Effects of Film Thickness of ALD-Deposited Al2O3, ZrO2 and HfO2 Nano-Layers on the Corrosion Resistance of Ti(N,O)-Coated Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Deposition
2.2. Coating Characterisation
3. Results
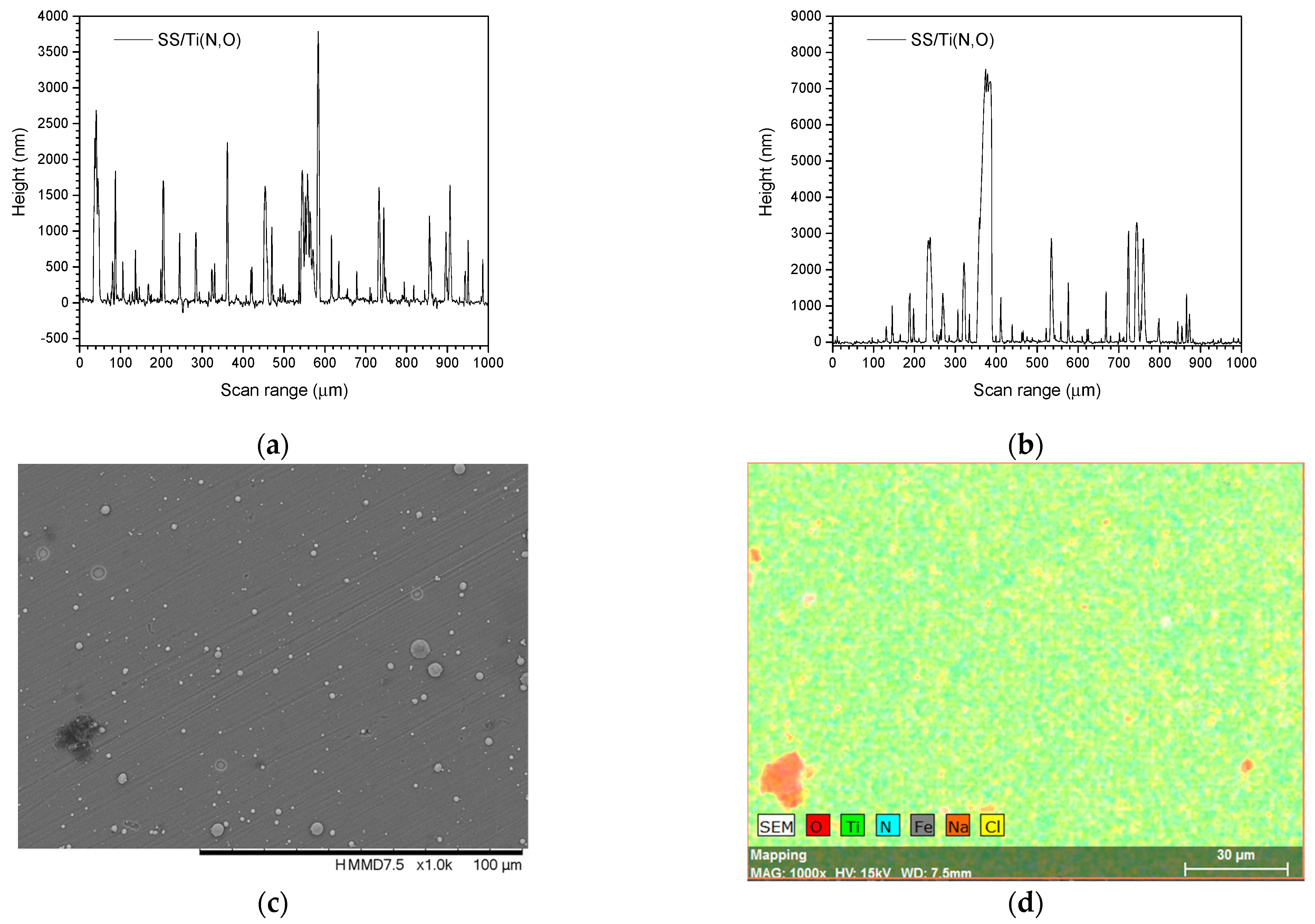
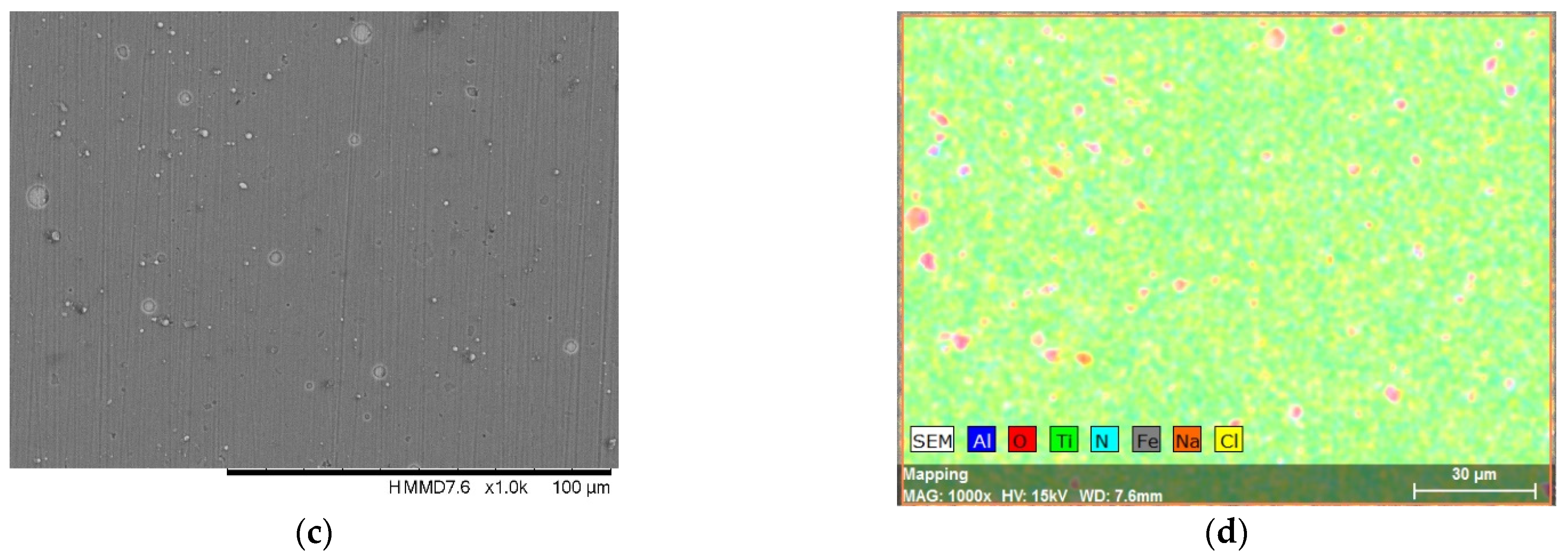
3.1. Surface Morphology and Elemental Composition
3.2. Phase Composition
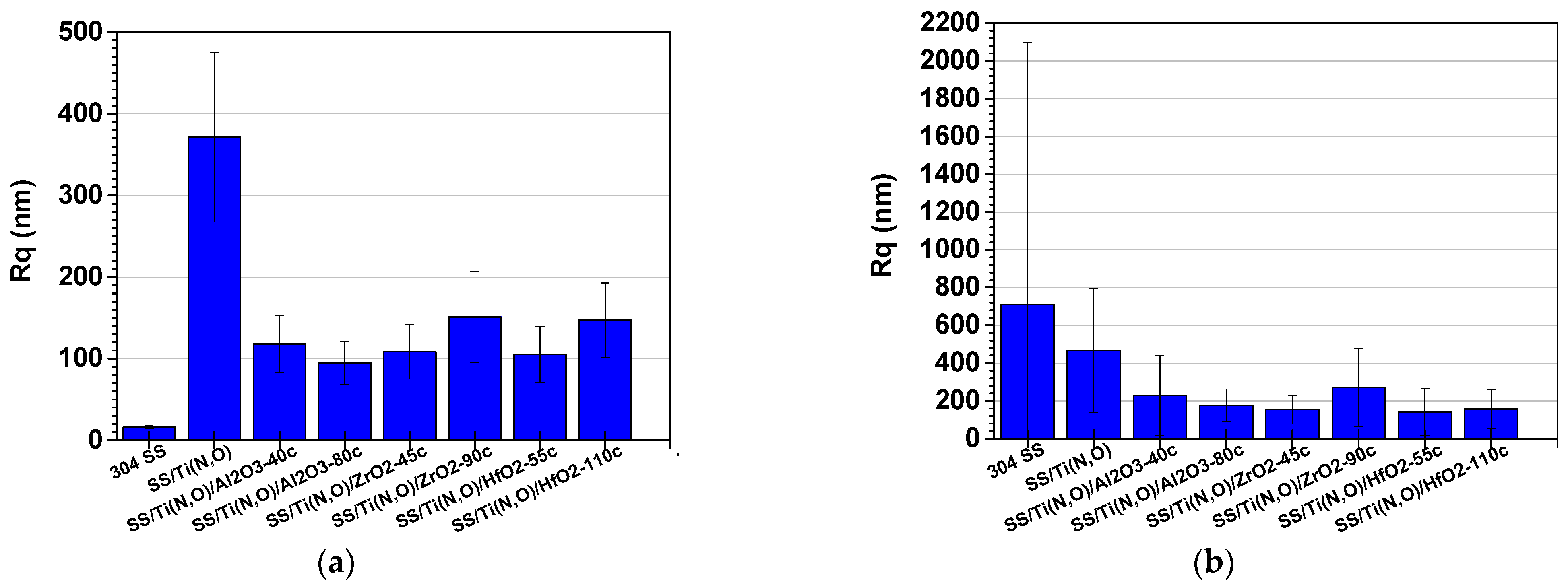
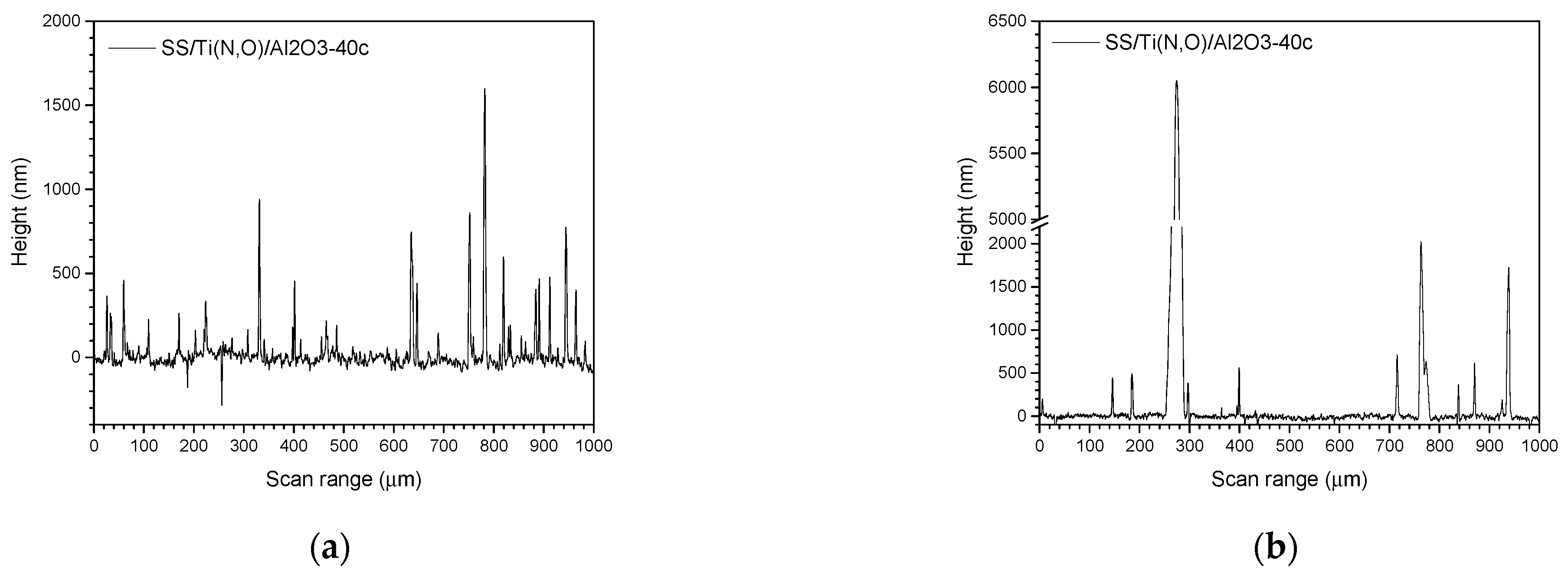
3.3. Surface Roughness before and after the Corrosive Attack
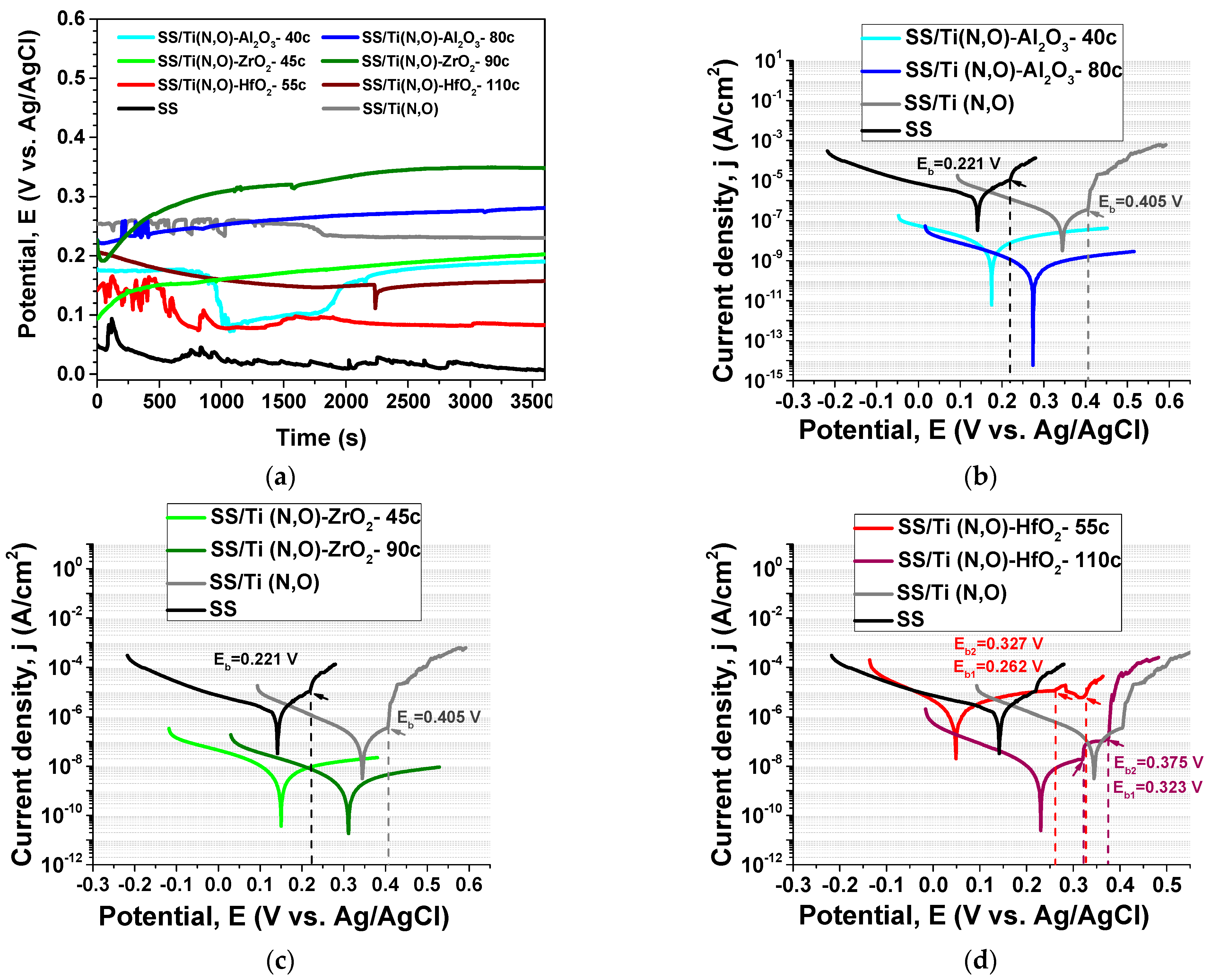
3.4. Electrochemical Evaluation—Tafel Plots
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marin, E.; Guzman, L.; Lanzutti, A.; Fedrizzi, L.; Saikkonen, M. Chemical and electrochemical characterization of hybrid PVD + ALD hard coatings on tool steel. Electrochem. Commun. 2009, 11, 2060–2063. [Google Scholar] [CrossRef]
- Perillo, P.M. Corrosion Behavior of Coatings of Titanium Nitride and Titanium-Titanium Nitride on Steel Substrates. Corrosion 2006, 62, 182–185. [Google Scholar] [CrossRef]
- Subramanian, B.; Muraleedharan, C.; Ananthakumar, R.; Jayachandran, M. A comparative study of titanium nitride (TiN), titanium oxy nitride (TiON) and titanium aluminum nitride (TiAlN), as surface coatings for bio implants. Surf. Coat. Technol. 2011, 205, 5014–5020. [Google Scholar] [CrossRef]
- El-Fattah, H.A.A.; El-Mahallawi, I.S.; Shazly, M.H.; Khalifa, W.A. Optical Properties and Microstructure of TiNxOy and TiN Thin Films before and after Annealing at Different Conditions. Coatings 2019, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Rtimi, S.; Baghriche, O.; Sanjines, R.; Pulgarin, C.; Bensimon, M.; Kiwi, J. TiON and TiON-Ag sputtered surfaces leading to bacterial inactivation under indoor actinic light. J. Photochem. Photobiol. A Chem. 2013, 256, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Duta, O.C.; Ficai, D.; Ficai, A.; Andronescu, E.; Beshchasna, N.; Saqib, M.; Opitz, J.; Kraśkiewicz, H.; Wasyluk, Ł.; Kuzmin, O.; et al. Titanium oxynitride coatings deposited by magnetron sputtering for improvement of cardiovascular stent design. In Proceedings of the World Congress on New Technologies, Madrid, Spain, 19–21 August 2018; pp. 18–20. [Google Scholar] [CrossRef]
- Braic, L.; Vasilantonakis, N.; Mihai, A.; Villar-Garcia, I.J.; Fearn, S.; Zou, B.; Alford, N.M.; Doiron, B.; Oulton, R.F.; Maier, S.A.; et al. Titanium Oxynitride Thin Films with Tunable Double Epsilon-Near-Zero Behavior for Nanophotonic Applications. ACS Appl. Mater. Interfaces 2017, 9, 29857–29862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Xinxin, G. A review of metal oxynitrides for photocatalysis. Inorg. Chem. Front. 2016, 3, 578–590. [Google Scholar] [CrossRef]
- Pana, I.; Braic, V.; Dinu, M.; Mouele, E.S.M.; Parau, A.C.; Petrik, L.F.; Braic, M. In Vitro Corrosion of Titanium Nitride and Oxynitride-Based Biocompatible Coatings Deposited on Stainless Steel. Coatings 2020, 10, 710. [Google Scholar] [CrossRef]
- Esaka, F.; Furuya, K.; Shimada, H.; Imamura, M.; Matsubayashi, N.; Sato, H.; Nishijima, A.; Kawana, A.; Ichimura, H.; Kikuchi, T. Comparison of surface oxidation of titanium nitride and chromium nitride films studied by x-ray absorption and photoelectron spectroscopy. J. Vac. Sci. Technol. A 1997, 15, 2521–2528. [Google Scholar] [CrossRef]
- Wang, L.; Northwood, D.; Nie, X.; Housden, J.; Spain, E.; Leyland, A.; Matthews, A. Corrosion properties and contact resistance of TiN, TiAlN and CrN coatings in simulated proton exchange membrane fuel cell environments. J. Power Sources 2010, 195, 3814–3821. [Google Scholar] [CrossRef]
- Mouele, E.M.; Tijani, J.; Badmus, K.; Pereao, O.; Babajide, O.; Zhang, C.; Shao, T.; Sosnin, E.; Tarasenko, V.; Fatoba, O.; et al. Removal of Pharmaceutical Residues from Water and Wastewater Using Dielectric Barrier Discharge Methods—A Review. Int. J. Environ. Res. Public Health 2021, 18, 1683. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Tijani, J.O.; Badmus, K.O.; Pereao, O.; Babajide, O.; Fatoba, O.O.; Zhang, C.; Shao, T.; Sosnin, E.; Tarasenko, V.; et al. A critical review on ozone and co-species, generation and reaction mechanisms in plasma induced by dielectric barrier discharge technologies for wastewater remediation. J. Environ. Chem. Eng. 2021, 9, 105758. [Google Scholar] [CrossRef]
- Badmus, K.O.; Irakoze, N.; Adeniyi, O.R.; Petrik, L. Synergistic advance Fenton oxidation and hydrodynamic cavitation treatment of persistent organic dyes in textile wastewater. J. Environ. Chem. Eng. 2019, 8, 103521. [Google Scholar] [CrossRef]
- Badmus, K.O.; Tijani, J.O.; Massima, E.; Petrik, L. Treatment of persistent organic pollutants in wastewater using hydrodynamic cavitation in synergy with advanced oxidation process. Environ. Sci. Pollut. Res. 2018, 25, 7299–7314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Jin, Z.-X.; Zhang, W.; Bai, Y.-H.; Cao, Y.-Q.; Li, W.-M.; Di Wu, D.; Li, A.-D. Comparison of chemical stability and corrosion resistance of group IV metal oxide films formed by thermal and plasma-enhanced atomic layer deposition. Sci. Rep. 2019, 9, 10438. [Google Scholar] [CrossRef] [Green Version]
- Díaz, B.; Härkönen, E.; Światowska, J.; Maurice, V.; Seyeux, A.; Marcus, P.; Ritala, M. Low-temperature atomic layer deposition of Al2O3 thin coatings for corrosion protection of steel: Surface and electrochemical analysis. Corros. Sci. 2011, 53, 2168–2175. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, C.; Ding, G.; Wu, J. Effects of aging on the characteristics of TiNiPd shape memory alloy thin films. Mater. Charact. 2008, 59, 957–960. [Google Scholar] [CrossRef]
- Abbas, A.; Hung, H.-Y.; Lin, P.-C.; Yang, K.-C.; Chen, M.-C.; Lin, H.-C.; Han, Y.-Y. Atomic layer deposited TiO2 films on an equiatomic NiTi shape memory alloy for biomedical applications. J. Alloys Compd. 2021, 886, 161282. [Google Scholar] [CrossRef]
- Chu, C.; Chung, C.; Lin, P. DSC study of the effect of aging temperature on the reverse martensitic transformation in porous Ni-rich NiTi shape memory alloy fabricated by combustion synthesis. Mater. Lett. 2005, 59, 404–407. [Google Scholar] [CrossRef]
- Baradaran, S.; Basirun, W.; Zalnezhad, E.; Hamdi, M.; Sarhan, A.A.; Alias, Y. Fabrication and deformation behaviour of multilayer Al2O3/Ti/TiO2 nanotube arrays. J. Mech. Behav. Biomed. Mater. 2013, 20, 272–282. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Marshall, C.L. Atomic layer deposition: Catalytic preparation and modification technique for the next generation. Chin. J. Catal. 2019, 40, 1311–1323. [Google Scholar] [CrossRef]
- Marin, E.; Guzman, L.; Lanzutti, A.; Ensinger, W.; Fedrizzi, L. Multilayer Al2O3/TiO2 Atomic Layer Deposition coatings for the corrosion protection of stainless steel. Thin Solid Films 2012, 522, 283–288. [Google Scholar] [CrossRef]
- Peron, M.; Cogo, S.; Bjelland, M.; Bin Afif, A.; Dadlani, A.; Greggio, E.; Berto, F.; Torgersen, J. On the evaluation of ALD TiO2, ZrO2 and HfO2 coatings on corrosion and cytotoxicity performances. J. Magnes. Alloy. 2021, 9, 1806–1819. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Luo, L.; Liu, Y.; Wan, J. Microstructure and corrosion resistance of TiN/TiO2 nano-composite film on AZ31 magnesium alloy. Surf. Coat. Technol. 2020, 406, 126681. [Google Scholar] [CrossRef]
- Shahmohammadi, M.; Sun, Y.; Yuan, J.C.-C.; Mathew, M.T.; Sukotjo, C.; Takoudis, C.G. In vitro corrosion behavior of coated Ti6Al4V with TiO2, ZrO2, and TiO2/ZrO2 mixed nanofilms using atomic layer deposition for dental implants. Surf. Coat. Technol. 2022, 444, 128686. [Google Scholar] [CrossRef]
- Peron, M.; Bertolini, R.; Cogo, S. On the corrosion, stress corrosion and cytocompatibility performances of ALD TiO2 and ZrO2 coated magnesium alloys. J. Mech. Behav. Biomed. Mater. 2021, 125, 104945. [Google Scholar] [CrossRef]
- Fedel, M.; Deflorian, F. Electrochemical characterization of atomic layer deposited Al2O3 coatings on AISI 316L stainless steel. Electrochim. Acta 2016, 203, 404–415. [Google Scholar] [CrossRef]
- Díaz, B.; Światowska, J.; Maurice, V.; Seyeux, A.; Härkönen, E.; Ritala, M.; Tervakangas, S.; Kolehmainen, J.; Marcus, P. Tantalum oxide nanocoatings prepared by atomic layer and filtered cathodic arc deposition for corrosion protection of steel: Comparative surface and electrochemical analysis. Electrochim. Acta 2013, 90, 232–245. [Google Scholar] [CrossRef]
- Daubert, J.S.; Hill, G.T.; Gotsch, H.N.; Gremaud, A.P.; Ovental, J.S.; Williams, P.S.; Oldham, C.J.; Parsons, G.N. Corrosion Protection of Copper Using Al2O3, TiO2, ZnO, HfO2, and ZrO2 Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2017, 9, 4192–4201. [Google Scholar] [CrossRef]
- Gao, Y.; Walsh, M.; Liang, X. Atomic layer deposited conformal ceramic coatings for anti-corrosion of Ag nanoparticles. Appl. Surf. Sci. 2020, 532, 147374. [Google Scholar] [CrossRef]
- Mondal, J.; Marques, A.; Aarik, L.; Kozlova, J.; Simões, A.; Sammelselg, V. Development of a thin ceramic-graphene nanolaminate coating for corrosion protection of stainless steel. Corros. Sci. 2016, 105, 161–169. [Google Scholar] [CrossRef]
- Díaz, B.; Światowska, J.; Maurice, V.; Seyeux, A.; Normand, B.; Härkönen, E.; Ritala, M.; Marcus, P. Electrochemical and time-of-flight secondary ion mass spectrometry analysis of ultra-thin metal oxide (Al2O3 and Ta2O5) coatings deposited by atomic layer deposition on stainless steel. Electrochim. Acta 2011, 56, 10516–10523. [Google Scholar] [CrossRef]
- Chai, Z.; Liu, Y.; Li, J.; Lu, X.; He, D. Ultra-thin Al2O3 films grown by atomic layer deposition for corrosion protection of copper. RSC Adv. 2014, 4, 50503–50509. [Google Scholar] [CrossRef]
- Behzadnasab, M.; Mirabedini, S.; Kabiri, K.; Jamali, S. Corrosion performance of epoxy coatings containing silane treated ZrO2 nanoparticles on mild steel in 3.5% NaCl solution. Corros. Sci. 2011, 53, 89–98. [Google Scholar] [CrossRef]
- Cai, Y.; Quan, X.; Li, G.; Gao, N. Anticorrosion and Scale Behaviors of Nanostructured ZrO2–TiO2 Coatings in Simulated Geothermal Water. Ind. Eng. Chem. Res. 2016, 55, 11480–11494. [Google Scholar] [CrossRef]
- Staišiūnas, L.; Kalinauskas, P.; Juzeliūnas, E.; Grigucevičienė, A.; Leinartas, K.; Niaura, G.; Stanionytė, S.; Selskis, A. Silicon Passivation by Ultrathin Hafnium Oxide Layer for Photoelectrochemical Applications. Front. Chem. 2022, 10, 859023. [Google Scholar] [CrossRef] [PubMed]
- Bodunrin, M.O.; Chown, L.H.; van der Merwe, J.W.; Alaneme, K.K.; Oganbule, C.; Klenam, D.E.; Mphasha, N.P. Corrosion behavior of titanium alloys in acidic and saline media: Role of alloy design, passivation integrity, and electrolyte modification. Corros. Rev. 2020, 38, 25–47. [Google Scholar] [CrossRef]
- Seethammaraju, S.; Rangarajan, M. Corrosion of stainless steels in acidic, neutral and alkaline saline media: Electrochemical and microscopic analysis. IOP Conf. Ser. Mater. Sci. Eng. 2019, 577, 012188. [Google Scholar] [CrossRef]
- Dinu, M.; Parau, A.C.; Vladescu, A.; Kiss, A.E.; Pana, I.; Mouele, E.S.M.; Petrik, L.F.; Braic, V. Corrosion Improvement of 304L Stainless Steel by ZrSiN and ZrSi(N,O) Mono- and Double-Layers Prepared by Reactive Cathodic Arc Evaporation. Coatings 2021, 11, 1257. [Google Scholar] [CrossRef]
- Bailey, R.; Sun, Y. Corrosion and Tribocorrosion Performance of Pack-Carburized Commercially Pure Titanium with Limited Oxygen Diffusion in a 0.9% NaCl Solution. J. Bio-Tribo-Corros. 2017, 4, 6. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Chen, B.; Wang, L.; Zhu, R.; Yang, J. Hydrogen peroxide formation in water during the VUV/UV irradiation process: Impacts and mechanisms of selected anions. Environ. Res. 2021, 195, 110751. [Google Scholar] [CrossRef]
- Gorey, T.J.; Dai, Y.; Anderson, S.L.; Lee, S.; Lee, S.; Seifert, S.; Winans, R.E. Selective growth of Al2O3 on size-selected platinum clusters by atomic layer deposition. Surf. Sci. 2020, 691, 121485. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Wu, J.; Sun, J. Structure and Dielectric Property of High-k ZrO2 Films Grown by Atomic Layer Deposition Using Tetrakis(Dimethylamido)Zirconium and Ozone. Nanoscale Res. Lett. 2019, 14, 154. [Google Scholar] [CrossRef] [Green Version]
- Gonta, C.J. Atomic Layer of Deposition of Ferroelectric HfO2. J. Microelectron. Eng. Conf. 2017, 23, 12. Available online: https://scholarworks.rit.edu/ritamec/vol23/iss1/12 (accessed on 30 December 2022).
- Jafari, A.; Alam, M.H.; Das-tan, D.; Ziakhodadadian, S.; Shi, Z.; Garmestani, H.; Weidenbach, A.S.; Ţălu, Ş. Statistical, morphological, and corrosion behavior of PECVD derived cobalt oxide thin films. J. Mater. Sci. Mater. Electron. 2019, 30, 21185–21198. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical Polarization I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Delabie, A.; Caymax, M.; Maes, J.W.; Bajolet, P.; Brijs, B.; Cartier, E.; Conard, T.; De Gendt, S.; Richard, O.; Vandervorst, W.; et al. ALD HfO2 surface preparation study. MRS Online Proc. Libr. 2002, 745, 511. [Google Scholar] [CrossRef]
- Lebeau, B.; Naboulsi, I.; Michelin, L.; Marichal, C.; Rigolet, S.; Carteret, C.; Brunet, S.; Bonne, M.; Blin, J.-L. Amorphous mesostructured zirconia with high (hydro)thermal stability. RSC Adv. 2020, 10, 26165–26176. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Hsu, C.-H.; Lien, S.-Y.; Wu, W.-Y.; Ou, S.-L.; Chen, S.-Y.; Huang, W.; Zhu, W.-Z.; Xiong, F.-B.; Zhang, S. Temperature-Dependent HfO2/Si Interface Structural Evolution and its Mechanism. Nanoscale Res. Lett. 2019, 14, 83. [Google Scholar] [CrossRef] [Green Version]
- Prokes, S.M.; Katz, M.B.; Twigg, M.E. Growth of crystalline Al2O3 via thermal atomic layer deposition: Nanomaterial phase stabilization. APL Mater. 2014, 2, 032105. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Leskela, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- Reclaru, L.; Eschler, P.-Y.; Lerf, R.; Blatter, A. Electrochemical corrosion and metal ion release from Co-Cr-Mo prosthesis with titanium plasma spray coating. Biomaterials 2005, 26, 4747–4756. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, K.; Keddam, M.; Srhiri, A.; Takenouti, H. Corrosion of copper in 3% NaCl solution polluted by sulphide ions. Corros. Sci. 2005, 47, 3249–3266. [Google Scholar] [CrossRef]
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control—An Introduction to Corrosion Science and Engineering, 4th ed.; Wiley Interscience, A John Wiley & Sons, Inc., Publication: Hoboken, NJ, USA, 2008. [Google Scholar]
- Khoshnaw, F.; Gubner, R. Corrosion Atlas Case Studies, Part II: Corrosion Topics. In Corrosion Atlas Series, 2021 ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. xliii–lxviii. [Google Scholar] [CrossRef]
- Ramón, J.E.; Martínez, I.; Gandía-Romero, J.M.; Soto, J. Improved Tafel-Based Potentiostatic Approach for Corrosion Rate Monitoring of Reinforcing Steel. J. Nondestruct. Eval. 2022, 41, 70. [Google Scholar] [CrossRef]
- Mobtaker, H.; Azadi, M.; Rassouli, M. The corrosion inhibition of carbon steel in 1 M HCl solution by Oestrus ovis larvae extract as a new bio–inhibitor. Heliyon 2022, 8, e12297. [Google Scholar] [CrossRef]
- Li, T.; Hu, T.; Dai, L.; Li, C.M. Metal-free photo- and electro-catalysts for hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 23674–23698. [Google Scholar] [CrossRef]
- Boudalia, M.; Fernández-Domene, R.M.; Guo, L.; Echihi, S.; Belghiti, M.E.; Zarrouk, A.; Bellaouchou, A.; Guenbour, A.; García-Antón, J. Experimental and Theoretical Tests on the Corrosion Protection of Mild Steel in Hydrochloric Acid Environment by the Use of Pyrazole Derivative. Materials 2023, 16, 678. [Google Scholar] [CrossRef] [PubMed]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef] [Green Version]
- Fouda, A.E.-A.S.; Al-Bonayan, A.M.; Eissa, M.; Eid, D.M. Electrochemical and quantum chemical studies on the corrosion inhibition of 1037 carbon steel by different types of surfactants. RSC Adv. 2022, 12, 3253–3273. [Google Scholar] [CrossRef] [PubMed]
- Baboian, R. Corrosion Tests and Standards: Application and Interpretation; ASTM Series; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Kendall, M.G. Rank Correlation Methods, 2nd ed.; Charles Griffin & Co.: London, UK, 1955. [Google Scholar]
- Chang, Y.; Ducroquet, F.; Gautier, E.; Renault, O.; Legrand, J.; Damlencourt, J.; Martin, F. Surface preparation and post thermal treatment effects on interface properties of thin Al2O3 films deposited by ALD. Microelectron. Eng. 2004, 72, 326–331. [Google Scholar] [CrossRef]
- Gosset, L.; Damlencourt, J.-F.; Renault, O.; Rouchon, D.; Holliger, P.; Ermolieff, A.; Trimaille, I.; Ganem, J.-J.; Martin, F.; Séméria, M.-N. Interface and material characterization of thin Al2O3 layers deposited by ALD using TMA/H2O. J. Non-Cryst. Solids 2002, 303, 17–23. [Google Scholar] [CrossRef]
- Kania, A.; Szindler, M.M.; Szindler, M. Structure and Corrosion Behavior of TiO2 Thin Films Deposited by ALD on a Biomedical Magnesium Alloy. Coatings 2021, 11, 70. [Google Scholar] [CrossRef]

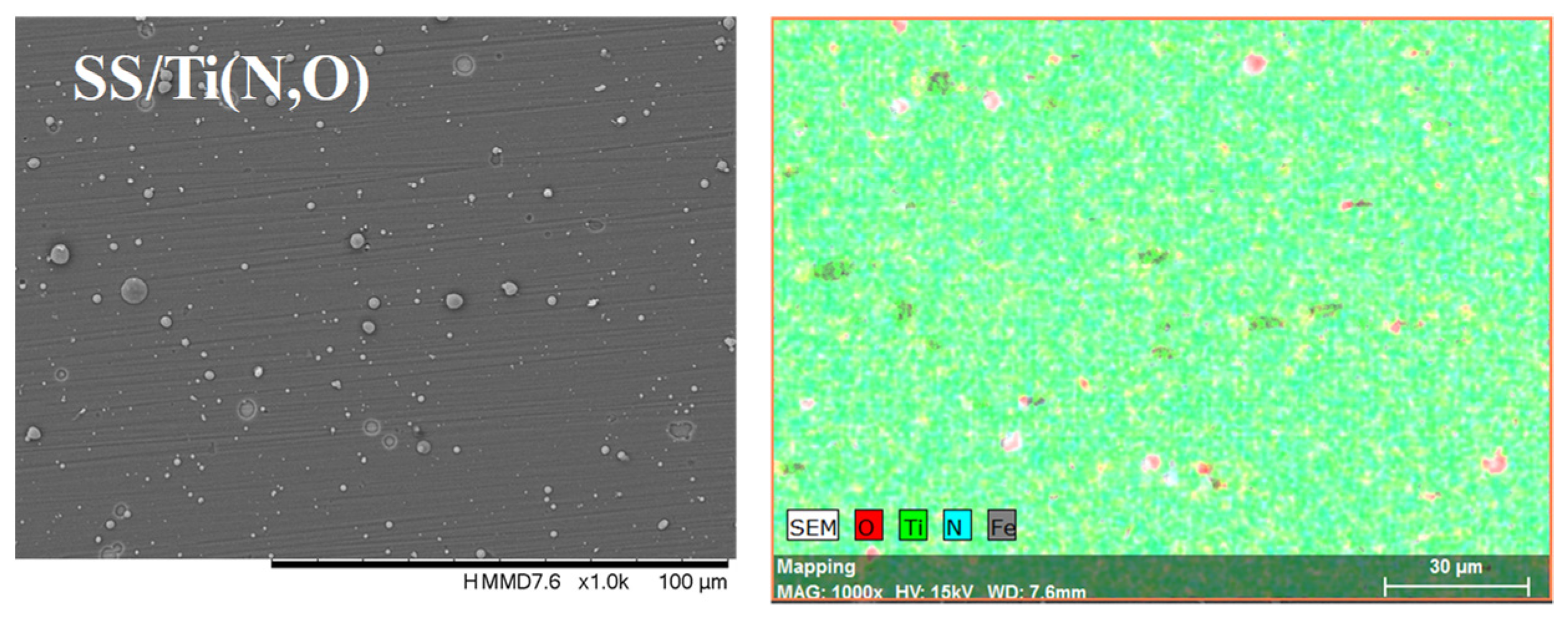
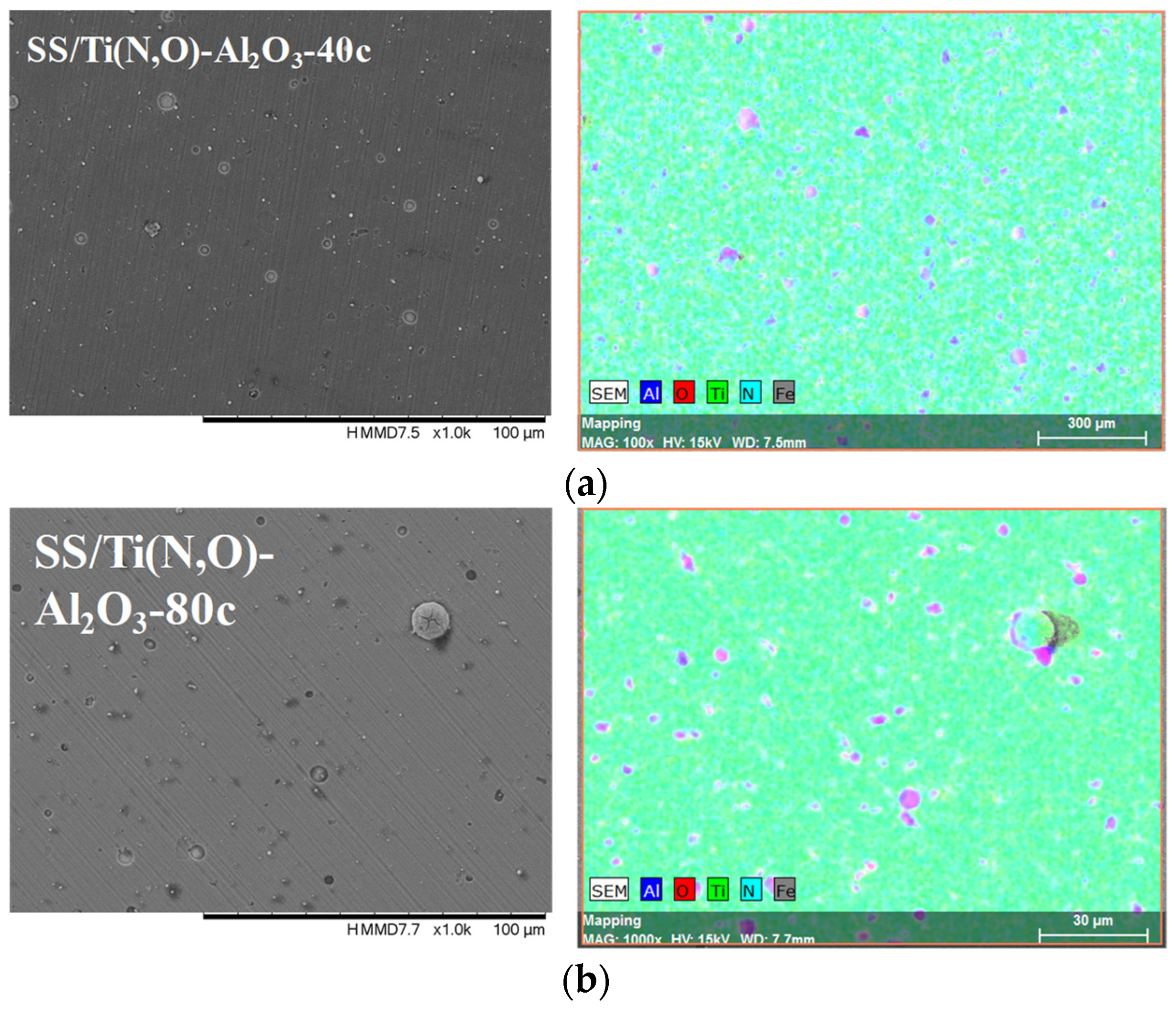
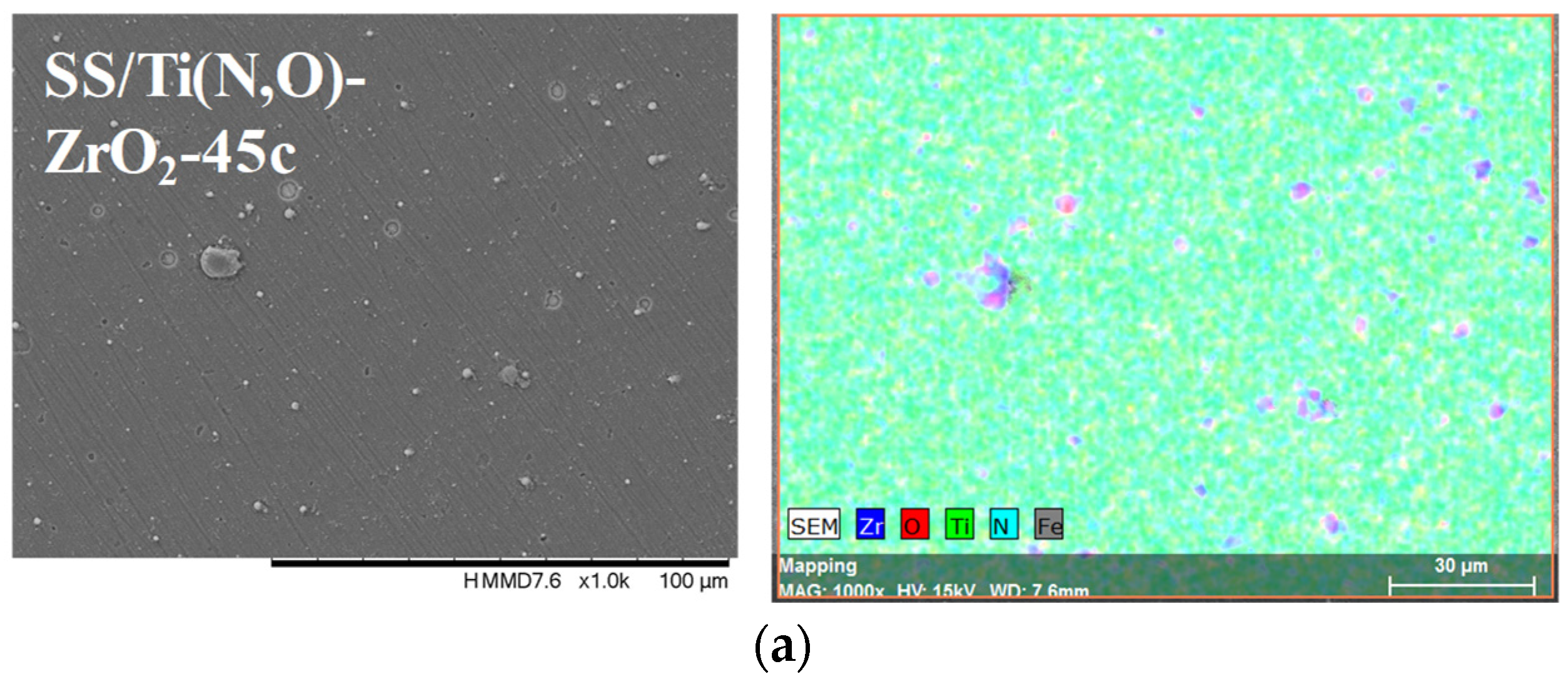

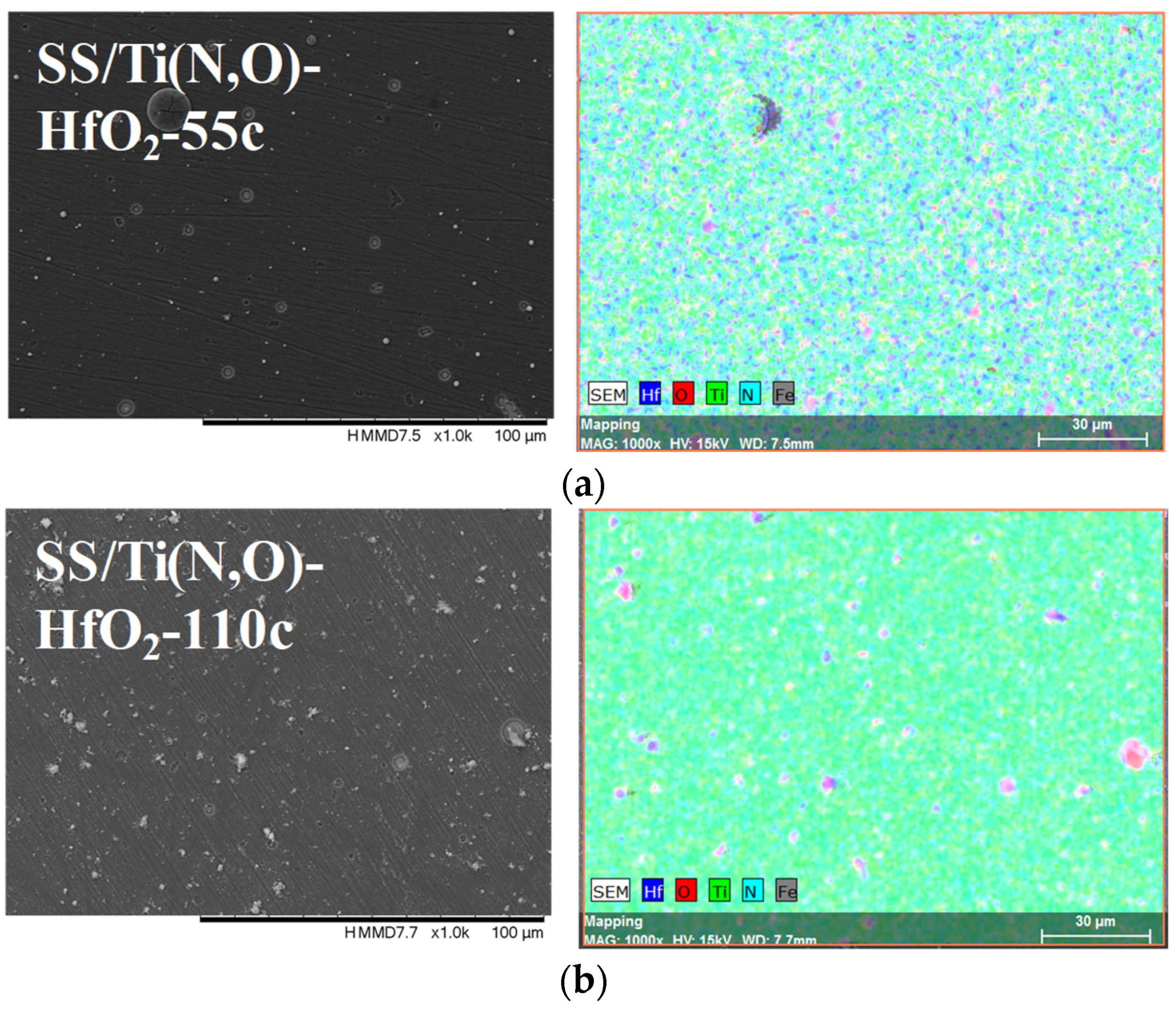



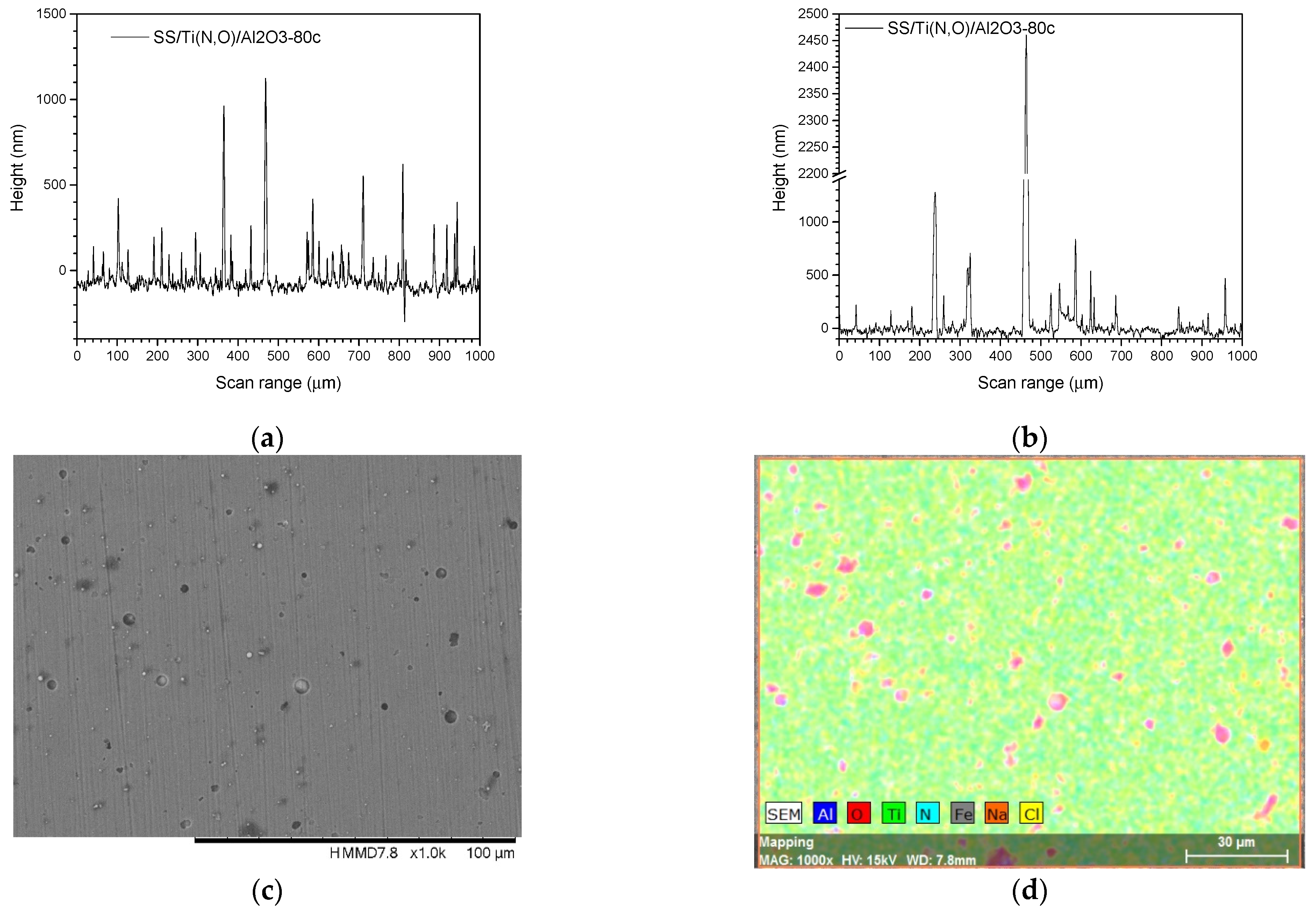
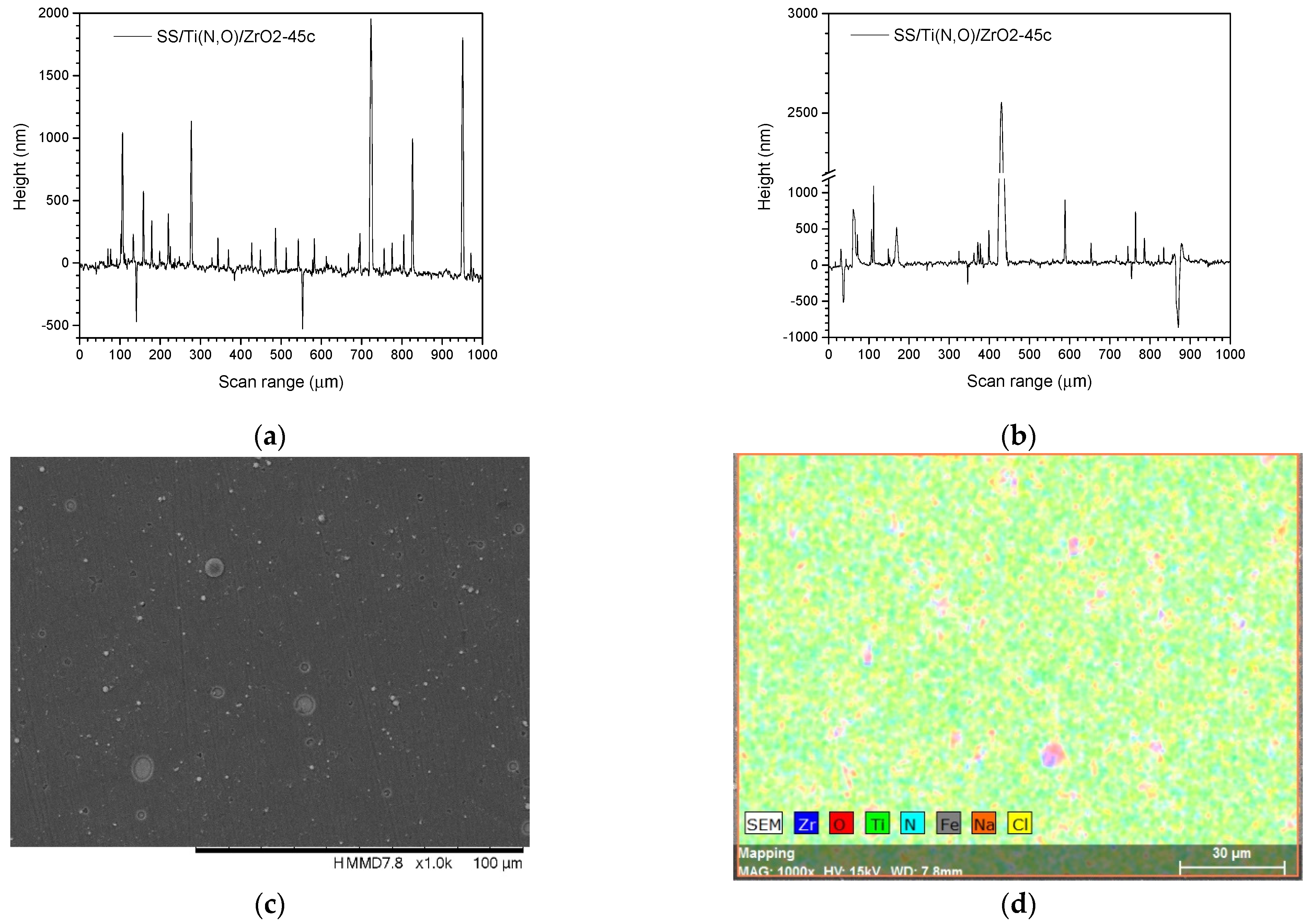
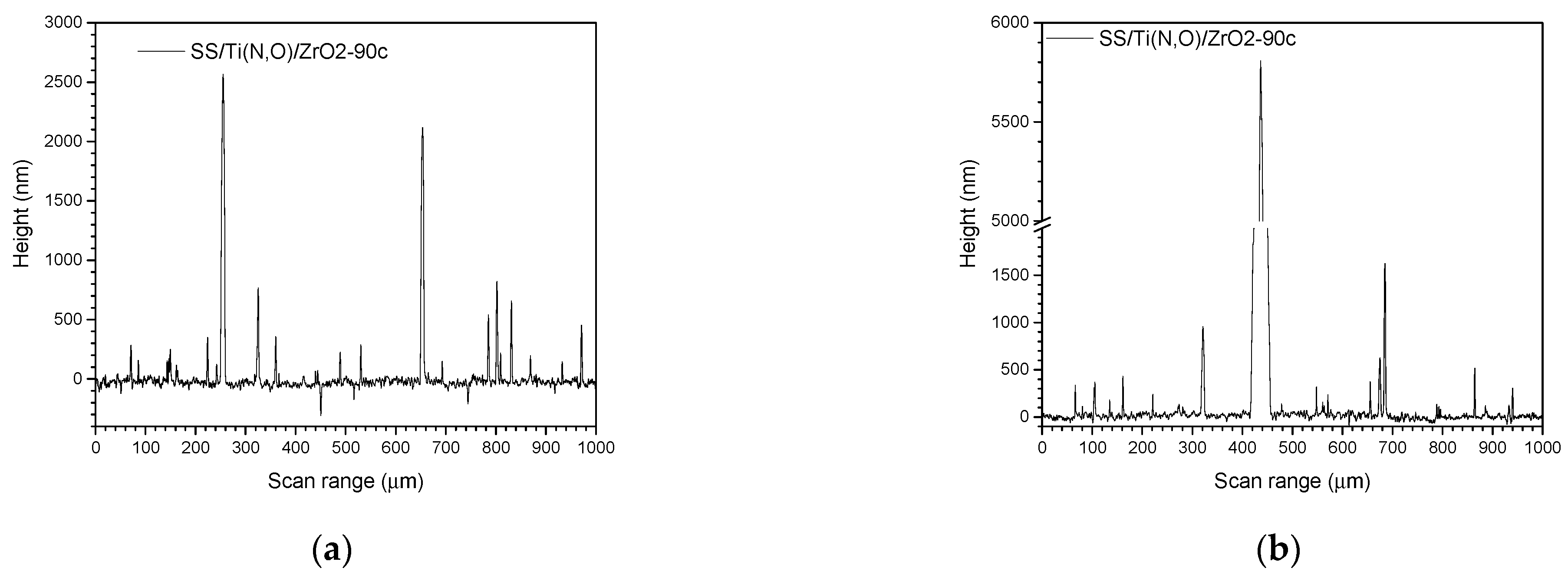
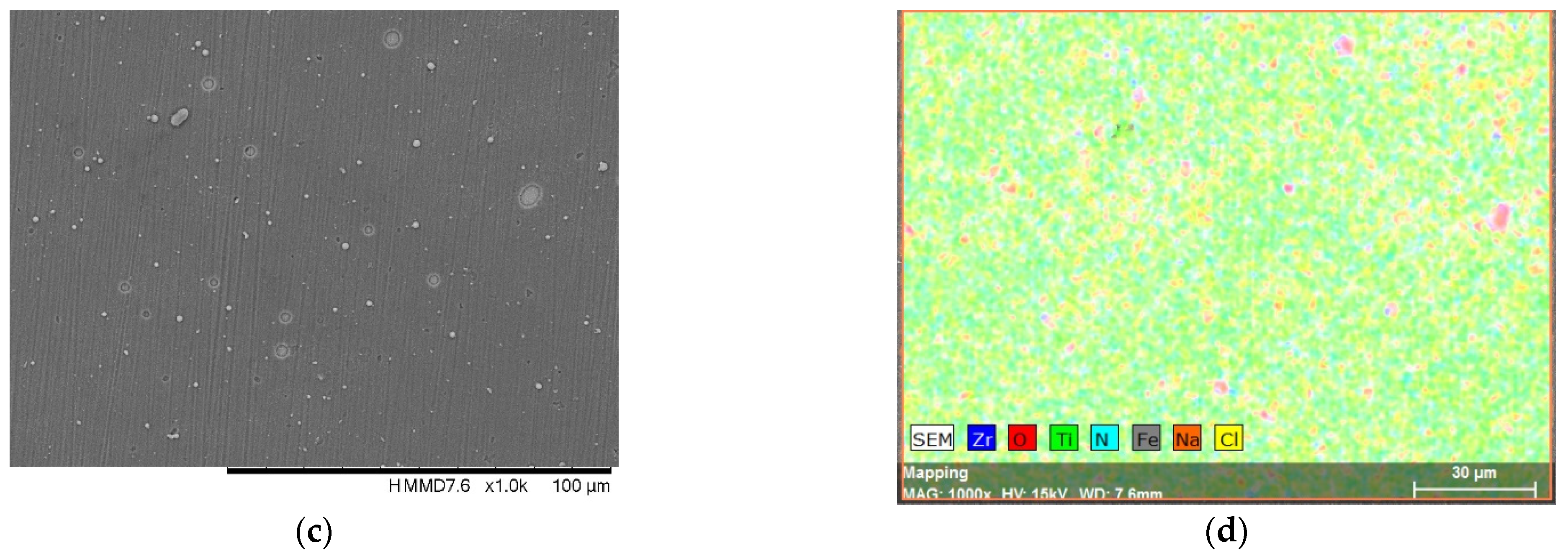
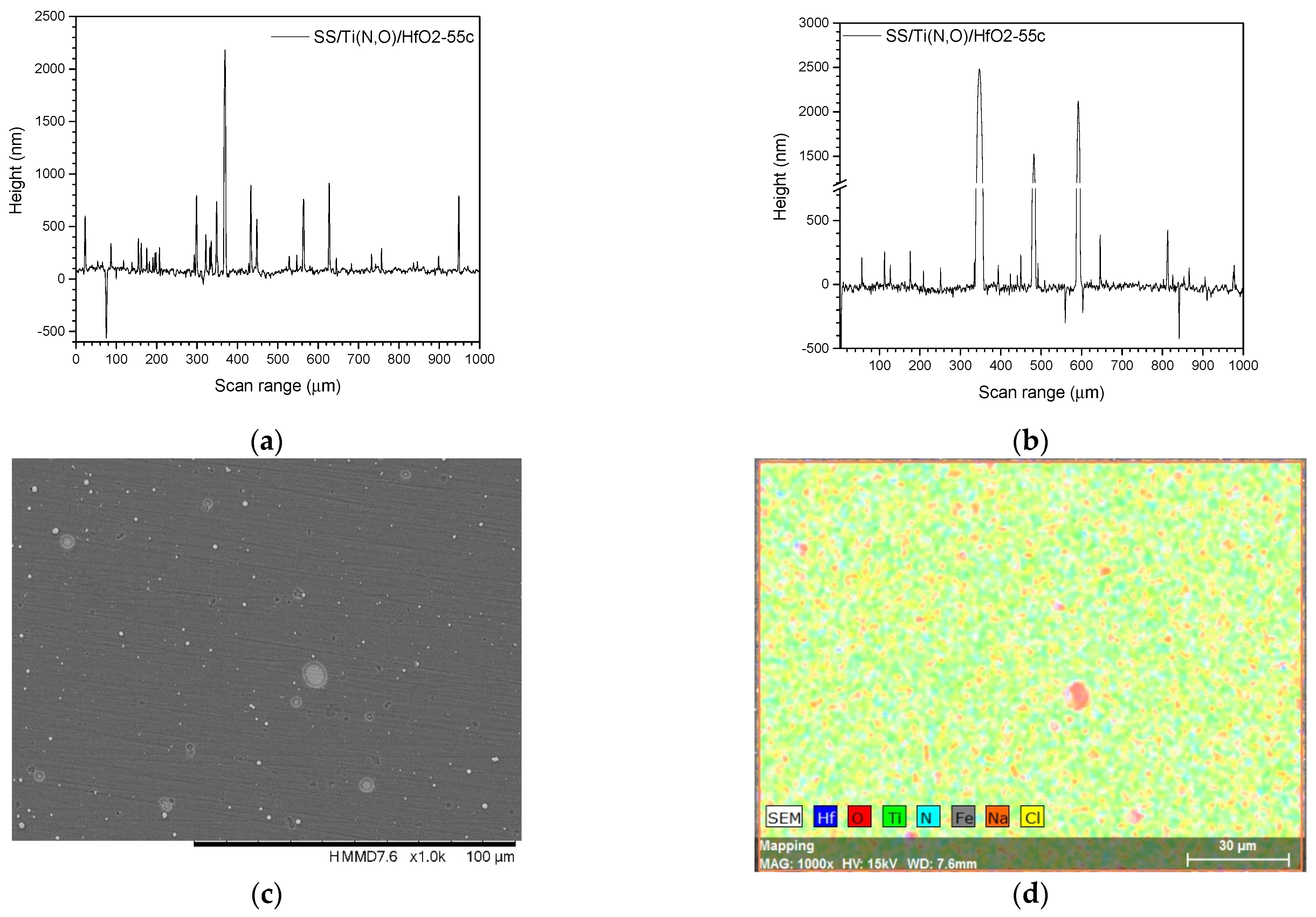
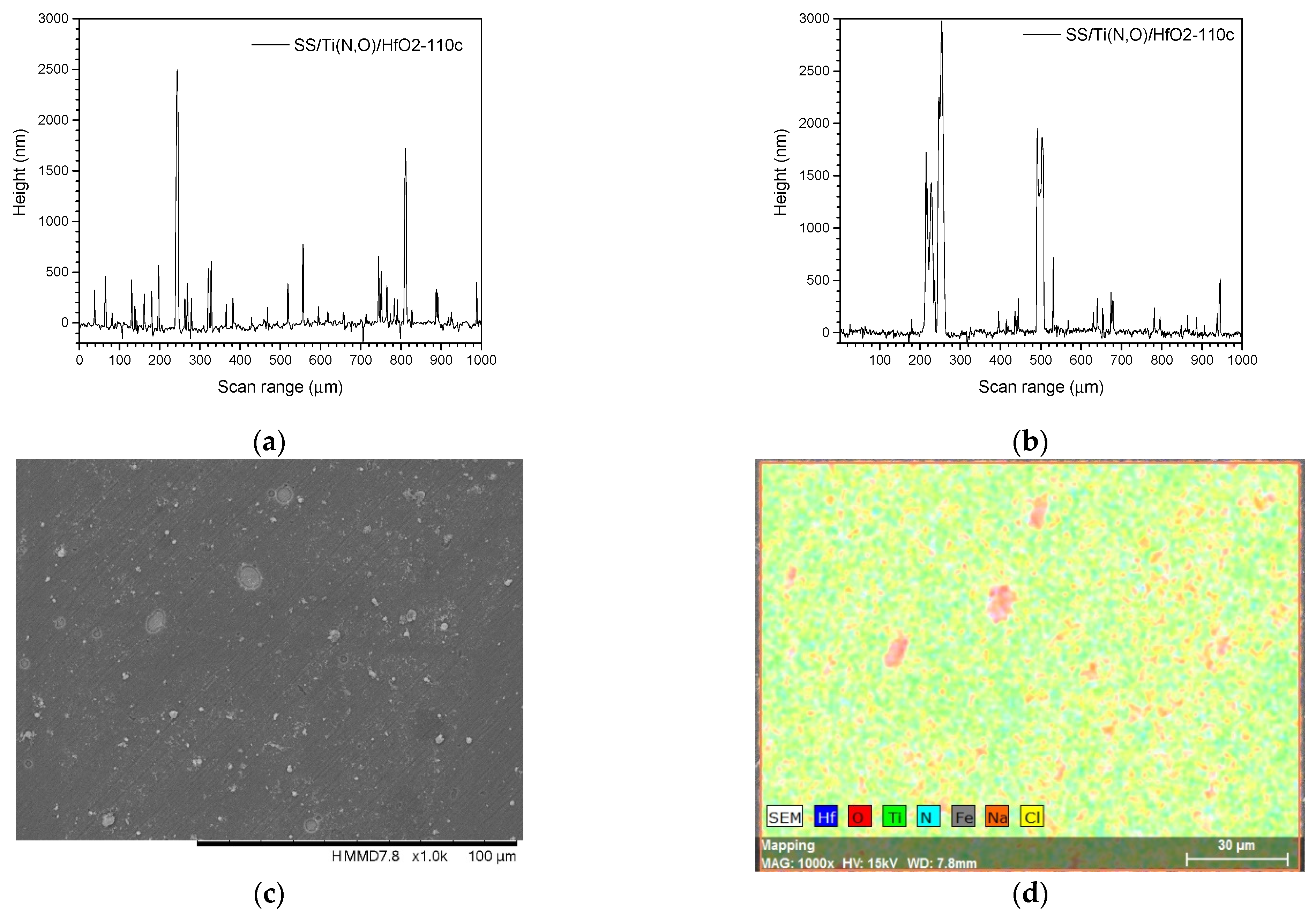
| Sample | Al (at.%) | Zr (at.%) | Hf (at.%) | O (at.%) | Ti (at.%) | N (at.%) | Fe (at.%) |
|---|---|---|---|---|---|---|---|
| SS/Ti(N,O) | - | - | - | 8.95 ± 0.53 | 52.60 ± 2.28 | 47.58 ± 1.72 | 0.87 ± 0.07 |
| SS/Ti(N,O)-Al2O3-40c | 1.802 ± 0.106 | 28.13 ± 1.72 | 41.61 ± 2.09 | 27.36 ± 1.44 | 1.09 ± 0.09 | ||
| SS/Ti(N,O)-Al2O3-80c | 2.887 ± 0.165 | 37.29 ± 2.43 | 37.11 ± 2.25 | 21.82 ± 1.25 | 0.89 ± 0.082 | ||
| SS/Ti(N,O)-ZrO2-45c | 0.710 ± 0.102 | 23.18 ± 1.28 | 48.06 ± 2.14 | 26.77 ± 1.26 | 1.288 ± 0.09 | ||
| SS/Ti(N,O)-ZrO2-90c | 1.832 ± 0.230 | 37.70 ± 2.10 | 44.28 ± 2.49 | 15.05 ± 0.75 | 1.14 ± 0.09 | ||
| SS/Ti(N,O)-HfO2-55c | 0.006 ± 0.001 | 8.05 ± 0.49 | 50.85 ± 2.18 | 39.70 ± 1.80 | 1.40 ± 0.10 | ||
| SS/Ti(N,O)-HfO2-110c | 0.540 ± 0.127 | 17.06 ± 1.01 | 47.99 ± 2.17 | 33.06 ± 1.60 | 1.36 ± 0.10 |
| Sample | Eoc (mV) | Ecorr (mV) | icorr (nA/cm2) | βc (mV) | βa (mV) | Rp (kΩ) |
|---|---|---|---|---|---|---|
| SS | 6 | 142 | 1362 | 182.98 | 67.75 | 16 |
| SS/Ti(N,O) | 230 | 345 | 174 | 144.961 | 132.523 | 173 |
| SS/Ti(N,O)-Al2O3-40c | 190 | 171 | 12.6 | 240 | 509 | 5627 |
| SS/Ti(N,O)-Al2O3-80c | 281 | 271 | 0.5 | 142 | 287 | 82,646 |
| SS/Ti(N,O)-ZrO2-45c | 202 | 152 | 10.3 | 220 | 613 | 6846 |
| SS/Ti(N,O)-ZrO2-90c | 348 | 311 | 4.5 | 231 | 654 | 16,503 |
| SS/Ti(N,O)-HfO2-55c | 83 | 48 | 4344 | 185 | 428 | 13 |
| SS/Ti(N,O)-HfO2-110c | 157 | 231 | 7.7 | 127 | 130 | 3627 |
| Sample | Rank-Ecorr | Rank-icorr | Rank-Rp | ΣRanks | Overall Rank |
|---|---|---|---|---|---|
| SS | 7 | 7 | 7 | 21 | 6 |
| SS/Ti(N,O) | 1 | 6 | 6 | 13 | 4 |
| SS/Ti(N,O)-Al2O3-40c | 5 | 5 | 4 | 14 | 5 |
| SS/Ti(N,O)-Al2O3-80c | 3 | 1 | 1 | 5 | 1 |
| SS/Ti(N,O)-ZrO2-45c | 6 | 4 | 3 | 13 | 4 |
| SS/Ti(N,O)-ZrO2-90c | 2 | 2 | 2 | 6 | 2 |
| SS/Ti(N,O)-HfO2-55c | 8 | 8 | 8 | 24 | 7 |
| SS/Ti(N,O)-HfO2-110c | 4 | 3 | 5 | 12 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, M.; Wang, K.; Mouele, E.S.M.; Parau, A.C.; Vladescu, A.; Liang, X.; Braic, V.; Petrik, L.F.; Braic, M. Effects of Film Thickness of ALD-Deposited Al2O3, ZrO2 and HfO2 Nano-Layers on the Corrosion Resistance of Ti(N,O)-Coated Stainless Steel. Materials 2023, 16, 2007. https://doi.org/10.3390/ma16052007
Dinu M, Wang K, Mouele ESM, Parau AC, Vladescu A, Liang X, Braic V, Petrik LF, Braic M. Effects of Film Thickness of ALD-Deposited Al2O3, ZrO2 and HfO2 Nano-Layers on the Corrosion Resistance of Ti(N,O)-Coated Stainless Steel. Materials. 2023; 16(5):2007. https://doi.org/10.3390/ma16052007
Chicago/Turabian StyleDinu, Mihaela, Kaiying Wang, Emile S. Massima Mouele, Anca C. Parau, Alina Vladescu (Dragomir), Xinhua Liang, Viorel Braic, Leslie Felicia Petrik, and Mariana Braic. 2023. "Effects of Film Thickness of ALD-Deposited Al2O3, ZrO2 and HfO2 Nano-Layers on the Corrosion Resistance of Ti(N,O)-Coated Stainless Steel" Materials 16, no. 5: 2007. https://doi.org/10.3390/ma16052007
APA StyleDinu, M., Wang, K., Mouele, E. S. M., Parau, A. C., Vladescu, A., Liang, X., Braic, V., Petrik, L. F., & Braic, M. (2023). Effects of Film Thickness of ALD-Deposited Al2O3, ZrO2 and HfO2 Nano-Layers on the Corrosion Resistance of Ti(N,O)-Coated Stainless Steel. Materials, 16(5), 2007. https://doi.org/10.3390/ma16052007





.jpg)



