Particle and Phase Analysis of Combusted Iron Particles for Energy Storage and Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Burner Setup
2.3. Particle Collection System
2.4. Operating Conditions and Stoichiometry
2.5. Iron Powder
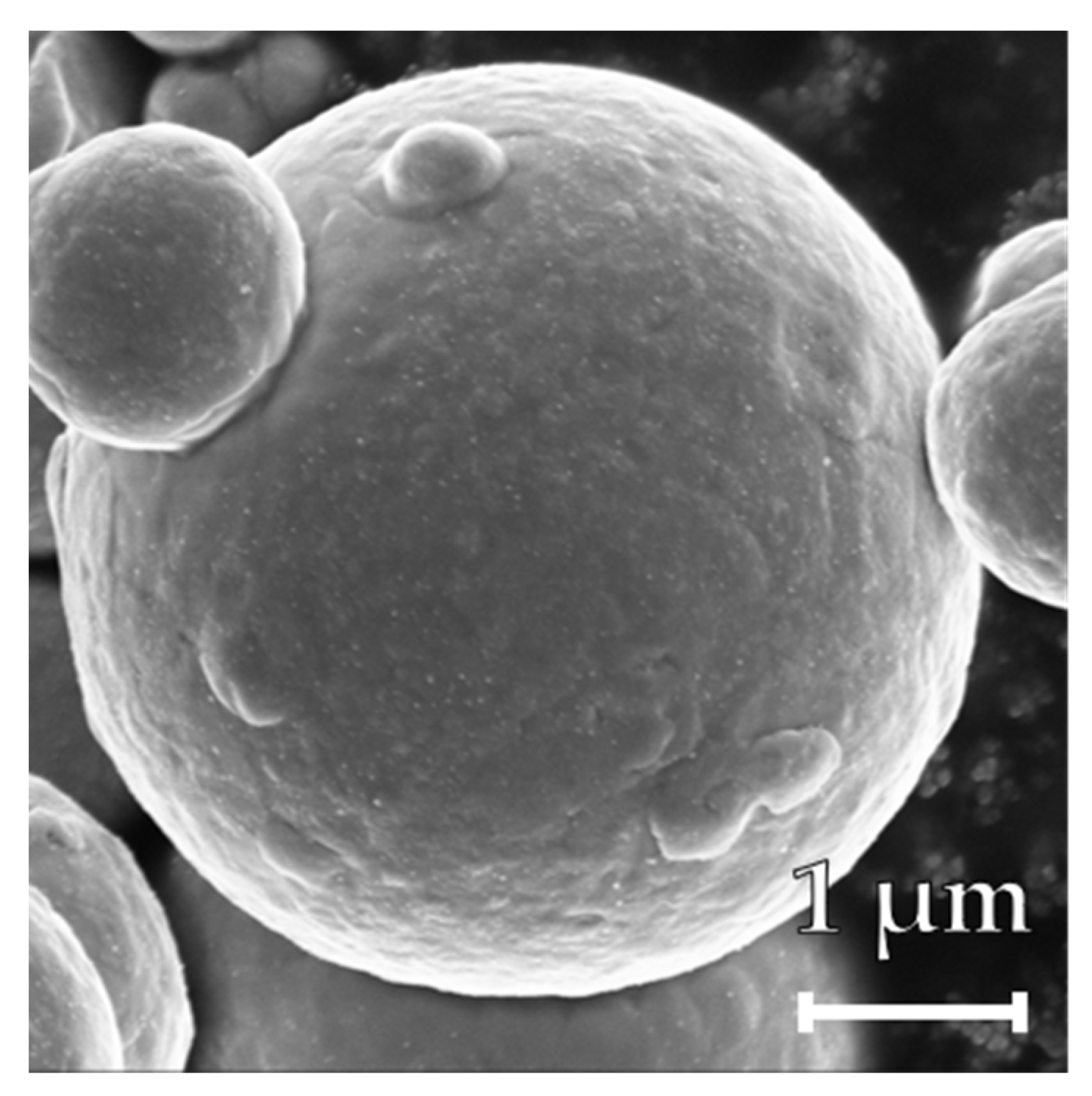
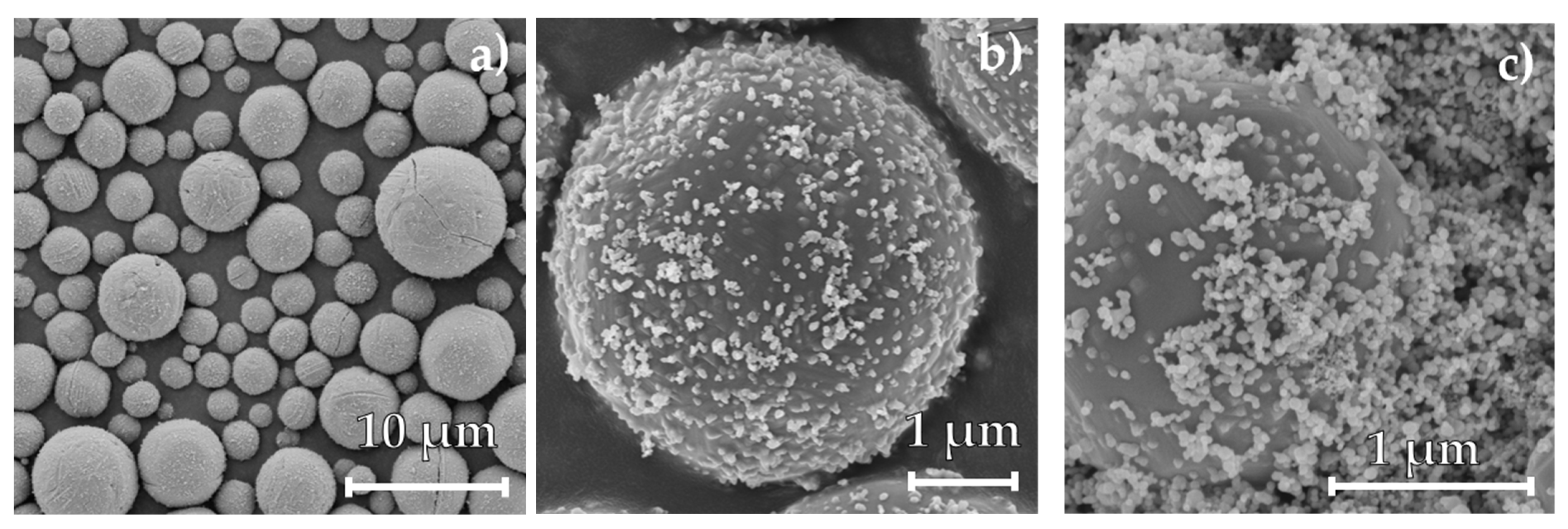
2.6. Particle Characterization
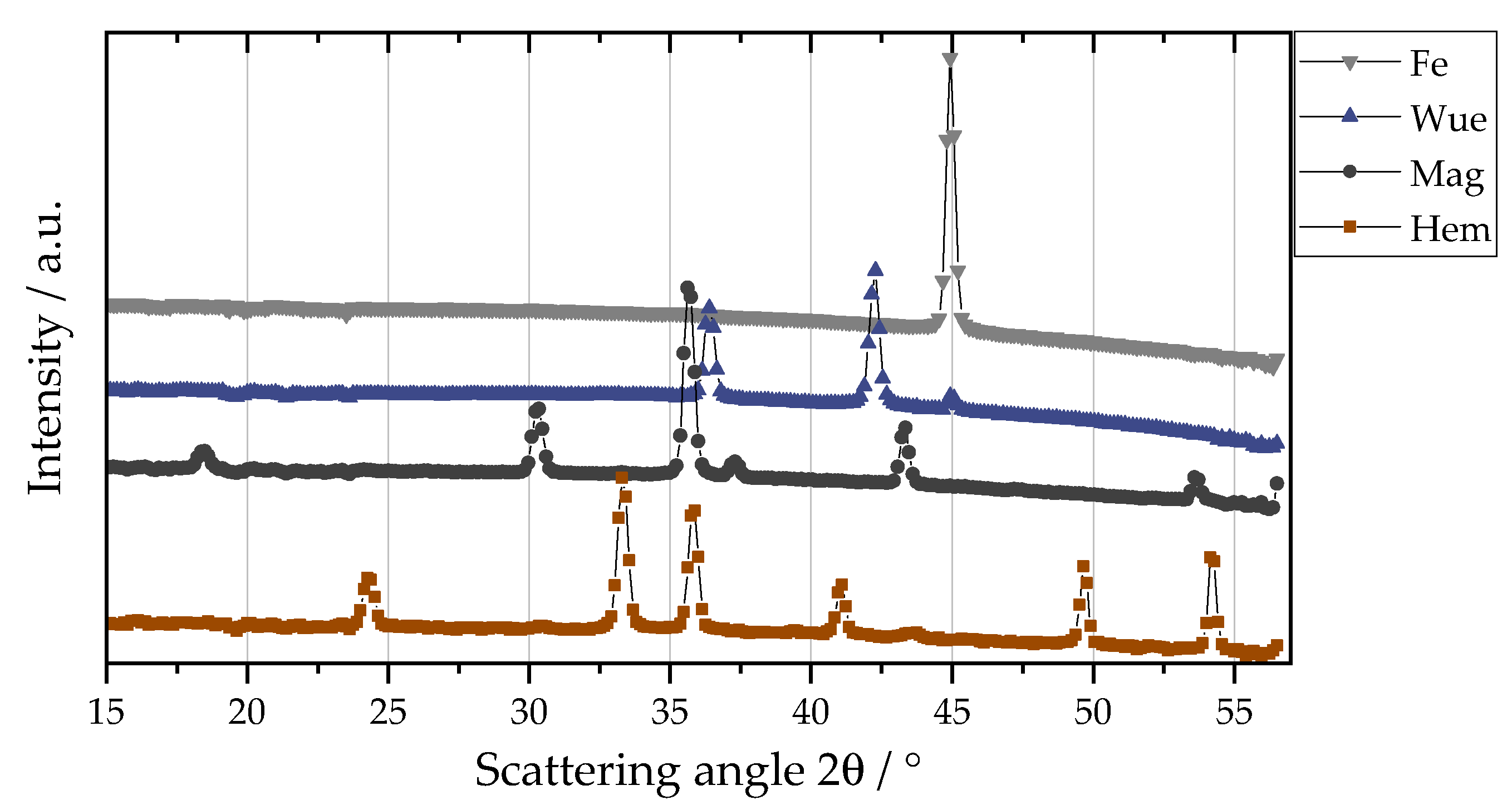
2.6.1. Calculation of the Weight Fractions of Iron and the Iron Oxide Phases in a Sample Using WAXS Calibration
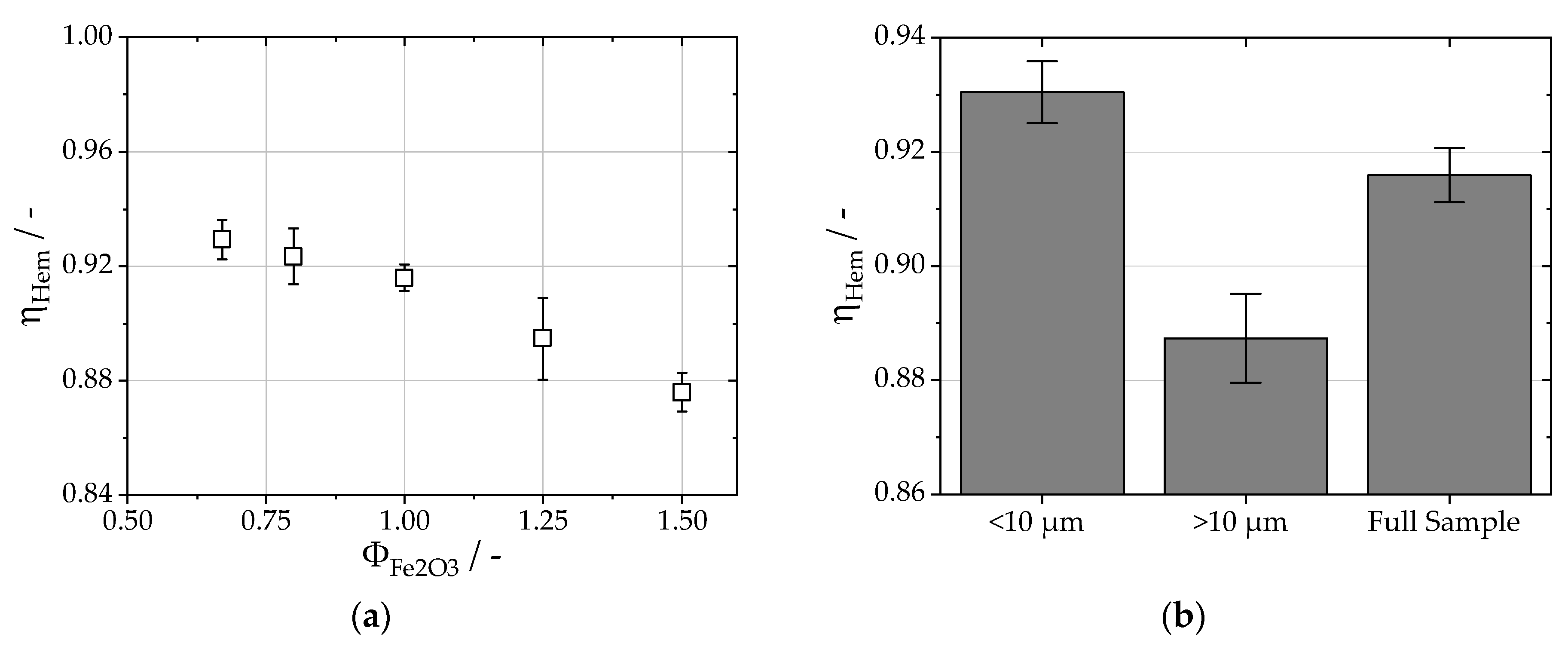
2.6.2. Calculation of the Fuel Usage Efficiency
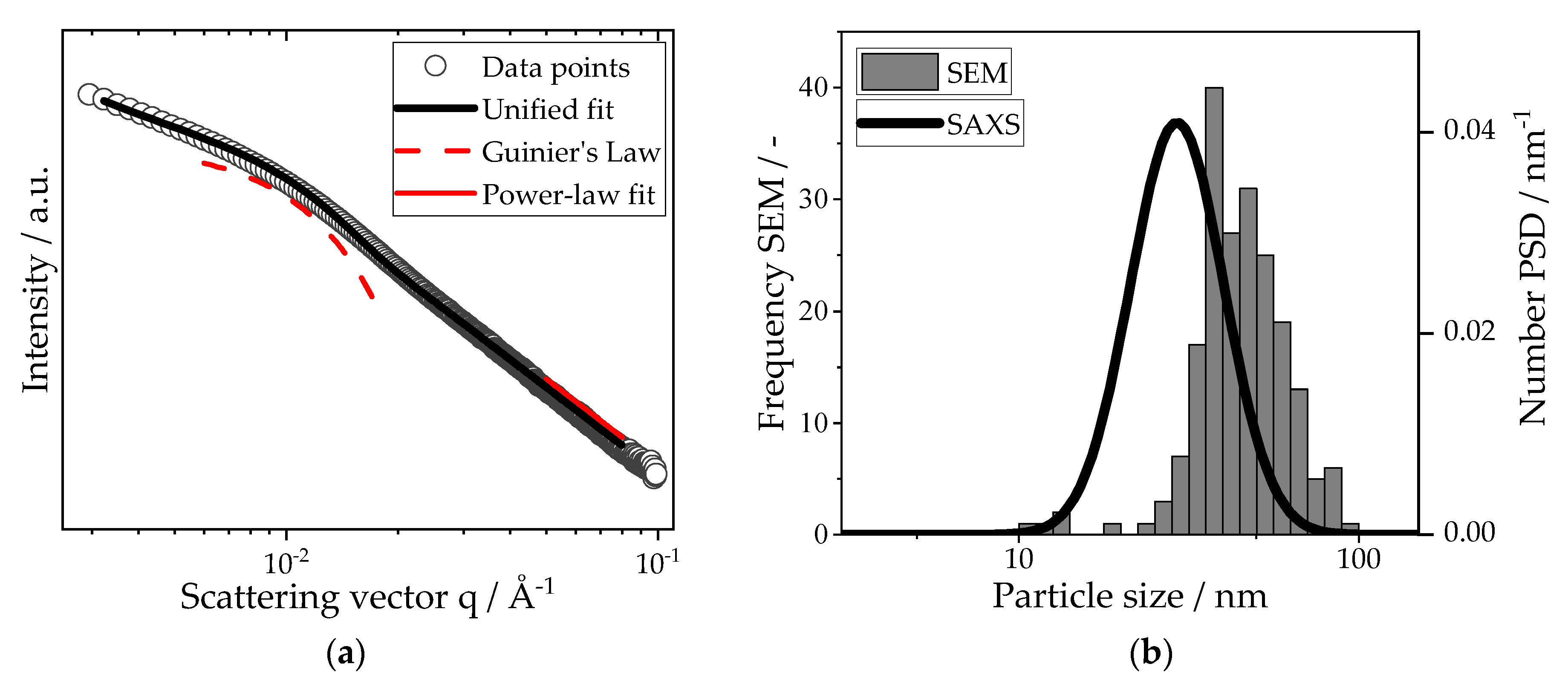
2.6.3. Small-Angle X-ray Scattering
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunt, J.; Zakeri, B.; Lopes, R.; Barbosa, P.; Nascimento, A.; de Castro, N.; Brandão, R.; Schneider, P.; Wada, Y. Existing and new arrangements of pumped-hydro storage plants. Renew. Sustain. Energy Rev. 2020, 129, 109914. [Google Scholar] [CrossRef]
- Steinmann, W.-D.; Jockenhöfer, H.; Bauer, D. Thermodynamic Analysis of High-Temperature Carnot Battery Concepts. Energy Technol. 2020, 8, 1900895. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Lei, J.; Shi, Y.; Ai, F.; Lu, Y.-C. Assessment methods and performance metrics for redox flow batteries. Nat. Energy 2021, 6, 582–588. [Google Scholar] [CrossRef]
- Bergthorson, J. Recyclable metal fuels for clean and compact zero-carbon power. Prog. Energy Combust. Sci. 2018, 68, 169–196. [Google Scholar] [CrossRef]
- Debiagi, P.; Rocha, R.; Scholtissek, A.; Janicka, J.; Hasse, C. Iron as a sustainable chemical carrier of renewable energy: Analysis of opportunities and challenges for retrofitting coal-fired power plants. Renew. Sustain. Energy Rev. 2022, 165, 112579. [Google Scholar] [CrossRef]
- Wen, D. Nanofuel as a potential secondary energy carrier. Energy Environ. Sci. 2010, 3, 591. [Google Scholar] [CrossRef]
- Bazyn, T.; Krier, H.; Glumac, N. Evidence for the transition from the diffusion-limit in aluminum particle combustion. Proc. Combust. Inst. 2007, 31, 2021–2028. [Google Scholar] [CrossRef]
- Auner, N.; Holl, S. Silicon as energy carrier—Facts and perspectives. Energy 2006, 31, 1395–1402. [Google Scholar] [CrossRef]
- Bardsley, W. The Sustainable Global Energy Economy: Hydrogen or Silicon? Nat. Resour. Res. 2008, 17, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Poletaev, N.; Khlebnikova, M. Combustion of Iron Particles Suspension in Laminar Premixed and Diffusion Flames. Combust. Sci. Technol. 2022, 194, 1356–1377. [Google Scholar] [CrossRef]
- Bergthorson, J.; Goroshin, S.; Soo, M.; Julien, P.; Palecka, J.; Frost, D.; Jarvis, D. Direct combustion of recyclable metal fuels for zero-carbon heat and power. Appl. Energy 2015, 160, 368–382. [Google Scholar] [CrossRef]
- Wiinikka, H.; Vikström, T.; Wennebro, J.; Toth, P.; Sepman, A. Pulverized Sponge Iron, a Zero-Carbon and Clean Substitute for Fossil Coal in Energy Applications. Energy Fuels 2018, 32, 9982–9989. [Google Scholar] [CrossRef]
- Tóth, P.; Ögren, Y.; Sepman, A.; Gren, P.; Wiinikka, H. Combustion behavior of pulverized sponge iron as a recyclable electrofuel. Powder Technol. 2020, 373, 210–219. [Google Scholar] [CrossRef]
- Sápi, A.; Rajkumar, T.; Kiss, J.; Kukovecz, Á.; Kónya, Z.; Somorjai, G. Metallic Nanoparticles in Heterogeneous Catalysis. Catal. Lett. 2021, 151, 2153–2175. [Google Scholar] [CrossRef]
- Hazenberg, T.; van Oijen, J. Structures and burning velocities of flames in iron aerosols. Proc. Combust. Inst. 2021, 38, 4383–4390. [Google Scholar] [CrossRef]
- Huang, Y.; Risha, G.; Yang, V.; Yetter, R. Effect of particle size on combustion of aluminum particle dust in air. Combust. Flame 2009, 156, 5–13. [Google Scholar] [CrossRef]
- Li, Y.-H.; Purwanto, A.; Chuang, B.-C. Micro-Explosion mechanism of iron hybrid Methane-Air premixed flames. Fuel 2022, 325, 124841. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Weng, W.; Qian, Y.; Lu, X.; Aldén, M.; Li, Z. Ignition and combustion behavior of single micron-sized iron particle in hot gas flow. Combust. Flame 2022, 241, 112099. [Google Scholar] [CrossRef]
- Li, S.; Sanned, D.; Huang, J.; Berrocal, E.; Cai, W.; Aldén, M.; Richter, M.; Li, Z. Stereoscopic high-speed imaging of iron microexplosions and nanoparticle-release. Opt. Express 2021, 29, 34465–34476. [Google Scholar] [CrossRef]
- Li, T.; Heck, F.; Reinauer, F.; Böhm, B.; Dreizler, A. Visualizing particle melting and nanoparticle formation during single iron particle oxidation with multi-parameter optical diagnostics. Combust. Flame 2022, 245, 112357. [Google Scholar] [CrossRef]
- Wainwright, E.; Lakshman, S.; Leong, A.; Kinsey, A.; Gibbins, J.; Arlington, S.; Sun, T.; Fezzaa, K.; Hufnagel, T.; Weihs, T. Viewing internal bubbling and microexplosions in combusting metal particles via x-ray phase contrast imaging. Combust. Flame 2019, 199, 194–203. [Google Scholar] [CrossRef]
- Tang, Y.; Kong, C.; Zong, Y.; Li, S.; Zhuo, J.; Yao, Q. Combustion of aluminum nanoparticle agglomerates: From mild oxidation to microexplosion. Proc. Combust. Inst. 2017, 36, 2325–2332. [Google Scholar] [CrossRef]
- Ning, D.; Shoshin, Y.; van Oijen, J.; Finotello, G.; de Goey, L. Burn time and combustion regime of laser-ignited single iron particle. Combust. Flame 2021, 230, 111424. [Google Scholar] [CrossRef]
- Huang, J.; Li, S.; Sanned, D.; Xu, L.; Xu, S.; Wang, Q.; Stiti, M.; Qian, Y.; Cai, W.; Berrocal, E.; et al. A detailed study on the micro-explosion of burning iron particles in hot oxidizing environments. Combust. Flame 2022, 238, 111755. [Google Scholar] [CrossRef]
- Fedoryk, M.; Stelzner, B.; Harth, S.; Trimis, D. Experimental investigation of the laminar burning velocity of iron-air flames in a tube burner. Appl. Energy Combust. Sci. 2023, 13, 100111. [Google Scholar] [CrossRef]
- Bloemacher, D. Carbonyl iron powders: Its production and new developments. Met. Powder Rep. 1990, 45, 117–119. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Beaucage, G.; Kammler, H.; Pratsinis, S. Particle size distributions from small-angle scattering using global scattering functions. J. Appl. Crystallogr. 2004, 37, 523–535. [Google Scholar] [CrossRef]
- Gröhn, A.; Pratsinis, S.; Sánchez-Ferrer, A.; Mezzenga, R.; Wegner, K. Scale-up of Nanoparticle Synthesis by Flame Spray Pyrolysis: The High-Temperature Particle Residence Time. Ind. Eng. Chem. Res. 2014, 53, 10734–10742. [Google Scholar] [CrossRef]
- Tischendorf, R.; Simmler, M.; Weinberger, C.; Bieber, M.; Reddemann, M.; Fröde, F.; Lindner, J.; Pitsch, H.; Kneer, R.; Tiemann, M.; et al. Examination of the evolution of iron oxide nanoparticles in flame spray pyrolysis by tailored in situ particle sampling techniques. J. Aerosol Sci. 2021, 154, 105722. [Google Scholar] [CrossRef]
- Hubbard, C.; Snyder, R. RIR-Measurement and Use in Quantitative XRD. Powder Diffr. 1988, 3, 74–77. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Suh, C.-Y.; Cho, S.-W.; Roh, K.-M.; Kwon, H.; Song, K.; Shon, I.-J. A new method for the identification and quantification of magnetite-maghemite mixture using conventional X-ray diffraction technique. Talanta 2012, 94, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Kontoyannis, C.; Vagenas, N. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. Analyst 2000, 125, 251–255. [Google Scholar] [CrossRef]
- Bragg, W.H. The Structure of Magnetite and the Spinels. Nature 1915, 95, 561. [Google Scholar] [CrossRef] [Green Version]
- El Mendili, Y.; Bardeau, J.-F.; Randrianantoandro, N.; Grasset, F.; Greneche, J.-M. Insights into the Mechanism Related to the Phase Transition from γ-Fe2O3 to α-Fe2O3 Nanoparticles Induced by Thermal Treatment and Laser Irradiation. J. Phys. Chem. C 2012, 116, 23785–23792. [Google Scholar] [CrossRef]
- Jette, E.; Foote, F. An X-Ray Study of the Wüstite (FeO) Solid Solutions. J. Chem. Phys. 1933, 1, 29–36. [Google Scholar] [CrossRef]
- Zhang, J.; Guyot, F. Thermal equation of state of iron and Fe 0.91 Si 0.09. Phys. Chem. Miner. 1999, 26, 206–211. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Cryst. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Allison, T.C. NIST-JANAF Thermochemical Tables-SRD 13. Natl. Inst. Stand. Technol. 2013, 10, T42S31. [Google Scholar]
- Porod, G. Die Röntgenkleinwinkelstreuung von dichtgepackten kolloiden Systemen. Kolloid-Z. 1951, 124, 83–114. [Google Scholar] [CrossRef]
- Ilavsky, J.; Jemian, P. Irena tool suite for modeling and analysis of small-angle scattering. J. Appl. Crystallogr. 2009, 42, 347–353. [Google Scholar] [CrossRef]
- Beaucage, G. Approximations Leading to a Unified Exponential/Power-Law Approach to Small-Angle Scattering. J. Appl. Crystallogr. 1995, 28, 717–728. [Google Scholar] [CrossRef]
- Sztucki, M.; Narayanan, T.; Beaucage, G. In situ study of aggregation of soot particles in an acetylene flame by small-angle x-ray scattering. J. Appl. Phys. 2007, 101, 114304. [Google Scholar] [CrossRef]
- Chen, Z.; Qu, Y.; Zeilstra, C.; van der Stel, J.; Sietsma, J.; Yang, Y. Prediction of density and volume variation of hematite ore particles during in-flight melting and reduction. J. Iron Steel Res. Int. 2019, 26, 1285–1294. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.; Goroshin, S.; Higgins, A. Combustion Time and Ignition Temperature of Iron Particles in Different Oxidizing Environments. Proceedings of the 25th International Colloquium on the Dynamics of Explosions and Reactive Systems, Paper 259. 2015. Available online: https://escholarship.mcgill.ca/concern/papers/5999n828k (accessed on 27 January 2023).
- Ning, D.; Shoshin, Y.; van Oijen, J.A.; Finotello, G.; de Goey, L.P. Critical temperature for nanoparticle cloud formation during combustion of single micron-sized iron particle. Combust. Flame 2022, 244, 112296. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Liu, G. Energy conversion and ignition of iron nanoparticles by flash. Sci. China Technol. Sci. 2017, 60, 1878–1884. [Google Scholar] [CrossRef]
- Zhao, B. Prediction of gas-particle separation efficiency for cyclones: A time-of-flight model. Sep. Purif. Technol. 2012, 85, 171–177. [Google Scholar] [CrossRef]



| AFR | kgAir/kgFe | 1.24 | 1.49 | 1.86 | 2.33 | 2.79 |
| - | 1.50 | 1.25 | 1.00 | 0.80 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchheiser, S.; Deutschmann, M.P.; Rhein, F.; Allmang, A.; Fedoryk, M.; Stelzner, B.; Harth, S.; Trimis, D.; Nirschl, H. Particle and Phase Analysis of Combusted Iron Particles for Energy Storage and Release. Materials 2023, 16, 2009. https://doi.org/10.3390/ma16052009
Buchheiser S, Deutschmann MP, Rhein F, Allmang A, Fedoryk M, Stelzner B, Harth S, Trimis D, Nirschl H. Particle and Phase Analysis of Combusted Iron Particles for Energy Storage and Release. Materials. 2023; 16(5):2009. https://doi.org/10.3390/ma16052009
Chicago/Turabian StyleBuchheiser, Simon, Max Philipp Deutschmann, Frank Rhein, Amanda Allmang, Michal Fedoryk, Björn Stelzner, Stefan Harth, Dimosthenis Trimis, and Hermann Nirschl. 2023. "Particle and Phase Analysis of Combusted Iron Particles for Energy Storage and Release" Materials 16, no. 5: 2009. https://doi.org/10.3390/ma16052009





