Lychee-like TiO2@Fe2O3 Core-Shell Nanostructures with Improved Lithium Storage Properties as Anode Materials for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Core-Shell Structure TiO2@Fe2O3 Microspheres
2.3. Structure Analysis and Characterization
2.4. Electrochemical Tests
2.5. Computational Details
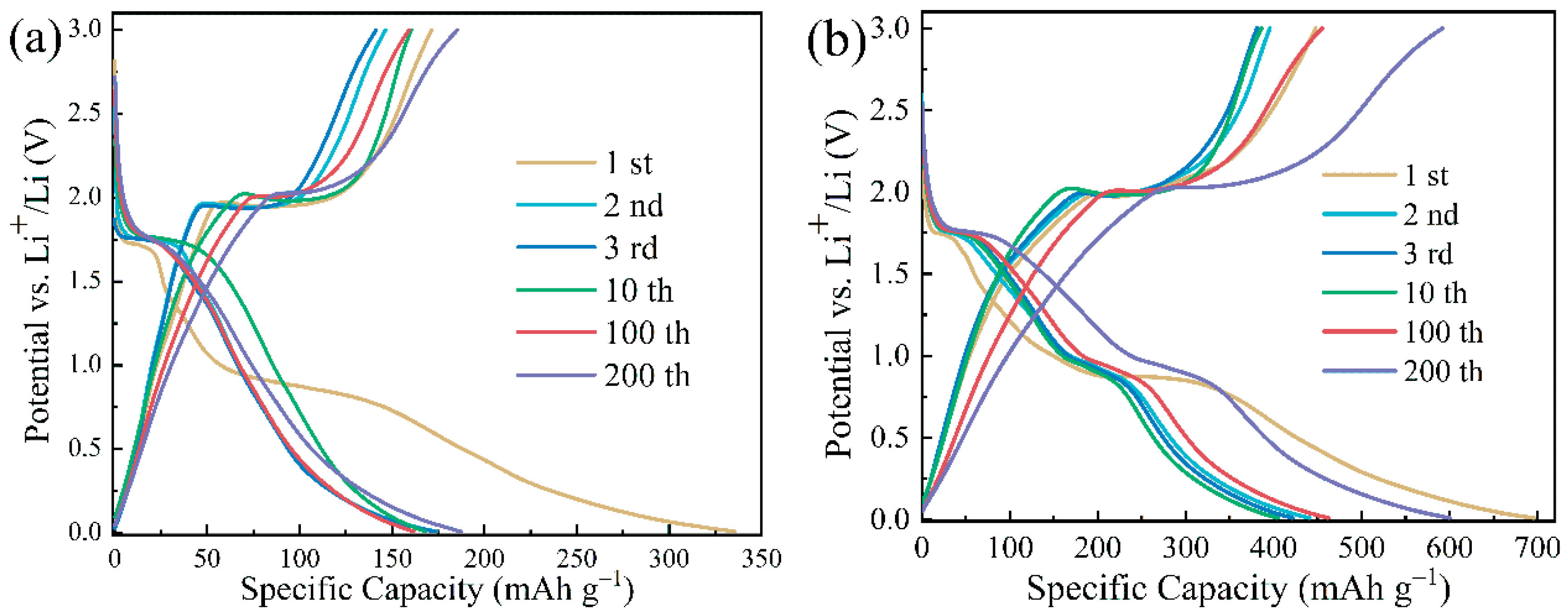
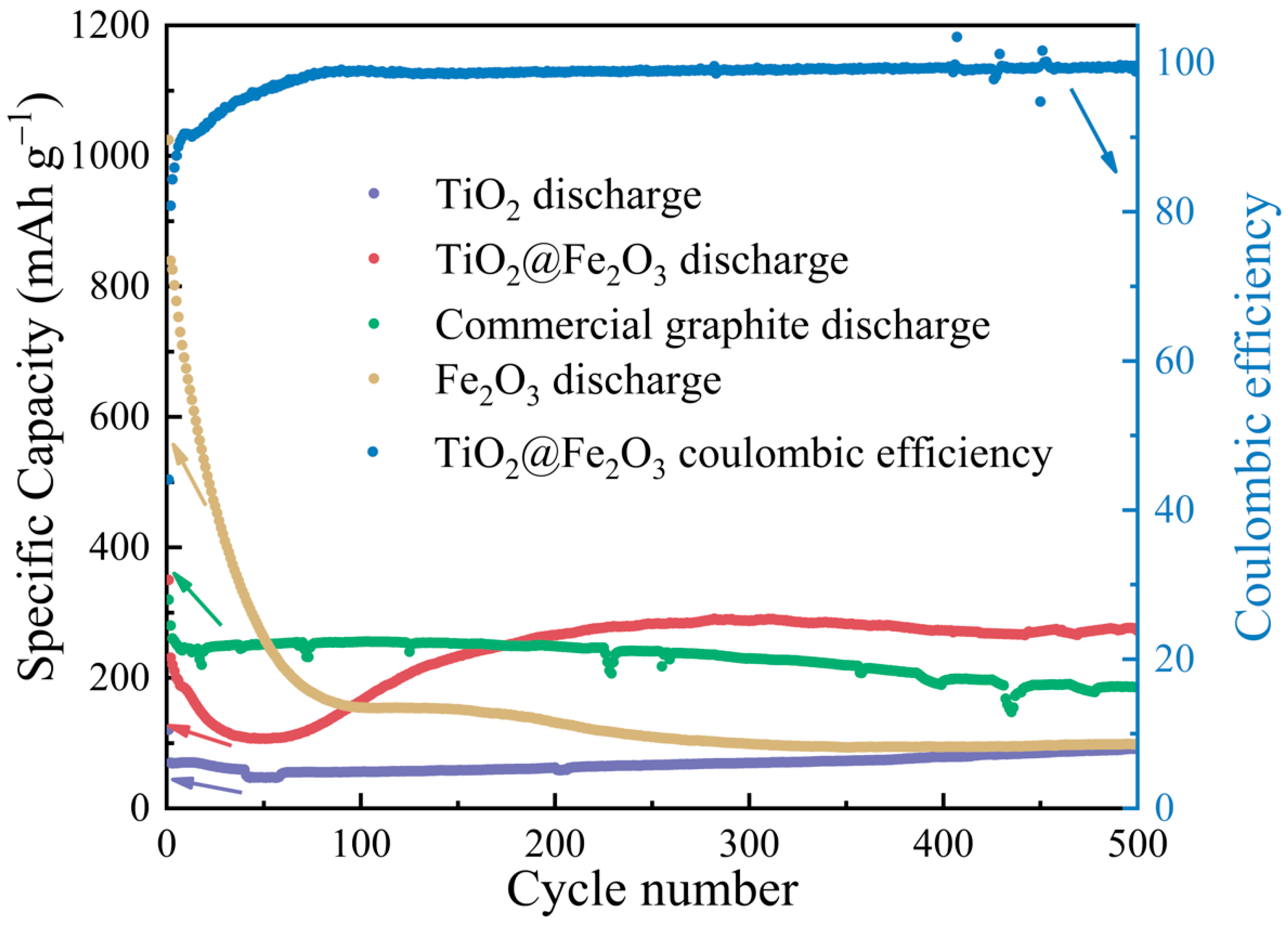
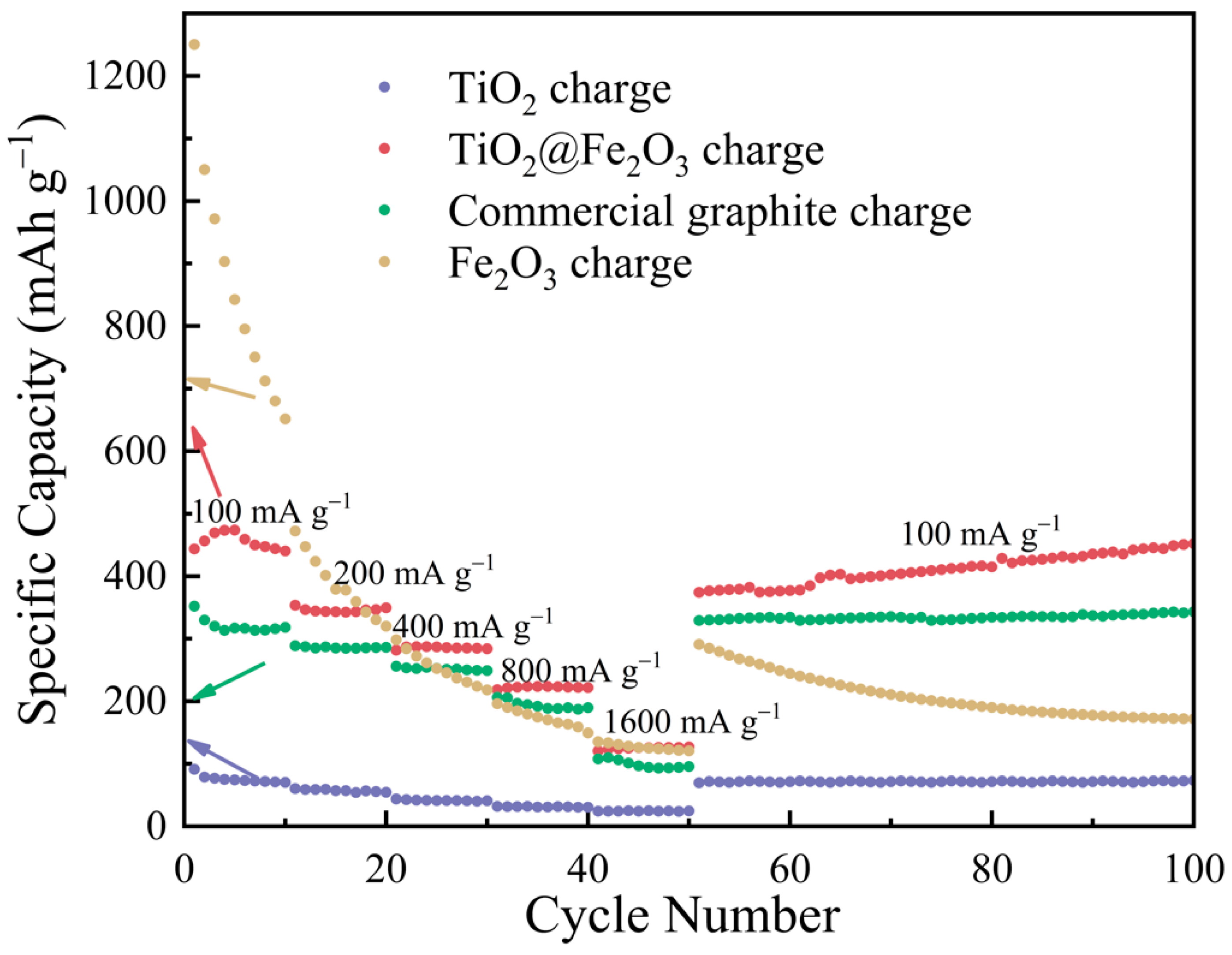
3. Results and Discussion
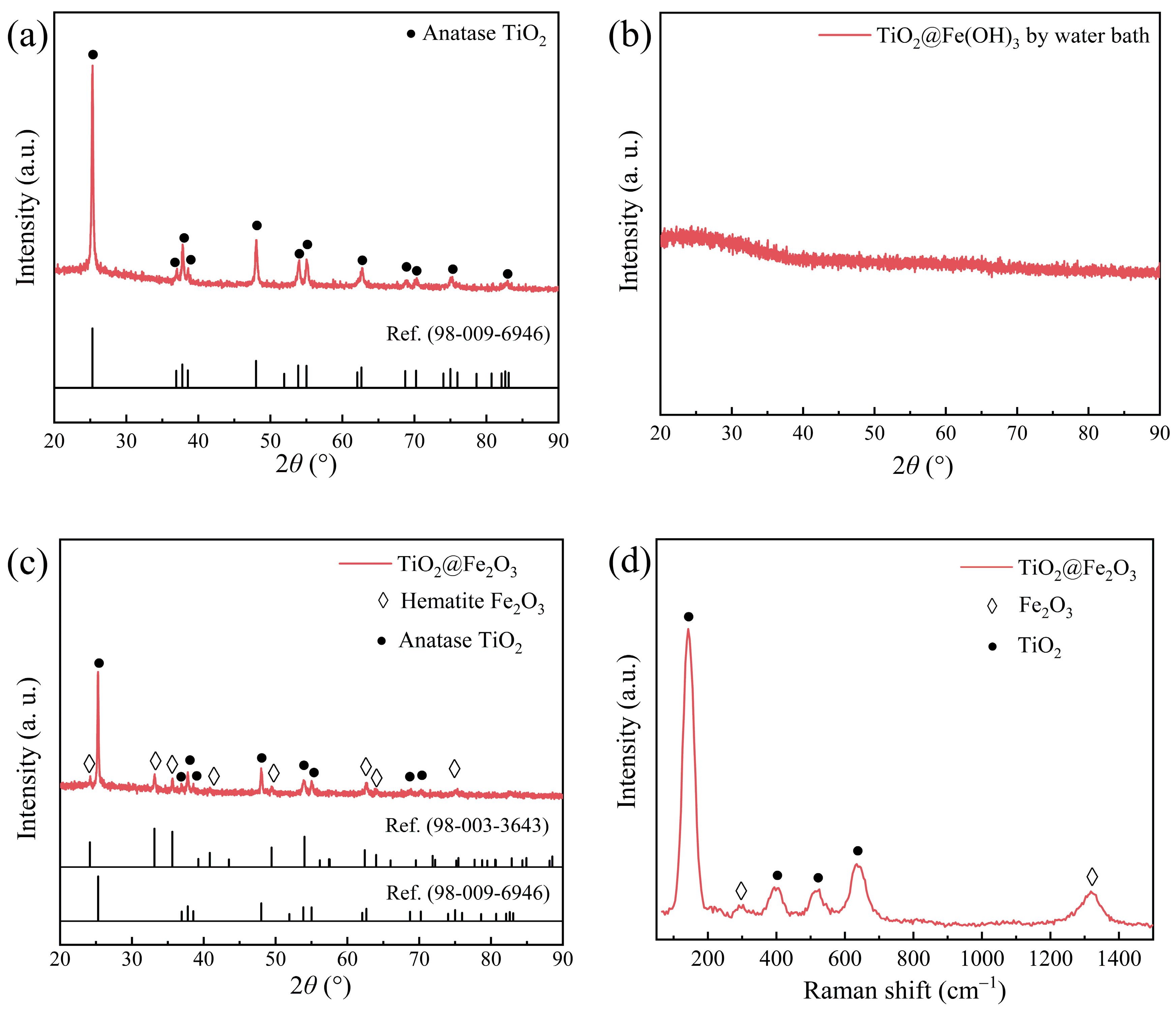
3.1. Structural Characterization
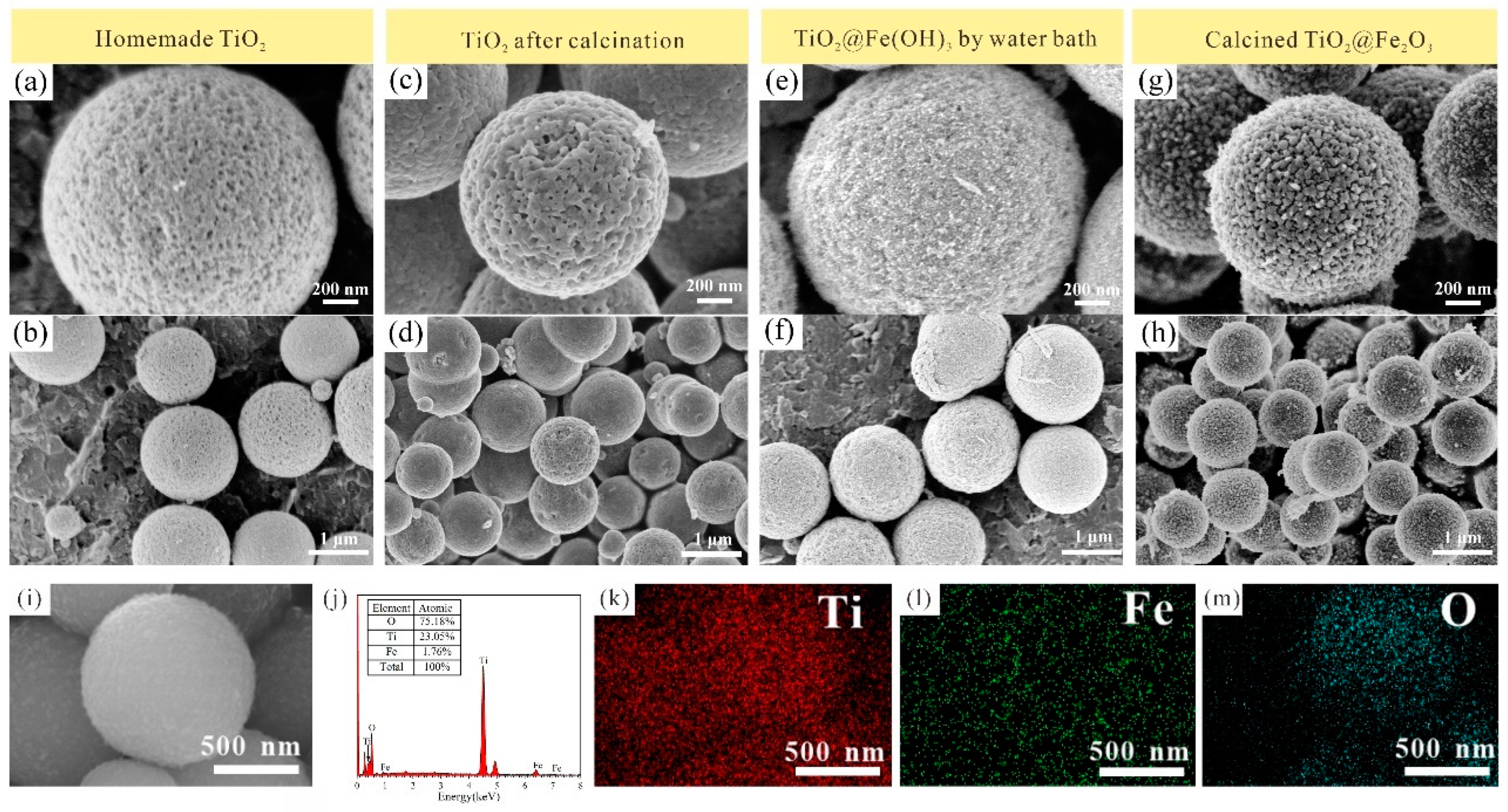
3.2. Morphological Characterization
= 335 × 92.95% + 1007 × 7.05% = 382.38 mAh g−1
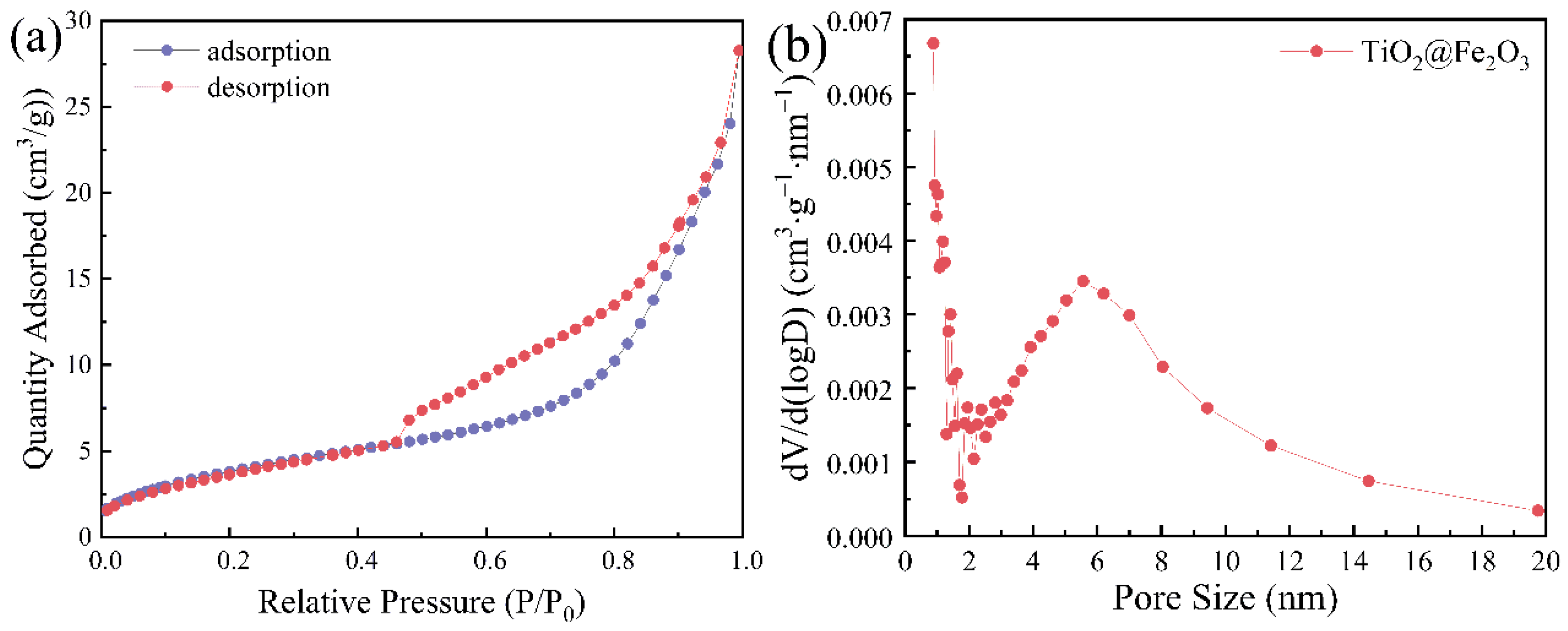
3.3. BET Analysis
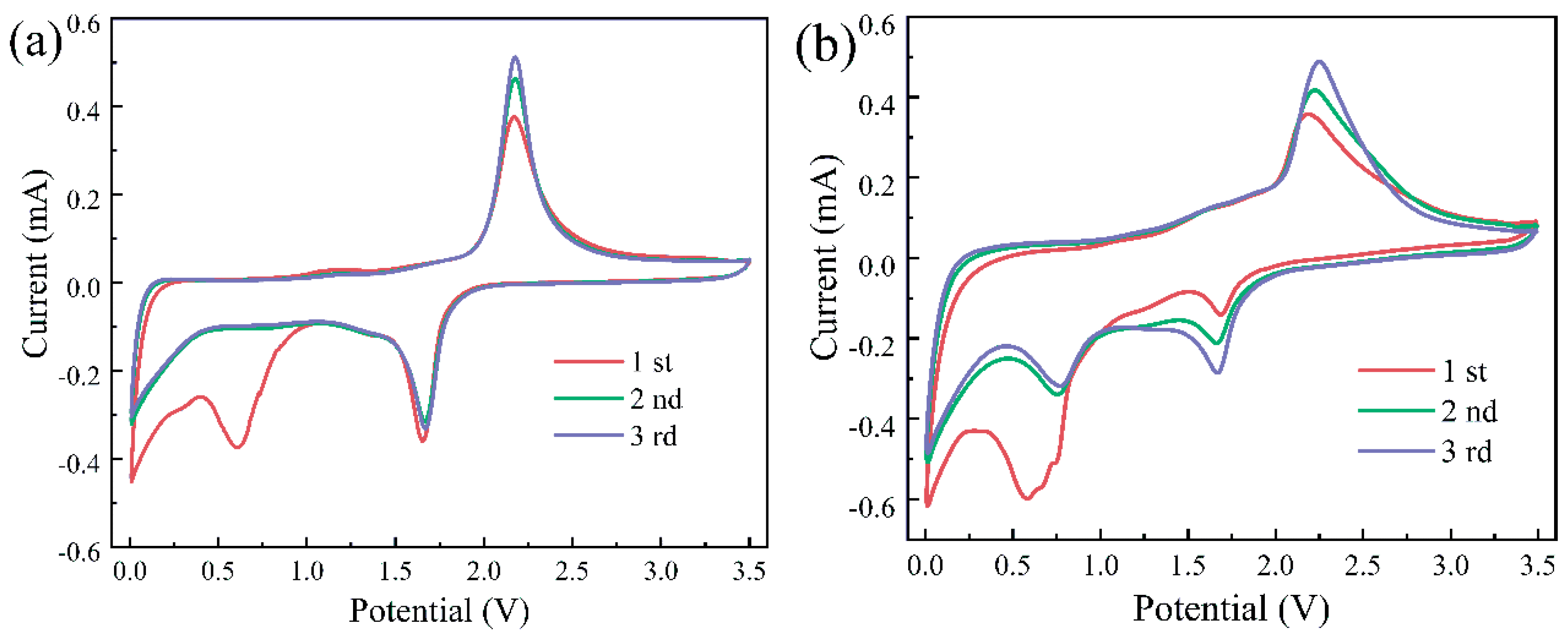
3.4. Electrochemical Performance
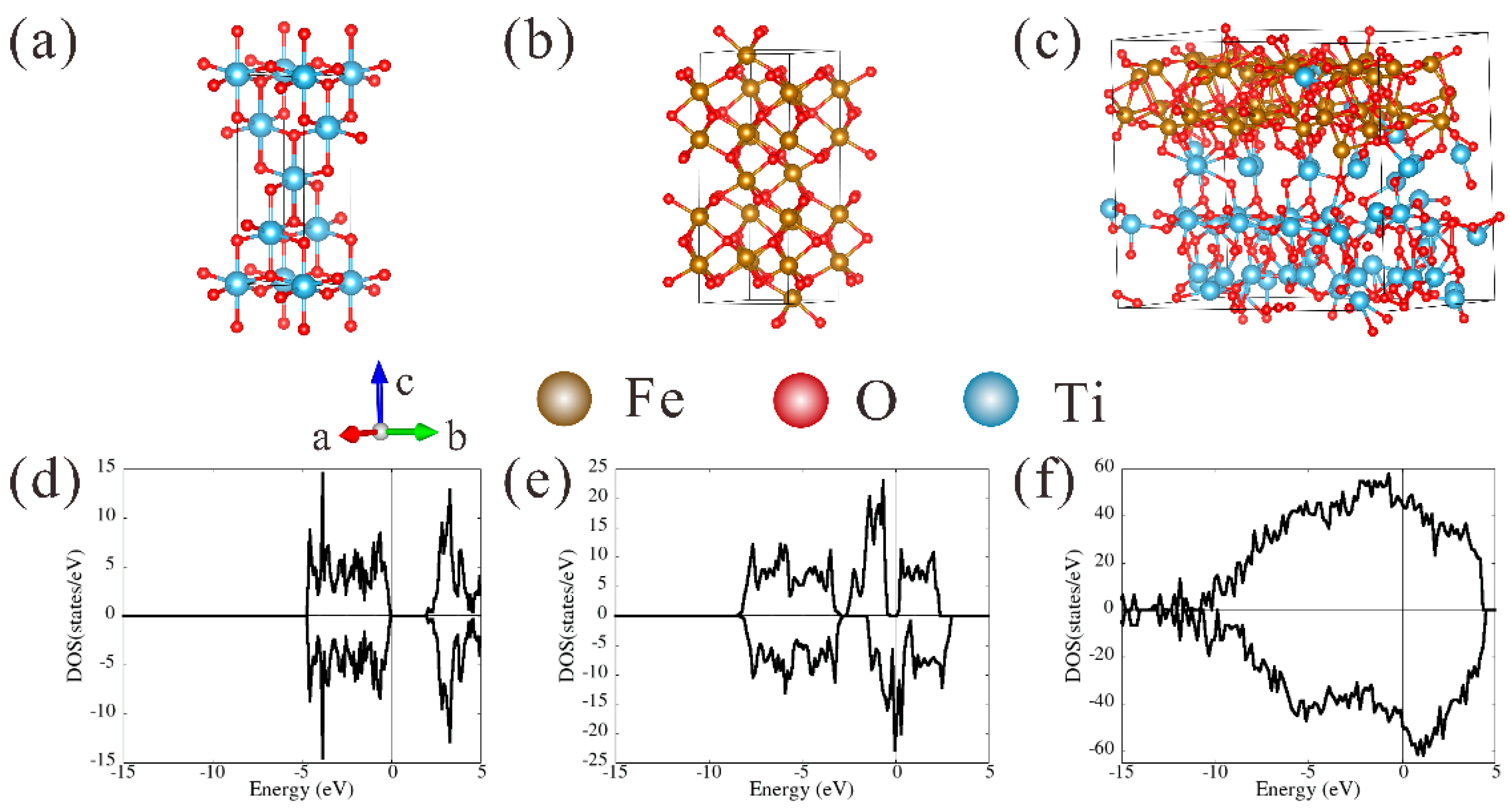
3.5. First-Principles Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, J.; Guo, X.; Yan, Y.; Xue, H.; Pang, H. FeOx-based materials for electrochemical energy storage. Adv. Sci. 2018, 5, 1700986. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ng, V.M.H.; Wang, F.; Xiao, Z.; Li, C.; Kong, L.B.; Que, W.; Zhou, K. Synthesis and application of iron-based nanomaterials as anodes of lithium-ion batteries and supercapacitors. J. Mater. Chem. A 2018, 6, 9332–9367. [Google Scholar] [CrossRef]
- Balogun, M.S.; Wu, Z.; Luo, Y.; Qiu, W.; Fan, X.; Long, B.; Huang, M.; Liu, P.; Tong, Y. High power density nitridated hematite (α-Fe2O3) nanorods as anode for high-performance flexible lithium ion batteries. J. Power Sources 2016, 308, 7–17. [Google Scholar] [CrossRef]
- Zuniga, L.; Gonzalez, G.; Orrostieta Chavez, R.; Myers, J.C.; Lodge, T.P.; Alcoutlabi, M. Centrifugally spun α-Fe2O3/TiO2/carbon composite fibers as anode materials for lithium-ion batteries. Appl. Sci. 2019, 9, 4032. [Google Scholar] [CrossRef]
- Han, M.; Mu, Y.; Yuan, F.; Bai, X.; Yu, J. Vapor pressure-assisted synthesis of chemically bonded TiO2/C nanocomposites with highly mesoporous structure for lithium-ion battery anode with high capacity, ultralong cycling lifetime, and superior rate capability. J. Power Sources 2020, 465, 228206. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Zhai, B.; Wang, X.; Zhao, J.; Wang, Z. Facile synthesis of porous anatase TiO2 nanomaterials with the assistance of biomass resource for lithium ion batteries with high-rate performance. J. Phys. Chem. Solids 2020, 145, 109552. [Google Scholar] [CrossRef]
- Guo, S.; Liu, J.; Qiu, S.; Wang, Y.; Yan, X.; Wu, N.; Wang, S.; Guo, Z. Enhancing electrochemical performances of TiO2 porous microspheres through hybridizing with FeTiO3 and nanocarbon. Electrochim. Acta 2016, 190, 556–565. [Google Scholar] [CrossRef]
- Yuan, L.; Song, K.; Liu, Z.; Yu, Y.; Yang, B.; Qiao, H.; Hu, X. Fe2O3 nanorods decorated with ultrafine CeO2 as binder-free cathode to improve the performance of LiO2 batteries. Electrochim. Acta 2021, 368, 137645. [Google Scholar] [CrossRef]
- Zhou, T.; Shen, Z.; Wu, Y.; Han, T.; Zhu, M.; Qiao, X.; Zhu, Y.; Zhang, H.; Liu, J. A yolk–shell Fe3O4@void@carbon nanochain as shuttle effect suppressive and volume-change accommodating sulfur host for long-life lithium–sulfur batteries. Nanoscale 2021, 13, 7744–7750. [Google Scholar] [CrossRef]
- Huang, A.; Chen, J.; Zhou, W.; Wang, A.; Chen, M.; Tian, Q.; Xu, J. Electrodeposition of MnO2 nanoflakes onto carbon nanotube film towards high-performance flexible quasi-solid-state Zn-MnO2 batteries. J. Electroanal. Chem. 2020, 873, 114392. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.; Yan, X.; Han, Q.; Dong, C.; Sun, X.; You, D.; Jiang, F. Spherical-graphite/nano-Mn2O3 composites as advanced anode materials for lithium half/full batteries. J. Alloy. Compd. 2021, 853, 157109. [Google Scholar] [CrossRef]
- Weng, S.C.; Brahma, S.; Huang, P.C.; Huang, Y.C.; Lee, Y.H.; Chang, C.C.; Huang, J.L. Enhanced capacity and significant rate capability of Mn3O4/reduced graphene oxide nanocomposite as high performance anode material in lithium-ion batteries. Appl. Surf. Sci. 2020, 505, 144629. [Google Scholar] [CrossRef]
- Fu, W.; Liu, T.; Hou, S.; Guo, Y.; Mei, C.; Zhao, L. Engineering MnO/C microsphere for enhanced lithium storage. J. Alloy. Compd. 2021, 861, 157961. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Li, L.; Wang, Y.; Zhang, L.; Zhang, B.; Wang, F. Formation of dumbbell and sphere-like CuO as high-performance anode materials for lithium ion batteries. Mater. Lett. 2020, 261, 127058. [Google Scholar] [CrossRef]
- Guo, R.; Huang, X.; Lin, Y.; Cao, Y. NiO/carbon aerogel microspheres with plum-pudding structure as anode materials for lithium ion batteries. Materials 2020, 13, 2363. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, C.; Wu, H.; Wang, S.; Wang, F.; Chen, Z.; Zhao, T.; Zhang, Z.; Zhang, L.Y.; Li, C.M. Synthesis of hollow Co3O4 nanocrystals in situ anchored on holey graphene for high rate lithium-ion batteries. Carbon 2020, 163, 137–144. [Google Scholar] [CrossRef]
- Qu, D.; Sun, Z.; Gan, S.; Gao, L.; Song, Z.; Kong, H.; Xu, J.; Dong, X.; Niu, L. Two-dimensional Fe2O3/TiO2 Composite Nanoplates with Improved Lithium Storage Properties as Anodic Materials for Lithium-Ion Full Cells. ChemElectroChem 2020, 7, 4963–4970. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef]
- Yao, M.; Wang, H.; Qian, R.; Yao, T.; Shi, J.W.; Cheng, Y. Robust hollow TiO2 spheres for lithium/sodium ion batteries with excellent cycling stability and rate capability. Inorg. Chem. Front. 2021, 8, 5024–5033. [Google Scholar] [CrossRef]
- Zhao, S.; Kang, D. Enhanced electrochemical performance via using interconnected TiO2 nanofibers to inhibit the shuttle effect. Ionics 2021, 27, 2073–2077. [Google Scholar] [CrossRef]
- Lu, X.; Luo, F.; Tian, Q.; Zhang, W.; Sui, Z.; Chen, J. Anatase TiO2 nanowires intertangled with CNT for conductive additive-free lithium-ion battery anodes. J. Phys. Chem. Solids 2021, 153, 110037. [Google Scholar] [CrossRef]
- Luo, J.; Xia, X.; Luo, Y.; Guan, C.; Liu, J.; Qi, X.; Ng, C.F.; Yu, T.; Zhang, H.; Fan, H.J. Rationally designed hierarchical TiO2@Fe2O3 hollow nanostructures for improved lithium ion storage. Adv. Energy Mater. 2013, 3, 737–743. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Yu, G.; Qin, L.; Jiang, Y.; Ren, L.; Chen, J. Nitrogen-doped carbon-coated TiO2/TiF3 heterostructure nanoboxes with enhanced lithium and sodium storage performance. ACS Appl. Energy Mater. 2020, 3, 4738–4745. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, Y.; Zhang, F.; Zhang, W.; Sui, Z.; Yang, L. Hierarchical carbon-riveted 2D@0D TiO2 nanosheets@SnO2 nanoparticles composite for a improved lithium-ion battery anode. Appl. Surf. Sci. 2020, 511, 145625. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Wang, X.; Shu, H.; Yang, X.; Sun, S. Porous hollow α-Fe2O3@TiO2 core–shell nanospheres for superior lithium/sodium storage capability. J. Mater. Chem. A 2015, 3, 13807–13818. [Google Scholar] [CrossRef]
- Guan, S.; Fan, Q.; Shen, Z.; Zhao, Y.; Sun, Y.; Shi, Z. Heterojunction TiO2@TiOF2 nanosheets as superior anode materials for sodium-ion batteries. J. Mater. Chem. A 2021, 9, 5720–5729. [Google Scholar] [CrossRef]
- Wang, H.; Guo, P.; Zhou, T.; Wu, X.; Zeng, M. Investigation of TiO2 surface-coating modification of MgCo2O4 nanorods anode material. Mater. Lett. 2021, 282, 128681. [Google Scholar] [CrossRef]
- Li, G.; Cao, S.; Fu, L.; Wan, S.; Liu, Q. A two-step hydrothermal synthesis of TiO2/C/FeS2 composite as high performance anode for lithium ion batteries. Electrochim. Acta 2021, 386, 138470. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Z.; Xiao, J.; Cen, W.; Yuan, S. Polypyrrole-encapsulated Fe2O3 nanotube arrays on a carbon cloth support: Achieving synergistic effect for enhanced supercapacitor performance. Electrochim. Acta 2021, 386, 138486. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Q.; Yang, X.; He, S.; Khan, J.; Meng, Y.; Zhu, X.; Tong, S.; Wu, M. Chestnut-like TiO2@α-Fe2O3 core–shell nanostructures with abundant interfaces for efficient and ultralong life lithium-ion storage. ACS Appl. Mater. Interfaces 2017, 9, 354–361. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, J.; Tian, Q.; Zhang, W.; Sui, Z. Facile construction of clustered Fe2O3/TiO2 composite for improved lithium storage performance. Synth. Met. 2020, 263, 116353. [Google Scholar] [CrossRef]
- Chang, Y.; He, P.; Wei, Z.; Chen, Y.; Wang, H.; Wu, C.; Zhou, Z.; Huang, H.; Kowalska, E.; Dong, S. Three-dimensional monodispersed TiO2 microsphere network formed by a sub-zero sol-gel method. Mater. Lett. 2020, 268, 127592. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple density functional for the electronic exchange energy: Generalized gradient approximation. Phys. Rev. B 1986, 33, 8800–8802. [Google Scholar] [CrossRef]
- Burke, K.; Perdew, J.P.; Wang, Y. Derivation of a Generalized Gradient Approximation: The PW91 Density Functional. In Electronic Density Functional Theory; Dobson, J.F., Vigale, G., Das, M.M., Eds.; Springer: Boston, MA, USA, 1998. [Google Scholar]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, W.; Zhou, Z.; Liu, X.; Zhang, H.; Wei, M. Hierarchical porous anatase TiO2 microspheres with high-rate and long-term cycling stability for sodium storage in ether-based electrolyte. ACS Appl. Energy Mater. 2020, 3, 3619–3627. [Google Scholar] [CrossRef]
- Paul, S.; Rahman, M.A.; Sharif, S.B.; Kim, J.H.; Siddiqui, S.E.T.; Hossain, M.A.M. TiO2 as an Anode of high-performance lithium-ion batteries: A Comprehensive Review towards Practical Application. Nanomaterials 2022, 12, 2034. [Google Scholar] [CrossRef]
- Li, Z.; Hu, X.; Shi, Z.; Lu, J.; Wang, Z. MOFs-derived metal oxides inlayed in carbon nanofibers as anode materials for high-performance lithium-ion batteries. Appl. Surf. Sci. 2020, 531, 147290. [Google Scholar] [CrossRef]
- Hou, S.; Wang, P.; Li, Y.; Pang, F.; Liu, M.; Luo, Y.; Zhuang, L.; Zhao, L. Podocarpus-like α-Fe2O3/TiO2 composite with balsam pear texture for enhanced lithium storage. Appl. Surf. Sci. 2019, 476, 959–965. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Xie, Y.; Guo, J. Ultralong life lithium-ion battery anode with superior high-rate capability and excellent cyclic stability from mesoporous Fe2O3@TiO2 core–shell nanorods. J. Mater. Chem. A 2014, 2, 3912–3918. [Google Scholar] [CrossRef]
- Gu, X.; Chen, L.; Ju, Z.; Xu, H.; Yang, J.; Qian, Y. Controlled growth of porous α-Fe2O3 branches on β-MnO2 nanorods for excellent performance in lithium-ion batteries. Adv. Funct. Mater. 2013, 23, 4049–4056. [Google Scholar] [CrossRef]
- Qin, G.; Zeng, M.; Wu, X.; Wen, J.; Li, J. Fabrication of Fe2O3@TiO2 core–shell nanospheres as anode materials for lithium-ion batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 12944–12950. [Google Scholar] [CrossRef]
- Pian, C.; Peng, W.; Ren, H.; Ma, C.; Su, Y.; Ti, R.; Chen, X.; Zhu, L.; Liu, J.; Sun, X.; et al. Robust α-Fe2O3@TiO2 Core–Shell Structures with Tunable Buffer Chambers for High-Performance Lithium Storage. Front. Chem. 2022, 10, 866369. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Yang, Y.; Li, Y.; Jiang, J.; Xiao, S.; Yang, J. Interconnected α-Fe2O3 nanoparticles prepared from leaching liquor of tin ore tailings as anode materials for lithium-ion batteries. J. Alloy. Compd. 2021, 855, 157288. [Google Scholar] [CrossRef]
- Xiao, S.; Li, X.; Li, T.; Xiang, Y.; Chen, J.S. Practical strategies for enhanced performance of anode materials in Na+/K+-ion batteries. J. Mater. Chem. A 2021, 9, 7317–7335. [Google Scholar] [CrossRef]
- Zhu, J.; Ng, K.Y.S.; Deng, D. Micro single crystals of hematite with nearly 100% exposed {104} facets: Preferred etching and lithium storage. Cryst. Growth Des. 2014, 14, 2811–2817. [Google Scholar] [CrossRef]

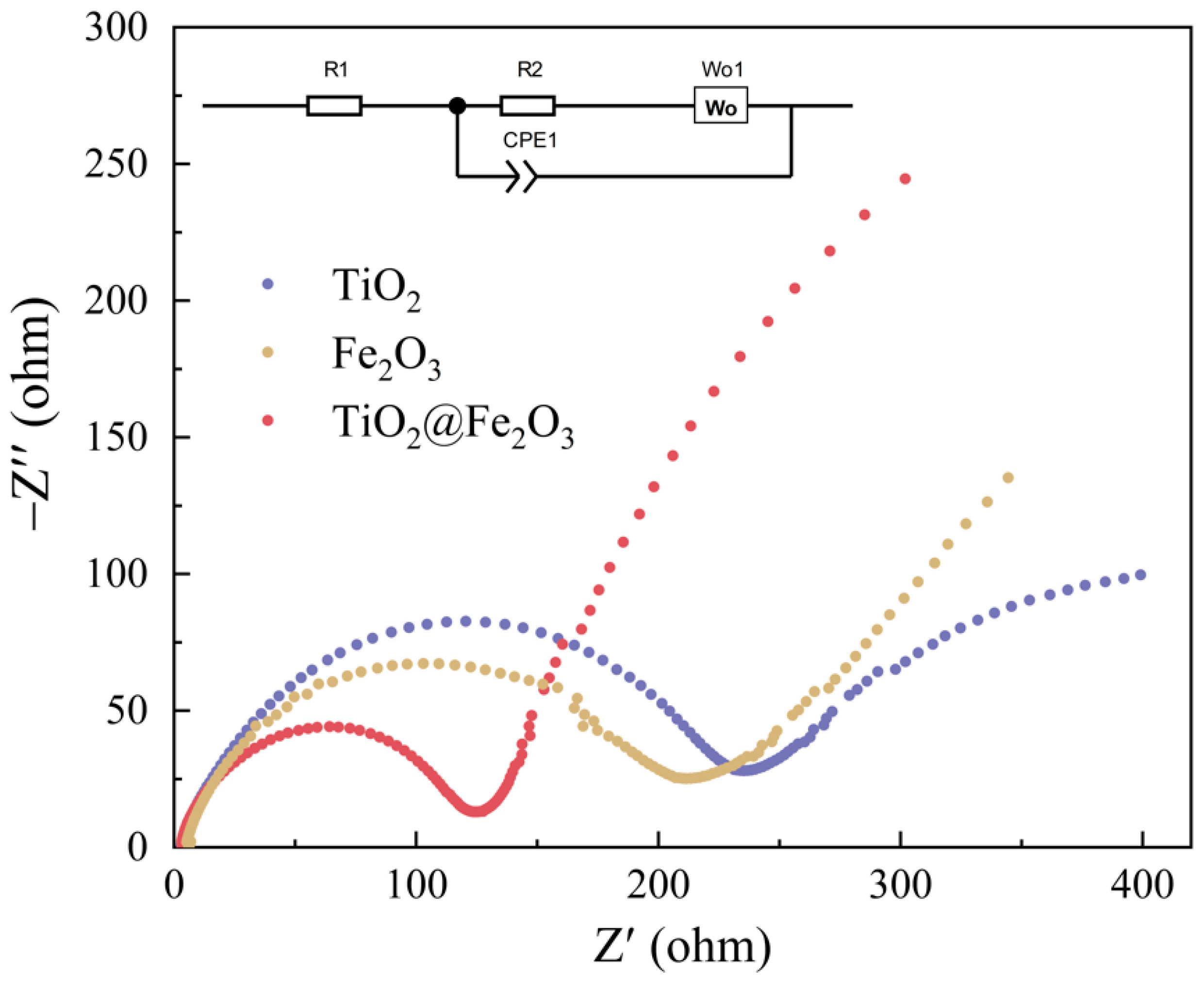
| Materials | R1 | R2 |
|---|---|---|
| TiO2 | 2.29 | 238.35 |
| TiO2@Fe2O3 | 2.08 | 119.81 |
| Fe2O3 | 4.03 | 202.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, F.; Zhao, Y.; Ding, M.; Wang, J.; Zheng, X.; Wang, H.; Record, M.-C.; Boulet, P. Lychee-like TiO2@Fe2O3 Core-Shell Nanostructures with Improved Lithium Storage Properties as Anode Materials for Lithium-Ion Batteries. Materials 2023, 16, 1945. https://doi.org/10.3390/ma16051945
Chen Y, Liu F, Zhao Y, Ding M, Wang J, Zheng X, Wang H, Record M-C, Boulet P. Lychee-like TiO2@Fe2O3 Core-Shell Nanostructures with Improved Lithium Storage Properties as Anode Materials for Lithium-Ion Batteries. Materials. 2023; 16(5):1945. https://doi.org/10.3390/ma16051945
Chicago/Turabian StyleChen, Yuan, Feihong Liu, Yufei Zhao, Mengdie Ding, Juan Wang, Xuan Zheng, Huihu Wang, Marie-Christine Record, and Pascal Boulet. 2023. "Lychee-like TiO2@Fe2O3 Core-Shell Nanostructures with Improved Lithium Storage Properties as Anode Materials for Lithium-Ion Batteries" Materials 16, no. 5: 1945. https://doi.org/10.3390/ma16051945
APA StyleChen, Y., Liu, F., Zhao, Y., Ding, M., Wang, J., Zheng, X., Wang, H., Record, M.-C., & Boulet, P. (2023). Lychee-like TiO2@Fe2O3 Core-Shell Nanostructures with Improved Lithium Storage Properties as Anode Materials for Lithium-Ion Batteries. Materials, 16(5), 1945. https://doi.org/10.3390/ma16051945






