Effect of Two-Step Sintering on the Mechanical and Electrical Properties of 5YSZ and 8YSZ Ceramics
Abstract
:1. Introduction
2. Experiment and Test Methods
2.1. Experiment
2.2. Test Methods
3. Results and Discussion
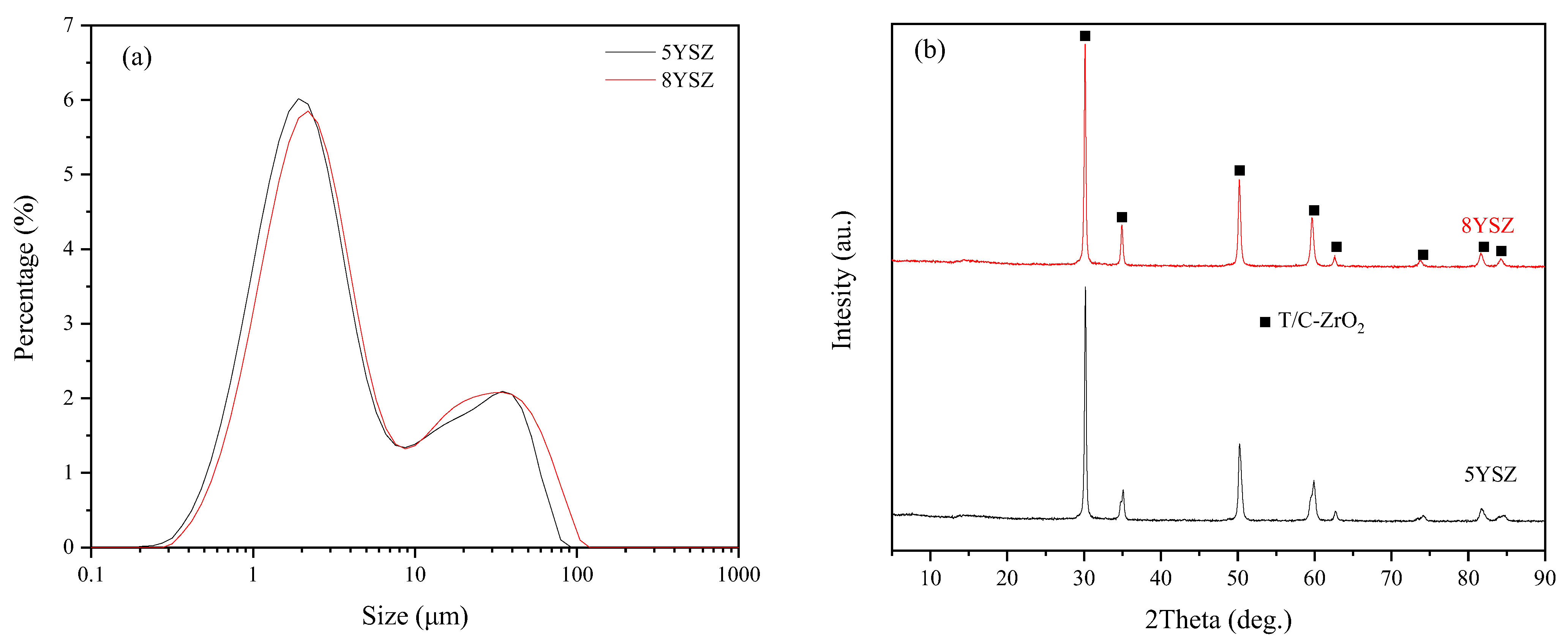
3.1. Analysis of YSZ Powders
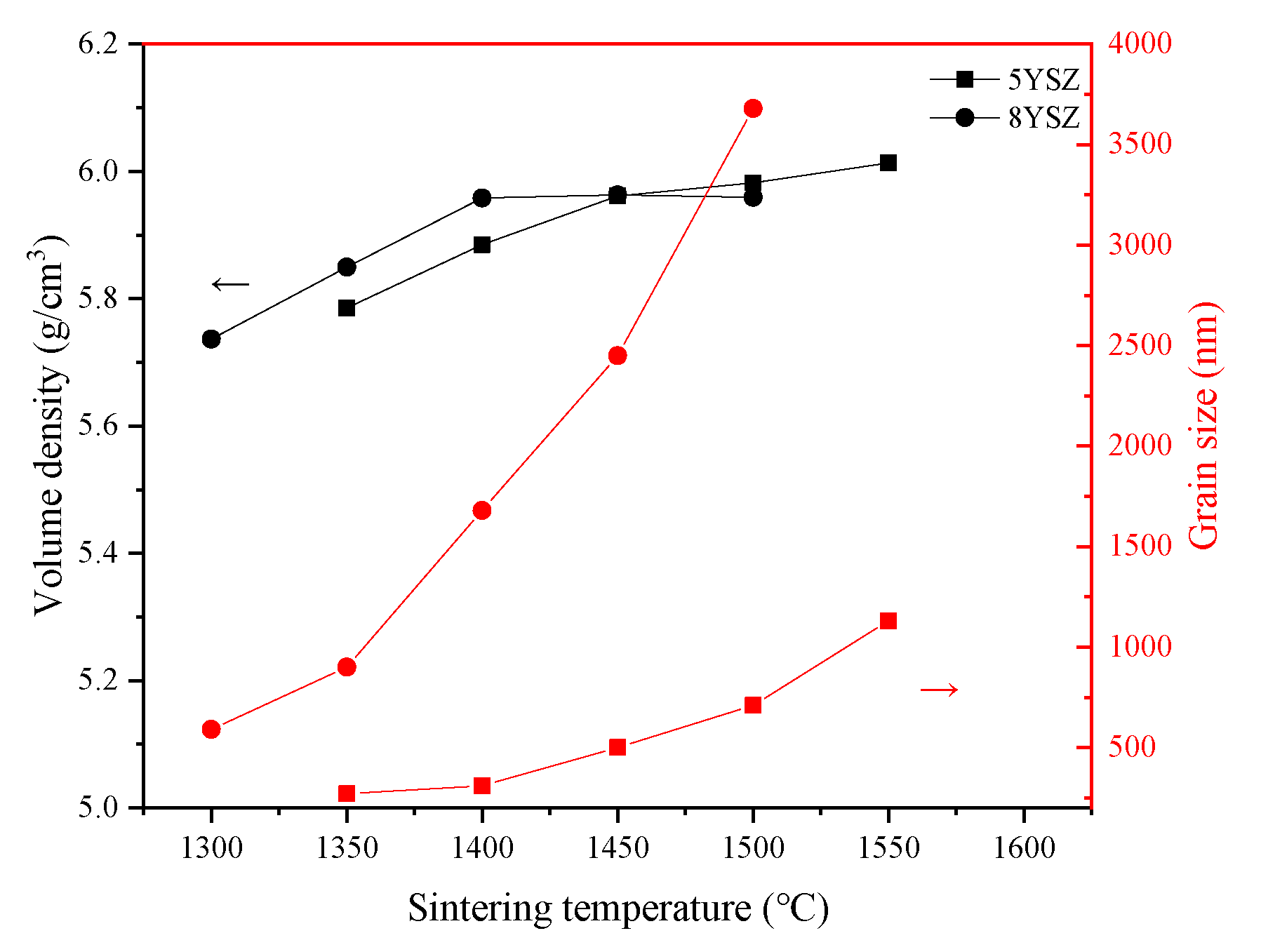
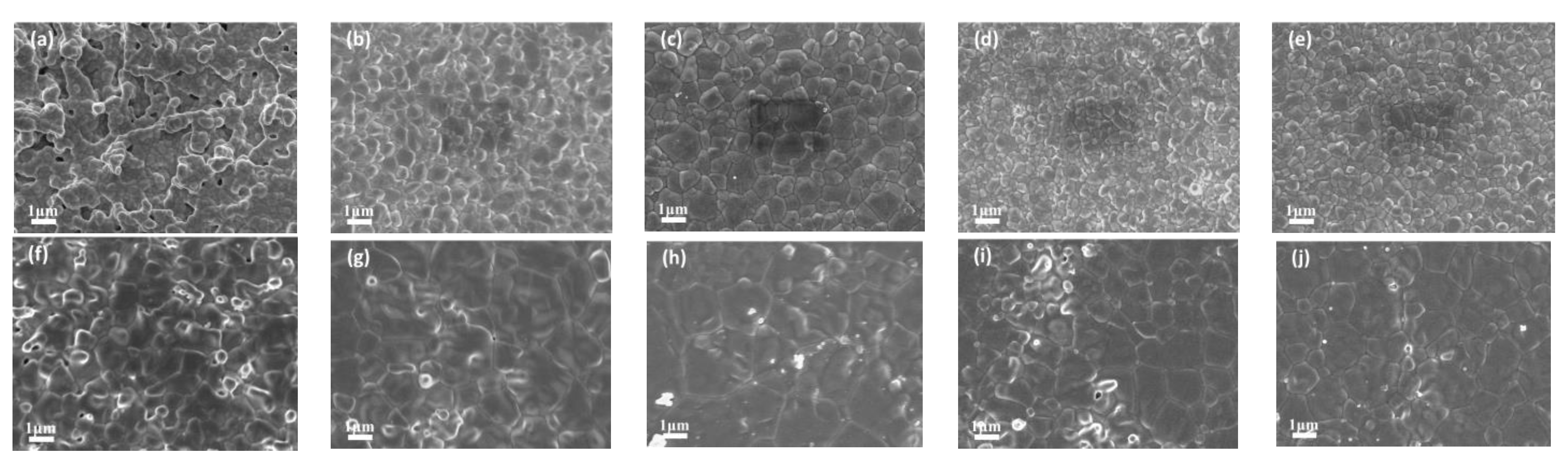
3.2. Volume Density and Average Grain Size of Ceramics
| T1 (°C) | T2 (°C) | t (h) | ρv (g/cm3) | ρr (%) | G (nm) | T1 (°C) | T2 (°C) | t (h) | ρv (g/cm3) | ρr (%) | G (nm) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5YSZ | 1500 | 1400 | 2 | 5.962 | 98.83 | 400 | 8YSZ | 1450 | 1350 | 2 | 5.906 | 98.60 | 960 |
| 5 | 5.981 | 99.14 | 410 | 5 | 5.924 | 98.90 | 1310 | ||||||
| 10 | 6.012 | 99.66 | 510 | 10 | 5.936 | 99.10 | 1480 | ||||||
| 1450 | 1400 | 2 | 5.946 | 98.56 | 370 | 1400 | 1350 | 2 | 5.804 | 96.89 | 860 | ||
| 5 | 5.969 | 98.95 | 390 | 5 | 5.874 | 98.06 | 1010 | ||||||
| 10 | 5.987 | 99.24 | 480 | 10 | 5.921 | 98.85 | 1060 | ||||||
| 1450 | 1350 | 2 | 5.891 | 97.65 | 270 | 1400 | 1300 | 2 | 5.791 | 96.67 | 470 | ||
| 5 | 5.937 | 98.42 | 310 | 5 | 5.862 | 97.86 | 510 | ||||||
| 10 | 5.968 | 98.93 | 335 | 10 | 5.919 | 98.81 | 580 | ||||||
| 1400 | 1350 | 2 | 5.846 | 96.91 | 200 | 1350 | 1300 | 2 | 5.622 | 93.86 | 280 | ||
| 5 | 5.891 | 97.65 | 250 | 5 | 5.776 | 96.43 | 370 | ||||||
| 10 | 5.945 | 98.55 | 280 | 10 | 5.901 | 98.51 | 410 |
3.3. Physical Phase Structure
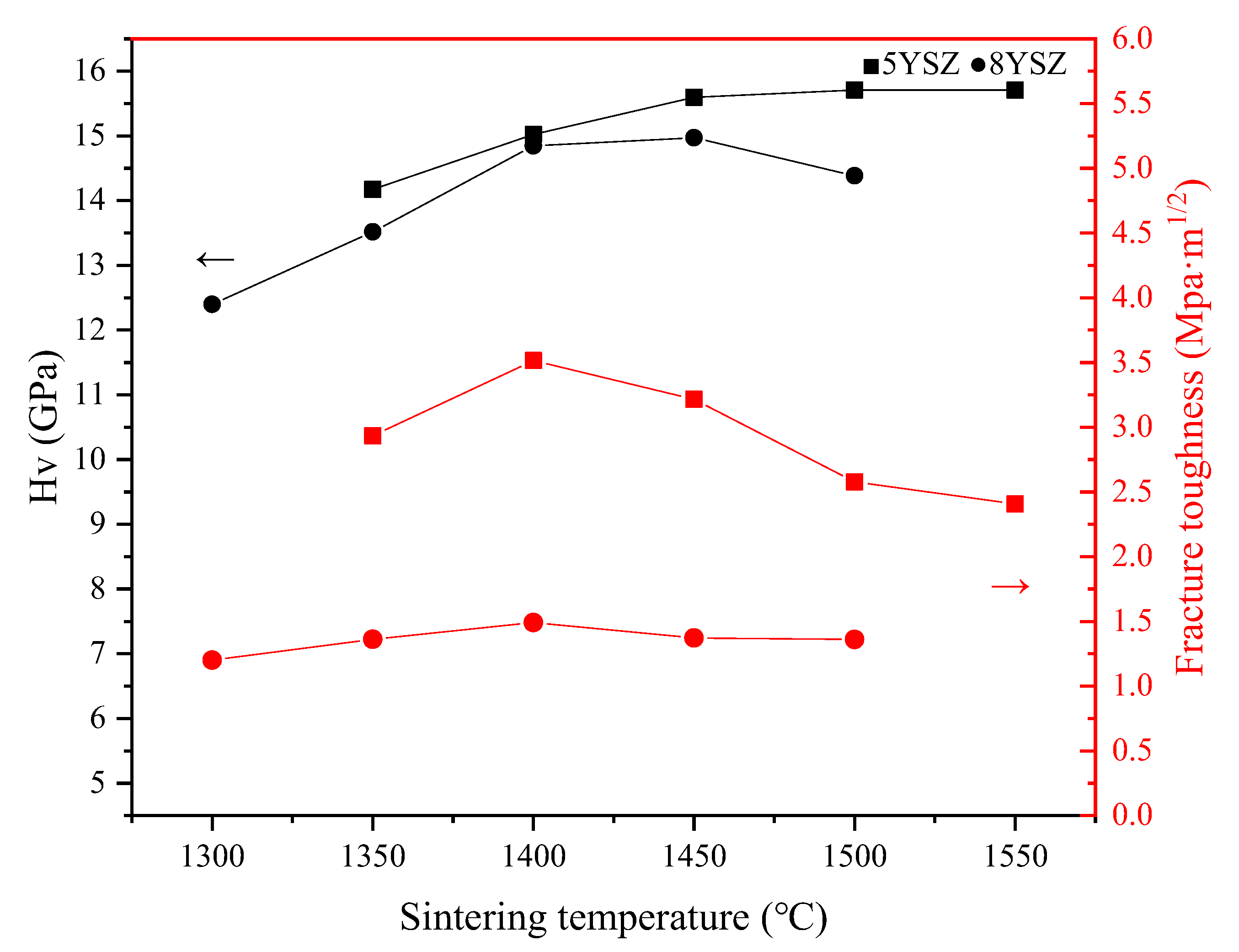
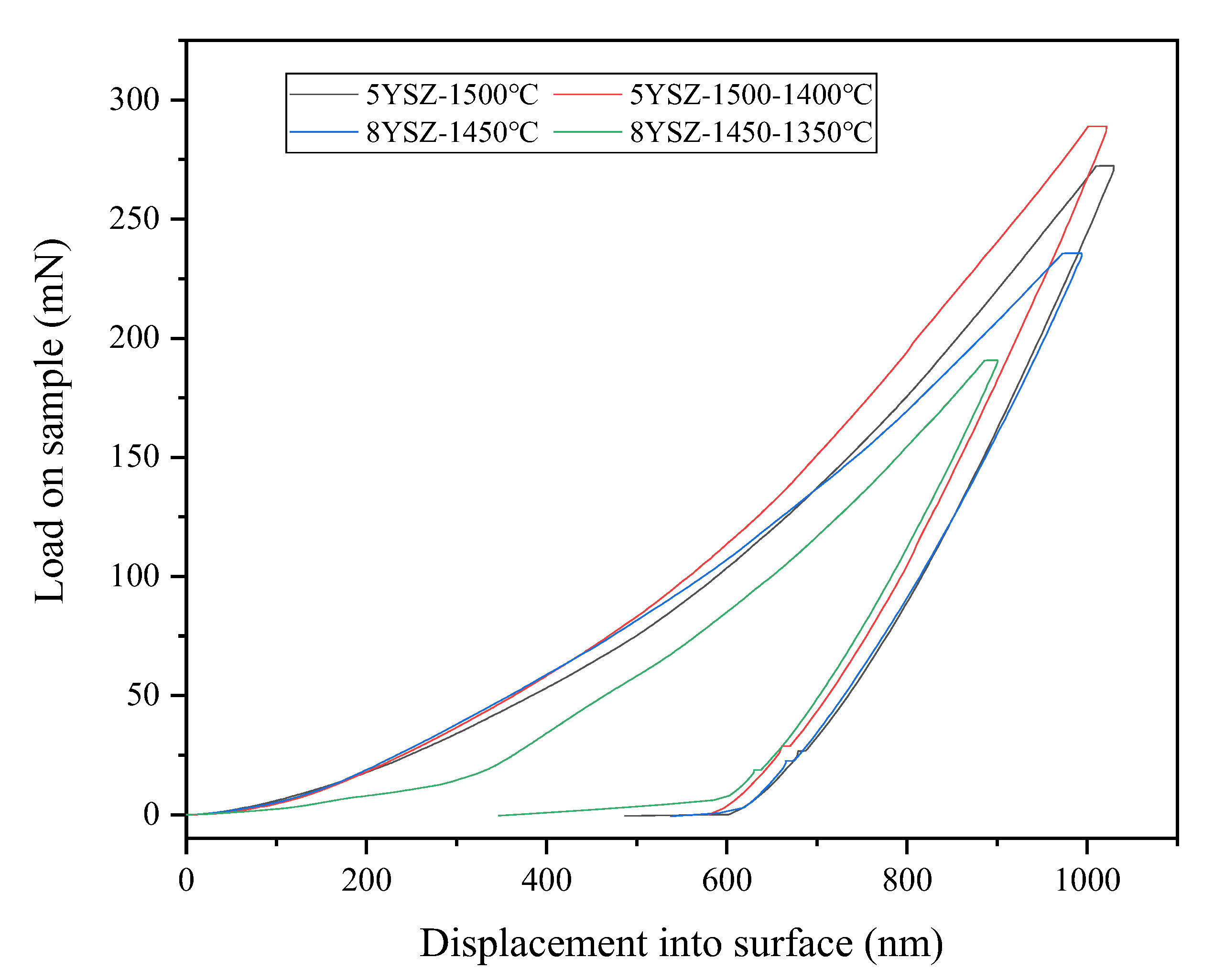
3.4. Mechanical Properties
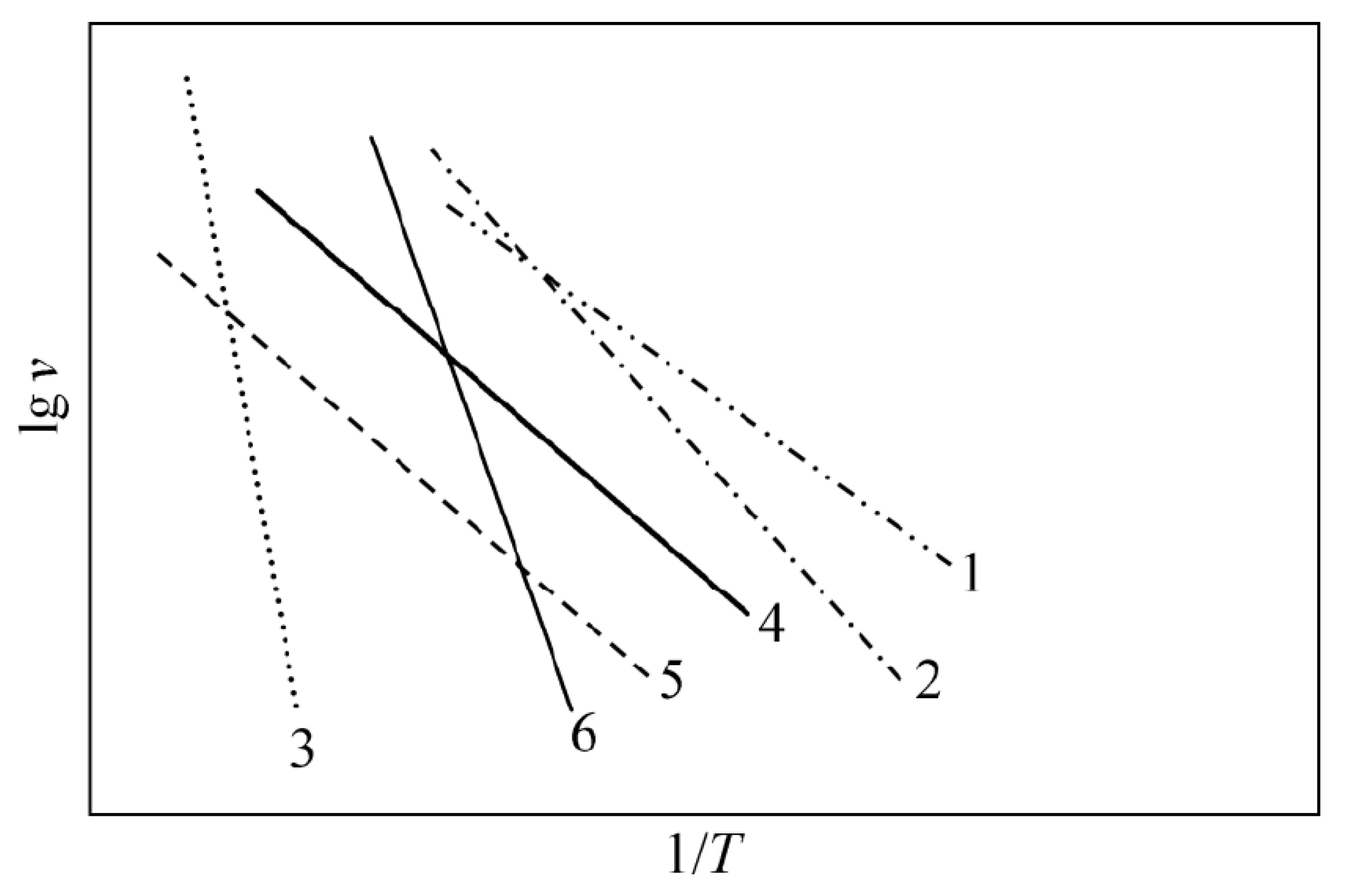
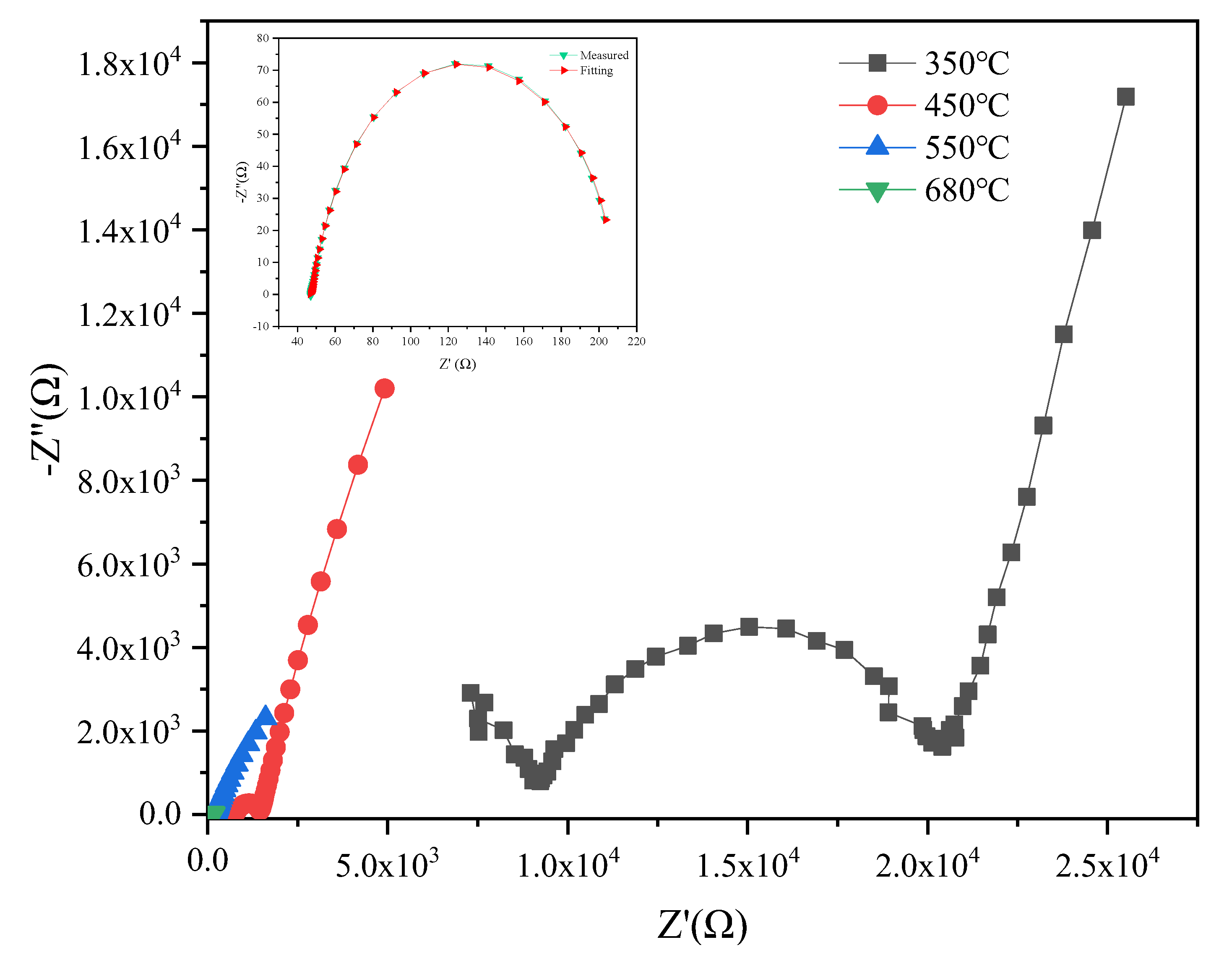
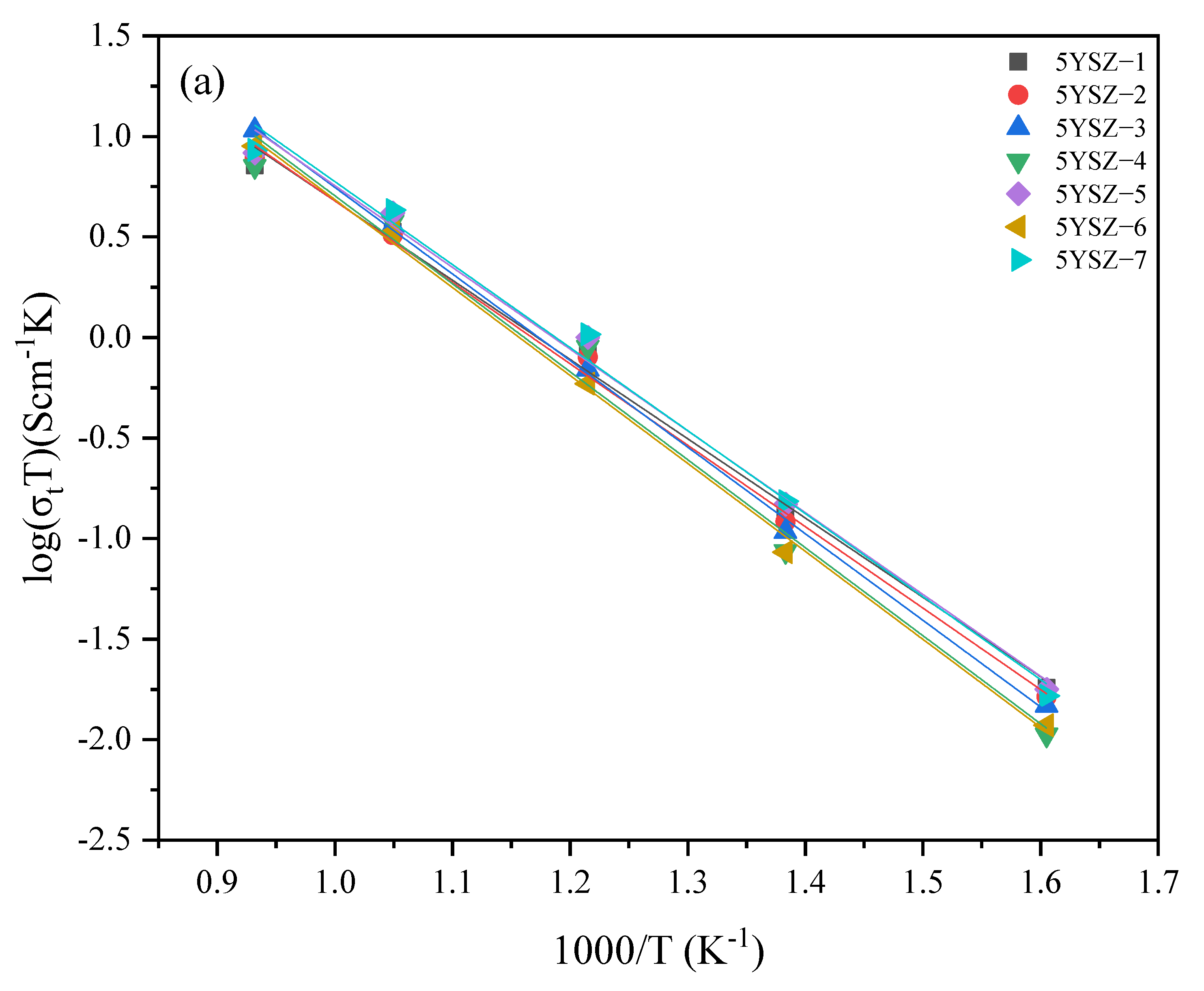
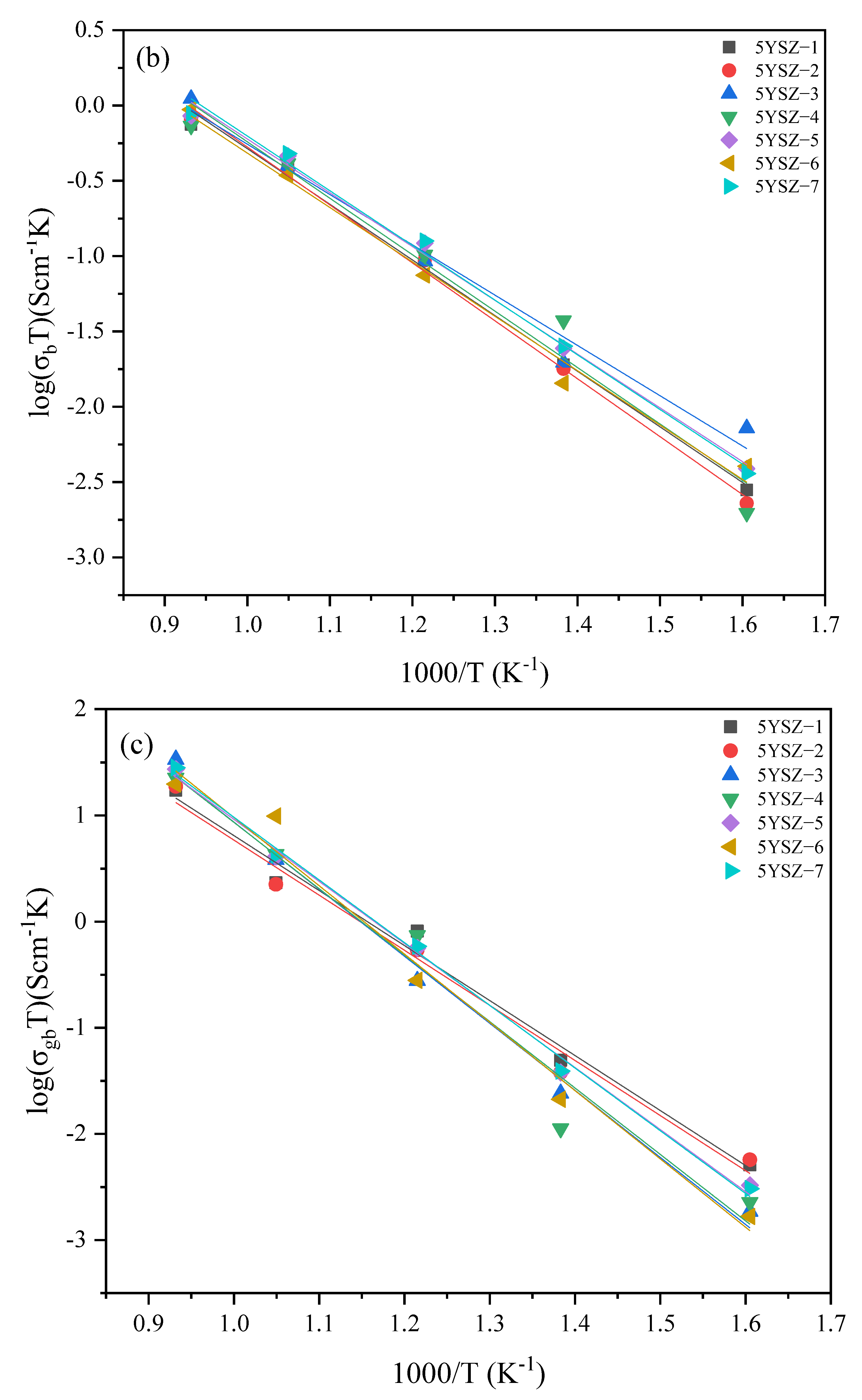
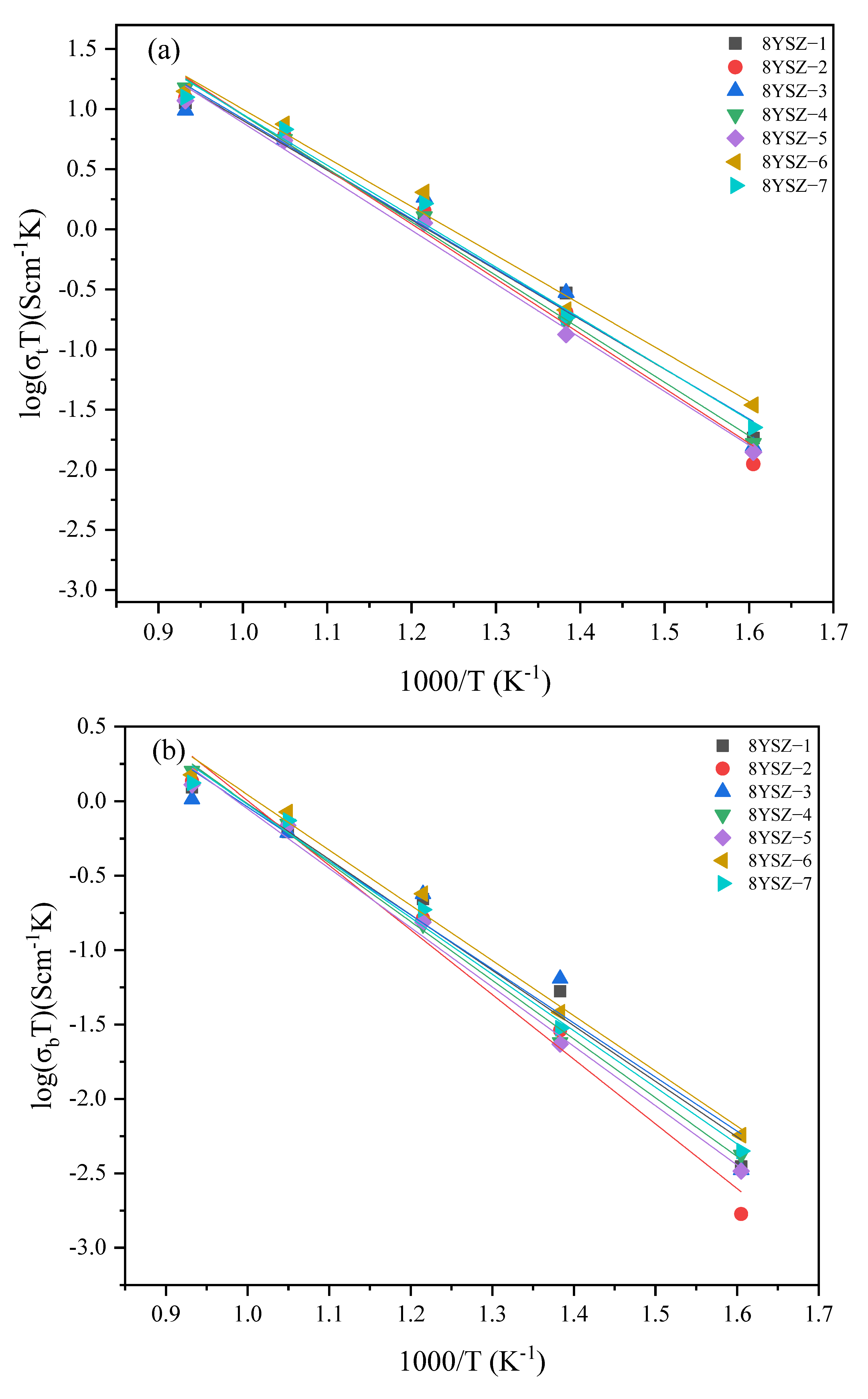
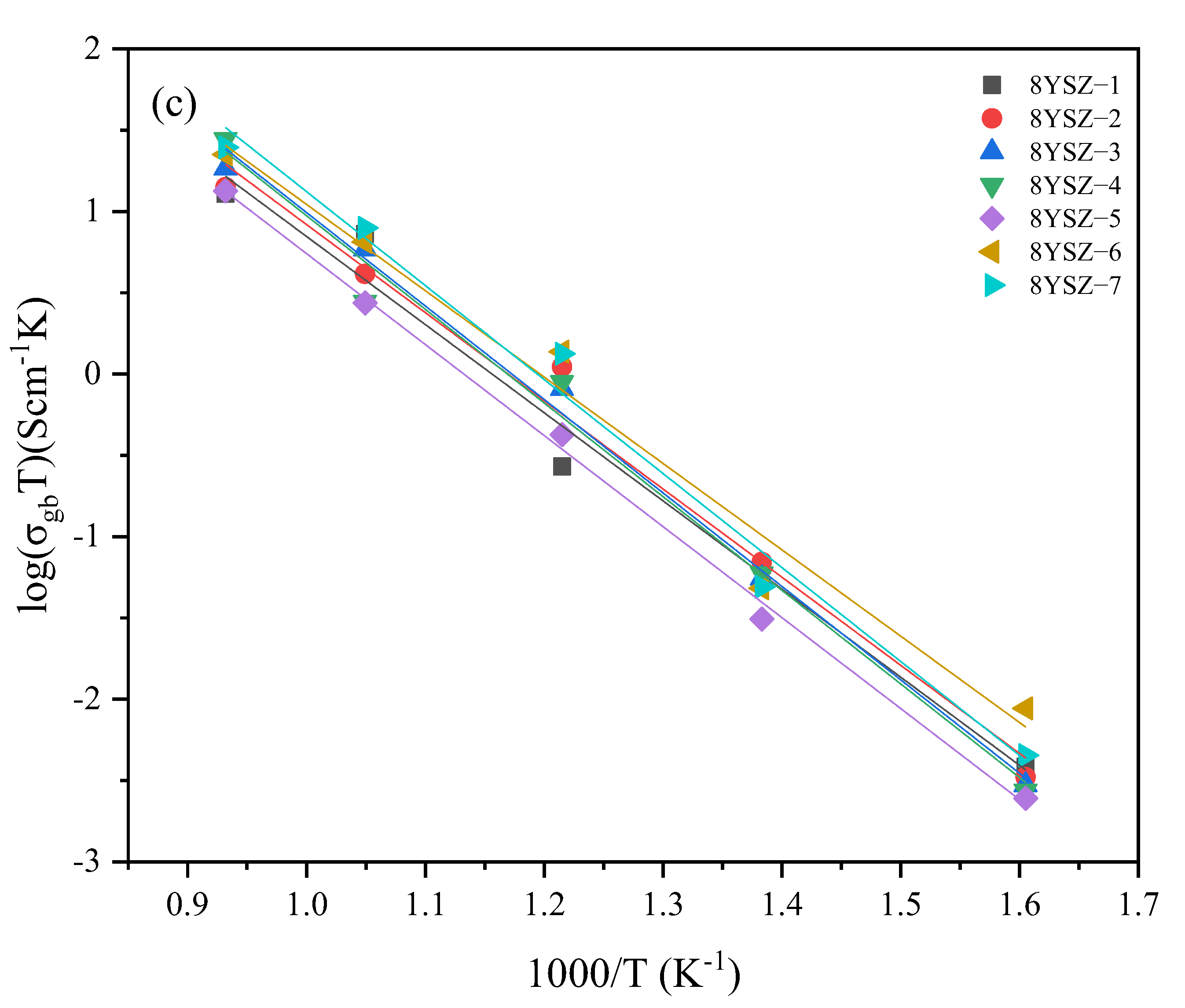
3.5. Electrical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nettleship, I.; Stevens, R. Tetragonal zirconia polycrystal (TZP)—A review. Int. J. High Technol. Ceram. 1987, 3, 1–32. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kuwajima, H.; Masaki, T. Phase change and mechanical properties of ZrO2-Y2O3 solid electrolyte after ageing. Solid State Ion. 1981, 3, 489–493. [Google Scholar] [CrossRef]
- Chen, X.J.; Khor, K.A.; Chan, S.H.; Yu, L.G. Influence of microstructure on the ionic conductivity of yttria-stabilized zirconia electrolyte. Mater. Sci. Eng. A 2002, 335, 246–252. [Google Scholar] [CrossRef]
- Kern, F.; Gommeringer, A. Mechanical Properties of 2Y-TZP Fabricated from Detonation Synthesized Powder. Ceramics 2020, 3, 440–452. [Google Scholar] [CrossRef]
- Liu, T.; Fang, J.; Li, S.; Li, S.; Wang, C.; Ji, C. Preparation of dense yttria stabilized zirconia ceramic by low-temperature sintering zirconium oxalate sol. J. Am. Ceram. Soc. 2015, 123, 554–560. [Google Scholar] [CrossRef] [Green Version]
- Ghatee, M.; Shariat, M.H.; Irvine, J.T.S. Investigation of electrical and mechanical properties of 3YSZ/8YSZ composite electrolytes. Solid State Ion. 2009, 180, 57–62. [Google Scholar] [CrossRef]
- Aragón-Duarte, M.C.; Nevarez-Rascón, A.; Esparza-Ponce, H.E.; Nevarez-Rascón, M.M.; Talamantes, R.P.; Ornelas, C.; Hurtado-Macías, A. Nanomechanical properties of zirconia-yttria and alμmina zirconia-yttria biomedical ceramics, subjected to low temperature aging. Ceram. Int. 2017, 43, 3931–3939. [Google Scholar] [CrossRef]
- Liu, X.Q.; Chen, X.M. Toughening of 8Y-FSZ Ceramics by Neodymiμm Titanate Secondary Phase. J. Am. Ceram. Soc. 2005, 88, 456–458. [Google Scholar] [CrossRef]
- Liu, X.Q.; Chen, X.M. Microstructures and mechanical properties of 8Y-FSZ ceramics with BaTiO3 additive. Ceram. Int. 2004, 30, 2269–2275. [Google Scholar] [CrossRef]
- Tredici, I.G.; Sebastiani, M.; Massimi, F.; Bemporad, E.; Resmini, A.; Merlati, G.; Anselmi-Tamburini, U. Low temperature degradation resistant nanostructured yttria-stabilized zirconia for dental applications. Ceram. Int. 2016, 42, 8190–8197. [Google Scholar] [CrossRef]
- Borrell, A.; Salvador, M.D.; Peñaranda-Foix, F.L.; Cátala-Civera, J.M. Microwave sintering of zirconia materials: Mechanical and microstructural properties. Int. J. Appl. Ceram. Technol. 2013, 10, 313–320. [Google Scholar] [CrossRef]
- Khor, K.A.; Yu, L.G.; Chan, S.H.; Chen, X.J. Densification of plasma sprayed YSZ electrolytes by spark plasma sintering (SPS). J. Eur. Ceram. 2003, 23, 1855–1863. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kondoh, I.; Tamari, N.; Balakrishnan, N.; Nomura, K.; Kageyama, H.; Takeda, Y. Improvement of mechanical strength of 8 mol% yttria-stabilized zirconia ceramics by spark-plasma sintering. J. Electrochem. Soc. 2002, 149, A455–A461. [Google Scholar] [CrossRef]
- Lin, F.J.; De Jonghe, L.C.; Rahaman, M.N. Microstructure refinement of sintered alμmina by a two-step sintering technique. J. Am. Ceram. Soc. 1997, 80, 2269–2277. [Google Scholar] [CrossRef]
- Chu, M.Y.; De Jonghe, L.C.; Lin, M.K.; Lin, F.J. Precoarsening to improve microstructure and sintering and sintering of powder compacts. J. Am. Ceram. Soc. 1991, 74, 2902–2911. [Google Scholar] [CrossRef]
- Chen, I.W.; Wang, X.H. Sintering dense nanocrystalline ceramics without final-stage grain growth. Nature 2000, 404, 168–171. [Google Scholar] [CrossRef]
- Hotza, D.; García, D.E.; Castro, R.H.R. Obtaining highly dense YSZ nanoceramics by pressureless, unassisted sintering. Int. Mater. Rev. 2015, 60, 353–375. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Qin, G. Two-step sintering of nano-yttria stabilized tetragonal zirconia. J. Chin. Ceram. Soc. 2012, 40, 335–339. [Google Scholar]
- Mazaheri, M.; Hesabi, Z.R.; Golestani-Fard, F.; Mollazadeh, S.; Jafari, S.; Sadrnezhaad, S.K. The effect of conformation method and sintering technique on the densification and grain growth of nanocrystalline 8 mol% Yttria-Stabilized Zirconia. J. Am. Ceram. Soc. 2009, 92, 990–995. [Google Scholar] [CrossRef]
- Han, M.; Tang, X.; Yin, H.; Peng, S. Fabrication, microstructure and properties of a YSZ electrolyte for SOFCs. J. Power Sources 2007, 165, 757–763. [Google Scholar] [CrossRef]
- Hesabi, Z.R.; Mazaheri, M.; Ebadzadeh, T. Enhanced electrical conductivity of ultrafine-grained 8Y2O3 stabilized ZrO2 produced by two-step sintering technique. J. Alloys Compd. 2010, 494, 362–365. [Google Scholar] [CrossRef]
- Porter, D.L.; Heuer, A.H. Microstructural development in MgO-partially stabilized zirconia (Mg-PSZ). J. Am. Ceram. Soc. 1979, 62, 298–305. [Google Scholar] [CrossRef]
- Bikyashev, E.A.; Belousov, P.A.; Anokhin, A.S.; Letovaltsev, A.O. Temperature-induced phase transitions and structural features of non-polar phases stabilizing below TC in the PbZrO3–LaMg2/3Nb1/3O3 system. Ferroelectrics 2021, 574, 53–64. [Google Scholar] [CrossRef]
- ASTM C1327-96; Standard Test Method for Vickers Indentation Hardness of Advanced Ceramics. Annual Book of ASTM Standards vol. 15.01. ASTM International: West Conshohocken, PA, USA, 2003.
- Mazaheri, M.; Zahedi, A.M.; Hejazi, M.M. Processing of nanocrystalline 8 mol% yttria-stabilized zirconia by conventional, microwave-assisted and two-step sintering. Mater. Sci. Eng. A 2008, 492, 261–267. [Google Scholar] [CrossRef]
- Kulyk, V.V.; Duriagina, Z.A.; Vasyliv, B.D.; Vavrukh, V.; Kovbasiuk, T.M.; Holovchuk, M. Effects of yttria content and sintering temperature on the microstructure and tendency to brittle fracture of yttria-stabilized zirconia. Arch. Mater. Sci. Eng. 2021, 109, 65–79. [Google Scholar] [CrossRef]
- Kulyk, V.; Duriagina, Z.; Vasyliv, B.; Vavrukh, V.; Kovbasiuk, T.; Lyutyy, P.; Vira, V. The effect of sintering temperature on the phase composition, microstructure, and mechanical properties of yttria-stabilized zirconia. Materials 2022, 15, 2707. [Google Scholar] [CrossRef]
- Ingel, R.P.; Rice, R.W.; Lewis, D. Room-Temperature Strength and Fracture of ZrO2-Y2O3 Single Crystals. J. Am. Ceram. Soc. 1982, 65, c108–c109. [Google Scholar] [CrossRef]
- Matsui, K.; Yoshida, H.; Ikuhara, Y. Grain-boundary structure and microstructure development mechanism in 2–8 mol% yttria-stabilized zirconia polycrystals. Acta Mater. 2008, 56, 1315–1325. [Google Scholar] [CrossRef]
- Wang, X.; Wang, R.; Peng, C.; Wang, Z. Two-step sintering of BeO nanopowder. J. Cent. South Univ. 2014, 45, 1043–1051. [Google Scholar]
- Mazaheri, M.; Zahedi, A.M.; Sadrnezhaad, S.K. Two-step sintering of nanocrystalline ZnO compacts: Effect of temperature on densification and grain growth. J. Am. Ceram. Soc. 2008, 91, 56–63. [Google Scholar] [CrossRef]
- Kingery, W.D.; Berg, M. Study of initial stages of sintering solid by viscous flow, evaporation-condensation and self-diffusion. J. Appl. Phys. 1955, 26, 1205–1212. [Google Scholar] [CrossRef]
- Ingel, R.P.; Lewis, D., III. Lattice parameters and density for Y2O3-stabilized ZrO2. J. Am. Ceram. Soc. 1986, 69, 325–332. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, I.W. Mobility transition at grain boundaries in two-step sintered 8 mol% yttria-stabilized zirconia. J. Am. Ceram. Soc. 2018, 101, 1857–1869. [Google Scholar] [CrossRef]
- Kontoyannis, C.G.; Orkoula, M. Quantitative determination of the cubic, tetragonal and monoclinic phases in partially stabilized zirconias by Raman spectroscopy. J. Mater Sci. 1994, 29, 5316–5320. [Google Scholar] [CrossRef]
- Hayashi, H.; Ueda, A.; Suino, A.; Hiro, K.; Hakuta, Y. Hydrothermal synthesis of yttria stabilized ZrO2 nanoparticles in subcritical and supercritical water using a flow reaction system. J. Solid State Chem. 2009, 182, 2985–2990. [Google Scholar] [CrossRef]
- Belichko, D.R.; Konstantinova, T.E.; Maletsky, A.V.; Volkova, G.K.; Doroshkevich, A.S.; Lakusta, M.V.; Cornei, N. Influence of hafnium oxide on the structure and properties of powders and ceramics of the YSZ–HfO2 composition. Ceram. Int. 2021, 47, 3142–3148. [Google Scholar] [CrossRef]
- Bauerle, J.E. Study of solid electrolyte polarization by a complex admittance method. J. Phys. Chem. Solids 1969, 30, 2657–2670. [Google Scholar] [CrossRef]
- Matsui, N. Equivalent circuit analysis of high temperature solid electrolyte. Solid State Ion. 1986, 18, 888–891. [Google Scholar] [CrossRef]
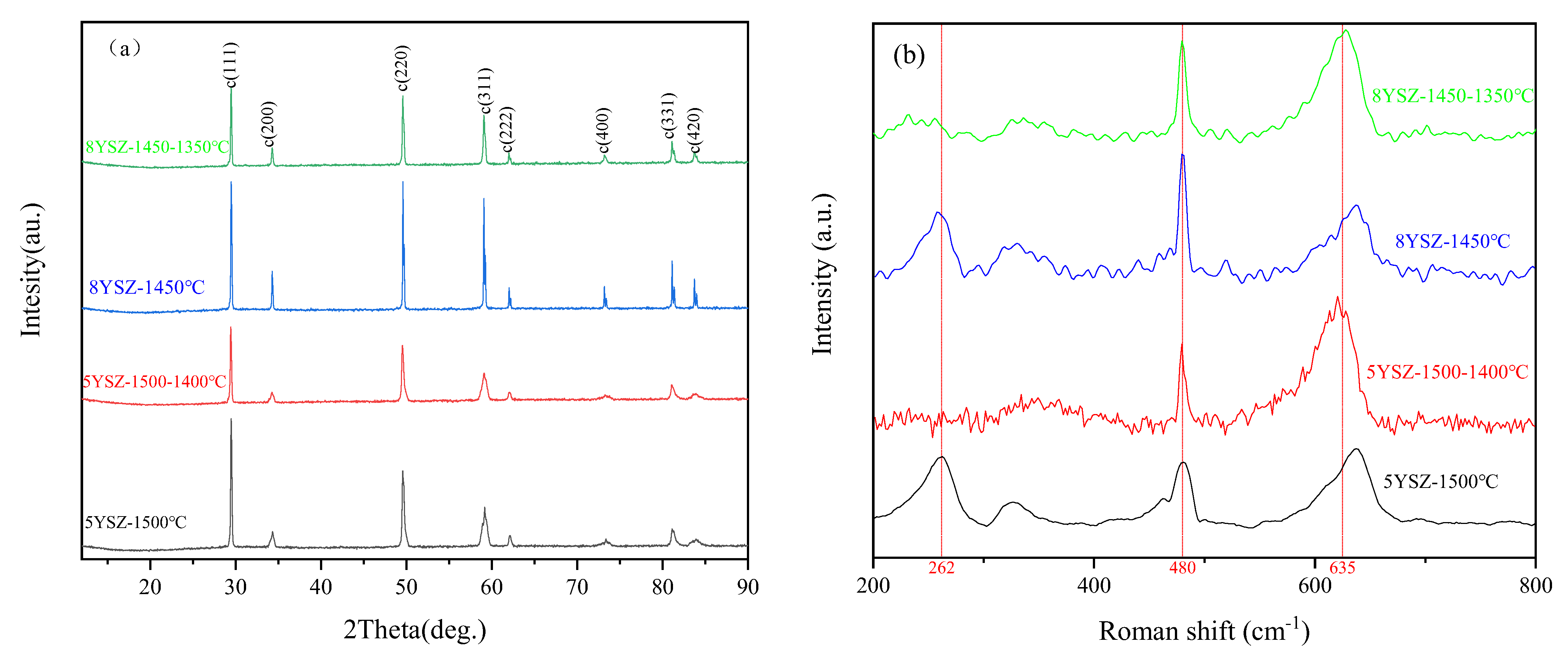
| Specimen | Phase Composition, % | Latticle Options, Å | c/a | Vcell, Å3 | |
|---|---|---|---|---|---|
| a | c | ||||
| 5YSZ-1500 °C | 47.52%C | - | 5.1705 | - | 138.23 |
| 52.48%T | 3.6589 | 5.1677 | 1.413 | 69.18 | |
| 5YSZ-1500-1400 °C | 59.05%C | - | 5.1641 | - | 137.72 |
| 40.95%T | 3.6513 | 5.1622 | 1.414 | 68.82 | |
| 8YSZ-1450 °C | 94%C | - | 5.1723 | - | 138.37 |
| 6%T | 3.6644 | 5.1803 | 1.414 | 69.56 | |
| 8YSZ-1450-1350 °C | 94.71%C | - | 5.163 | - | 137.63 |
| 5.29%T | 3.6427 | 5.1732 | 1.42 | 68.64 | |
| Sample | Vickers Hardness GPa | Fracture Toughness Mpa·m1/2 | Elastic Modulus GPa |
|---|---|---|---|
| 5YSZ-1500-1400 °C | 15.301 | 3.289 | 238.075 |
| 5YSZ-1450-1400 °C | 14.828 | 4.034 | - |
| 5YSZ-1450-1350 °C | 14.076 | 2.797 | - |
| 5YSZ-1400-1350 °C | 12.861 | 2.902 | - |
| 8YSZ-1450-1350 °C | 15.134 | 1.585 | 233.408 |
| 8YSZ-1400-1350 °C | 14.320 | 2.126 | - |
| 8YSZ-1400-1300 °C | 13.636 | 1.817 | - |
| 8YSZ-1350-1300 °C | 12.507 | 1.36 | - |
| Specimen | Sintering Conditions | σt (680 °C) | Activation Energy Ea (eV) | Specimen | Sintering Conditions | σt (680 °C) | Activation Energy Ea (eV) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | ×10−3 S/cm | σt | σb | σgb | °C | ×10−3 S/cm | σt | σb | σgb | ||
| 5YSZ-1 | 1500-2 h | 3.52 | 0.72 | 0.67 | 0.96 | 8YSZ-1 | 14502 h | 6.09 | 0.75 | 0.67 | 1.01 |
| 5YSZ-2 | 1400-2 h | 3.39 | 0.74 | 0.70 | 0.96 | 8YSZ-2 | 1400-2 h | 6.24 | 0.84 | 0.80 | 1.01 |
| 5YSZ-3 | 1500-1400-2 h | 3.76 | 0.79 | 0.60 | 1.19 | 8YSZ-3 | 1450-1350-2 h | 5.84 | 0.76 | 0.65 | 1.07 |
| 5YSZ-4 | 1450-1400-5 h | 3.95 | 0.80 | 0.68 | 1.18 | 8YSZ-4 | 1400-1350-5 h | 5.91 | 0.82 | 0.72 | 1.08 |
| 5YSZ-5 | 1450-1350-5 h | 4.37 | 0.74 | 0.64 | 1.09 | 8YSZ-5 | 1400-1300-5 h | 5.77 | 0.82 | 0.72 | 1.04 |
| 5YSZ-6 | 1450-1350-10 h | 3.49 | 0.80 | 0.65 | 1.21 | 8YSZ-6 | 1400-1300-10 h | 7.87 | 0.74 | 0.67 | 0.99 |
| 5YSZ-7 | 1400-1350-10 h | 4.52 | 0.75 | 0.65 | 1.11 | 8YSZ-7 | 1350-1300-10 h | 7.2 | 0.77 | 0.69 | 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Sun, H.; Song, J.; Zhang, Z.; Lan, H.; Tian, L.; Xie, K. Effect of Two-Step Sintering on the Mechanical and Electrical Properties of 5YSZ and 8YSZ Ceramics. Materials 2023, 16, 2019. https://doi.org/10.3390/ma16052019
Li Y, Sun H, Song J, Zhang Z, Lan H, Tian L, Xie K. Effect of Two-Step Sintering on the Mechanical and Electrical Properties of 5YSZ and 8YSZ Ceramics. Materials. 2023; 16(5):2019. https://doi.org/10.3390/ma16052019
Chicago/Turabian StyleLi, Yunpeng, Hongqian Sun, Jing Song, Zhiyu Zhang, Hao Lan, Liangliang Tian, and Keqiang Xie. 2023. "Effect of Two-Step Sintering on the Mechanical and Electrical Properties of 5YSZ and 8YSZ Ceramics" Materials 16, no. 5: 2019. https://doi.org/10.3390/ma16052019
APA StyleLi, Y., Sun, H., Song, J., Zhang, Z., Lan, H., Tian, L., & Xie, K. (2023). Effect of Two-Step Sintering on the Mechanical and Electrical Properties of 5YSZ and 8YSZ Ceramics. Materials, 16(5), 2019. https://doi.org/10.3390/ma16052019







