Optimising the Performance of CO2-Cured Alkali-Activated Aluminosilicate Industrial By-Products as Precursors
Abstract
1. Introduction
2. Materials and Methods
2.1. Binders
2.2. Aggregates
2.3. Alkaline Activator
2.4. Water Reducing Admixture
2.5. Setting Time Retarder
2.6. Mix Design
2.7. Production Method and Curing Regime
2.8. Characterisation and Testing Methods
3. Results
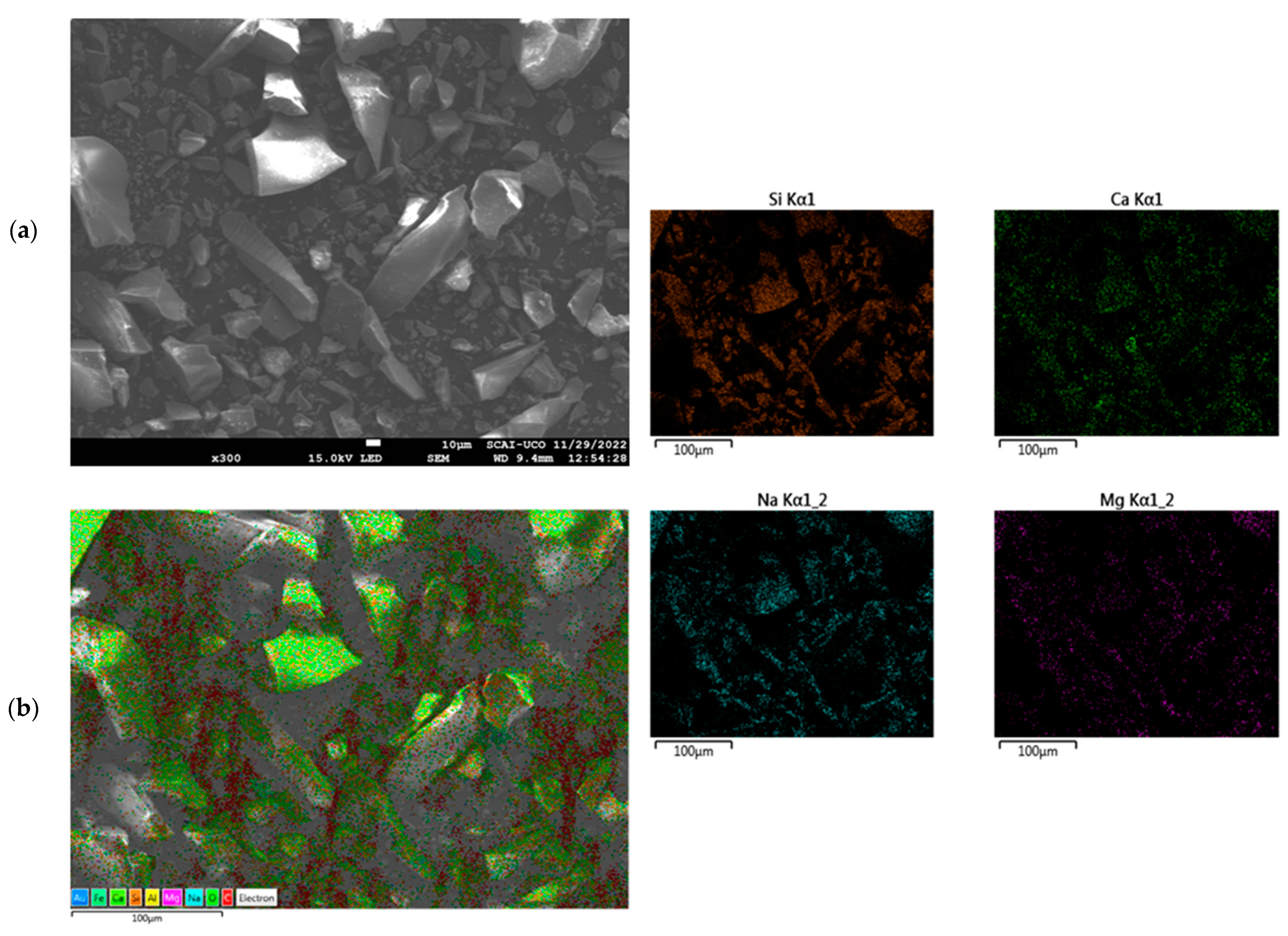
3.1. Materials Characterisation
3.1.1. Binder Density
3.1.2. X-ray Fluorescence
3.1.3. X-ray Diffraction
3.1.4. Particle Size Distribution
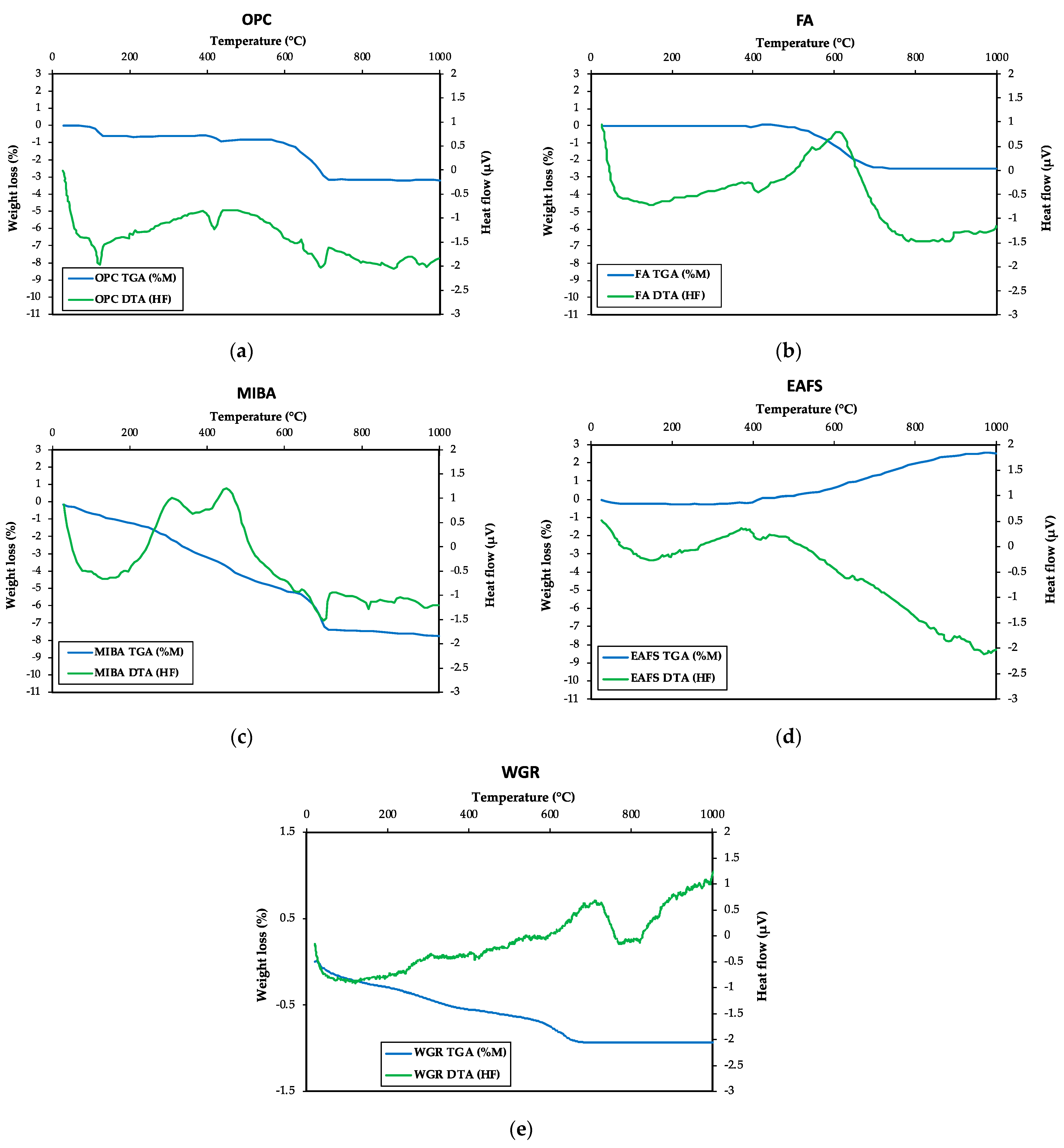
3.1.5. Thermogravimetric Analysis
3.1.6. Fourier-Transform Infrared Analyses
3.2. Fresh-State Performance
3.3. Hardened-State Performance
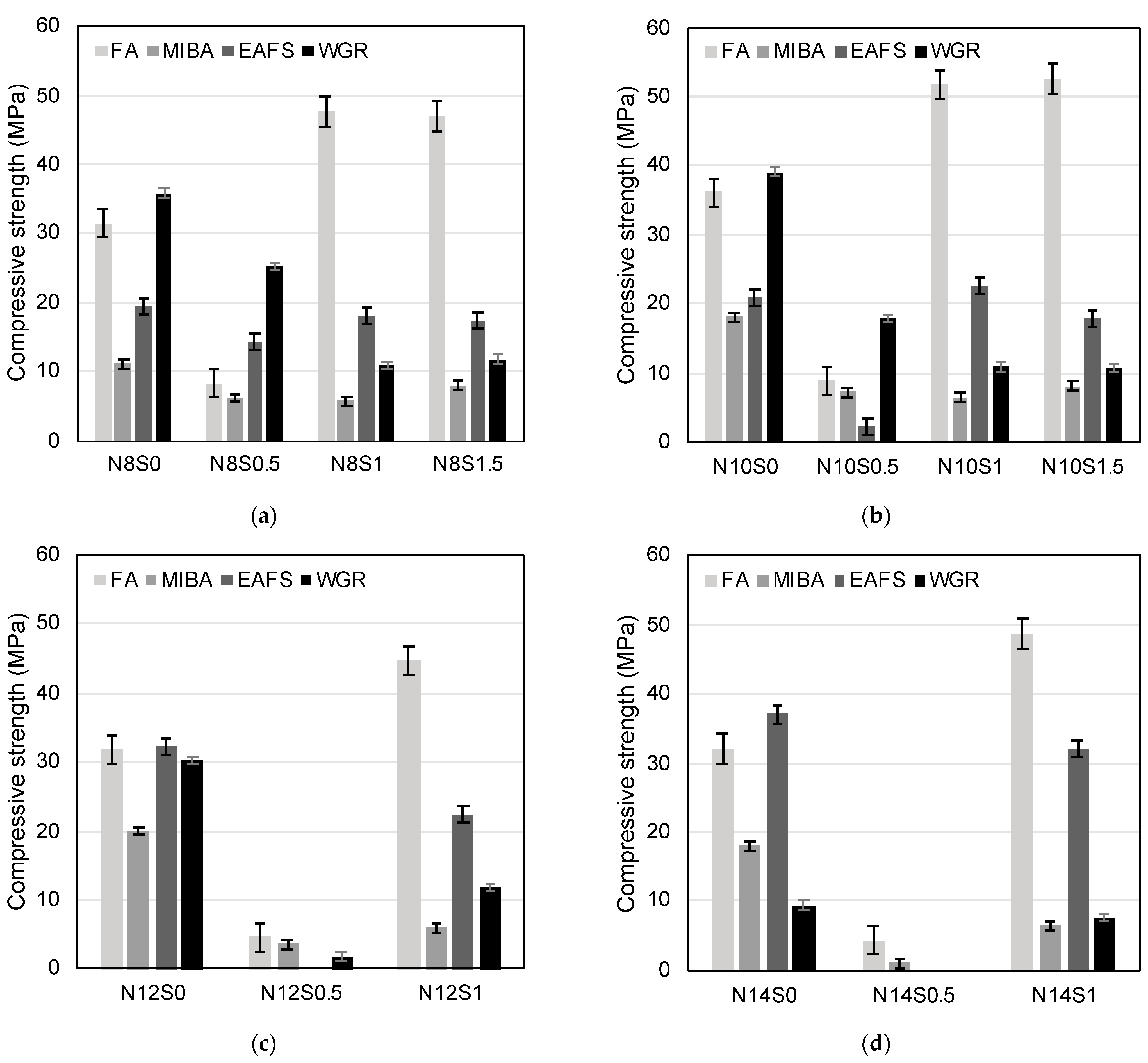
3.3.1. Compressive Strength
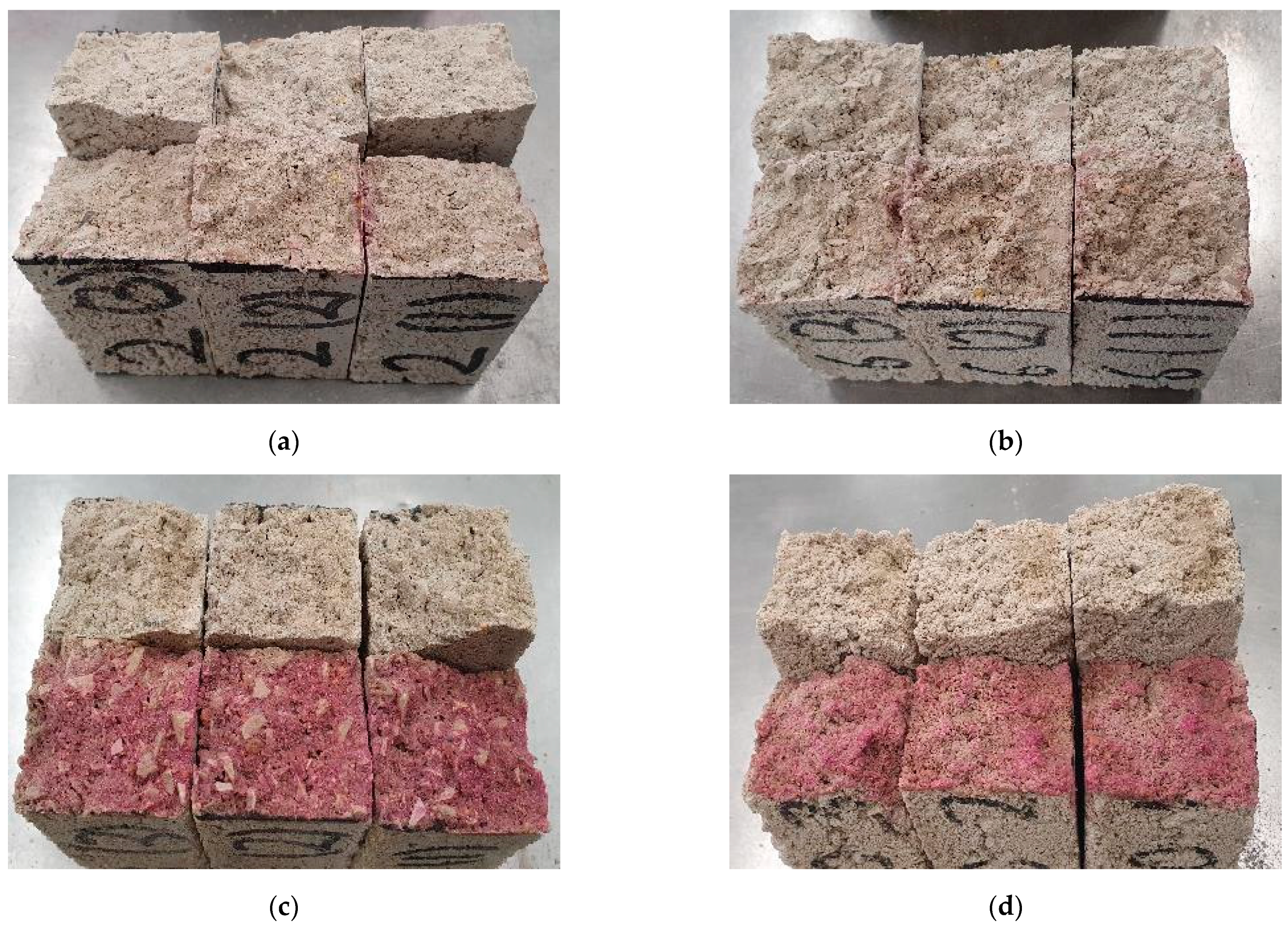
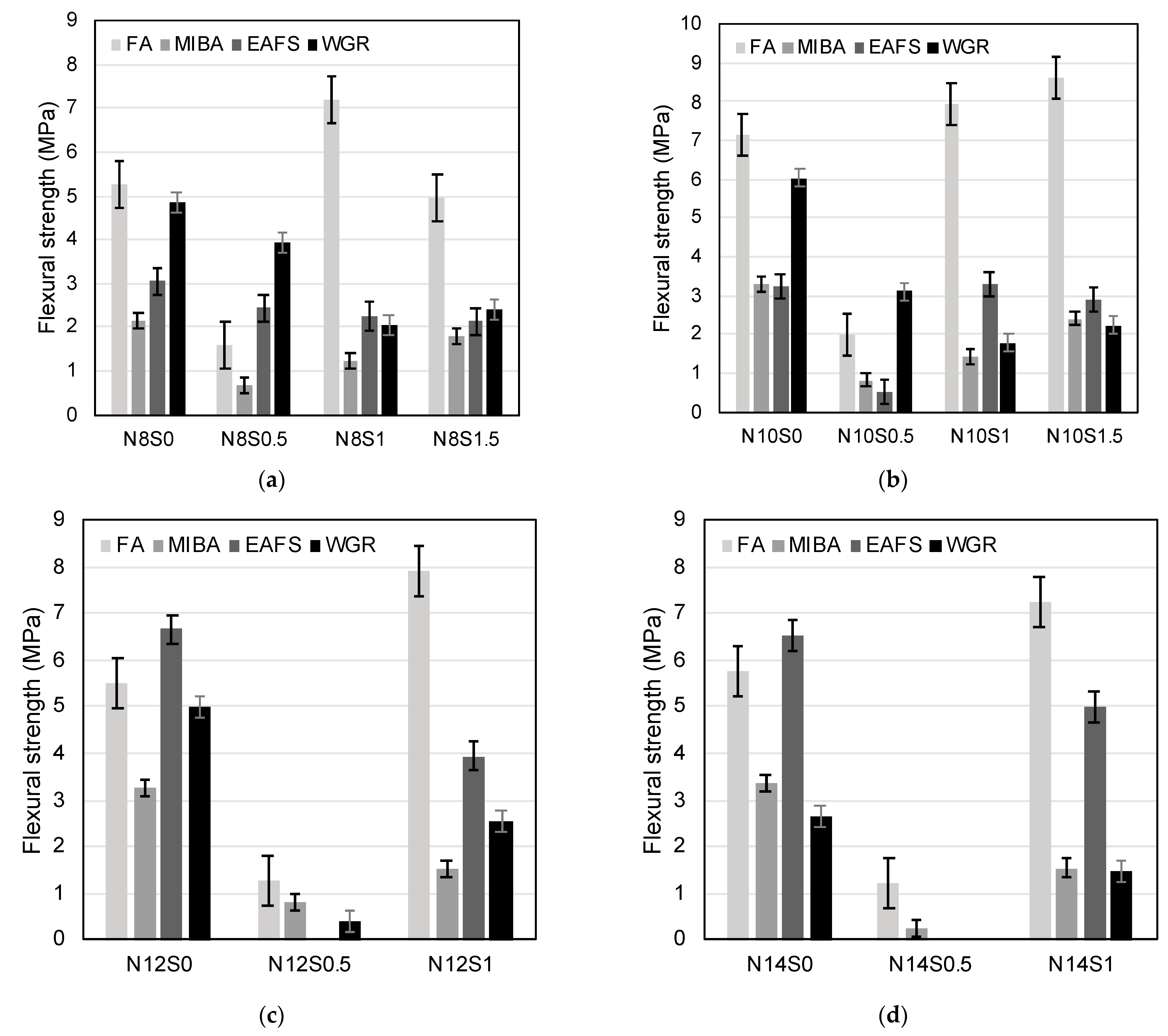
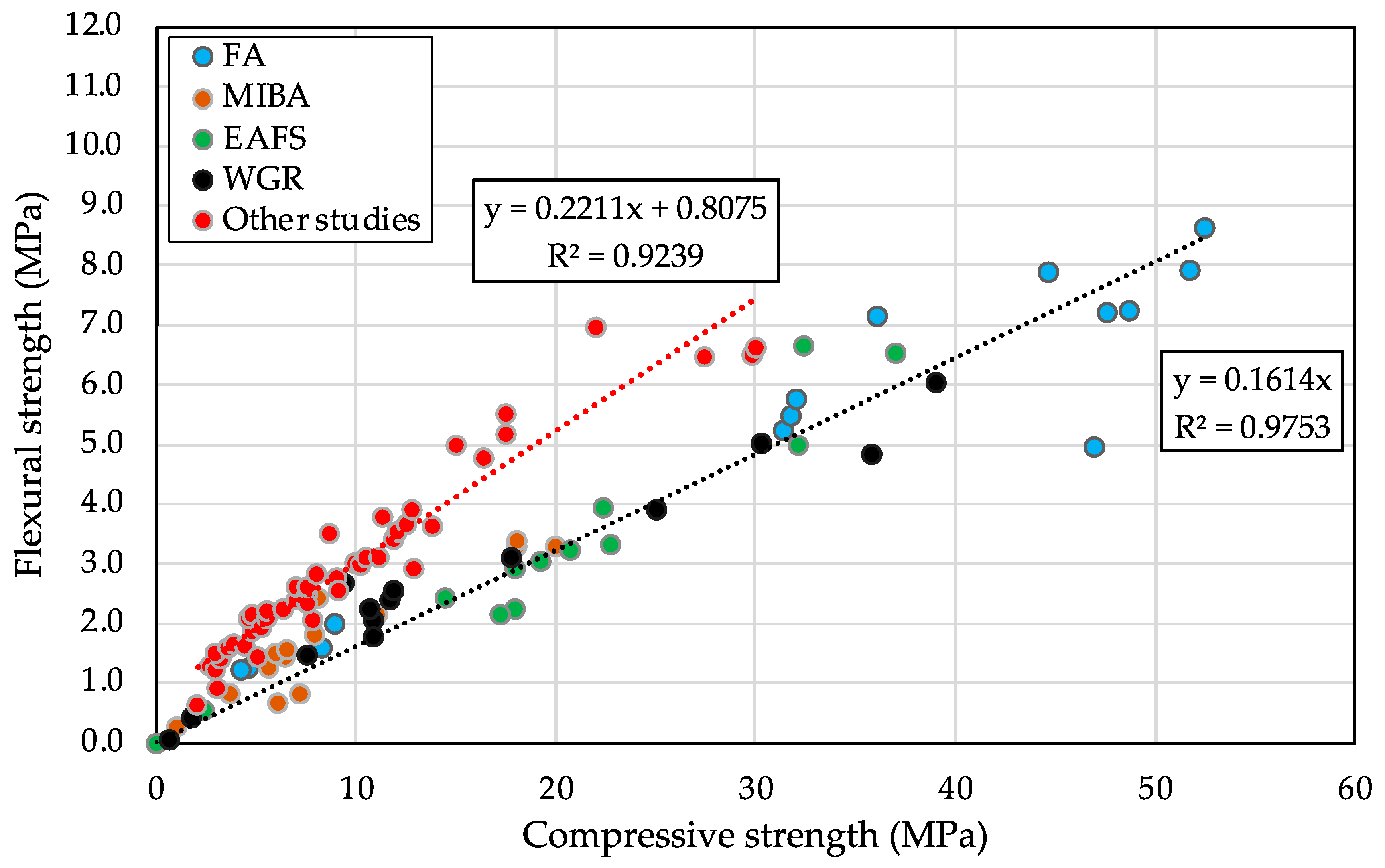
3.3.2. Flexural Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mindess, S.; Young, J.F.; Darwin, D. Concrete, 2nd ed.; Prentice Hall, Pearson Education, Inc.: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Nobis, R. Illustrated History of Cement and Concrete: The Exciting Development of Two Outstanding Building Materials; Rainer Nobis: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- U.S. Geological Survey. Cement Production Worldwide from 1995 to 2020, USGS—Mineral Commodity Summaries 2021; U.S. Geological Survey Data Series 140; U.S. Geological Survey: Reston, VA, USA, 2019.
- IEA. Cement Roadmap. Available online: http://www.iea.org/publications/freepublications/publication/Cement_Roadmap_Foldout_WEB.pdf (accessed on 15 November 2022).
- Conners, C. Empire State Building Fact Sheet. Available online: https://www.esbnyc.com (accessed on 16 November 2022).
- Klappholz, S. Cement and Construction Industries Gather Together to Find Solutions for a Net Zero CO2 Emissions Future; World Cement: London, UK, 2022. [Google Scholar]
- Pierrehumbert, R. There is no Plan B for dealing with the climate crisis. Bull. At. Sci. 2019, 75, 215–221. [Google Scholar] [CrossRef]
- Mikulčić, H.; Vujanović, M.; Fidaros, D.K.; Priesching, P.; Minić, I.; Tatschl, R.; Duić, N.; Stefanović, G. The application of CFD modelling to support the reduction of CO2 emissions in cement industry. Energy 2012, 45, 464–473. [Google Scholar] [CrossRef]
- Ashraf, W. Carbonation of cement-based materials: Challenges and opportunities. Constr. Build. Mater. 2016, 120, 558–570. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Yang, K.-H.; Jung, Y.-B.; Cho, M.-S.; Tae, S.-H. Effect of supplementary cementitious materials on reduction of CO2 emissions from concrete. J. Clean. Prod. 2015, 103, 774–783. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C.J. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related alkali-activated materials. In Annual Review of Materials Research; Clarke, D.R., Ed.; Annual Reviews: San Mateo, CA, USA, 2014; Volume 44, pp. 299–327. [Google Scholar]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Setting and mechanical properties of alkali-activated fly ash/slag concrete manufactured at room temperature. Constr. Build. Mater. 2013, 47, 1201–1209. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.M.; Palomo, A.; Lopez-Hombrados, C. Engineering properties of alkali-activated fly ash concrete. ACI Mater. J. 2006, 103, 106–112. [Google Scholar]
- Chi, M.; Huang, R. Binding mechanism and properties of alkali-activated fly ash/slag mortars. Constr. Build. Mater. 2013, 40, 291–298. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag–fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef]
- Puertas, F.; Martínez-Ramírez, S.; Alonso, S.; Vázquez, T. Alkali-activated fly ash/slag cements: Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Wang, R.; Mirza, N.; Vasbieva, D.G.; Abbas, Q.; Xiong, D. The nexus of carbon emissions, financial development, renewable energy consumption, and technological innovation: What should be the priorities in light of COP 21 Agreements? J. Environ. Manag. 2020, 271, 111027. [Google Scholar] [CrossRef]
- Casanova, S.; Silva, R.V.; de Brito, J.; Pereira, M.F.C. Mortars with alkali-activated municipal solid waste incinerator bottom ash and fine recycled aggregates. J. Clean. Prod. 2021, 289, 125707. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Bravo, M.; Silva, R.V.; Jiménez, J.R.; Fernandez-Rodriguez, J.M.; de Brito, J. Effect of reactive magnesium oxide in alkali-activated fly ash mortars exposed to accelerated CO2 curing. Constr. Build. Mater. 2022, 342, 127999. [Google Scholar] [CrossRef]
- Gumbau, A. What Europe Can Learn from Portugal’s Accelerated Coal Exit. Energy Monitor, 29 March 2022. [Google Scholar]
- Liu, J.; Hu, L.; Tang, L.; Ren, J. Utilisation of municipal solid waste incinerator (MSWI) fly ash with metakaolin for preparation of alkali-activated cementitious material. J. Hazard. Mater. 2021, 402, 123451. [Google Scholar] [CrossRef] [PubMed]
- Kurda, R.; Silva, R.; de Brito, J. Incorporation of alkali-activated municipal solid waste incinerator bottom ash in mortar and concrete: A critical review. Materials 2020, 13, 3428. [Google Scholar] [CrossRef]
- Ren, J.; Hu, L.; Dong, Z.J.; Tang, L.P.; Xing, F.; Liu, J. Effect of silica fume on the mechanical property and hydration characteristic of alkali-activated municipal solid waste incinerator (MSWI) fly ash. J. Clean. Prod. 2021, 295, 126317. [Google Scholar] [CrossRef]
- Ozturk, M.; Bankir, M.B.; Bolukbasi, O.S.; Sevim, U.K. Alkali activation of electric arc furnace slag: Mechanical properties and micro analyzes. J. Build. Eng. 2019, 21, 97–105. [Google Scholar] [CrossRef]
- Traven, K.; Češnovar, M.; Ducman, V. Particle size manipulation as an influential parameter in the development of mechanical properties in electric arc furnace slag-based AAM. Ceram. Int. 2019, 45, 22632–22641. [Google Scholar] [CrossRef]
- Kassim, D.; Lamaa, G.; Silva, R.V.; de Brito, J. Performance enhancement of alkali-activated electric arc furnace slag mortars through an accelerated CO2 curing process. Appl. Sci. 2022, 12, 1662. [Google Scholar] [CrossRef]
- Hafez, H.; Kassim, D.; Kurda, R.; Silva, R.V.; de Brito, J. Assessing the sustainability potential of alkali-activated concrete from electric arc furnace slag using the ECO2 framework. Constr. Build. Mater. 2021, 281, 122559. [Google Scholar] [CrossRef]
- Nedeljković, M.; Zuo, Y.; Arbi, K.; Ye, G. Carbonation resistance of alkali-activated slag under natural and accelerated conditions. J. Sustain. Metall. 2018, 4, 33–49. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Vázquez, T. Carbonation process of alkali-activated slag mortars. J. Mater. Sci. 2006, 41, 3071–3082. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F. Effect of carbonation on alkali-activated slag paste. J. Am. Ceram. Soc. 2006, 89, 3211–3221. [Google Scholar] [CrossRef]
- Bernal, S.A.; Mejia de Gutierrez, R.; Pedraza, A.L.; Provis, J.L.; Rodriguez, E.D.; Delvasto, S. Effect of binder content on the performance of alkali-activated slag concretes. Cem. Concr. Res. 2011, 41, 1–8. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, L.; Yang, Y.; Gao, Y.; Yang, C. Effect of early age-curing methods on drying shrinkage of alkali-activated slag concrete. Materials 2019, 12, 1633. [Google Scholar] [CrossRef]
- Jun, Y.B.; Han, S.H.; Kim, J.H. Early-age strength of CO2 cured alkali-activated blast furnace slag pastes. Constr. Build. Mater. 2021, 288, 123075. [Google Scholar] [CrossRef]
- Jun, Y.; Han, S.H.; Shin, T.Y.; Kim, J.H. Effects of CO2 curing on alkali-activated slag paste cured in different curing conditions. Materials 2019, 12, 3513. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Resistance of alkali-activated slag concrete to carbonation. Cem. Concr. Res. 2001, 31, 1277–1283. [Google Scholar] [CrossRef]
- Carvalho, R.; Silva, R.V.; de Brito, J.; Pereira, M.F.C. Alkali activation of bottom ash from municipal solid waste incineration: Optimization of NaOH- and Na2SiO3-based activators. J. Clean. Prod. 2021, 291, 125930. [Google Scholar] [CrossRef]
- Morone, M.; Cizer, O.; Costa, G.; Baciocchi, R. Effects of alkali activation and co2 curing on the hydraulic reactivity and carbon storage capacity of bof slag in view of its use in concrete. Waste Biomass Valori. 2020, 11, 3007–3020. [Google Scholar] [CrossRef]
- Bernal, S.A.; de Gutierrez, R.M.; Provis, J.L.; Rose, V. Effect of silicate modulus and metakaolin incorporation on the carbonation of alkali silicate-activated slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]
- Shi, Z.G.; Shi, C.J.; Wan, S.; Li, N.; Zhang, Z.H. Effect of of alkali dosage and silicate modulus on carbonation of alkali-activated slag mortars. Cem. Concr. Res. 2018, 113, 55–64. [Google Scholar] [CrossRef]
- Al Bakri Abdullah, M.M.; Kamarudin, H.; Abdulkareem, O.A.K.A.; Ghazali, C.M.R.; Rafiza, A.R.; Norazian, M.N. Optimization of alkaline activator/fly ash ratio on the compressive strength of manufacturing fly ash-based geopolymer. Appl. Mech. Mater. 2011, 110–116, 734–739. [Google Scholar] [CrossRef]
- EN 197-1; Cement. Composition, Specifications and Conformity Criteria for Common Cements. European Committee for Standardization: Brussels, Belgium, 2000; p. 50.
- EN-12620:2002+A1:2008; Aggregates for Concrete. European Committee for Standardization: Brussels, Belgium, 2008; p. 56.
- European Union. Diretiva 98/83/CE do Conselho, de 3 de novembro de 1998, relativa à qualidade da água destinada ao consumo humano. Off. J. Eur. Union 1998, 330, 32–54. [Google Scholar]
- Bernal, S.A.; Provis, J.L. Durability of Alkali- activated materials: Progress and perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- EN-196-1; Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2005; p. 36.
- JCPDS. Joint Committee on International Centre for Diffraction Data. Powder Diffraction File, PDF-4/Organics 2003; International Centre for Diffraction Data: Newtown Square, PA, USA, 2003. [Google Scholar]
- EN-1015-3; Methods of Test for Mortar for Masonry—Part 3: Determination of Consistence of Fresh Mortar (by Flow Table). European Committee for Standardization: Brussels, Belgium, 1999; p. 10.
- EN-1015-11; Methods of Test for Mortar for Masonry—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar. European Committee for Standardization: Brussels, Belgium, 1999; p. 12.
- Ozturk, M.; Akgol, O.; Sevim, U.K.; Karaaslan, M.; Demirci, M.; Unal, E. Experimental work on mechanical, electromagnetic and microwave shielding effectiveness properties of mortar containing electric arc furnace slag. Constr. Build. Mater. 2018, 165, 58–63. [Google Scholar] [CrossRef]
- ASTM-C618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2015; p. 5.
- Zhang, Y.; Xiao, R.; Jiang, X.; Li, W.; Zhu, X.; Huang, B. Effect of particle size and curing temperature on mechanical and microstructural properties of waste glass-slag-based and waste glass-fly ash-based geopolymers. J. Clean. Prod. 2020, 273, 122970. [Google Scholar] [CrossRef]
- Islam, G.M.S.; Rahman, M.H.; Kazi, N. Waste glass powder as partial replacement of cement for sustainable concrete practice. Int. J. Sustain. Built Environ. 2017, 6, 37–44. [Google Scholar] [CrossRef]
- Khmiri, A.; Chaabouni, M.; Samet, B. Chemical behaviour of ground waste glass when used as partial cement replacement in mortars. Constr. Build. Mater. 2013, 44, 74–80. [Google Scholar] [CrossRef]
- Rashad, A.M.; Zeedan, S.R. The effect of activator concentration on the residual strength of alkali-activated fly ash pastes subjected to thermal load. Constr. Build. Mater. 2011, 25, 3098–3107. [Google Scholar] [CrossRef]
- Cho, Y.-K.; Yoo, S.-W.; Jung, S.-H.; Lee, K.-M.; Kwon, S.-J. Effect of Na2O content, SiO2/Na2O molar ratio, and curing conditions on the compressive strength of FA-based geopolymer. Constr. Build. Mater. 2017, 145, 253–260. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Gopala Krishna, G.V.T.; Saraswathy, V.; Srinivasan, K. Experimental investigation on concrete partially replaced with waste glass powder and waste E-plastic. Constr. Build. Mater. 2021, 278, 122400. [Google Scholar] [CrossRef]
- Kim, J.; Yi, C.; Zi, G. Waste glass sludge as a partial cement replacement in mortar. Constr. Build. Mater. 2015, 75, 242–246. [Google Scholar] [CrossRef]
- Jiang, X.; Xiao, R.; Bai, Y.; Huang, B.; Ma, Y. Influence of waste glass powder as a supplementary cementitious material (SCM) on physical and mechanical properties of cement paste under high temperatures. J. Clean. Prod. 2022, 340, 130778. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Silva, R.V.; Bravo, M.; Jiménez, J.R.; Fernández-Rodríguez, J.M.; de Brito, J. Effect of incorporating municipal solid waste incinerated bottom ash in alkali-activated fly ash concrete subjected to accelerated CO2 curing. J. Clean. Prod. 2022, 370, 133533. [Google Scholar] [CrossRef]
- Fanijo, E.O.; Kassem, E.; Ibrahim, A. ASR mitigation using binary and ternary blends with waste glass powder. Constr. Build. Mater. 2021, 280, 122425. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Jimenez, J.R.; Fernandez-Rodriguez, J.M. Use of carbonated water as kneading in mortars made with recycled aggregates. Materials 2022, 15, 4876. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Leite-Costa, J. Use of bottom ash from olive pomace combustion in the production of eco-friendly fired clay bricks. Waste Manag 2016, 48, 323–333. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Fernández-Rodríguez, J.M.; Jiménez, J.R. Use of carbonated water to improve the mechanical properties and reduce the carbon footprint of cement-based materials with recycled aggregates. J. CO2 Util. 2022, 57, 101886. [Google Scholar] [CrossRef]
- Hidalgo López, A.; García Calvo, J.L.; García Olmo, J.; Petit, S.; Alonso, M.C. Microstructural evolution of calcium aluminate cements hydration with silica fume and fly ash additions by scanning electron microscopy, and mid and near-infrared spectroscopy. J. Am. Ceram. Soc. 2008, 91, 1258–1265. [Google Scholar] [CrossRef]
- Lucia, F.-C.; Torrens-Martín, D.; Morales, L.M.; Sagrario, M.-R. Infrared spectroscopy in the analysis of building and construction materials. In Infrared Spectroscopy; Theophile, T., Ed.; IntechOpen: Rijeka, Croatia, 2012; p. 19. [Google Scholar]
- Garcia Calvo, J.L.; Sanchez Moreno, M.; Alonso Alonso, M.C.; Hidalgo Lopez, A.; Garcia Olmo, J. Study of the microstructure evolution of low-ph cements based on ordinary portland cement (opc) by mid- and near-infrared spectroscopy, and their influence on corrosion of steel reinforcement. Materials 2013, 6, 2508–2521. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratio. Microporous Mesoporous Mater. 2007, 106, 180–191. [Google Scholar] [CrossRef]
- Aziz, I.H.; Zulkifly, K.; Sakkas, K.; Panias, D.; Tsaousi, G.M.; Bakri, M.M.A.A.; Yong, H.C. The characterization of steel slag by alkali activation. OALib 2017, 4, 1–13. [Google Scholar] [CrossRef]
- García Lodeiro, I.; Macphee, D.E.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalis on fresh C–S–H gels. FTIR analysis. Cem. Concr. Res. 2009, 39, 147–153. [Google Scholar] [CrossRef]
- Jumrat, S.; Chatveera, B.; Rattanadecho, P. Dielectric properties and temperature profile of fly ash-based geopolymer mortar. Int. Commun. Heat Mass Transf. 2011, 38, 242–248. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, Y.; Wang, K.; Zhang, J. A review on properties of fresh and hardened geopolymer mortar. Compos. Part B Eng. 2018, 152, 79–95. [Google Scholar] [CrossRef]
- Bhowmick, A.; Ghosh, S. Effect of synthesizing parameters on workability and compressive strength of fly ash based geopolymer mortar. Int. J. Civ. Struct. Eng 2012, 3, 168–177. [Google Scholar]
- Li, X.; Ma, X.; Zhang, S.; Zheng, E. Mechanical properties and microstructure of class C fly ash-based geopolymer paste and mortar. Materials 2013, 6, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Musaddiq Laskar, S.; Talukdar, S. Development of ultrafine slag-based geopolymer mortar for use as repairing mortar. J. Mater. Civ. Eng. 2017, 29, 04016292. [Google Scholar] [CrossRef]
- Al-Majidi, M.H.; Lampropoulos, A.; Cundy, A.; Meikle, S. Development of geopolymer mortar under ambient temperature for in situ applications. Constr. Build. Mater. 2016, 120, 198–211. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L.; Pratt, P.L. Factors affecting the strength of alkali-activated slag. Cem. Concr. Res. 1994, 24, 1033–1043. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A. High strength geopolymer binder based on waste-glass powder. Adv. Powder Technol. 2017, 28, 215–222. [Google Scholar] [CrossRef]
- Allahverdi, A.; Najafi Kani, E.; Hossain, K.M.A.; Lachemi, M. Methods to control efflorescence in alkali-activated cement-based materials. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Pacheco-Torgal, F., Labrincha, J.A., Leonelli, C., Palomo, A., Chindaprasirt, P., Eds.; Woodhead Publishing: Oxford, UK, 2015; Chapter 17; pp. 463–483. [Google Scholar]
- Avila, Y.; Silva, R.V.; de Brito, J. Alkali-activated materials with pre-treated municipal solid waste incinerator bottom ash. Appl. Sci. 2022, 12, 3535. [Google Scholar] [CrossRef]
- Lee, N.K.; Koh, K.T.; Kim, M.O.; An, G.H.; Ryu, G.S. Physicochemical changes caused by reactive MgO in alkali-activated fly ash/slag blends under accelerated carbonation. Ceram. Int. 2017, 43, 12490–12496. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Z.; Wang, J.; Guo, J.; Hu, S.; Ling, Y. Properties of fresh and hardened fly ash/slag based geopolymer concrete: A review. J. Clean. Prod. 2020, 270, 122389. [Google Scholar] [CrossRef]
- Silva, R.V.; de Brito, J.; Dhir, R.K. Performance of cementitious renderings and masonry mortars containing recycled aggregates from construction and demolition wastes. Constr. Build. Mater. 2016, 105, 400–415. [Google Scholar] [CrossRef]
- Corinaldesi, V. Mechanical behavior of masonry assemblages manufactured with recycled-aggregate mortars. Cem. Concr. Compos. 2009, 31, 505–510. [Google Scholar] [CrossRef]
- Corinaldesi, V.; Moriconi, G. Behaviour of cementitious mortars containing different kinds of recycled aggregate. Constr. Build. Mater. 2009, 23, 289–294. [Google Scholar] [CrossRef]
- Vegas, I.; Azkarate, I.; Juarrero, A.; Frias, M. Design and performance of masonry mortars made with recycled concrete aggregates. Mater. Construcción 2009, 59, 5–18. [Google Scholar] [CrossRef]
- Jiménez, J.R.; Ayuso, J.; Lopez, M.; Fernandez, J.M.; de Brito, J. Use of fine recycled aggregates from ceramic waste in masonry mortar manufacturing. Constr. Build. Mater. 2013, 40, 679–690. [Google Scholar] [CrossRef]
- Ledesma, E.F.; Jiménez, J.R.; Ayuso, J.; Fernandez, J.M.; de Brito, J. Maximum feasible use of recycled sand from construction and demolition waste for eco-mortar production—Part-I: Ceramic masonry waste. J. Clean. Prod. 2015, 87, 692–706. [Google Scholar] [CrossRef]
- Corinaldesi, V. Environmentally-friendly bedding mortars for repair of historical buildings. Constr. Build. Mater. 2012, 35, 778–784. [Google Scholar] [CrossRef]
- Cuenca-Moyano, G.M.; Martin-Morales, M.; Valverde-Palacios, I.; Valverde-Espinosa, I.; Zamorano, M. Influence of pre-soaked recycled fine aggregate on the properties of masonry mortar. Constr. Build. Mater. 2014, 70, 71–79. [Google Scholar] [CrossRef]





| Na2O/Binder (%) | 8 | 10 | 12 | 14 | |
|---|---|---|---|---|---|
| SiO2/Na2O | |||||
| 0 | N8S0 | N10S0 | N12S0 | N14S0 | |
| 0.5 | N8S0.5 | N10S0.5 | N12S0.5 | N14S0.5 | |
| 1.0 | N8S1.0 | N10S1.0 | N12S1.0 | N14S1.0 | |
| 1.5 | N8S1.5 | N10S1.5 | N12S1.5 | N14S1.5 | |
| Type of Binder | Mix Code | Binder | WRA | Water | Fine Sand | Coarse Sand | Sand Gravel | NaOH | Na2SiO3 Solution | Borax |
|---|---|---|---|---|---|---|---|---|---|---|
| OPC | - | 350 | 5.3 | 136.1 | 300.3 | 581.3 | 1035.3 | - | - | - |
| AAMs | N8S0 | 350 | 5.3 | 135.2 | 300.3 | 581.3 | 1035.3 | 36.1 | 0.0 | 14 |
| N8S0.5 | 350 | 5.3 | 100.6 | 300.3 | 581.3 | 1035.3 | 30.4 | 53.0 | 14 | |
| N8S1.0 | 350 | 5.3 | 66.0 | 300.3 | 581.3 | 1035.3 | 24.6 | 106.1 | 14 | |
| N8S1.5 | 350 | 5.3 | 31.4 | 300.3 | 581.3 | 1035.3 | 18.9 | 159.1 | 14 | |
| N10S0 | 350 | 5.3 | 135.1 | 300.3 | 581.3 | 1035.3 | 45.5 | 0.0 | 14 | |
| N10S0.5 | 350 | 5.3 | 91.9 | 300.3 | 581.3 | 1035.3 | 38.0 | 66.3 | 14 | |
| N10S1.0 | 350 | 5.3 | 48.6 | 300.3 | 581.3 | 1035.3 | 30.8 | 132.6 | 14 | |
| N10S1.5 | 350 | 5.3 | 5.4 | 300.3 | 581.3 | 1035.3 | 23.6 | 198.9 | 14 | |
| N12S0 | 350 | 5.3 | 135.0 | 300.3 | 581.3 | 1035.3 | 54.5 | 0.0 | 14 | |
| N12S0.5 | 350 | 5.3 | 83.1 | 300.3 | 581.3 | 1035.3 | 45.6 | 79.5 | 14 | |
| N12S1.0 | 350 | 5.3 | 31.2 | 300.3 | 581.3 | 1035.3 | 37.0 | 159.1 | 14 | |
| N14S0 | 350 | 5.3 | 135.0 | 300.3 | 581.3 | 1035.3 | 63.6 | 0.0 | 14 | |
| N14S0.5 | 350 | 5.3 | 74.4 | 300.3 | 581.3 | 1035.3 | 53.2 | 92.8 | 14 | |
| N14S1.0 | 350 | 5.3 | 13.8 | 300.3 | 581.3 | 1035.3 | 43.1 | 185.6 | 14 |
| Binders | Stage 1 | Stage 2 | Stage 3 |
|---|---|---|---|
| 24 h | 21 Days | 7 Days | |
| OPC | Spray with water | Dry chamber (23 ± 2 °C and 65% RH). Sprayed with water twice a day for the first 2 days | Carbonation chamber (23 ± 2 °C, 65% RH, and 5% CO2) |
| FA, MIBA, EAFS, and WGR | Thermal curing (70 °C) | Dry chamber (23 ± 2 °C and 65% RH) | Carbonation chamber (23 ± 2 °C, 65% RH, and 5% CO2) |
| Materials | OPC (%) | FA (%) | MIBA (%) | EAFS (%) | WGR (%) |
|---|---|---|---|---|---|
| Al2O3 | 5.42 | 25.5 | 8.82 | 10.2 | 1.01 |
| CaO | 64.8 | 2.27 | 18.3 | 28.2 | 8.74 |
| Fe2O3 | 2.92 | 6.90 | 6.68 | 28.5 | 0.67 |
| K2O | 0.74 | 2.74 | 1.59 | 0.03 | 0.40 |
| MgO | 2.12 | 1.83 | 4.01 | 5.67 | 3.55 |
| Na2O | 0.14 | 1.29 | 6.53 | 0.18 | 11.8 |
| SiO2 | 18.1 | 56.3 | 48.8 | 17.7 | 71.4 |
| SO3 | 4.81 | 0.80 | 1.36 | 0.33 | 0.30 |
| Cl- | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cr2O3 | 0.51 | 0.48 | 0.06 | 2.38 | 0.03 |
| TiO2 | 0.34 | 1.14 | 0.48 | 0.65 | 0.05 |
| ZnO | - | - | - | - | - |
| P2O5 | 0.03 | 0.44 | 2.51 | 0.42 | - |
| V2O5 | 0.02 | 0.05 | - | 0.11 | - |
| CuO | - | - | - | - | 0.02 |
| MnO2 | - | - | 0.12 | 5.45 | - |
| Combination | FA | MIBA | EAFS | WGR |
|---|---|---|---|---|
| (mm) | (mm) | (mm) | (mm) | |
| N8S0 | 125.4 | – | – | 104.7 |
| N8S0.5 | 101.4 | – | 103.2 | 102.5 |
| N8S1.0 | 131.8 | 103.7 | 107.8 | 103.2 |
| N8S1.5 | 128.8 | 103.2 | 104.7 | 104.6 |
| N10S0 | 122.4 | 104.1 | – | 105.6 |
| N10S0.5 | – | – | – | – |
| N10S1.0 | 135.8 | 106.1 | 102.6 | 105.7 |
| N10S1.5 | 122.5 | 103.0 | 102.7 | 102.1 |
| N12S0 | 128.5 | 105.8 | 104.7 | 127.4 |
| N12S0.5 | – | – | – | – |
| N12S1.0 | 124.0 | 104.2 | 103.1 | 102.2 |
| N14S0 | 131.3 | 102.3 | 103.8 | 150.9 |
| N14S0.5 | – | – | – | – |
| N14S1.0 | 126.5 | 103.3 | 103.4 | 103.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamaa, G.; Suescum-Morales, D.; Duarte, A.P.C.; Silva, R.V.; de Brito, J. Optimising the Performance of CO2-Cured Alkali-Activated Aluminosilicate Industrial By-Products as Precursors. Materials 2023, 16, 1923. https://doi.org/10.3390/ma16051923
Lamaa G, Suescum-Morales D, Duarte APC, Silva RV, de Brito J. Optimising the Performance of CO2-Cured Alkali-Activated Aluminosilicate Industrial By-Products as Precursors. Materials. 2023; 16(5):1923. https://doi.org/10.3390/ma16051923
Chicago/Turabian StyleLamaa, Ghandy, David Suescum-Morales, António P. C. Duarte, Rui Vasco Silva, and Jorge de Brito. 2023. "Optimising the Performance of CO2-Cured Alkali-Activated Aluminosilicate Industrial By-Products as Precursors" Materials 16, no. 5: 1923. https://doi.org/10.3390/ma16051923
APA StyleLamaa, G., Suescum-Morales, D., Duarte, A. P. C., Silva, R. V., & de Brito, J. (2023). Optimising the Performance of CO2-Cured Alkali-Activated Aluminosilicate Industrial By-Products as Precursors. Materials, 16(5), 1923. https://doi.org/10.3390/ma16051923











