A Newly Designed High-Strength Tool Steel with High Wear and Corrosion Resistance
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
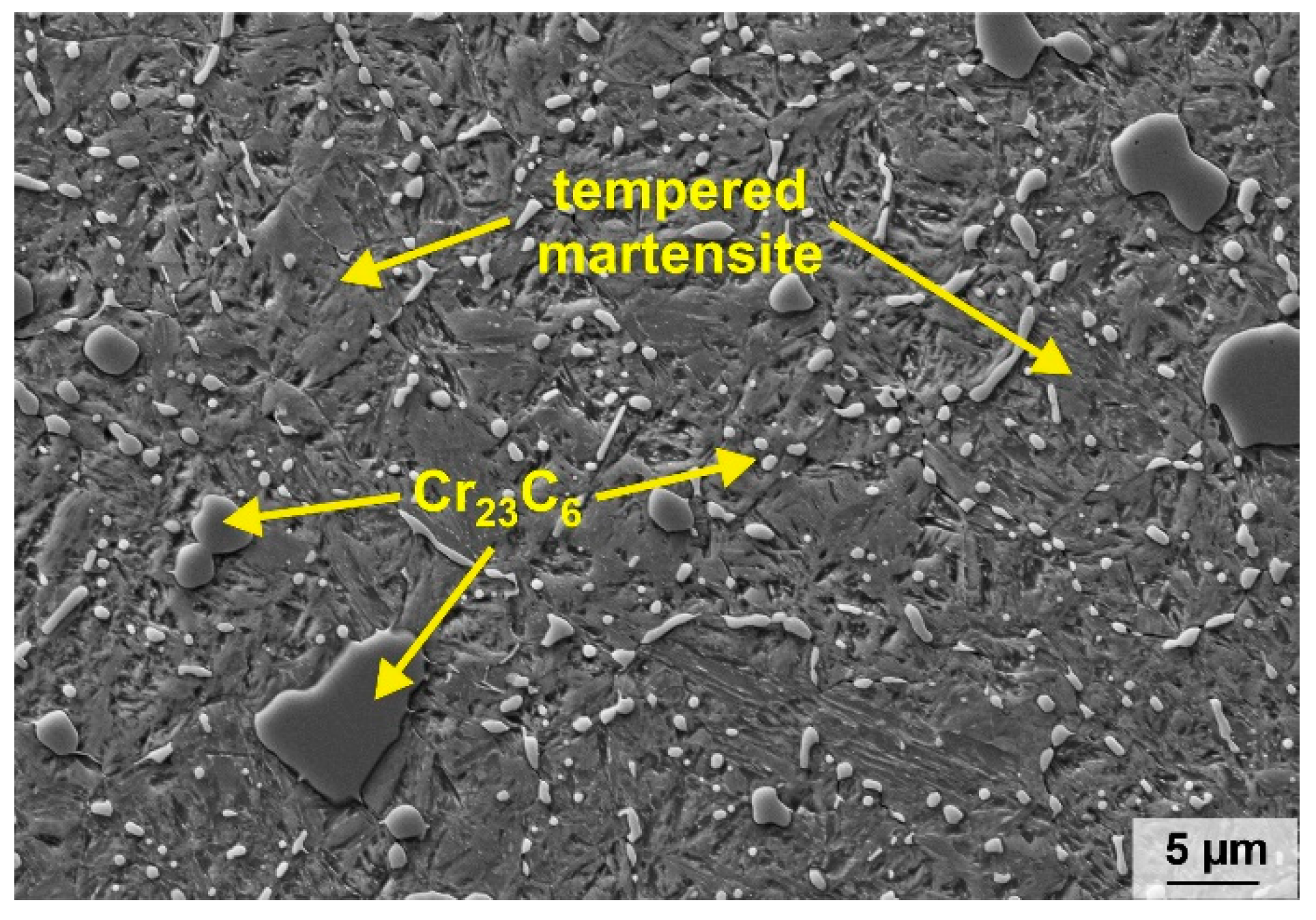
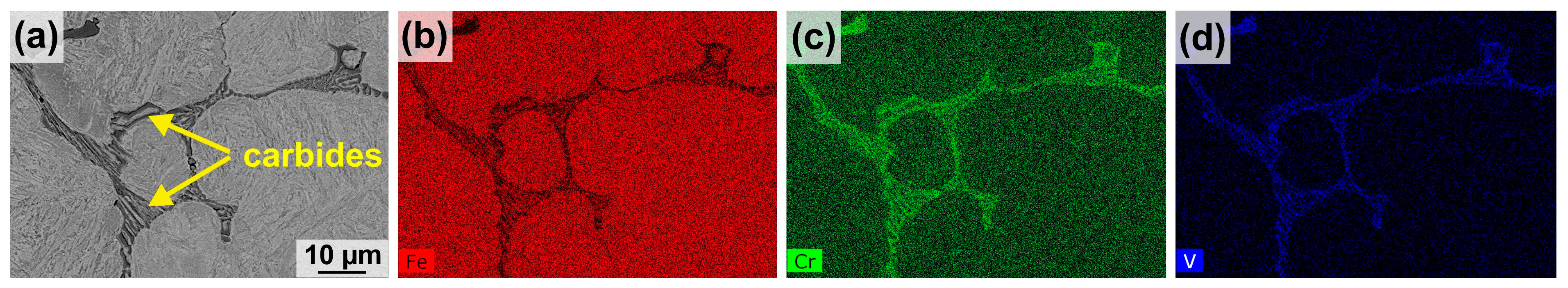
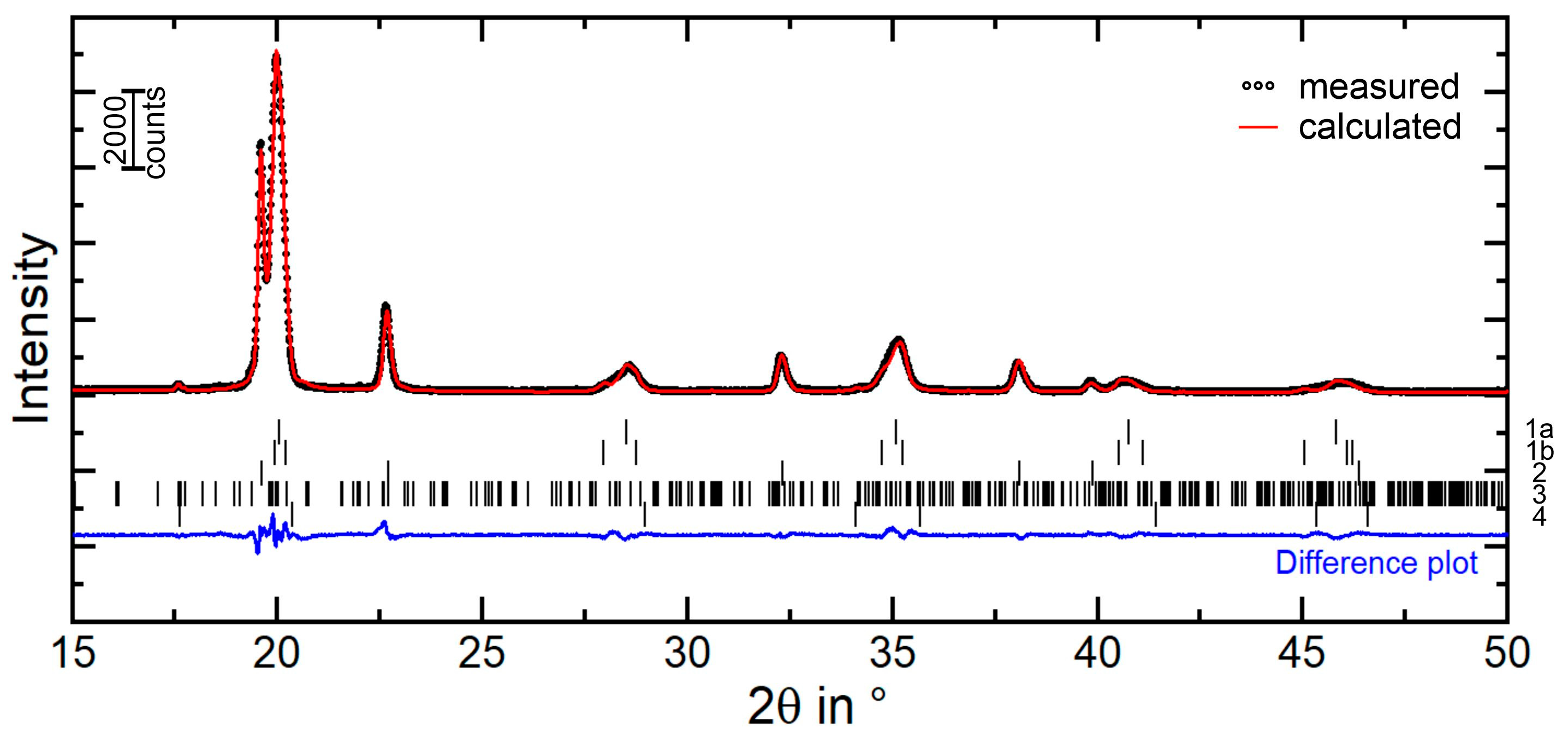
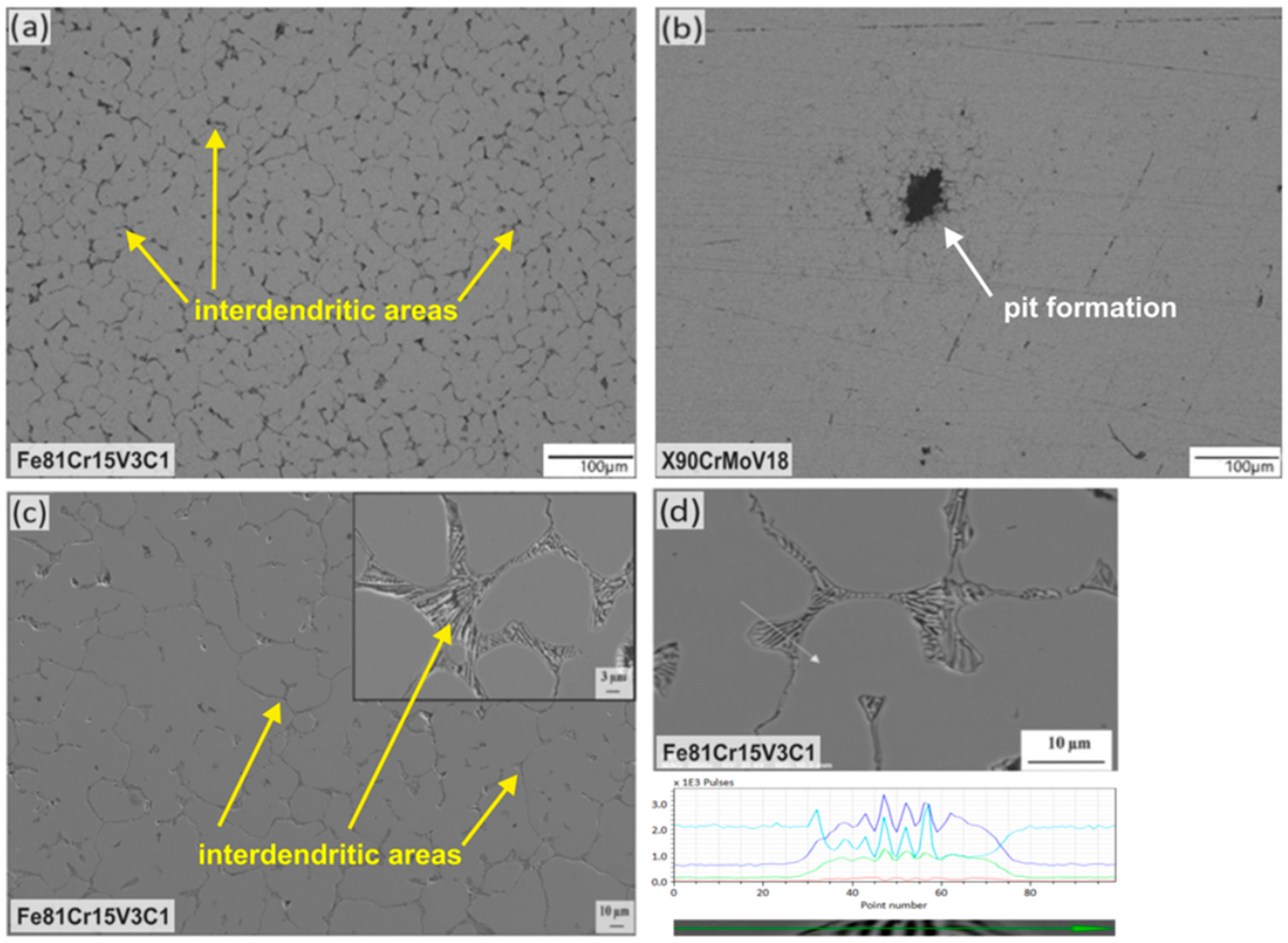
3.1. Microstructural Characterization
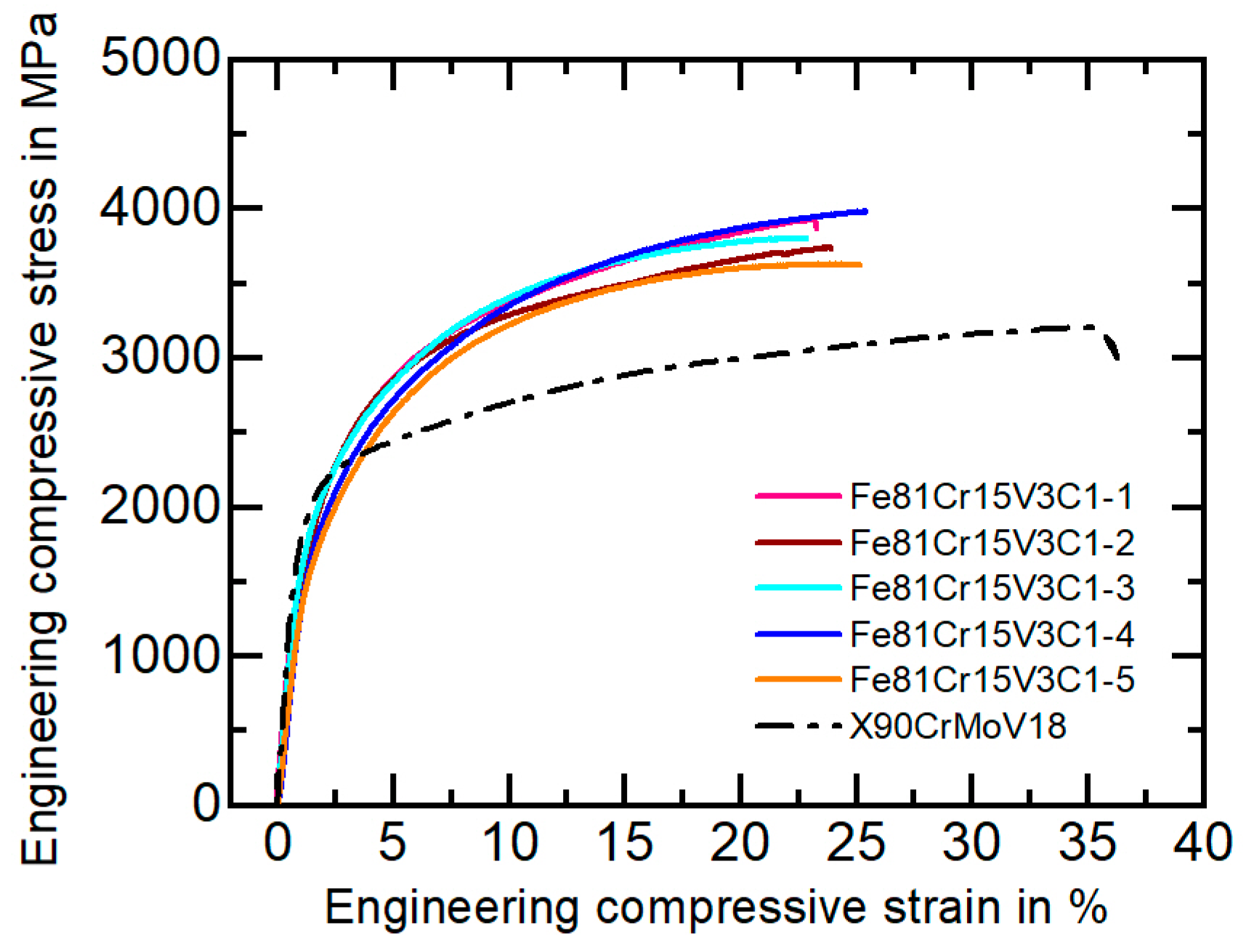
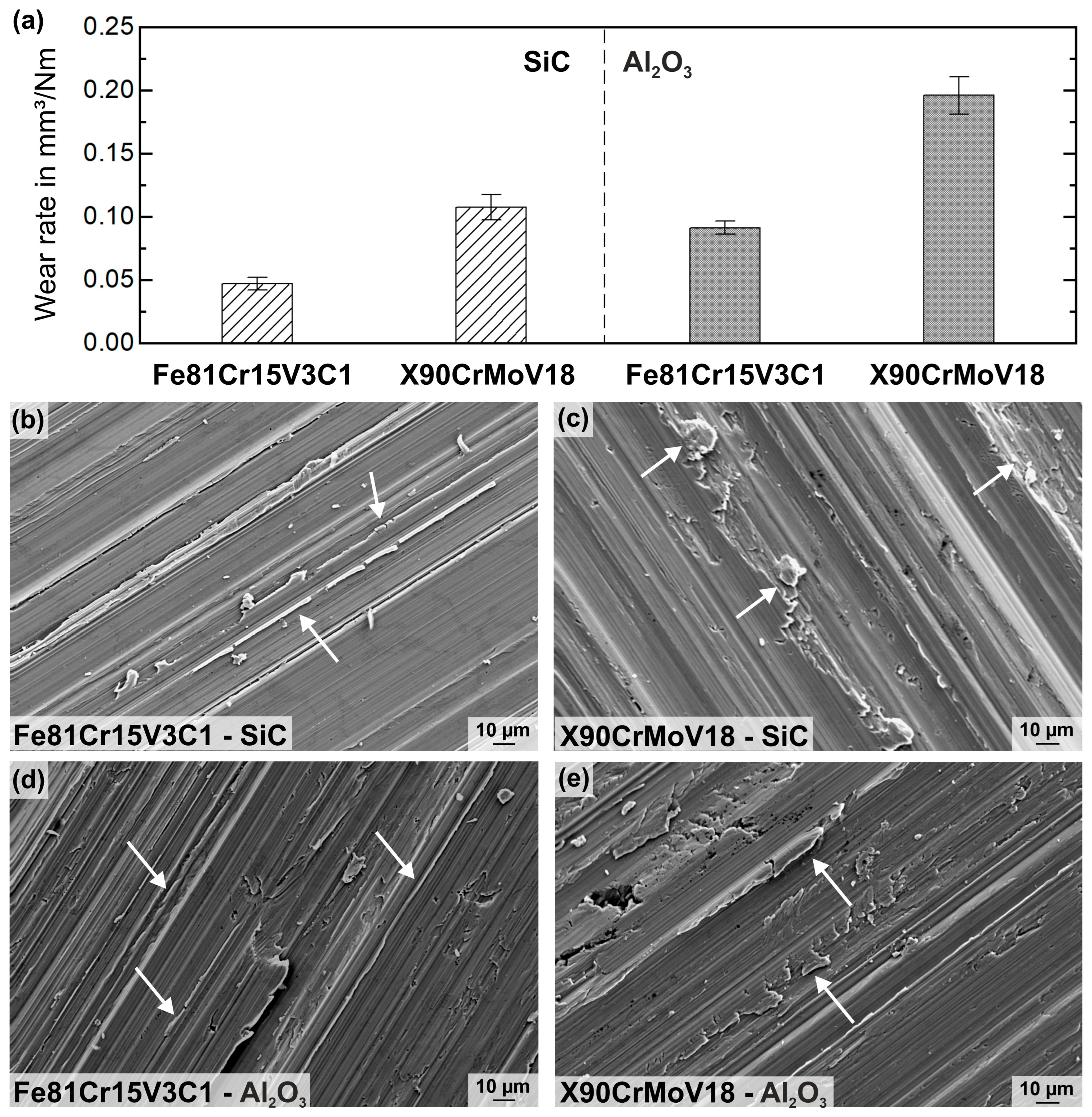
3.2. Mechanical Characterization and Wear Behavior
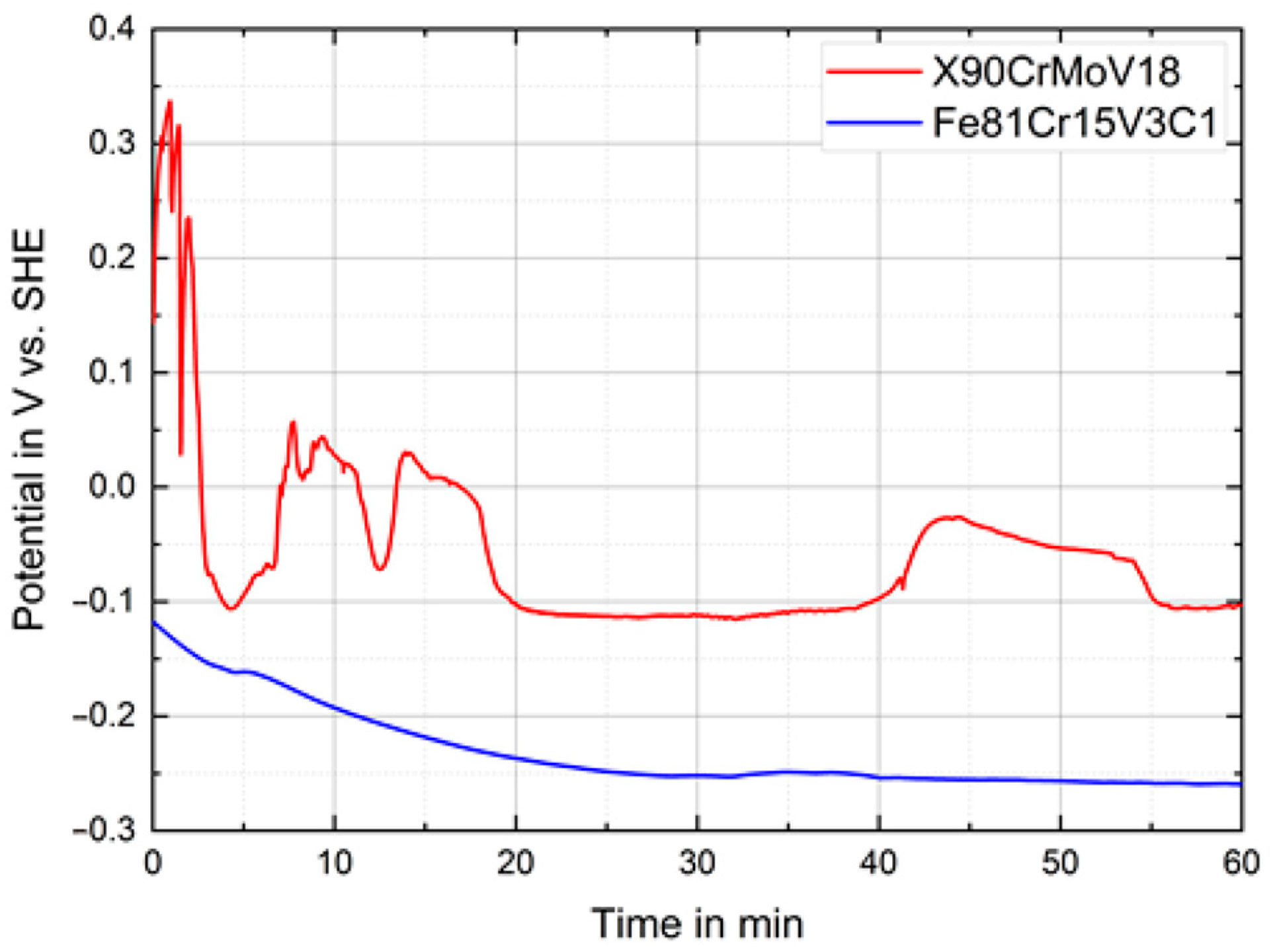
3.3. Corrosion Behavior
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, J.R. ASM Specialty Handbook: Tool Materials; ASM International: Almere, The Netherlands, 1995; ISBN 978-0- 87170-545-7. [Google Scholar]
- Berns, H.; Theisen, W. Eisenwerkstoffe—Stahl und Gusseisen; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Mesquita, R.A. Tool Steels: Properties and Performance; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9780367782573. [Google Scholar]
- Huth, S.; Krasokha, N.; Theisen, W. Development of wear and corrosion resistant cold-work tool steels produced by diffusion alloying. Wear 2009, 267, 449–457. [Google Scholar] [CrossRef]
- Mc Collum, J.M.; Serrano Delgado, I. Manufacturing strategies in fluorinated polymers and composites. In Opportunities for Fluoropolymers—Synthesis, Characterization, Processing, Simulation and Recycling; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-821966-9. [Google Scholar]
- Blutmager, A.; Varga, M.; Schmidt, T.; Pock, A.; Friesenbichler, W. Abrasive/Erosive Wear on MMCs in Plastic Molds as a Function of Volumetric Flow Rates and Glass Fiber Distribution. Polym. Eng. Sci. 2019, 59, 302–311. [Google Scholar] [CrossRef] [Green Version]
- Forke, E.; Niederhofer, P.; Albrecht, M.; Hüllmann, A.; Kräusel, V.; Schneiders, T.; Gehde, M. Profile Cross Rolling of High-Interstitial Austenitic Stainless Steels for Application in Plastics Extrusion. Steel Res. Int. 2020, 91, 1900417. [Google Scholar] [CrossRef] [Green Version]
- Hill, H.; Huth, S.; Weber, S.; Theisen, W. Corrosion properties of a plastic mould steel with special focus on the processing route. Mater. Corr. 2011, 62, 436–443. [Google Scholar] [CrossRef]
- Davis, J.R. ASM Specialty Handbook: Stainless Steels; ASM International: Almere, The Netherlands, 1994; ISBN 978-0-87170-503-7. [Google Scholar]
- Wendl, F.; Wupper, K.-D. Wear resistance of different plastic mould steels. J. Mater. Process. Tech. 1990, 24, 355–364. [Google Scholar] [CrossRef]
- Kaesche, H. Corrosion of Metals; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-540-00626-8. [Google Scholar]
- Zum Gahr, K.-H. Microstructure and Wear of Materials; Elsevier Science Publishers: Amsterdam, The Netherlands, 1987; ISBN 978-0-08-087574-3. [Google Scholar]
- Seifert, M.; Wieskämper, D.; Tonfeld, T.; Huth, S. Corrosion properties of a complex multi-phase martensitic stainless steel depending on the tempering temperature. Mater. Corros. 2015, 66, 1290–1298. [Google Scholar] [CrossRef]
- Capello, E.; Colombo, D.; Previtali, B. Repairing of sintered tools using laser cladding by wire. J. Mater. Process. Technol. 2005, 164–165, 990–1000. [Google Scholar] [CrossRef]
- Hufenbach, J.; Kohlar, S.; Kühn, U.; Giebeler, L.; Eckert, J. Microstructural and mechanical characterization of an ultra-high-strength Fe86.7Cr4.4Mo0.6V1.1W2.5C4.7 alloy. J. Mater. Sci. 2012, 47, 267–271. [Google Scholar] [CrossRef]
- Hufenbach, J.; Kunze, K.; Giebeler, L.; Gemming, T.; Wendrock, H.; Baldauf, C.; Kühn, U.; Hufenbach, W.; Eckert, J. The effect of boron on microstructure and mechanical properties of high-strength cast FeCrVC. Mater. Sci. Eng. A 2013, 586, 267–275. [Google Scholar] [CrossRef]
- Zeisig, J.; Schädlich, N.; Hufenbach, J.; Wendrock, H.; Kimme, J.; Kühn, U. Effect of cooling rate on precipitation behaviour and transformation characteristics of a novel FeCrVBC cast alloy. J. Alloys Compd. 2020, 816, 152544. [Google Scholar] [CrossRef]
- Zeisig, J.; Giebeler, L.; Gebert, A.; Oswald, S.; Kühn, U.; Hufenbach, J. Novel Corrosion-Resistant Tool Steels with Superior Wear Properties. Adv. Eng. Mater. 2022, 24, 2200293. [Google Scholar] [CrossRef]
- Laurent, B.; Gruet, N.; Gwinner, B.; Miserque, F.; Rousseau, K.; Ogle, K. Dissolution and passivation of a silicon-rich austenitic stainless steel during active-passive cycles in sulfuric and nitric acid. J. Electrochem. Soc. 2017, 164, 892–900. [Google Scholar] [CrossRef]
- Tao, X.; Gu, J.; Han, L. Characterization of precipitates in X12CrMoWVNbN10-1-1 steel during heat treatment. J. Nucl. Mater. 2014, 452, 557–564. [Google Scholar] [CrossRef]
- Li, H.; Dong, C.; Xiao, K.; Li, X.; Zhong, P. Effects of chloride ion concentration and pH values on the corrosion behavior of Cr12Ni3Co12Mo4W ultra-high-strength martensitic stainless steel. Int. J. Miner. Metall. Mater. 2016, 23, 1286–1293. [Google Scholar] [CrossRef]
- Kietov, V.; Mandel, M.; Krüger, L. Combination of Electrochemical Noise and Acoustic Emission for Analysis of the Pitting Corrosion Behavior of an Austenitic Stainless Cast Steel. Adv. Eng. Mater. 2019, 21, 1800682. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Yao, K.; Chen, Y.; Wang, M.; Liu, X.; Ge, X. The effect of tempering temperature on the microstructure and electrochemical properties of a 13 wt.% Cr-type martensitic stainless Steel. Electrochim. Acta 2015, 165, 45–55. [Google Scholar] [CrossRef]
- Yan, F.; Tao, N.; Pan, C.; Liu, L. Microstructures and Corrosion Behaviors of an Austenitic Stainless Steel Strengthened by Nanotwinned Austenitic Grains. Adv. Eng. Mater. 2016, 18, 650–656. [Google Scholar] [CrossRef]
- Vignal, V.; Ringeval, S.; Thiébaut, S.; Tabalaiev, K.; Dessolin, C.; Heintz, O.; Herbst, F.; Chassagnon, R. Influence of the microstructure on the corrosion behaviour of low-carbon martensitic stainless steel after tempering treatment. Corros. Sci. 2014, 85, 42–51. [Google Scholar] [CrossRef]
- Ahn, S.-Y.; Kang, N. The Effects of δ-ferrite on Weldment of 9–12% Cr Steels. J. Weld. Join. 2013, 31, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, L. Influence of delta ferrite and dendritic carbides on the impact and tensile properties of a martensitic chromium steel. J. Nucl. Mater. 1998, 258–263, 1336–1339. [Google Scholar] [CrossRef]
- Bleckmann, M.; Gleinig, J.; Hufenbach, J.; Wendrock, H.; Giebeler, L.; Zeisig, J.; Diekmann, U.; Eckert, J.; Kühn, U. Effect of cooling rate on the microstructure and properties of FeCrVC. J. Alloys Compd. 2015, 634, 200–207. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríguez-Carvajal, J. WinPLOTR: A Windows Tool for Powder Diffraction Pattern Analysis. Mater. Sci. Forum 2001, 378, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Filipović, M.; Kamberovic, Z.; Korac, M. Solidification of High Chromium White Cast Iron Alloyed with Vanadium. Mater. Trans. 2011, 52, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Filipović, M.; Romhanji, E.; Kamberovic, Z. Chemical Composition and Morphology of M7C3 Eutectic Carbide in High Chromium White Cast Iron Alloyed with Vanadium. ISIJ Int. 2012, 52, 2200–2204. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Liu, L.; Li, Q.; Sun, Y.L.; Gao, Y.K.; Ren, X.J.; Yang, Q.X. Characterization on carbide of a novel steel for cold work roll during solidification process. Mater. Charact. 2013, 79, 100–109. [Google Scholar] [CrossRef]
- DeMello, J.D.B.; Durand-Charre, M. Phase equilibria and solidification sequences of white cast irons containing vanadium and chromium. Mater. Sci. Eng. 1984, 67, 109–117. [Google Scholar] [CrossRef]
- Schaaf, P.; Krämer, A.; Wiesen, S.; Gonser, U. Mössbauer study of iron carbides: Mixed carbides M7C3 and M23C6. Acta Metall. Mater. 1994, 42, 3077–3081. [Google Scholar] [CrossRef]
- Fruchart, M.R.; Rouault, M.A. Sur l’existence de macles dans les carbures orthorhombiques isomorphes Cr7C3, Mn7C3, Fe7C3. Ann. Chim. Fr. 1969, 4, 143–145. [Google Scholar]
- Rouault, M.A.; Herpin, P.; Fruchart, M.R. Crystallographic Study of Carbides Cr7C3 and Mn7C3. Ann. Chim. Fr. 1970, 5, 461–470. [Google Scholar]
- Vegard, L. Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Z. Phys. 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Colaço, R.; Vilar, R. On the influence of retained austenite in the abrasive wear behaviour of a laser surface melted tool steel. Wear 2005, 258, 225–231. [Google Scholar] [CrossRef]
- Pleterski, M.; Muhič, T.; Podgornik, B.; Tušek, J. Blanking punch life improvement by laser cladding. Eng. Fail. Anal. 2011, 18, 1527–1537. [Google Scholar] [CrossRef]
- Maier, B.; Frankel, G.S. Pitting Corrosion of Bare Stainless Steel 304 under Chloride Solution Droplets. J. Electrochem. Soc. 2010, 157, 302–312. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.; Zhao, X.; Chen, H.; Huang, B.; Ma, J.; Wang, C.; Yang, Y. Comparative study on corrosion behaviors of ferrite-pearlite steel with dual-phase steel in the simulated bottom plate environment of cargo oil tanks. J. Mater. Res. Technol. 2021, 12, 399–411. [Google Scholar] [CrossRef]
- Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions—A Review. Int. J. Hydrogen Energy 2015, 40, 256–274. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, Y. Inclusions in Stainless Steels–A Review. Steel Res. Int. 2017, 88, 1700130. [Google Scholar] [CrossRef]


| Materials | Fe | Cr | Mo | V | C | |
|---|---|---|---|---|---|---|
| Fe81Cr15V3C1 | nominal analysed | 81 81.92 ± 0.14% | 15 15.13 ± 0.13% | - - | 3 2.94 ± 0.32% | 1 0.92 ± 0.02% |
| Phase | Space Group | a (nm) | b (nm) | c (nm) | V (nm³) | Phase Content (wt%) |
|---|---|---|---|---|---|---|
| Fe81Cr15V3C1 | ||||||
| Austenite | Fm-3m (fcc) | 0.36033(4) | 0.04678(2) | 31 | ||
| Martensite | I4/mm (bct) Im-3m (bcc) | 0.28575(6) 0.28811(2) | 0.28575 | 0.29362(7) | 0.02398(2) 0.023914(6) | 15 51 |
| Cr7C3 | Pnma | 0.4487(3) | 0.7059(7) | 1.228(1) | 0.3889(9) | 2 |
| VC | Fm-3m | 0.4011(2) | 0.7059(7) | 1.228(1) | 0.06452(9) | 1 |
| σy0.2 in MPa | σmax in MPa | εf in % | Rp0.2 in MPa | Rm in MPa | At in % | |
|---|---|---|---|---|---|---|
| Fe81Cr15V3C1 | 1463 ± 64 | 3815 ± 144 | 24 ± 1.1 | 822 ± 13 | 1292 ± 64 | 1.4 ± 0.2 |
| X90CrMoV18 | 1681 ± 22 | 3255 ± 154 | 30 ± 2.9 | 1056 ± 33 | 1912 ± 10 | 4.5 ± 0.9 |
| t in Hours | ba in mV/dec | bc in mV/dec | Rp in kOm∙cm2 | Icorr in µA/cm2 | Ecorr in mV | Epit in mV | Epit − Ecorr in mV | |
|---|---|---|---|---|---|---|---|---|
| Fe81Cr15V3C1 | 1 24 48 72 | 92 ± 2 118 ± 3 124 ± 3 179 ± 5 | 85 ± 1 62 ±0.3 62 ± 0.4 54 ± 0.8 | 13.0 ± 2 11.9 ± 1 5.55 ± 0.3 5.58 ± 0.2 | 1.471 ± 0.016 1.488 ± 0.081 3.241 ± 0.132 3.246 ± 0.136 | −263 ± 7 −244 ± 3 −224 ± 5 −230 ± 3 | 0.092 ± 5 0.268 ± 8 0.375 ± 6 0.379 ± 5 | 0.355 ± 6 0.512 ± 5 0.599 ± 5 0.612 ± 4 |
| X90CrMoV18 | 1 24 48 72 | 81 ± 2.3 112 ± 0.5 113 ± 0.3 117 ± 2 | 70 ± 1 59 ± 2 50 ± 3 41 ± 2 | 14.3 ± 2 9.7 ± 1 7.3 ± 1 4.5 ± 1 | 1.154 ± 0.026 1.812 ± 0.103 2.083 ± 0.152 2.962 ± 0.320 | −86 ± 12 −216 ± 8 −221 ± 5 −218 ± 3 | 0.221 ± 10 0.279 ± 7 0.241 ± 6 0.269 ± 7 | 0.307 ± 11 0.495 ± 7 0.462 ± 5 0.487 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeisig, J.; Shtefan, V.; Giebeler, L.; Kühn, U.; Gebert, A.; Hufenbach, J.K. A Newly Designed High-Strength Tool Steel with High Wear and Corrosion Resistance. Materials 2023, 16, 1941. https://doi.org/10.3390/ma16051941
Zeisig J, Shtefan V, Giebeler L, Kühn U, Gebert A, Hufenbach JK. A Newly Designed High-Strength Tool Steel with High Wear and Corrosion Resistance. Materials. 2023; 16(5):1941. https://doi.org/10.3390/ma16051941
Chicago/Turabian StyleZeisig, Josephine, Viktoriia Shtefan, Lars Giebeler, Uta Kühn, Annett Gebert, and Julia Kristin Hufenbach. 2023. "A Newly Designed High-Strength Tool Steel with High Wear and Corrosion Resistance" Materials 16, no. 5: 1941. https://doi.org/10.3390/ma16051941
APA StyleZeisig, J., Shtefan, V., Giebeler, L., Kühn, U., Gebert, A., & Hufenbach, J. K. (2023). A Newly Designed High-Strength Tool Steel with High Wear and Corrosion Resistance. Materials, 16(5), 1941. https://doi.org/10.3390/ma16051941







