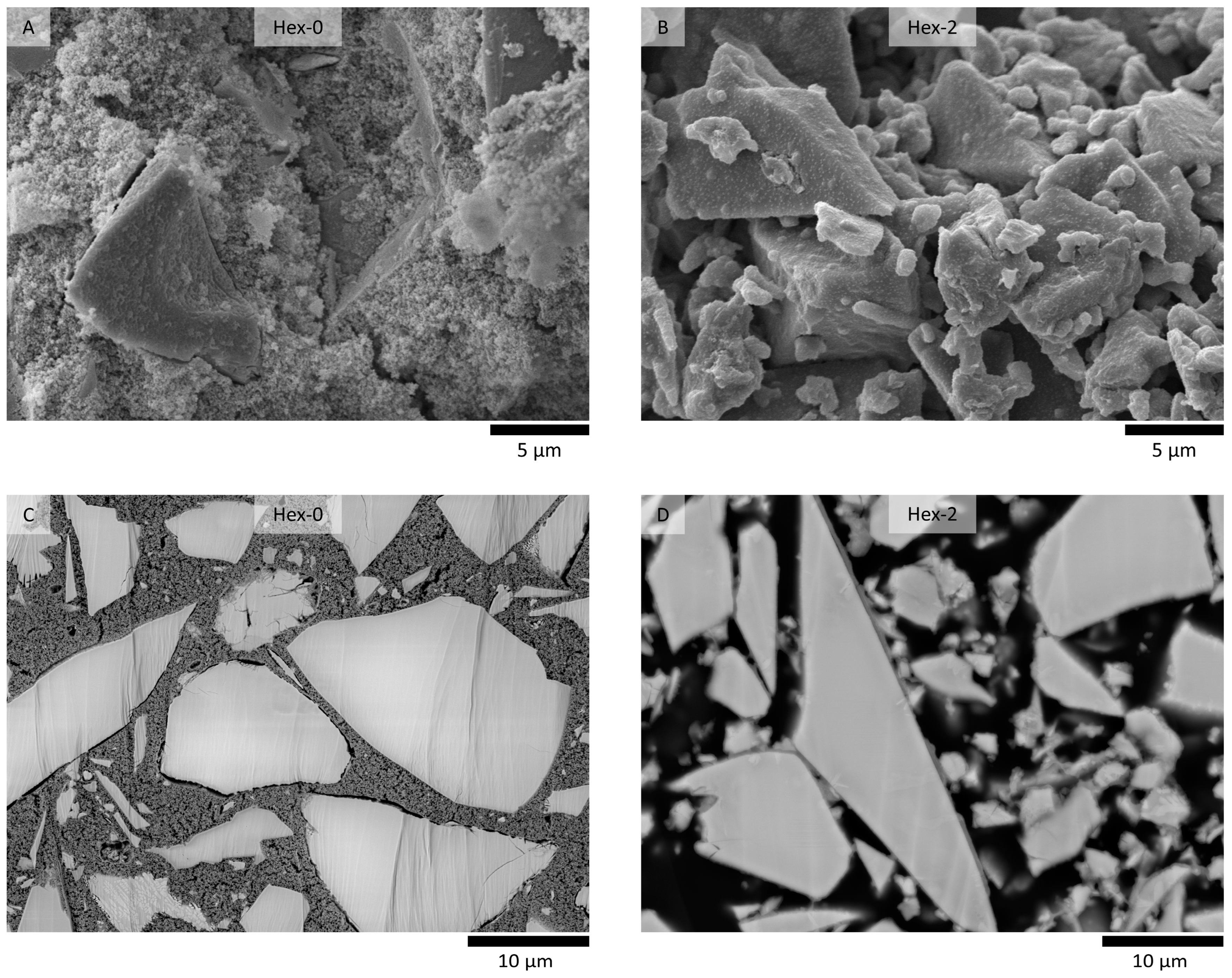
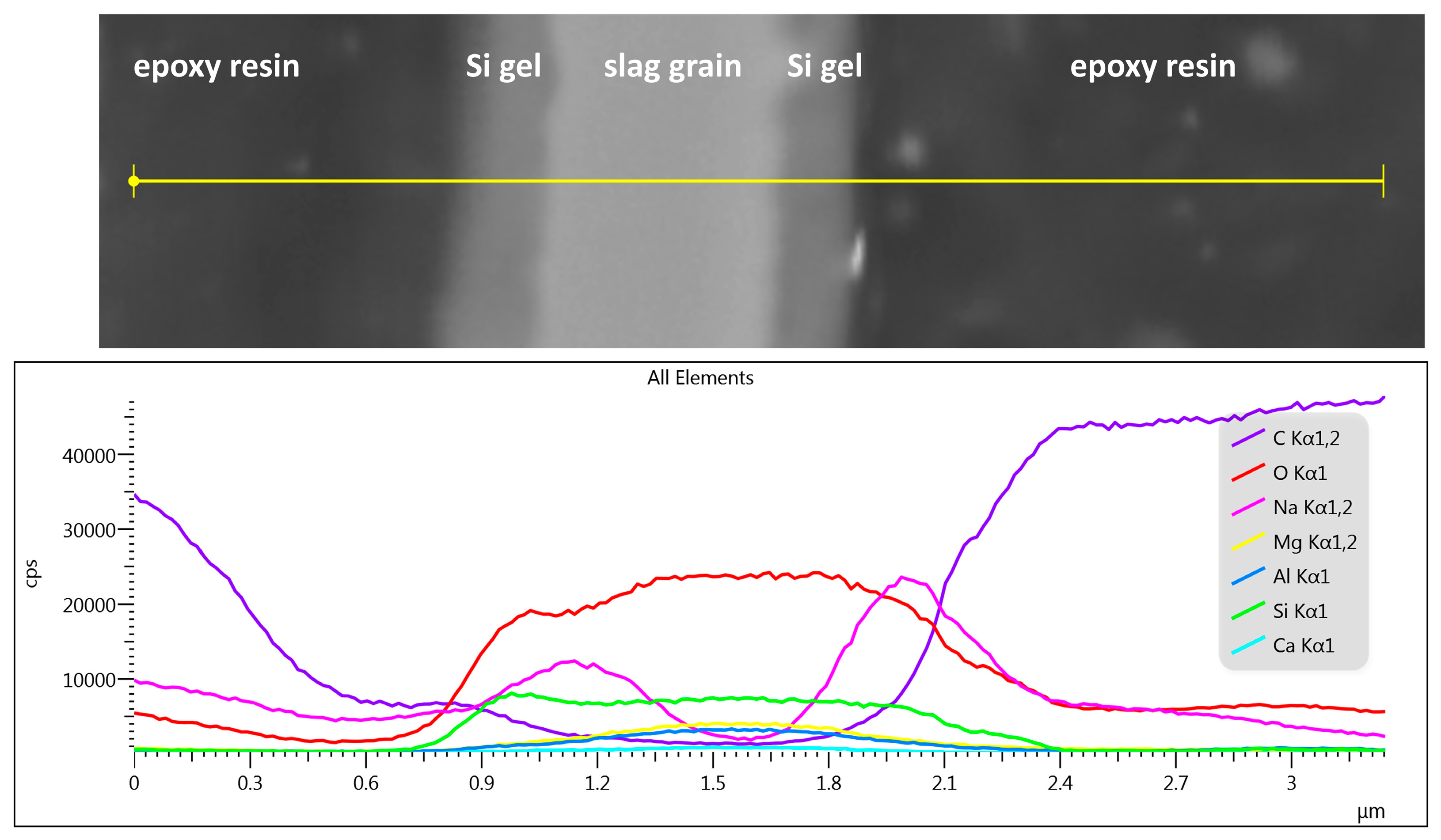
- The influence of organic additives lies in their action during the gelation stages of hydration. Hexylene glycol caused the formation of a few hundred nanometer thick layer of silicate-based gel on the slag surface instead of the formation of the continuous matrix in the bulk of the interstitial space, typical for waterglass-activated slag without additives. This seems to be the main reason for both the decreased rate of slag dissolution and the decrease in the formation of reaction products, resulting in a prolonged induction period.
- Despite the strong retardation effect of hexylene glycol on AAS hydration, the overall reaction process did not change distinctively. No new crystalline phase nor the significant changes observed by XRD and thermogravimetry, respectively. The latter only confirmed the retardation effect by very low and almost the same weight losses of the paste with hexylene glycol after 24 h and 7 days.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Effect of Various Organic Admixtures on AAS Hydration
- Brief summary of results
- Various alcohols can modify the process of alkaline activation of slag.
- Generally: The lower the second peak (or double peak), the more delayed the third peak
- Effect of alcohol structure on the delay of the maximum of the third calorimetric peak
- It is hard to find general trends, but some partial trends are observable as follows.
- Number of hydroxyl groups: Monoalcohols are stronger retarders than the corresponding diols
- Length of the carbon chain of diols
- Polymerization degree


References
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Shi, C.; Krivenko, P.V.; Roy, D. Alkali-Activated Cements and Concretes; Taylor & Francis: London, UK, 2006. [Google Scholar]
- Song, S.; Jennings, H.M. Pore solution chemistry of alkali-activated ground granulated blast-furnace slag. Cem. Concr. Res. 1999, 29, 159–170. [Google Scholar] [CrossRef]
- Zuo, Y.; Ye, G. Preliminary Interpretation of the Induction Period in Hydration of Sodium Hydroxide/Silicate Activated Slag. Materials 2020, 13, 4796. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.J.; Bernal, S.A.; San Nicolas, R.; Provis, J.L. Generalized structural description of calcium-sodium aluminosilicate hydrate gels: The cross-linked substituted tobermorite model. Langmuir 2013, 29, 5294–5306. [Google Scholar] [CrossRef]
- Lothenbach, B.; Gruskovnjak, A. Hydration of alkali-activated slag: Thermodynamic modelling. Adv. Cem. Res. 2007, 19, 81–92. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. A calorimetric study of early hydration of alkali-slag cement. Cem. Concr. Res. 1995, 25, 1333–1346. [Google Scholar] [CrossRef]
- Ravikumar, D.; Neithalath, N. Reaction kinetics in sodium silicate powder and liquid activated slag binders evaluated using isothermal calorimetry. Thermochim. Acta 2012, 546, 32–43. [Google Scholar] [CrossRef]
- Gebregziabiher, B.S.; Thomas, R.J.; Peethamparan, S. Temperature and activator effect on early-age reaction kinetics of alkali-activated slag binders. Constr. Build. Mater. 2016, 113, 783–793. [Google Scholar] [CrossRef] [Green Version]
- Fernández Jiménez, A.; Puertas, F. Setting of alkali-activated slag cement. Influence of activator nature. Adv. Cem. Res. 2001, 13, 115–121. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Myers, R.J.; San Nicolas, R.; van Deventer, J.S.J. Role of carbonates in the chemical evolution of sodium carbonate-activated slag binders. Mater. Struct. 2014, 48, 517–529. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Z.; Shi, C.; Li, N.; Jiao, D.; Yuan, Q. Rheology of alkali-activated materials: A review. Cem. Concr. Compos. 2021, 121, 104061. [Google Scholar] [CrossRef]
- Melo Neto, A.A.; Cincotto, M.A.; Repette, W. Drying and autogenous shrinkage of pastes and mortars with activated slag cement. Cem. Concr. Res. 2008, 38, 565–574. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Effect of pore size distribution on drying shrinkage of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1401–1406. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F. Effect of shrinkage-reducing admixtures on the properties of alkali-activated slag mortars and pastes. Cem. Concr. Res. 2007, 37, 691–702. [Google Scholar] [CrossRef]
- Bilek, V.; Kalina, L.; Novotny, R.; Tkacz, J.; Parizek, L. Some Issues of Shrinkage-Reducing Admixtures Application in Alkali-Activated Slag Systems. Materials 2016, 9, 462. [Google Scholar] [CrossRef]
- Kalina, L.; Bílek, V.; Bartoníčková, E.; Kalina, M.; Hajzler, J.; Novotný, R. Doubts over capillary pressure theory in context with drying and autogenous shrinkage of alkali-activated materials. Constr. Build. Mater. 2020, 248, 118620. [Google Scholar] [CrossRef]
- Bílek, V.; Kalina, L.; Novotný, R. Polyethylene glycol molecular weight as an important parameter affecting drying shrinkage and hydration of alkali-activated slag mortars and pastes. Constr. Build. Mater. 2018, 166, 564–571. [Google Scholar] [CrossRef]
- Qu, Z.Y.; Yu, Q.; Ji, Y.D.; Gauvin, F.; Voets, I.K. Mitigating shrinkage of alkali activated slag with biofilm. Cem. Concr. Res. 2020, 138, 106234. [Google Scholar] [CrossRef]
- Li, Z.; Wyrzykowski, M.; Dong, H.; Granja, J.; Azenha, M.; Lura, P.; Ye, G. Internal curing by superabsorbent polymers in alkali-activated slag. Cem. Concr. Res. 2020, 135, 106123. [Google Scholar] [CrossRef]
- Li, Z.; Lu, T.; Liang, X.; Dong, H.; Ye, G. Mechanisms of autogenous shrinkage of alkali-activated slag and fly ash pastes. Cem. Concr. Res. 2020, 135, 106107. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Duxson, P.; Brice, D.G.; Kilcullen, A. Settable Composition Comprising Slag. WO 2012/083384 A1, 2012. [Google Scholar]
- Chaouche, M.; Gao, X.X.; Cyr, M.; Cotte, M.; Frouin, L. On the origin of the blue/green color of blast-furnace slag-based materials: Sulfur K-edge XANES investigation. J. Am. Ceram. Soc. 2017, 100, 1707–1716. [Google Scholar] [CrossRef]
- Ballekere Kumarappa, D.; Peethamparan, S.; Ngami, M. Autogenous shrinkage of alkali activated slag mortars: Basic mechanisms and mitigation methods. Cem. Concr. Res. 2018, 109, 91. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, H.; Chen, H.; Ni, Y.; Xu, X.; Huo, Q. Shrinkage-reducing measures and mechanisms analysis for alkali-activated coal gangue-slag mortar at room temperature. Constr. Build. Mater. 2020, 252, 119001. [Google Scholar] [CrossRef]
- Beersaerts, G.; Ascensão, G.; Pontikes, Y. Modifying the pore size distribution in Fe-rich inorganic polymer mortars: An effective shrinkage mitigation strategy. Cem. Concr. Res. 2021, 141, 106330. [Google Scholar] [CrossRef]
- ČSN EN 196-3 (722100); Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness. Czech Office for Standards, Metrology and Testing: Prague, Czech Republic, 2017.
- Dakhane, A.; Peng, Z.; Marzke, R.; Neithalath, N. Comparative Analysis of the Influence of Sodium and Potassium Silicate Solutions on the Kinetics and Products of Slag Activation. Adv. Civ. Eng. Mater. 2014, 3, 371–387. [Google Scholar] [CrossRef]
- Tänzer, R.; Jin, Y.; Stephan, D. Alkali activated slag binder: Effect of cations from silicate activators. Mater. Struct. 2016, 50, 1–9. [Google Scholar] [CrossRef]
- Brough, A.R.; Atkinson, A. Sodium silicate-based, alkali-activated slag mortars Part I. Strength, hydration and microstructure. Cem. Concr. Res. 2002, 32, 865–879. [Google Scholar] [CrossRef]
- Brough, A.R.; Holloway, M.; Sykes, J.; Atkinson, A. Sodium silicate-based alkali-activated slag mortars Part II. The retarding effect of additions of sodium chloride or malic acid. Cem. Concr. Res. 2000, 30, 1375–1379. [Google Scholar] [CrossRef]
- Bílek Jr., V.; Kadlec, M.; Novotný, R.; Kalina, L.; Hrubý, P. Novel perspective to explain the retarding effect of organic admixtures in silicate-activated slag. In Proceedings of the SP-354: Superplasticizers and Other Chemical Admixtures in Concrete, Milan, Italy, 10–13 July 2022; pp. 49–60. [Google Scholar]
- Lu, Z.; Merkl, J.P.; Pulkin, M.; Firdous, R.; Wache, S.; Stephan, D. A Systematic Study on Polymer-Modified Alkali-Activated Slag-Part II: From Hydration to Mechanical Properties. Materials 2020, 13, 3418. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.l. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Ben Haha, M.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Yan, Y.; Ma, B.; Miron, G.D.; Kulik, D.A.; Scrivener, K.; Lothenbach, B. Al uptake in calcium silicate hydrate and the effect of alkali hydroxide. Cem. Concr. Res. 2022, 162, 106957. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Kotwica, Ł.; Różycka, A.; Gołek, Ł. Incorporation of Al in C-A-S-H gels with various Ca/Si and Al/Si ratio: Microstructural and structural characteristics with DTA/TG, XRD, FTIR and TEM analysis. Constr. Build. Mater. 2017, 155, 643–653. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Ben Haha, M.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- Ben Haha, M.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part II: Effect of Al2O3. Cem. Concr. Res. 2012, 42, 74–83. [Google Scholar] [CrossRef]
- Myers, R.J.; L’Hôpital, E.; Provis, J.L.; Lothenbach, B. Effect of temperature and aluminium on calcium (alumino)silicate hydrate chemistry under equilibrium conditions. Cem. Concr. Res. 2015, 68, 83–93. [Google Scholar] [CrossRef]
- Zhang, J.; Scherer, G.W. Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 2011, 41, 1024–1036. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Rahul, A.V.; Tao, Y.; Van Bockstaele, F.; Dewettinck, K.; Ye, G.; De Schutter, G. Rheology of alkali-activated slag pastes: New insight from microstructural investigations by cryo-SEM. Cem. Concr. Res. 2022, 157, 106806. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; De Schutter, G. Early structural build-up, setting behavior, reaction kinetics and microstructure of sodium silicate-activated slag mixtures with different retarder chemicals. Cem. Concr. Res. 2022, 159, 106872. [Google Scholar] [CrossRef]
- Criado, M.; Walkley, B.; Ke, X.; Provis, J.; Bernal, S. Slag and Activator Chemistry Control the Reaction Kinetics of Sodium Metasilicate-Activated Slag Cements. Sustainability 2018, 10, 4709. [Google Scholar] [CrossRef] [Green Version]
- Le Cornec, D.; Wang, Q.; Galoisy, L.; Renaudin, G.; Izoret, L.; Calas, G. Greening effect in slag cement materials. Cem. Concr. Compos. 2017, 84, 93–98. [Google Scholar] [CrossRef]


| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Na2O | P2O5 | TiO2 | Mn2O3 | SrO | ZnO | Cl | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 37.2 | 9.32 | 0.27 | 39.1 | 9.50 | 1.53 | 0.43 | 0.40 | 0.02 | 0.03 | 0.61 | 0.06 | 0.02 | 0.03 | 1.24 |
| Sample | 40–550 °C | 270–470 °C | 550–720 °C | 720–860 °C | 550–990 °C | 40–990 °C |
|---|---|---|---|---|---|---|
| Hex-0: 24 h | 6.64 | 1.67 | 1.45 | 0.39 | 1.87 | 8.51 |
| Hex-0: 7 d | 9.13 | 2.50 | 1.61 | 0.28 | 1.98 | 11.1 |
| Hex-0: 28 d | 12.1 | 2.36 | 1.51 | 0.32 | 1.97 | 14.1 |
| Hex-2: 24 h | 4.03 | 0.71 | 1.30 | 0.31 | 1.48 | 5.51 |
| Hex-2: 7 d | 4.35 | 0.80 | 1.34 | 0.31 | 1.53 | 5.88 |
| Hex-2: 28 d | 10.1 | 2.05 | 1.58 | 0.53 | 2.24 | 12.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bílek, V., Jr.; Kalina, L.; Dvořák, R.; Novotný, R.; Švec, J.; Másilko, J.; Šoukal, F. Correlating Hydration of Alkali-Activated Slag Modified by Organic Additives to the Evolution of Its Properties. Materials 2023, 16, 1908. https://doi.org/10.3390/ma16051908
Bílek V Jr., Kalina L, Dvořák R, Novotný R, Švec J, Másilko J, Šoukal F. Correlating Hydration of Alkali-Activated Slag Modified by Organic Additives to the Evolution of Its Properties. Materials. 2023; 16(5):1908. https://doi.org/10.3390/ma16051908
Chicago/Turabian StyleBílek, Vlastimil, Jr., Lukáš Kalina, Richard Dvořák, Radoslav Novotný, Jiří Švec, Jiří Másilko, and František Šoukal. 2023. "Correlating Hydration of Alkali-Activated Slag Modified by Organic Additives to the Evolution of Its Properties" Materials 16, no. 5: 1908. https://doi.org/10.3390/ma16051908






