Effect of Temperature and Impurity Content to Control Corrosion of 316 Stainless Steel in Molten KCl-MgCl2 Salt
Abstract
:1. Introduction
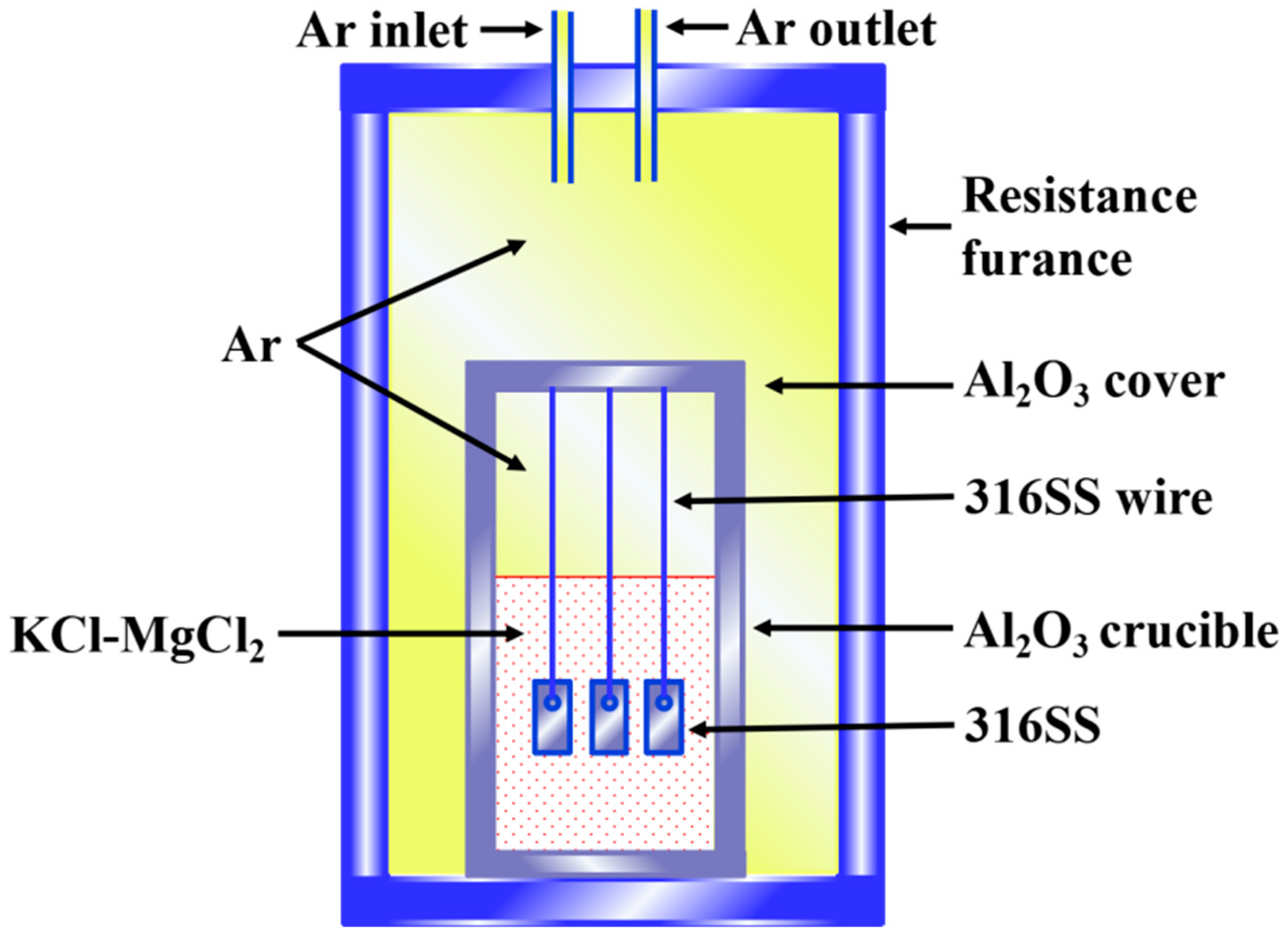
2. Experimental
2.1. Materials
2.2. Static Corrosion Test
2.3. Characterisation
3. Results and Discussion
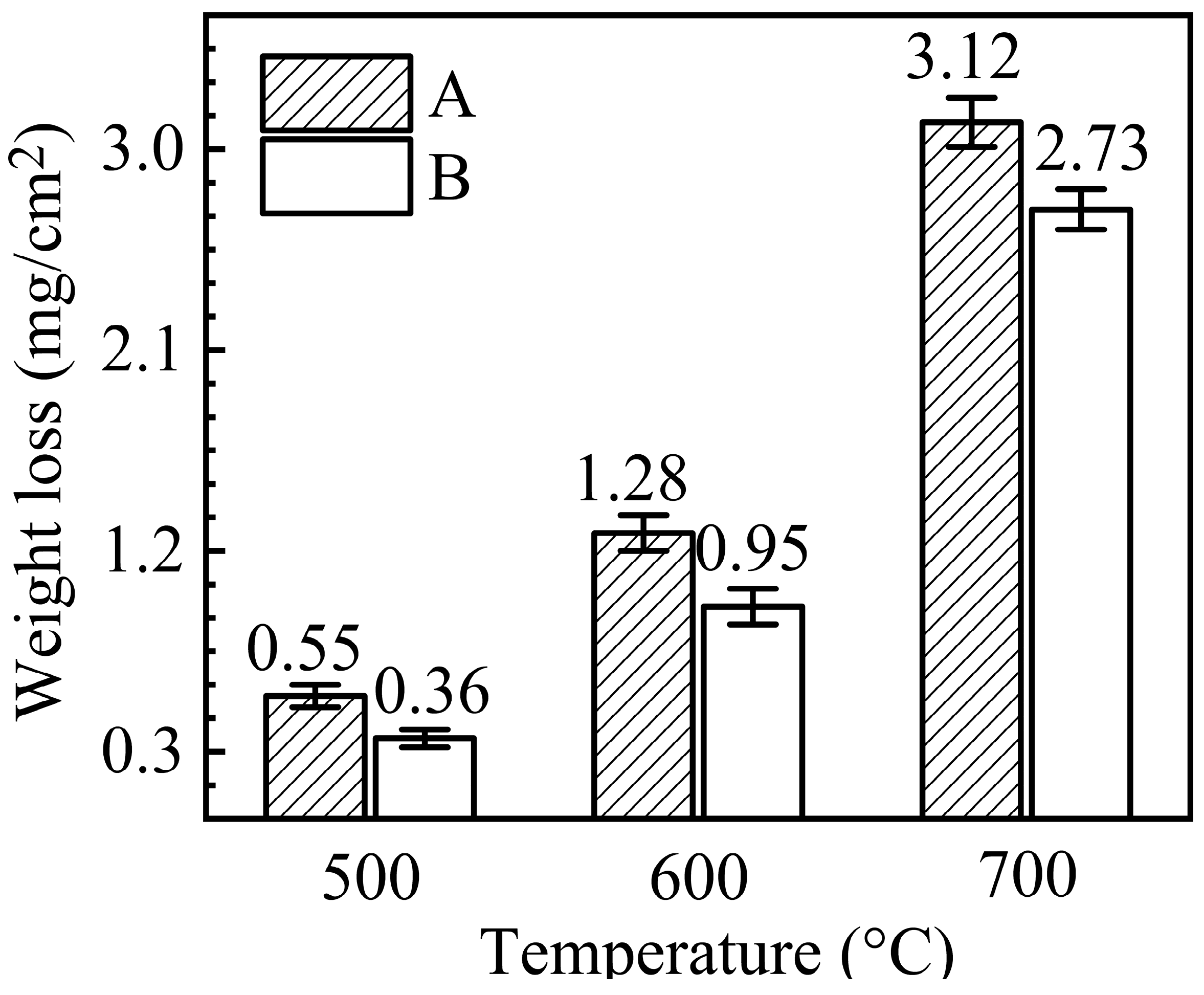
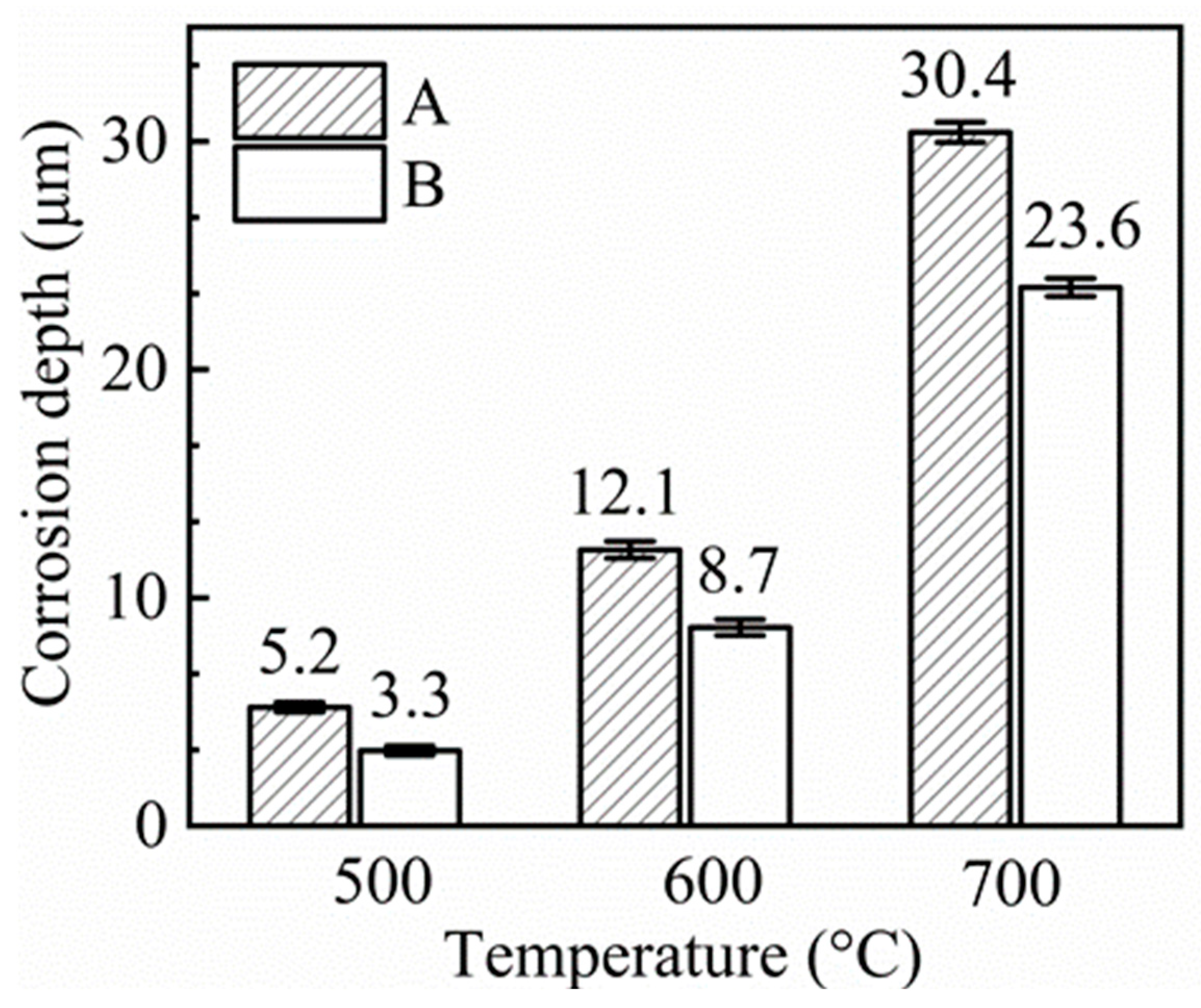
3.1. Weight Change
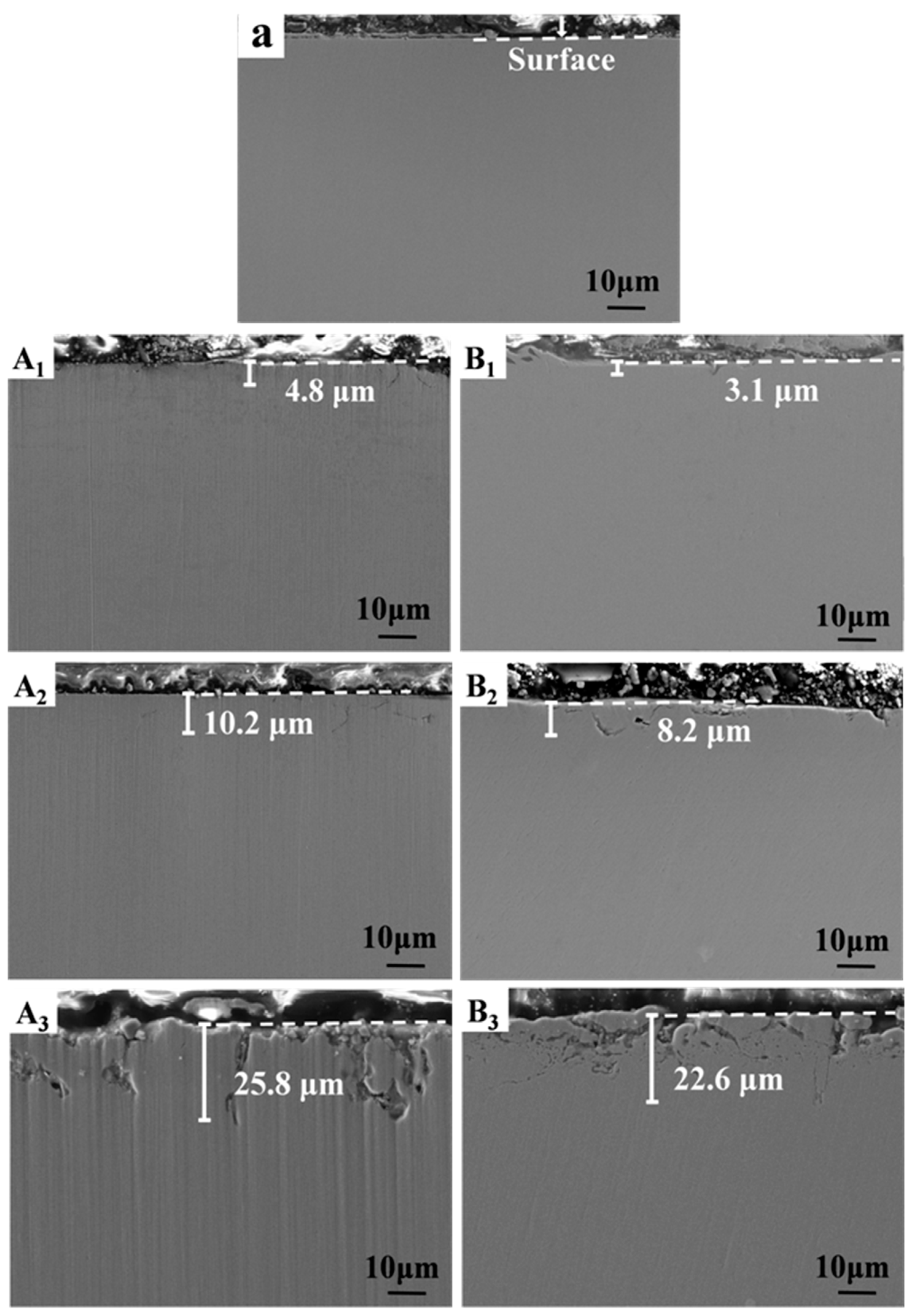
3.2. Microscopic Morphology Analysis
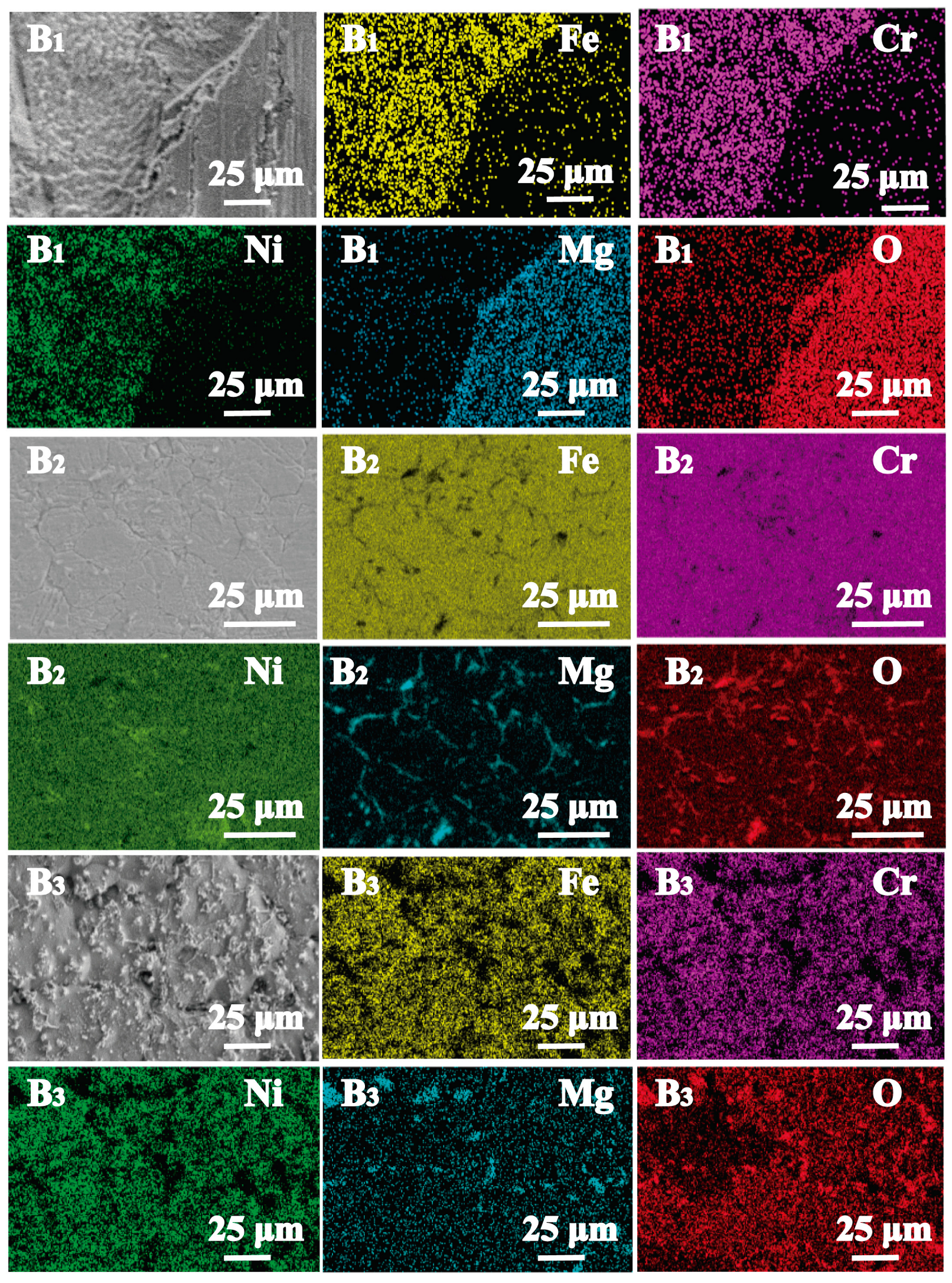
3.3. Element Distribution Analysis
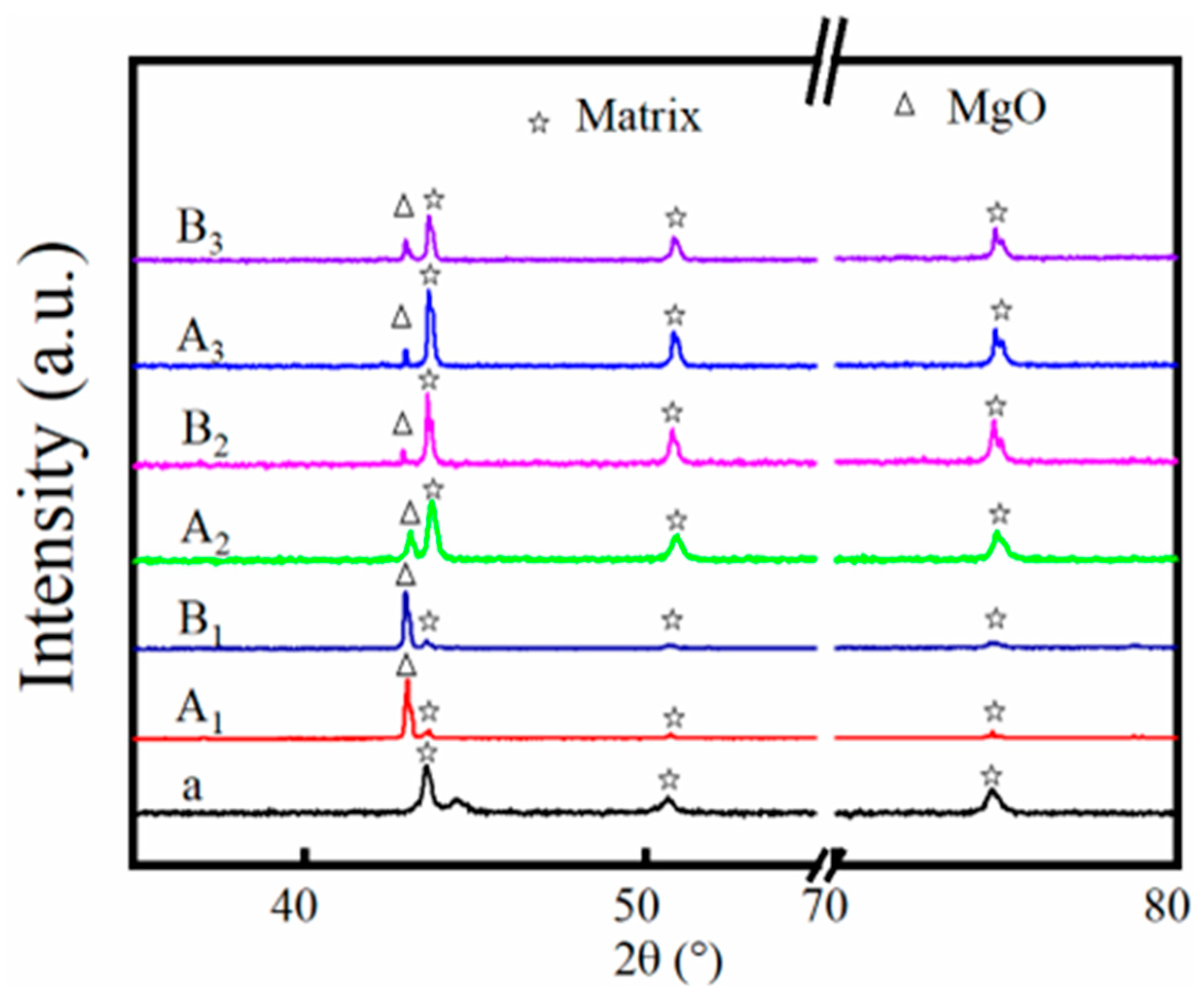
3.4. Crystalline Structure Variation
3.5. Analysis of Corrosion Products
3.6. Corrosion Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Wang, X.; Li, P.; Li, Y.; Hao, Q.; Xiao, B.; Elsentriecy, H.; Gervasio, D. Experimental test of properties of KCl-MgCl2 eutectic molten salt for heat transfer and thermal storage fluid in concentrated solar power systems. J. Sol. Energy Eng. 2018, 140, 051011–051019. [Google Scholar] [CrossRef]
- Xu, T.; Li, X.; Li, N.; Liu, M.; Wang, F.; Tang, Z. In-depth explorations on the microstructural, thermodynamic and kinetic characteristics of MgCl2-KCl eutectic salt. J. Mol. Liq. 2022, 347, 118275. [Google Scholar] [CrossRef]
- Williams, D. Assessment of Candidate Molten Salt Coolants for the NGNP/NHI Heat-Transfer Loop; 2006 Report ORNL/TM-2006/69; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2006. [Google Scholar]
- Li, Y.; Xu, X.; Wang, X.; Li, P.; Hao, Q.; Xiao, B. Survey and evaluation of equations for thermophysical properties of binary/ternary eutectic salts from NaCl, KCl, MgCl2, CaCl2, ZnCl2 for heat transfer and thermal storage fluids in CSP. Sol. Energy 2017, 152, 57–79. [Google Scholar] [CrossRef]
- Peng, Y.; Reddy, R. Effect of Ni on the corrosion behavior of Haynes 230 alloy in MgCl2-KCl salt, Flavor Physics and the TeV Scale. In Proceedings of the TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings, San Antonio, TX, USA, 10–14 March 2019; Springer: Cham, Switzerland, 2019; pp. 1313–1321. [Google Scholar]
- Ding, W.; Bauer, T. Progress in Research and Development of Molten Chloride Salt Technology for Next Generation Concentrated Solar Power Plants. Engineering 2021, 7, 334–347. [Google Scholar] [CrossRef]
- Lin, M.; Cheng, M.-S.; Dai, Z.-M. Feasibility of an innovative long-life molten chloride-cooled reactor. Nucl. Sci. Tech. 2020, 31, 33. [Google Scholar] [CrossRef]
- Anderson, N.; Sabharwall, P. Molten salt mixture properties (KF-ZrF4 and KCl-MgCl2) for use in RELAP5-3D for high-temperature reactor application. Nucl. Technol. 2011, 178, 335–340. [Google Scholar] [CrossRef]
- Bauer, T.; Pfleger, N.; Breidenbach, N.; Eck, M.; Laing, D.; Kaesche, S. Material aspects of Solar Salt for sensible heat storage. Appl. Energy 2013, 111, 1114–1119. [Google Scholar] [CrossRef]
- Ronne, A.; He, L.; Dolzhnikov, D.; Xie, Y.; Ge, M.; Halstenberg, P.; Wang, Y.; Manard, B.T.; Xiao, X.; Lee, W.-K.; et al. Revealing 3D Morphological and Chemical Evolution Mechanisms of Metals in Molten Salt by Multimodal Microscopy. ACS Appl. Mater. Interfaces 2020, 12, 17321–17333. [Google Scholar] [CrossRef]
- Yang, T.; Su, Y.; Liu, H.; Dai, Z.; Liang, X.; Wu, X. Corrosion behavior of Inconel 625 deposited metal in molten KCl-MgCl2. Mater. Res. Express 2020, 12, 126505. [Google Scholar] [CrossRef]
- Yang, T.; Su, Y.; Dai, Z.; Wang, Y.; Liang, X.; Wei, Z. Corrosion behavior of nitrogen-containing low-nickel weld cladding in KCl-MgCl2 eutectic molten salt at 900 °C. Materials 2022, 15, 8831. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.; Liu, W.; Yin, H.; Tang, Z.; Qian, Y. Ni-Mo-Cr alloy corrosion in molten NaCl-KCl-MgCl2 salt and vapour. Corros. Sci. 2021, 180, 109183. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, H.; Yin, H.; Li, N.; Wang, W.R.; Li, L.; Tang, Z.; Qian, Y. Corrosion behaviour of 316 stainless steel in NaCl-KCl-MgCl2 salt vapour at 700 °C. Corros. Sci. 2022, 194, 109921. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Tang, Z.; Xu, T.; Wang, J. Insight into dynamic corrosion mechanism of hydrous MgCl2-NaCl-KCl via FPMD simulations at high temperature. J. Mol. Liq. 2020, 314, 113596. [Google Scholar] [CrossRef]
- Lu, P.; Guo, L.; Liu, Q.; Liu, L.; Pan, T.; Tang, Z. Excellent high temperature corrosion resistance of 304 stainless steel immersed in purified NaCl-MgCl2 eutectic salts. Mater. Chem. Phys. 2023, 296, 127216. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Q.; Yin, H.; Pan, T.; Tang, Z. Excellent corrosion resistance of 316 stainless steel in purified NaCl-MgCl2 eutectic salt at high temperature. Corros. Sci. 2020, 166, 108473. [Google Scholar] [CrossRef]
- Li, X.; Xu, T.; Liu, M.; Song, Y.; Zuo, Y.; Tang, Z.; Yan, L.; Wang, J. Thermodynamic and kinetic corrosion behavior of alloys in molten MgCl2-NaCl eutectic: FPMD simulations and electrochemical technologies. Sol. Energy Mater. Sol. Cells 2022, 238, 111624. [Google Scholar] [CrossRef]
- Yin, H.; Wang, Z.; Lai, X.; Wang, Y.; Tang, Z. Optimum design and key thermal property of NaCl-KCl-CaCl2 eutectic salt for ultra-high-temperature thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 236, 111541. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, L.; Lu, P.; Liu, W.; Zhang, F.; Tang, Z. Corrosion behavior and mechanism of 316 stainless steel in NaCl-KCl-ZnCl2 molten salts at high temperature. Mater. Today Commun. 2022, 31, 103297. [Google Scholar] [CrossRef]
- Walczak, M.; Pineda, F.; Fernández, A.G.; Mata-Torres, C.; Escobar, R.A. Materials corrosion for thermal energy storage systems in concentrated solar power plants. Renew. Sustain. Energy Rev. 2018, 86, 22–44. [Google Scholar] [CrossRef]
- Lai, X.; Yin, H.; Li, P.; Liu, B.; Gao, L.; Tang, Z. The corrosion behavior of 304 stainless steel in NaNO3-NaCl-NaF molten salt and vapor. RSC Adv. 2022, 12, 7157–7163. [Google Scholar] [CrossRef]
- Ding, W.; Shi, H.; Xiu, Y.; Bonk, A.; Weisenburger, A.; Jianu, A.; Bauer, T. Hot corrosion behavior of commercial alloys in thermal energy storage material of molten MgCl2/KCl/NaCl under inert atmosphere. Sol. Energy Mater. Sol. Cells 2018, 184, 22–30. [Google Scholar] [CrossRef]
- Kurley, J.M.; Halstenberg, P.W.; McAlister, A.; Raiman, S.; Dai, S.; Mayes, R.T. Enabling chloride salts for thermal energy storage: Implications of salt purity. RSC Adv. 2019, 9, 25602–25608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Wang, J.; Tang, Z.; Liu, Y.; Wang, C. Assessment of effects of Mg treatment on corrosivity of molten NaCl-KCl-MgCl2 salt with Raman and Infrared spectra. Corros. Sci. 2020, 164, 108350. [Google Scholar] [CrossRef]
- Hanson, K.; Sankar, K.M.; Weck, P.F.; Startt, J.K.; Dingreville, R.; Deo, C.S.; Sugar, J.D.; Singh, P.M. Effect of excess Mg to control corrosion in molten MgCl2 and KCl eutectic salt mixture. Corros. Sci. 2022, 194, 109914. [Google Scholar] [CrossRef]
- Caldwell, A.; Itskos, G.; Sandhage, K.H. Air-stable, earth-abundant molten chloride sand corrosion-resistant containment for chemically-robust, high-temperature thermal energy storage for concentrated solar power. Mater. Today 2021, 46, 9–17. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, P.; Wang, J. Effects of alloying elements on the corrosion behavior of Ni-based alloys in molten NaCl-KCl-MgCl2 salt at different temperatures. Corros. Sci. 2018, 143, 187–199. [Google Scholar] [CrossRef]
- Shi, H.; Wu, T.; Gong, Q.; Ding, W.; Chai, Y.; Weisenburger, A.; Chang, L.; Zhang, Z.; Wang, K.; Richter, J.; et al. Hot corrosion behavior of additively manufactured stainless steel 316L and Inconel 718 in MgCl2/KCl/NaCl chloride salts at 700 °C. Corros. Sci. 2022, 207, 110561. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Su, X.; Yin, H.; Tang, Z. Corrosion behaviors and protective layer stabilization mechanism of 316 stainless steel in NaCl–KCl–NaF reciprocal salts at high temperatures. Corros. Sci. 2023, 211, 110893. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, C.; Wu, Y.; Lu, Y. Comparative review of different influence factors on molten salt corrosion characteristics for thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 235, 111485. [Google Scholar] [CrossRef]


| Impurity | Fe | Cr | Ni | Mn | Si | SO42− | NO3− | NO2− | PO43− | |
|---|---|---|---|---|---|---|---|---|---|---|
| Salts | ||||||||||
| A | 24.69 | 0.38 | 0.32 | 0.36 | 1.35 | 155.3 | 188.1 | 152.4 | 130.6 | |
| B | 0.12 | N.D | N.D | N.D | 0.02 | N.D | N.D | N.D | N.D | |
| Alloy | Fe | Cr | Ni | Mn | Si | P | N | Cu | Mo | C |
|---|---|---|---|---|---|---|---|---|---|---|
| 316SS | 69.04 | 16.84 | 10.12 | 1.284 | 0.472 | 0.021 | 0.008 | 0.09 | 2.08 | 0.0375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Wang, H.; Yin, H.; Liu, Q.; Tang, Z. Effect of Temperature and Impurity Content to Control Corrosion of 316 Stainless Steel in Molten KCl-MgCl2 Salt. Materials 2023, 16, 2025. https://doi.org/10.3390/ma16052025
Li N, Wang H, Yin H, Liu Q, Tang Z. Effect of Temperature and Impurity Content to Control Corrosion of 316 Stainless Steel in Molten KCl-MgCl2 Salt. Materials. 2023; 16(5):2025. https://doi.org/10.3390/ma16052025
Chicago/Turabian StyleLi, Na, Huaiyou Wang, Huiqin Yin, Qi Liu, and Zhongfeng Tang. 2023. "Effect of Temperature and Impurity Content to Control Corrosion of 316 Stainless Steel in Molten KCl-MgCl2 Salt" Materials 16, no. 5: 2025. https://doi.org/10.3390/ma16052025






