Viscoelastic Properties of Human Facial Skin and Comparisons with Facial Prosthetic Elastomers
Abstract
1. Introduction
2. Materials and Methods
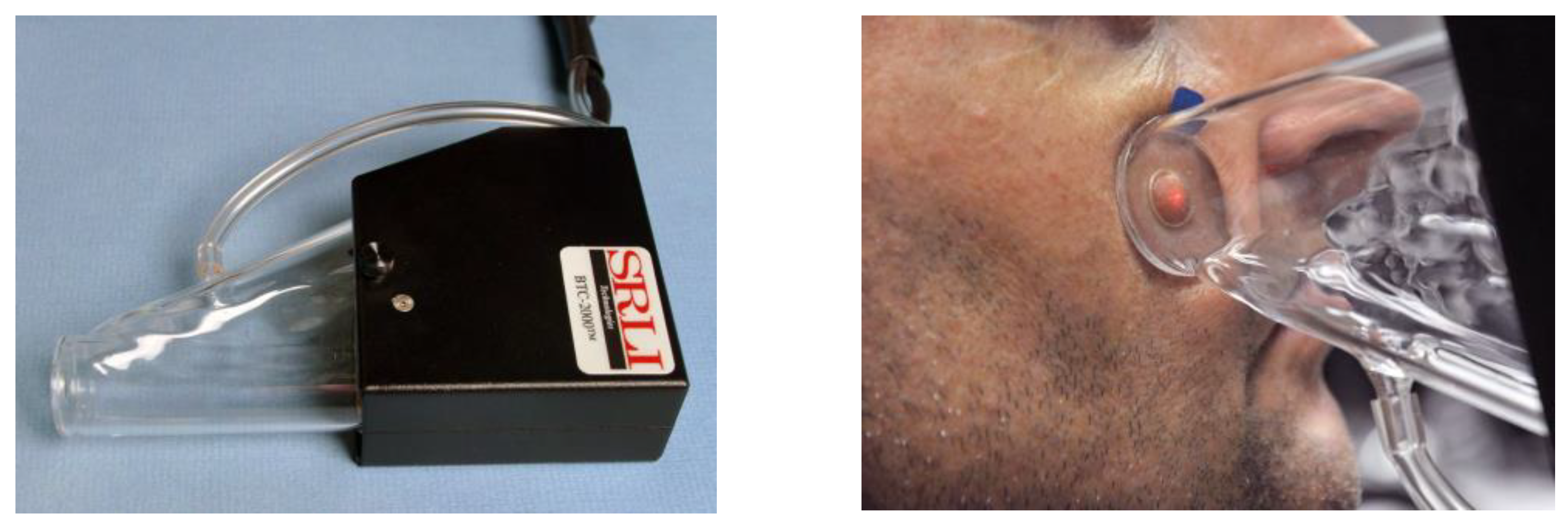
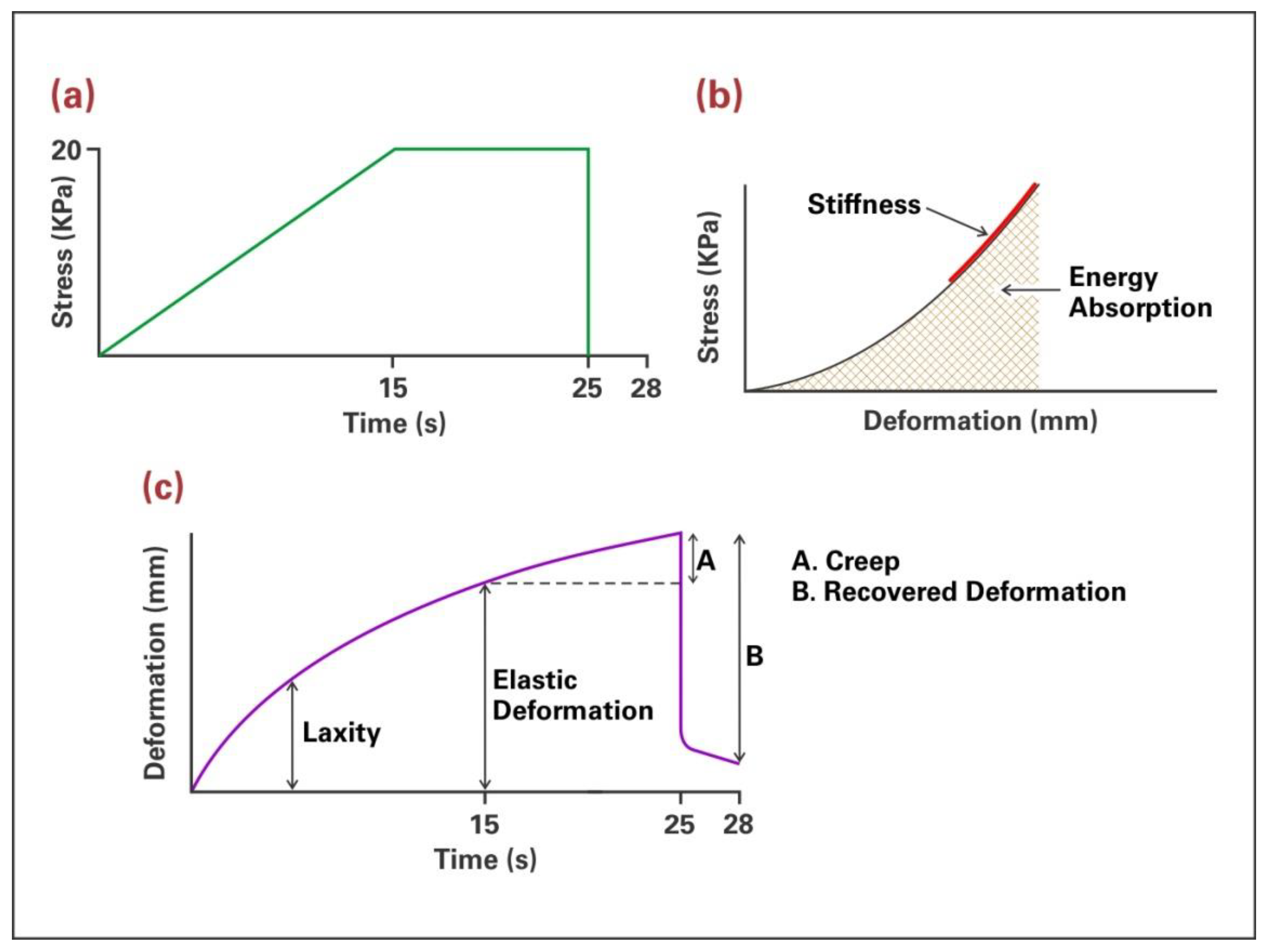
2.1. Facial Skin Measurements
2.2. Prosthetic Elastomer Measurements
3. Results
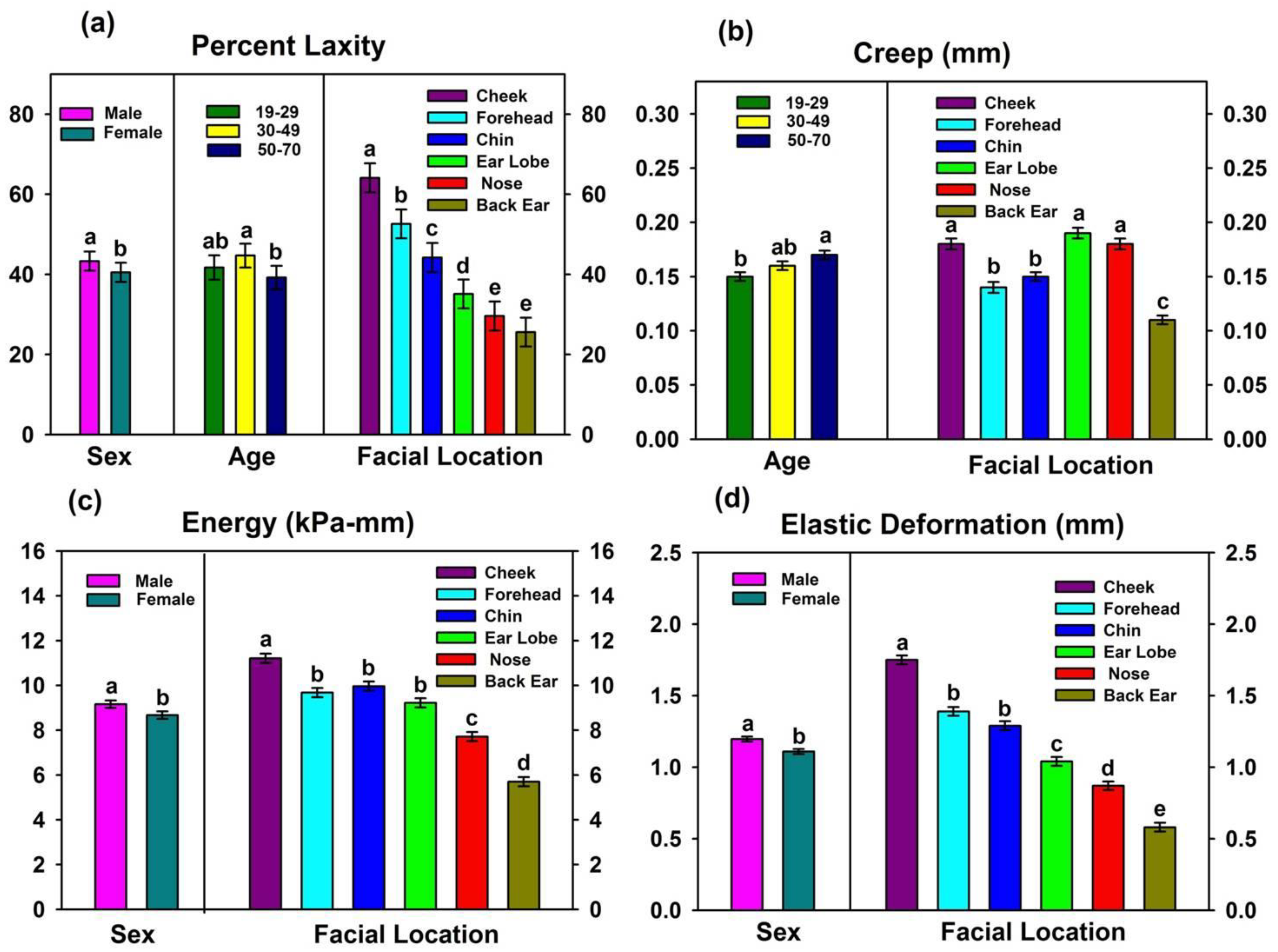
3.1. Facial Skin Properties
3.1.1. Results from Biomechanical Measurements
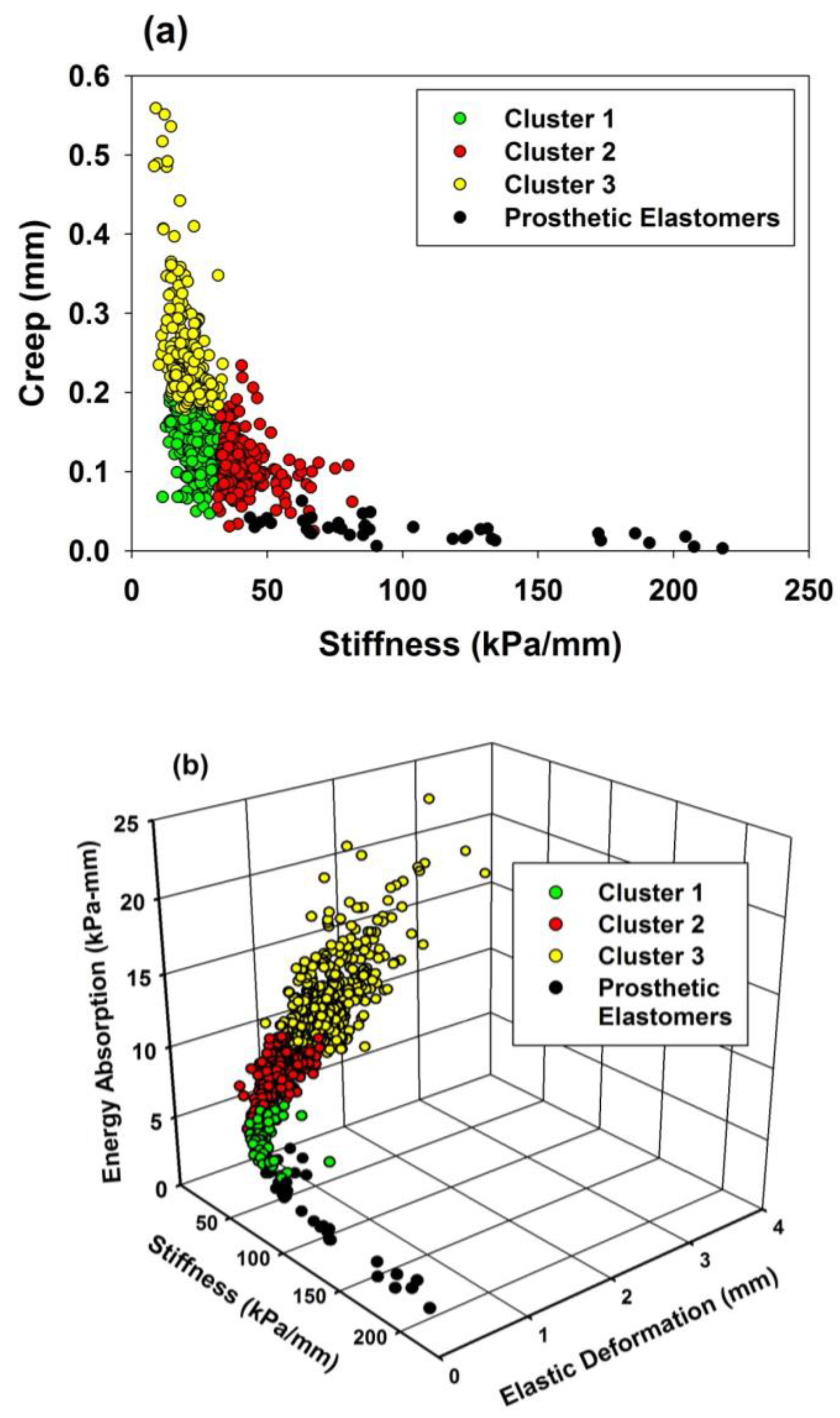
3.1.2. Results from Clustering Analyses
3.2. Comparisons of Facial Skin Properties with Prosthetic Elastomer Properties
3.2.1. Results from Biomechanical Measurements
3.2.2. Comparisons with Clustering Analyses Results
4. Discussion
4.1. Biomechanical Measurements of Facial Skin
4.1.1. Facial Location
4.1.2. Age
4.1.3. Sex
4.1.4. Race
4.2. Facial Prosthetic Elastomers
4.3. Engineering Design Considerations
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arda, O.; Goksugur, N.; Tuzun, Y. Basic histological structure and functions of facial skin. Clin. Derm. 2014, 32, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ha, R.Y.; Nojima, K.; Adams, W.P., Jr.; Brown, S.A. Analysis of facial skin thickness: Defining the relative thickness index. Plast. Reconstr. Surg. 2005, 115, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, K.W.; Kim, J.S.; Gil, Y.C.; Tanvaa, T.; Shin, D.H.; Kim, H.J. Regional thickness of facial skin and superficial fat: Application to the minimally invasive procedures. Clin. Anat. 2019, 32, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, G.; Seidenari, S. Variations in facial skin thickness and echogenicity with site and age. Acta Derm. Venereol. 1999, 79, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Takema, Y.; Yorimoto, Y.; Kawai, M.; Imokawa, G. Age-related changes in the elastic properties and thickness of human facial skin. Br. J. Derm. 1994, 131, 641–648. [Google Scholar] [CrossRef]
- Chopra, K.; Calva, D.; Sosin, M.; Tadisina, K.K.; Banda, A.; De La Cruz, C.; Chaudhry, M.R.; Legesse, T.; Drachenberg, C.B.; Manson, P.N.; et al. A comprehensive examination of topographic thickness of skin in the human face. Aesthet. Surg. J. 2015, 35, 1007–1013. [Google Scholar] [CrossRef]
- Barel, A.O.; Lambrecht, R.; Clarys, P. Mechanical function of the skin: State of the art. Curr. Probl. Derm. 1998, 26, 69–83. [Google Scholar] [CrossRef]
- Allareddy, V.; Allareddy, V.; Nalliah, R.P. Epidemiology of facial fracture injuries. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 2011, 69, 2613–2618. [Google Scholar] [CrossRef]
- Hoogewerf, C.J.; van Baar, M.E.; Hop, M.J.; Bloemen, M.C.; Middelkoop, E.; Nieuwenhuis, M.K. Burns to the head and neck: Epidemiology and predictors of surgery. Burns 2013, 39, 1184–1192. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rankin, M.; Borah, G.L. Perceived functional impact of abnormal facial appearance. Plast. Reconstr. Surg. 2003, 111, 2140–2146, discussion 2147–2148. [Google Scholar] [CrossRef] [PubMed]
- van Doorne, J.M.; van Waas, M.A.; Bergsma, J. Facial disfigurement after cancer resection: A problem with an extra dimension. J. Investig. Surg. Off. J. Acad. Surg. Res. 1994, 7, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Alsberg, E.; Hill, E.E.; Mooney, D.J. Craniofacial tissue engineering. Crit. Rev. Oral. Biol. Med. Off. Publ. Am. Assoc. Oral. Biol. 2001, 12, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; de Carvalho Dekon, S.F.; de Faria Almeida, D.A.; Sanchez, D.M.; dos Santos, D.M.; Pellizzer, E.P. Patients’ satisfaction after surgical facial reconstruction or after rehabilitation with maxillofacial prosthesis. J. Craniofacial. Surg. 2011, 22, 766–769. [Google Scholar] [CrossRef]
- Lemon, J.C.; Kiat-amnuay, S.; Gettleman, L.; Martin, J.W.; Chambers, M.S. Facial prosthetic rehabilitation: Preprosthetic surgical techniques and biomaterials. Curr. Opin. Otolaryngol. Head Neck Surg. 2005, 13, 255–262. [Google Scholar] [CrossRef]
- Griffin, M.F.; Leung, B.C.; Premakumar, Y.; Szarko, M.; Butler, P.E. Comparison of the mechanical properties of different skin sites for auricular and nasal reconstruction. J. Otolaryngol. Head Neck Surg. 2017, 46, 33. [Google Scholar] [CrossRef]
- Bellamy, K.E.; Waters, M.G. Designing a prosthesis to simulate the elastic properties of skin. Biomed. Mater. Eng. 2005, 15, 21–27. [Google Scholar]
- Farah, J.W.; Robinson, J.C.; Hood, J.A.; Koran, A.; Craig, R.G. Force-displacement properties of a modified cross-linked silicone compared with facial tissues. J. Oral. Rehabil. 1988, 15, 277–283. [Google Scholar] [CrossRef]
- Luebberding, S.; Krueger, N.; Kerscher, M. Mechanical properties of human skin in vivo: A comparative evaluation in 300 men and women. Ski. Res. Technol. 2014, 20, 127–135. [Google Scholar] [CrossRef]
- Pierard, G.E.; Hermanns-Le, T.; Gaspard, U.; Pierard-Franchimont, C. Asymmetric facial skin viscoelasticity during climacteric aging. Clin. Cosmet. Investig. Derm. 2014, 7, 111–118. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Montgomery, P.C.; Kiat-Amnuay, S. Survey of currently used materials for fabrication of extraoral maxillofacial prostheses in North America, Europe, Asia, and Australia. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2010, 19, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Willett, E.S.; Beatty, M.W. Outdoor weathering of facial prosthetic elastomers differing in Durometer hardness. J. Prosthet. Dent. 2015, 113, 228–235. [Google Scholar] [CrossRef]
- Hendrickson, E.L.; Lamont, R.J.; Hackett, M. Tools for interpreting large-scale protein profiling in microbiology. J. Dent. Res. 2008, 87, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.; Lowe, A.; Al-Jumaily, A.M. Mechanical Behavior of Skin: A Review. J. Mater. Sci. Eng. 2016, 5, 254. [Google Scholar] [CrossRef]
- Pierard, G.E.; Pierard, S.L.; Hermanns-Le, T. Facial Skin Rheology. In Textbook of Aging Skin; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin, Germany, 2010; pp. 351–361. [Google Scholar]
- Smalls, L.K.; Randall Wickett, R.; Visscher, M.O. Effect of dermal thickness, tissue composition, and body site on skin biomechanical properties. Ski. Res. Technol. 2006, 12, 43–49. [Google Scholar] [CrossRef]
- Ryu, H.S.; Joo, Y.H.; Kim, S.O.; Park, K.C.; Youn, S.W. Influence of age and regional differences on skin elasticity as measured by the Cutometer. Ski. Res. Technol. 2008, 14, 354–358. [Google Scholar] [CrossRef]
- Cua, A.B.; Wilhelm, K.P.; Maibach, H.I. Elastic properties of human skin: Relation to age, sex, and anatomical region. Arch. Derm. Res. 1990, 282, 283–288. [Google Scholar] [CrossRef]
- Diridollou, S.; Black, D.; Lagarde, J.M.; Gall, Y.; Berson, M.; Vabre, V.; Patat, F.; Vaillant, L. Sex- and site-dependent variations in the thickness and mechanical properties of human skin in vivo. Int. J. Cosmet. Sci. 2000, 22, 421–435. [Google Scholar] [CrossRef]
- Krueger, N.; Luebberding, S.; Oltmer, M.; Streker, M.; Kerscher, M. Age-related changes in skin mechanical properties: A quantitative evaluation of 120 female subjects. Ski. Res. Technol. 2011, 17, 141–148. [Google Scholar] [CrossRef]
- Fazio, M.J.; Olsen, D.R.; Uitto, J.J. Skin aging: Lessons from cutis laxa and elastoderma. Cutis 1989, 43, 437–444. [Google Scholar] [PubMed]
- Pierard, G.E.; Henry, F.; Castelli, D.; Ries, G. Ageing and rheological properties of facial skin in women. Gerontology 1998, 44, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Pailler-Mattei, C.; Bec, S.; Zahouani, H. In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med. Eng. Phys. 2008, 30, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Thieulin, C.; Pailler-Mattei, C.; Abdouni, A.; Djaghloul, M.; Zahouani, H. Mechanical and topographical anisotropy for human skin: Ageing effect. J. Mech. Behav. Biomed. Mater. 2020, 103, 103551. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Hansen, B.; Jemec, G.B.E. Mechanical properties of the skin: A comparison between two suction cup methods. Ski. Res. Technol. 2003, 9, 111–115. [Google Scholar] [CrossRef]
- Ishikawa, T.; Ishikawa, O.; Miyachi, Y. Measurement of skin elastic properties with a new suction device (I): Relationship to age, sex and the degree of obesity in normal individuals. J. Derm. 1995, 22, 713–717. [Google Scholar] [CrossRef]
- Diridollou, S.; Vabre, V.; Berson, M.; Vaillant, L.; Black, D.; Lagarde, J.M.; Gregoire, J.M.; Gall, Y.; Patat, F. Skin ageing: Changes of physical properties of human skin in vivo. Int. J. Cosmet. Sci. 2001, 23, 353–362. [Google Scholar] [CrossRef]
- Malm, M.; Samman, M.; Serup, J. In vivo skin elasticity of 22 anatomical sites: The vertical gradient of skin extensibility and implications in gravitational aging. Ski. Res. Technol. 1995, 1, 61–67. [Google Scholar] [CrossRef]
- Salter, D.C.; McArthur, H.C.; Crosse, J.E.; Dickens, A.D. Skin mechanics measured in vivo using torsion: A new and accurate model more sensitive to age, sex and moisturizing treatment. Int. J. Cosmet. Sci. 1993, 15, 200–218. [Google Scholar] [CrossRef]
- Berardesca, E.; de Rigal, J.; Leveque, J.L.; Maibach, H.I. In vivo biophysical characterization of skin physiological differences in races. Dermatologica 1991, 182, 89–93. [Google Scholar] [CrossRef]
- Warrier, A.G.; Kligman, A.M.; Harper, R.A.; Bowman, J.; Wickett, R.R. A comparison of Black and white skin using noninvasive methods. J. Soc. Cosmet. Chem. 1996, 47, 229–240. [Google Scholar]
- Wesley, N.O.; Maibach, H.I. Racial (ethnic) differences in skin properties: The objective data. Am. J. Clin. Derm. 2003, 4, 843–860. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.Y.; Dedhia, R.; Cervenka, B.; Tollefson, T.T. 3D Printing: Current use in facial plastic and reconstructive surgery. Curr. Opin. Otolaryngol. Head Neck Surg. 2017, 25, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Unkovskiy, A.; Spintzyk, S.; Brom, J.; Huettig, F.; Keutel, C. Direct 3D printing of silicone facial prostheses: A preliminary experience in digital workflow. J. Prosthet. Dent. 2018, 120, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Jindal, S.; Manzoor, F.; Haslam, N.; Mancuso, E. 3D printed composite materials for craniofacial implants: Current concepts, challenges and future directions. Int. J. Adv. Manuf. Technol. 2021, 112, 635–653. [Google Scholar] [CrossRef]
- Fay, C.D.; Jeiranikhameneh, A.; Sayyar, S.; Talebian, S.; Nagle, A.; Cheng, K.; Fleming, S.; Mukherjee, P.; Wallace, G.G. Development of a customised 3D printer as a potential tool for direct printing of patient-specific facial prosthesis. Int. J. Adv. Manuf. Technol. 2022, 120, 7143–7155. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, F.; Zhang, Y.; You, Y.; Wang, Z.; Guan, Z. Bioinspired design of nanostructured elastomers with cross-linked soft matrix grafting on the oriented rigid nanofibers to mimic mechanical properties of human skin. ACS Nano 2015, 9, 271–278. [Google Scholar] [CrossRef]
- Jindal, S.K.; Sherriff, M.; Waters, M.G.; Coward, T.J. Development of a 3D printable maxillofacial silicone: Part I. Optimization of polydimethylsiloxane chains and cross-linker concentration. J. Prosthet. Dent. 2016, 116, 617–622. [Google Scholar] [CrossRef]
- Jindal, S.K.; Sherriff, M.; Waters, M.G.; Smay, J.E.; Coward, T.J. Development of a 3D printable maxillofacial silicone: Part II. Optimization of moderator and thixotropic agent. J. Prosthet. Dent. 2018, 119, 299–304. [Google Scholar] [CrossRef]
- Pagano, S.; Lombardo, G.; Caponi, S.; Costanzi, E.; Di Michele, A.; Bruscoli, S.; Xhimitiku, I.; Coniglio, M.; Valenti, C.; Mattarelli, M.; et al. Bio-mechanical characterization of a CAD/CAM PMMA resin for digital removable prostheses. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2021, 37, e118–e130. [Google Scholar] [CrossRef]
| Product | Batch Number |
|---|---|
| A2000 | A54615, B54615 |
| A2006 | S120511 |
| A2186 | F62846 |
| A588-1 | S01112 |
| A223-30 | S 14U132L06 |
| A225-50 | F 51110110 |
| A225-70 | S 14U109L03 |
| RTV-40 | S41112136 |
| Mechanical Property | Facial Location | Race | Age | Sex |
|---|---|---|---|---|
| Laxity | <0.0001 * | 0.60 | 0.008 * | 0.049 * |
| Stiffness | <0.0001 * | 0.43 | 0.72 | 0.11 |
| Elastic Deformation | <0.0001 * | 0.85 | 0.68 | 0.02 * |
| Creep | <0.0001 * | 0.96 | 0.04 * | 0.14 |
| Energy | <0.0001 * | 0.67 | 0.84 | 0.049 * |
| Recovered deformation | <0.0001 * | 0.13 | 0.175 | 0.09 |
| Facial Location | Stiffness (kPa/mm) | Percent Elasticity |
|---|---|---|
| Back of Ear | 39.8 ± 0.72 A | 47.1 ± 1.04 B |
| Nose | 27.3 ± 0.73 B | 31.7 ± 1.03 D |
| Forehead | 26.2 ± 0.71 B | 38.2 ± 1.03 C |
| Ear Lobe | 25.4 ± 0.72 BC | 28.7 ± 1.02 D |
| Chin | 23.7 ± 0.72 CD | 50.6 ± 1.03 A |
| Cheek | 22.4 ± 0.71 D | 48.0 ± 1.04 AB |
| Viscoelastic Properties of Stiffness and Creep | |||||
|---|---|---|---|---|---|
| Cluster | Observations | Property | Mean ± SE | Minimum | Maximum |
| 1 | 442 | Stiffness (kPa/mm) Creep (mm) | 24.8 ± 0.21 0.14 ± 0.001 | 11.3 0.05 | 33.5 0.20 |
| 2 | 184 | Stiffness (kPa/mm) Creep (mm) | 42.5 ± 0.79 0.11 ± 0.003 | 31.7 0.02 | 81.4 0.19 |
| 3 | 232 | Stiffness (kPa/mm) Creep (mm) | 20.7 ± 0.35 0.25 ± 0.005 | 8.3 0.18 | 33.6 0.56 |
| Stretchability/Texture Properties of Stiffness, Elastic Deformation, and Energy | |||||
| Cluster | Observations | Property | Mean ± SE | Minimum | Maximum |
| 1 | 113 | Stiffness (kPa/mm) | 46.7 ± 0.91 | 36.7 | 81.4 |
| Elastic Defm (mm) | 0.5 ± 0.01 | 0.2 | 0.8 | ||
| Energy (kPa·mm) | 4.8 ± 0.06 | 3.5 | 6.0 | ||
| 2 | 340 | Stiffness (kPa/mm) | 28.7 ± 0.24 | 19.6 | 39.9 |
| Elastic Defm (mm) | 0.8 ± 0.01 | 0.4 | 1.4 | ||
| Energy (kPa·mm) | 7.3 ± 0.06 | 4.8 | 9.8 | ||
| 3 | 405 | Stiffness (kPa/mm) | 20.9 ± 0.23 | 8.3 | 38.0 |
| Elastic Defm (mm) | 1.6 ± 0.02 | 0.9 | 3.6 | ||
| Energy (kPa·mm) | 11.1 ± 0.11 | 8.0 | 22.7 | ||
| Stiffness, Creep | Stiffness, Elastic Deformation, and Energy | |||||
|---|---|---|---|---|---|---|
| Facial Location | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 1 | Cluster 2 | Cluster 3 |
| Cheek | 87 | 2 | 54 | 0 | 10 | 133 |
| Forehead | 136 | 17 | 20 | 6 | 40 | 97 |
| Chin | 102 | 14 | 27 | 3 | 51 | 89 |
| Ear Lobe | 52 | 28 | 63 | 22 | 60 | 61 |
| Nose | 56 | 24 | 63 | 11 | 110 | 22 |
| Back of Ear | 40 | 100 | 6 | 71 | 69 | 3 |
| Skin/Elastomer | Laxity 1 (Percent) | Stiffness (kPa/mm) | Elastic Deformation (mm) | Energy (kPa-mm) | Creep (mm) | Percent Elasticity |
|---|---|---|---|---|---|---|
| Cheek | 64.1 ± 1.49 A | 22.4 ± 0.71 F | 1.75 ± 0.031 A | 11.2 ± 0.21 A | 0.18 ± 0.005 A | 48.0 ± 0.94 B |
| Forehead | 52.6 ± 1.48 B | 26.2 ± 0.71 E | 1.39 ± 0.030 B | 9.7 ± 0.22 B | 0.14 ± 0.005 B | 38.2 ± 1.19 C |
| Chin | 44.2 ± 1.49 C | 23.7 ± 0.72 E | 1.29 ± 0.031 B | 10.0 ± 0.21 B | 0.15 ± 0.004 B | 50.6 ± 1.33 B |
| Ear Lobe | 35.1 ± 1.47 D | 25.4 ± 0.72 E | 1.04 ± 0.031 C | 9.2 ± 0.19 B | 0.19 ± 0.005 A | 28.7 ± 0.82 D |
| Nose | 29.6 ± 1.49 E | 27.3 ± 0.73 E | 0.87 ± 0.030 D | 7.7 ± 0.23 C | 0.18 ± 0.005 A | 31.7 ± 1.03 D |
| Back of Ear | 25.6 ± 1.48 E | 39.8 ± 0.72 D | 0.58 ± 0.031 E | 5.7 ± 0.21 D | 0.11 ± 0.004 C | 47.1 ± 1.12 B |
| A2000 | 58.1 ± 7.32 ABCD | 73.6 ± 3.74 C | 0.47 ± 0.047 E | 3.9 ± 0.19 D | 0.03 ± 0.002 D | 46.2 ± 5.63 B |
| A2006 | 22.1 ± 9.60 CDE | 60.0 ± 8.13 C | 0.34 ± 0.043 E | 4.0 ± 0.11 D | 0.04 ± 0.009 D | 82.9 ± 4.70 A |
| A2186 | 36.1 ± 3.43 BCDE | 80.9 ± 4.22 C | 0.23 ± 0.002 E | 3.2 ± 0.14 D | 0.02 ± 0.002 D | 84.5 ± 3.03 A |
| A588-1 | 23.4 ± 9.38 CDE | 64.1 ± 8.43 C | 0.32 ± 0.035 E | 3.7 ± 0.20 D | 0.04 ± 0.002 D | 81.0 ± 0.47 A |
| A223-30 | 18.0 ± 9.00 E | 78.9 ± 7.34 C | 0.23 ± 0.019 E | 3.0 ± 0.10 D | 0.04 ± 0.003 D | 74.5 ± 1.42 A |
| A225-50 | 14.0 ± 8.63 E | 151.5 ± 12.87 B | 0.12 ± 0.014 E | 2.3 ± 0.78 D | 0.02 ± 0.004 D | 79.0 ± 3.57 A |
| A225-70 | 22.1 ± 7.08 E | 218.0 ± 30.8 A | 0.08 ± 0.006 E | 3.0 ± 0.23 D | 0.01 ± 0.003 D | 75.9 ± 5.90 A |
| RTV-40 | 27.2 ± 4.87 E | 144.6 ± 14.23 B | 0.15 ± 0.008 E | 2.8 ± 0.05 D | 0.02 ± 0.001 D | 77.7 ± 4.13 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beatty, M.W.; Wee, A.G.; Marx, D.B.; Ridgway, L.; Simetich, B.; De Sousa, T.C.; Vakilzadian, K.; Schulte, J. Viscoelastic Properties of Human Facial Skin and Comparisons with Facial Prosthetic Elastomers. Materials 2023, 16, 2023. https://doi.org/10.3390/ma16052023
Beatty MW, Wee AG, Marx DB, Ridgway L, Simetich B, De Sousa TC, Vakilzadian K, Schulte J. Viscoelastic Properties of Human Facial Skin and Comparisons with Facial Prosthetic Elastomers. Materials. 2023; 16(5):2023. https://doi.org/10.3390/ma16052023
Chicago/Turabian StyleBeatty, Mark W., Alvin G. Wee, David B. Marx, Lauren Ridgway, Bobby Simetich, Thiago Carvalho De Sousa, Kevin Vakilzadian, and Joel Schulte. 2023. "Viscoelastic Properties of Human Facial Skin and Comparisons with Facial Prosthetic Elastomers" Materials 16, no. 5: 2023. https://doi.org/10.3390/ma16052023
APA StyleBeatty, M. W., Wee, A. G., Marx, D. B., Ridgway, L., Simetich, B., De Sousa, T. C., Vakilzadian, K., & Schulte, J. (2023). Viscoelastic Properties of Human Facial Skin and Comparisons with Facial Prosthetic Elastomers. Materials, 16(5), 2023. https://doi.org/10.3390/ma16052023








