Study on the Binary Hydraulic Kinetics Model of Glass Powder-Cement: Numerical Simulation
Abstract
:1. Introduction
2. Binary Hydraulic Kinetics Model of Glass Powder-Cement
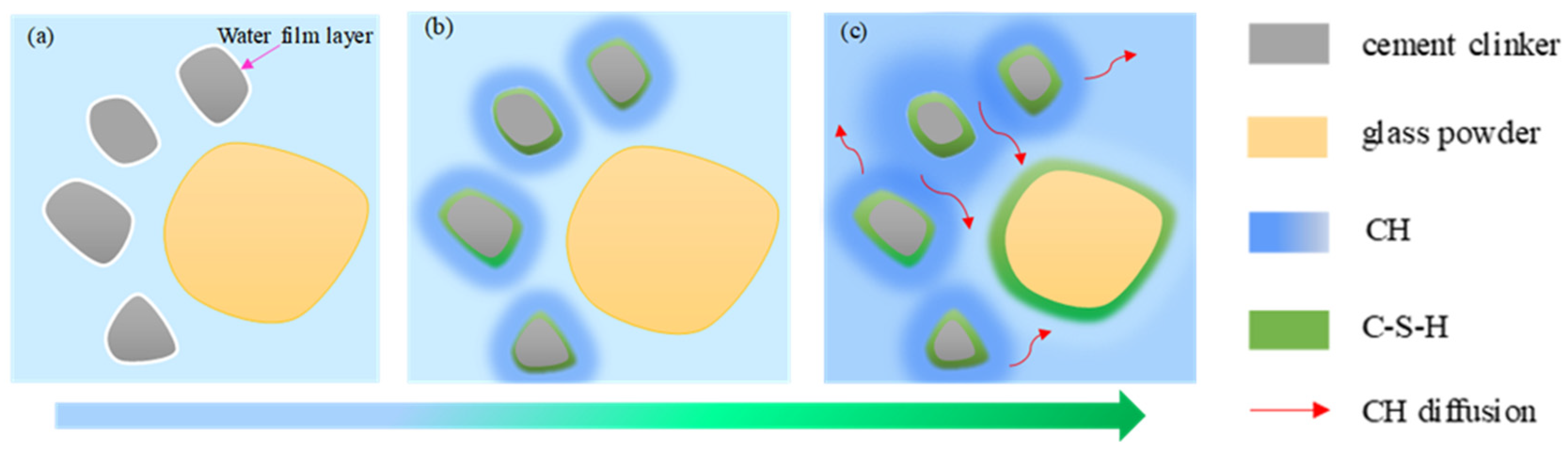
2.1. Hydration Mechanism
2.2. Cement Hydration Model
2.3. Pozzolanic Reaction of Glass Powder
2.4. Hydration Couple of Glass Powder and Cement
3. Results and Discussion
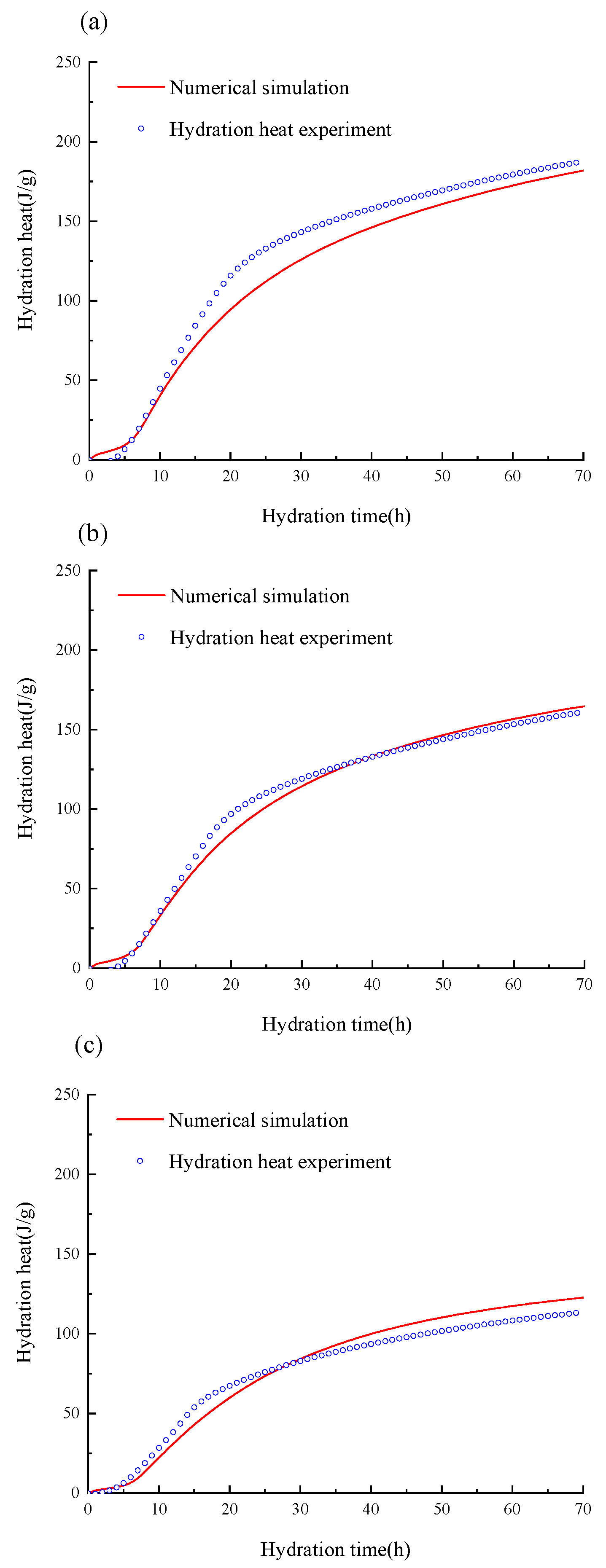
3.1. Validation
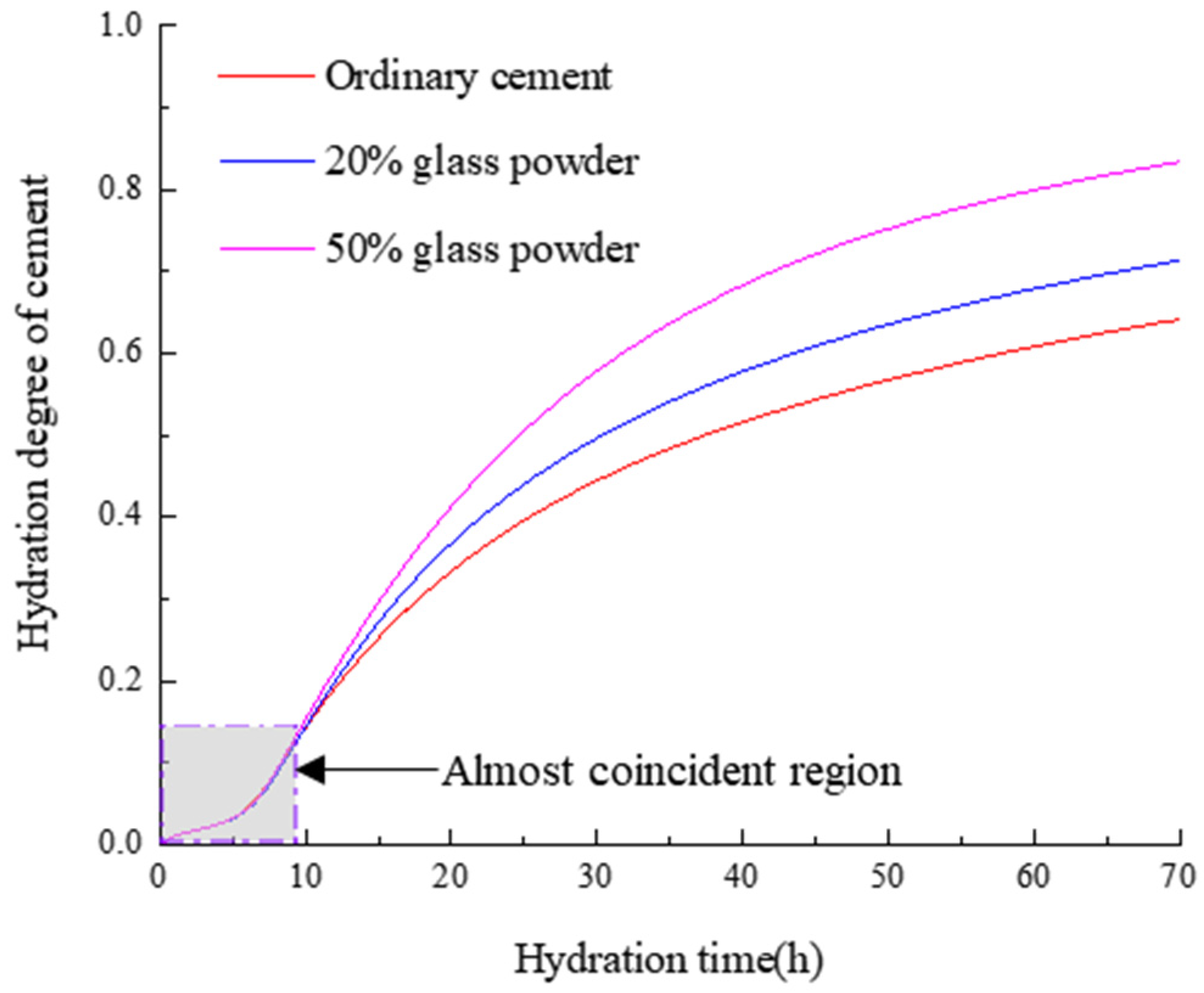
3.2. Hydration Reaction of Glass Powder-Cement Mixed Cementitious Material
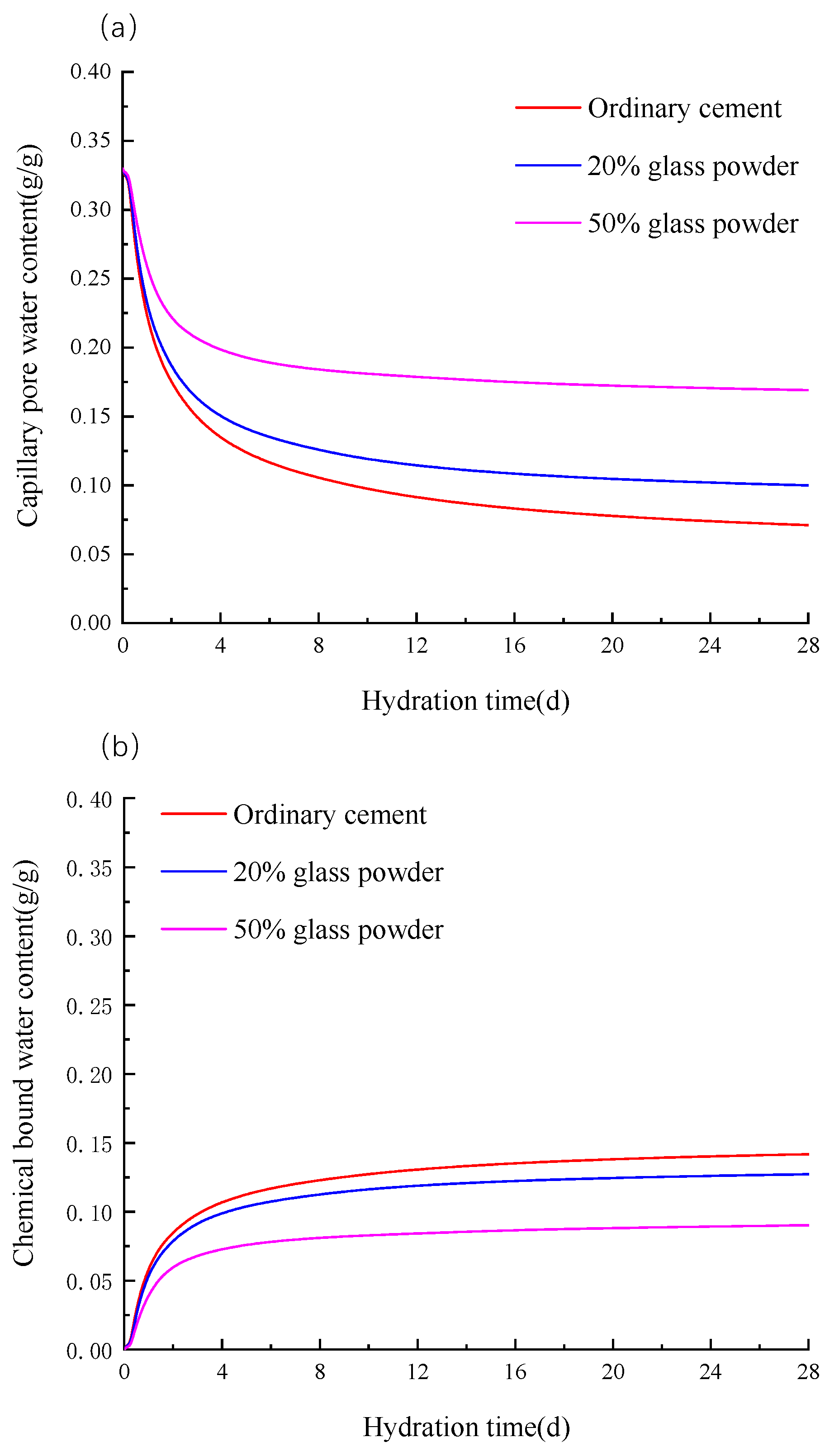
3.3. Chemically Bound Water and Capillary Water Content
3.4. Analysis of Influencing Factors
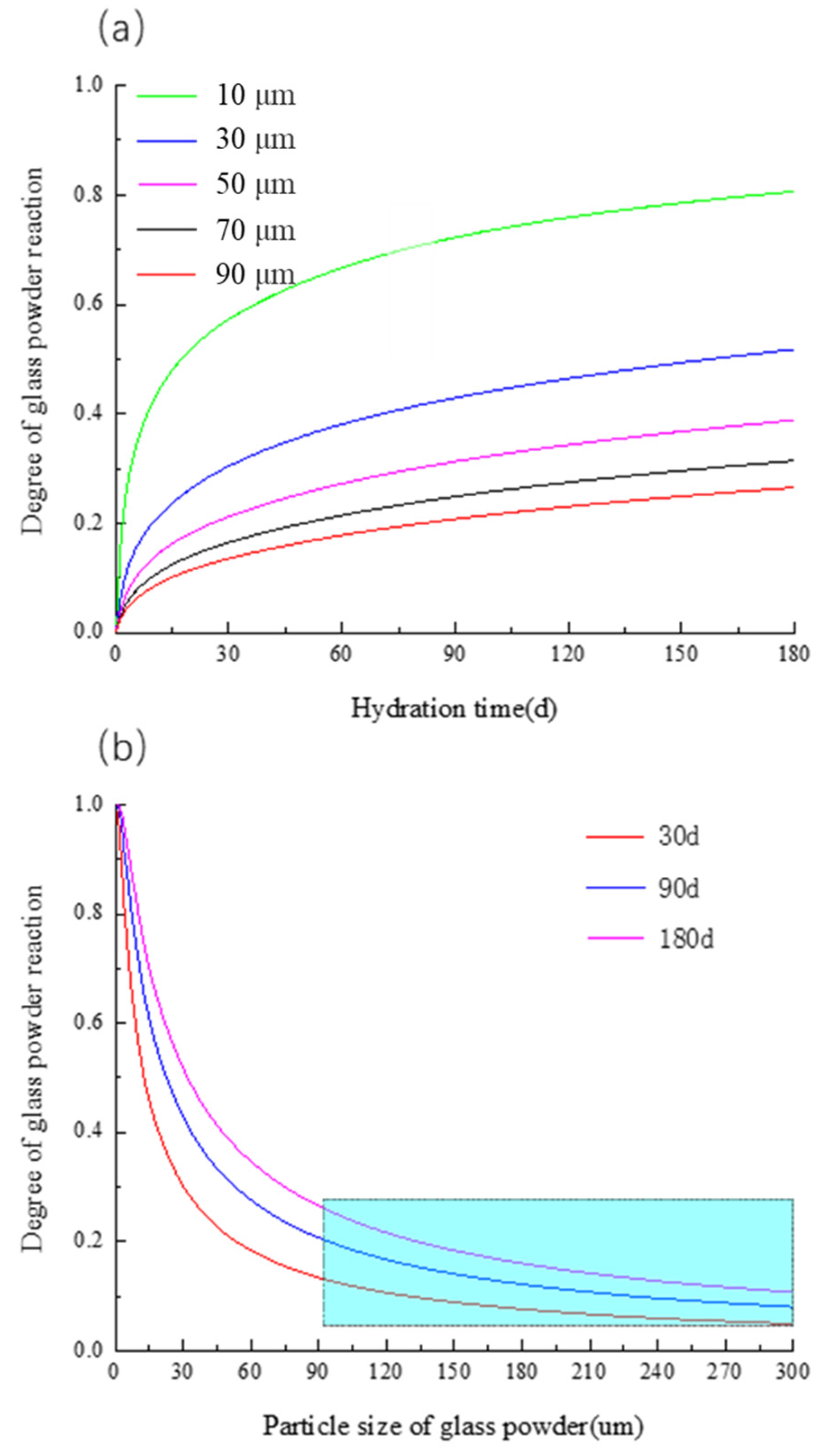
3.4.1. Influence of Particle Size of Glass Power
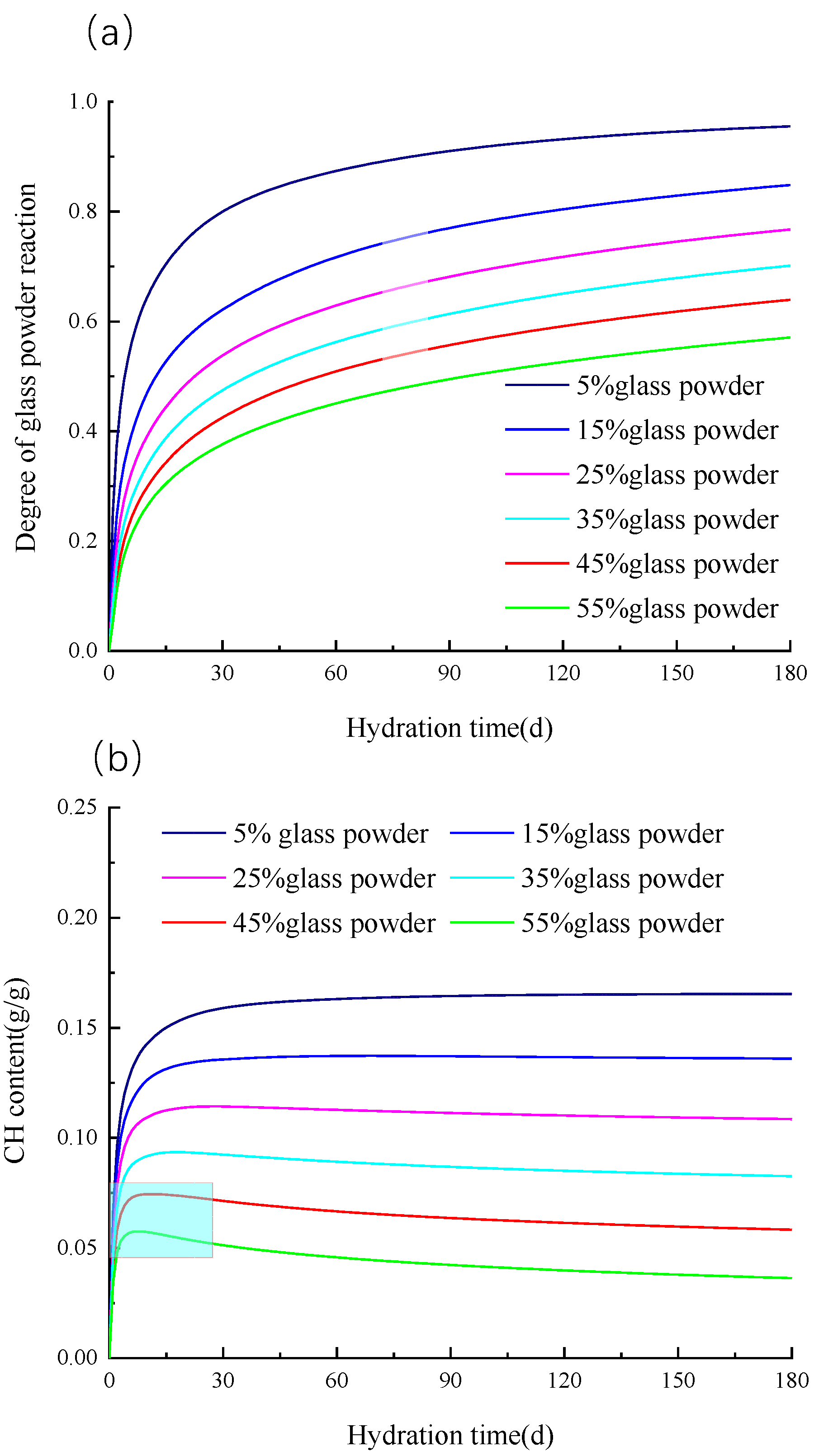
3.4.2. Influence of Glass Powder Content
4. Conclusions
- By comparing the hydration degree of cement with ordinary cement, 20% glass powder and 50% glass powder has a dilution effect on cement hydration and accelerates cement hydration. This dilution effect is mainly caused by the hydration reaction and water diffusion; however, at the initial hydration delay stage, the dilution effect of the glass powder can be ignored.
- The particle size of the glass powder has a great effect on the hydration of the glass powder, and the hydration degree of the glass powder decreases exponentially with the increase in the particle size. It is noteworthy that when the glass particle size is greater than 90 μm, the reaction activity of the glass powder is less than 0.2, and the glass powder mainly acts as a filler.
- The reactivity of glass powder decreases with the increase in the glass powder content. Compared with the sample with 5% glass powder content, the hydration degree of the glass powder decreased by 42.3% for the sample with 50% glass powder content. The CH concentration in the pore solution decreases with the increase in the glass powder content, which is the reason for the decrease in the hydration degree of the glass powder with the increase in the glass powder content.
- When the content of glass powder exceeds 45%, the CH concentration has a peak value, which indicates that the CH amount produced by cement hydration is less than the CH amount consumed by the pozzolanic reaction of the glass powder.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Topcu, I.B.; Canbaz, M. Properties of concrete containing waste glass. Cem. Concr. Res. 2003, 34, 267–274. [Google Scholar] [CrossRef]
- Nasir, M.; Al-Kutti, W. Performance of Date Palm Ash as a Cementitious Material by Evaluating Strength, Durability, and Characterization. Buildings 2018, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhu, P.; Shi, Y.; Xu, F.; Jiang, L.; Chu, H.; Xu, N.; Liu, M.; Jia, Y.; Peng, T. Prediction of Temperature Development of Concrete with Set-Controlling Admixture Based on a New Hydration Kinetics Model. Materials 2023, 16, 497. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Jia, Y. Experimental Study on the Mechanical Properties, Water Absorption, and Fiber Degradation of Naturally Aged Glass Fiber and Polypropylene Fiber-Reinforced Concrete. Materials 2022, 15, 3760. [Google Scholar] [CrossRef]
- Chen, W.; Dong, S.; Liu, Y.; Liang, Y.; Skoczylas, F. Effect of Waste Glass as Fine Aggregate on Properties of Mortar. Materials 2022, 15, 8499. [Google Scholar] [CrossRef]
- Liu, G.; Fan, L.; Gao, P.; Jin, D. Mechanical Properties of Glass powder Concrete. J. Henan Univ. Sci. Technol. (Nat. Sci.) 2018, 39, 59–62+7-8. [Google Scholar]
- Tamanna, N.; Tuladhar, R.; Sivakugan, N. Performance of recycled waste glass sand as partial replacement of sand in concrete. Constr. Build. Mater. 2020, 239, 117804. [Google Scholar] [CrossRef]
- Htet, P.; Chen, W.; Hao, H.; Shaikh, F. Physical and mechanical properties of quaternary blended concrete with recycled coarse aggregates and crushed waste glass. Constr. Build. Mater. 2022, 353, 129016. [Google Scholar] [CrossRef]
- Adesina, A.; de Azevedo, A.R.; Amin, M.; Hadzima-Nyarko, M.; Agwa, I.S.; Zeyad, A.M.; Tayeh, B.A. Fresh and mechanical properties overview of alkali-activated materials made with glass powder as precursor. Clean. Mater. 2022, 3, 100036. [Google Scholar] [CrossRef]
- Amin, M.; El-Aziz, A.; Agwa, I.S.; Abu El-Hassan, K. Properties and microstructure of high strength concrete incorporating different supplementary cementitious materials. Key Eng. Mater. 2022, 921, 247–257. [Google Scholar] [CrossRef]
- Nasir, M.; Megat Johari, M.A.; Yusuf, M.O.; Maslehuddin, M.; Al-Harthi, M.A.; Dafalla, H. Impact of Slag Content and Curing Methods on the Strength of Alkaline-Activated Silico-Manganese Fume/Blast Furnace Slag Mortars. Arab. J. Sci. Eng. 2019, 44, 8325–8335. [Google Scholar] [CrossRef]
- Patel, R.A.; Perko, J.; Jacques, D.; De Schutter, G.; Ye, G.; Van Breugel, K. A three-dimensional lattice Boltzmann method based reactive transport model to simulate changes in cement paste microstructure due to calcium leaching. Constr. Build. Mater. 2018, 166, 158–170. [Google Scholar] [CrossRef]
- Watts, B.; Tao, C.; Ferraro, C.; Masters, F. Proficiency analysis of VCCTL results for heat of hydration and mortar cube strength. Constr. Build. Mater. 2018, 161, 606–617. [Google Scholar] [CrossRef]
- Bentz, D.P.; Bentz, D.P. CEMHYD3D: A Three-Dimensional Cement Hydration and Microstructure Development Modeling Package; Version 3.0. NISTIR 7232; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2005.
- Tomosawa, F. Development of a kinetic model for hydration of cement. In Proceedings of the 10th International Congress on the Chemistry of Cement, Gothenburg, Sweden, 2–6 June 1997; pp. 51–58. [Google Scholar]
- Park, K.; Jee, N.; Yoon, I.; Lee, H. Prediction of temperature distribution in high-strength concrete using hydration model. ACI Mater. J. 2008, 105, 180–186. [Google Scholar]
- Wang, X.-Y. Effect of fly ash on properties evolution of cement-based materials. Constr. Build. Mater. 2014, 69, 32–40. [Google Scholar] [CrossRef]
- Wang, X.-Y. Analysis of hydration and strength optimization of cement-fly ash-limestone ternary blended concrete. Constr. Build. Mater. 2018, 166, 130–140. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Park, K.-B. Analysis of the compressive strength development of concrete considering the interactions between hydration and drying. Cem. Concr. Res. 2017, 102, 1–15. [Google Scholar] [CrossRef]
- Papadakis, V.G. Effect of supplementary cementing materials on concrete resistance against carbonation and chloride ingress. Cem. Concr. Res. 2000, 30, 291–299. [Google Scholar] [CrossRef]
- Papadakis, V.G. Effect of fly ash on Portland cement systems, Part II: High calcium fly ash. Cem. Concr. Res. 2000, 30, 1647–1654. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Lee, H.-S. Modeling the hydration of concrete incorporating fly ash or slag. Cem. Concr. Res. 2010, 40, 984–996. [Google Scholar] [CrossRef]
- Wang, X.-Y. A hydration-Based Integrated System for Blended Cement to Predict the Early-Age Properties and Durability of Concrete. Ph.D. Thesis, Hanyang University, Seoul, Republic of Korea, 2010. [Google Scholar]
- Wang, X.-Y.; Lee, H.-S. Evaluation of the mechanical properties of concrete considering the effects of temperature and aging. Constr. Build. Mater. 2012, 29, 581–590. [Google Scholar] [CrossRef]
- Maekawa, K.; Ishida, T.; Kishi, T. Multi-Scale Modeling of Structural Concrete; Taylor & Francis: London, UK; New York, NY, USA, 2009. [Google Scholar]
- Tahwia, A.; Essam, A.; Tayeh, B.; Elrahman, M. Enhancing sustainability of ultra-high-performance concrete utilizing high-volume waste glass powder. Case Stud. Constr. Mater 2022, 17, e01648. [Google Scholar] [CrossRef]
- Onaizi, A.; Lim, N.; Huseien, G.; Amran, M.; Ma, C. Effect of the addition of nano glass powder on the compressive strength of high volume fly ash modified concrete. Mater. Today Proc. 2022, 48, 1789–1795. [Google Scholar] [CrossRef]

| Produce Chemical. Combined Water | Consume Capillary Water | Consume CH |
|---|---|---|
| 0.24~0.4 | 0.16~0.2 | 0.18~0.3 |
| Compositions | SiO2 | Al2O3 | CaO | Na2O | K2O |
| Cement | 21.56 | 4.78 | 59.64 | 0.21 | 0.85 |
| Glass powder | 70.23 | 3.13 | 8.95 | 13.64 | 0.86 |
| Compositions | Fe2O3 | MgO | SO3 | TiO2 | Other |
| Cement | 3.12 | 2.21 | 3.62 | 0.15 | 3.86 |
| Glass powder | 1.37 | 0.78 | 0.05 | 0.10 | 0.89 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Bcem | 8.5 × 10−10 | Bglass | 7.98 × 10−8 |
| Ccem | 0.034 | Cglass | 0.1 |
| Krcem | 4.037 × 10−6 | Krglass | 9.8 × 10−7 |
| De20 | 4.03 × 10−10 | De20 | 5.92 × 10−11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ming, Y.; Li, L.; Ren, H.; Chen, P.; Chen, X. Study on the Binary Hydraulic Kinetics Model of Glass Powder-Cement: Numerical Simulation. Materials 2023, 16, 1957. https://doi.org/10.3390/ma16051957
Ming Y, Li L, Ren H, Chen P, Chen X. Study on the Binary Hydraulic Kinetics Model of Glass Powder-Cement: Numerical Simulation. Materials. 2023; 16(5):1957. https://doi.org/10.3390/ma16051957
Chicago/Turabian StyleMing, Yang, Ling Li, Hao Ren, Ping Chen, and Xuandong Chen. 2023. "Study on the Binary Hydraulic Kinetics Model of Glass Powder-Cement: Numerical Simulation" Materials 16, no. 5: 1957. https://doi.org/10.3390/ma16051957
APA StyleMing, Y., Li, L., Ren, H., Chen, P., & Chen, X. (2023). Study on the Binary Hydraulic Kinetics Model of Glass Powder-Cement: Numerical Simulation. Materials, 16(5), 1957. https://doi.org/10.3390/ma16051957






