Direct Imaging of the Kinetic Crystallization Pathway: Simulation and Liquid-Phase Transmission Electron Microscopy Observations
Abstract
:1. Introduction
2. Description and Analysis of Nucleation Pathways in Experiments and Simulation
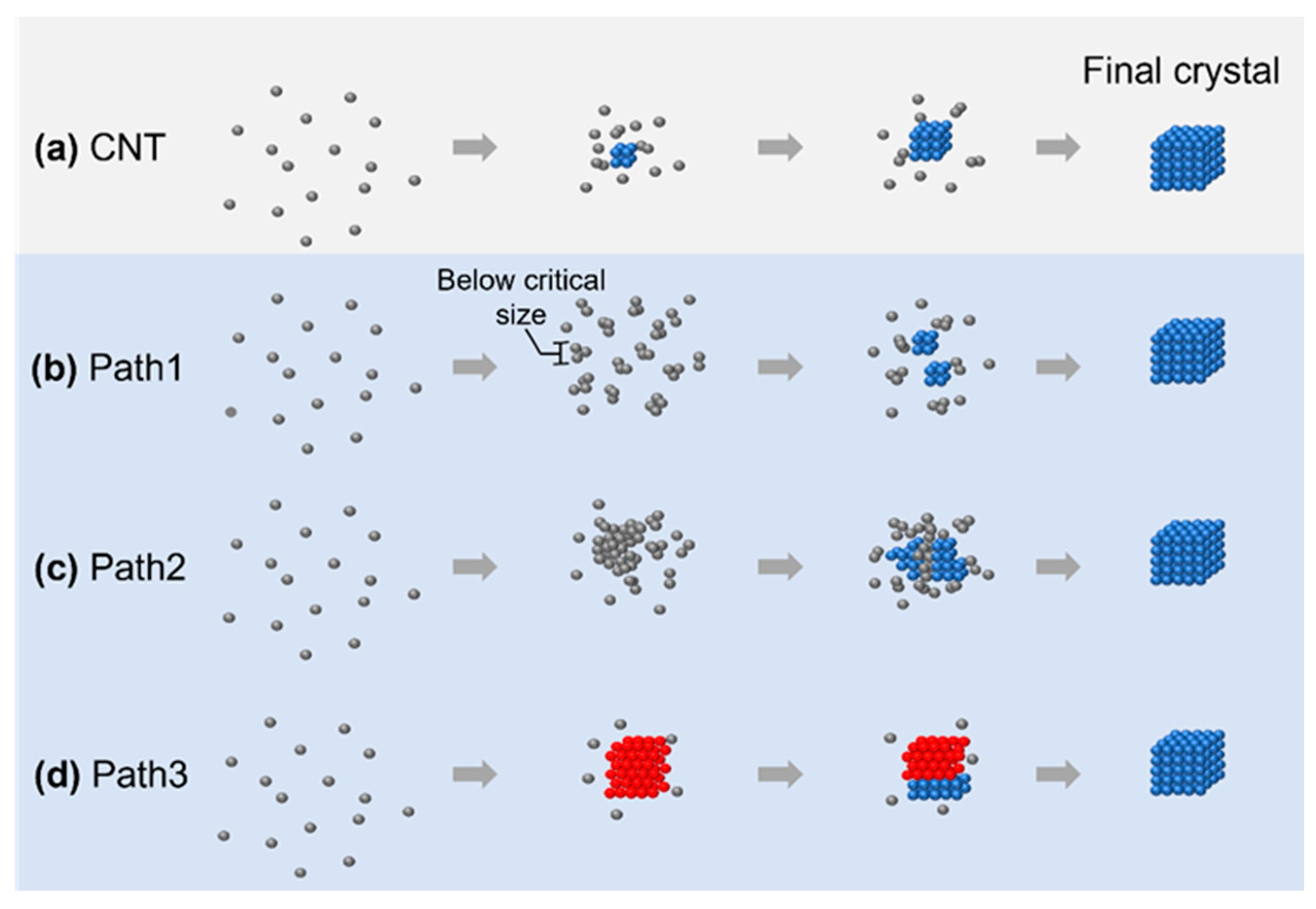
2.1. Classical and Nonclassical Nucleation Pathways
2.2. Data Processing Methods for Liquid-Phase Crystallization Observations
2.3. Computer Simulation for Crystallization Kinetics
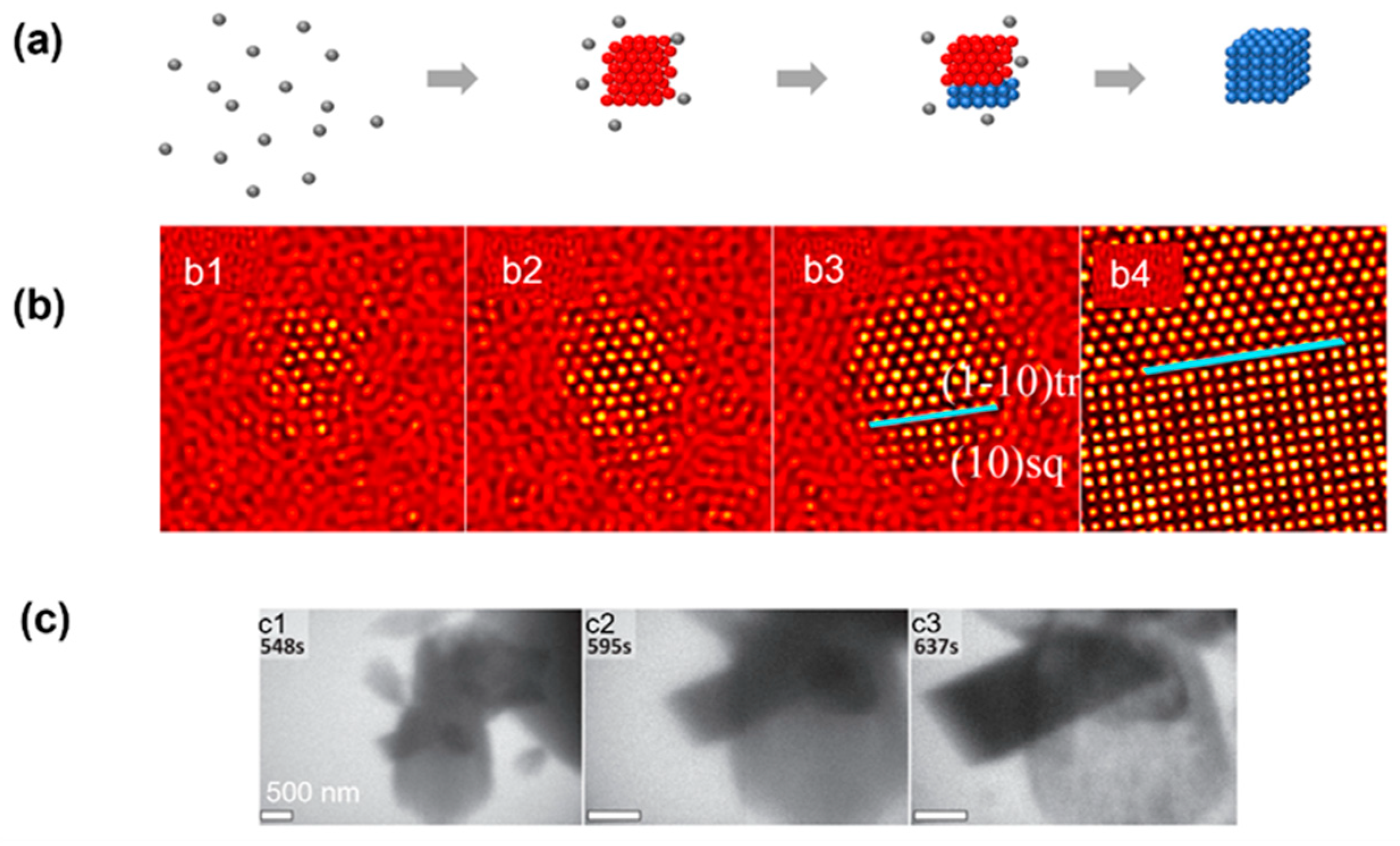
3. Experimental Observations of Classical Nucleation Pathway
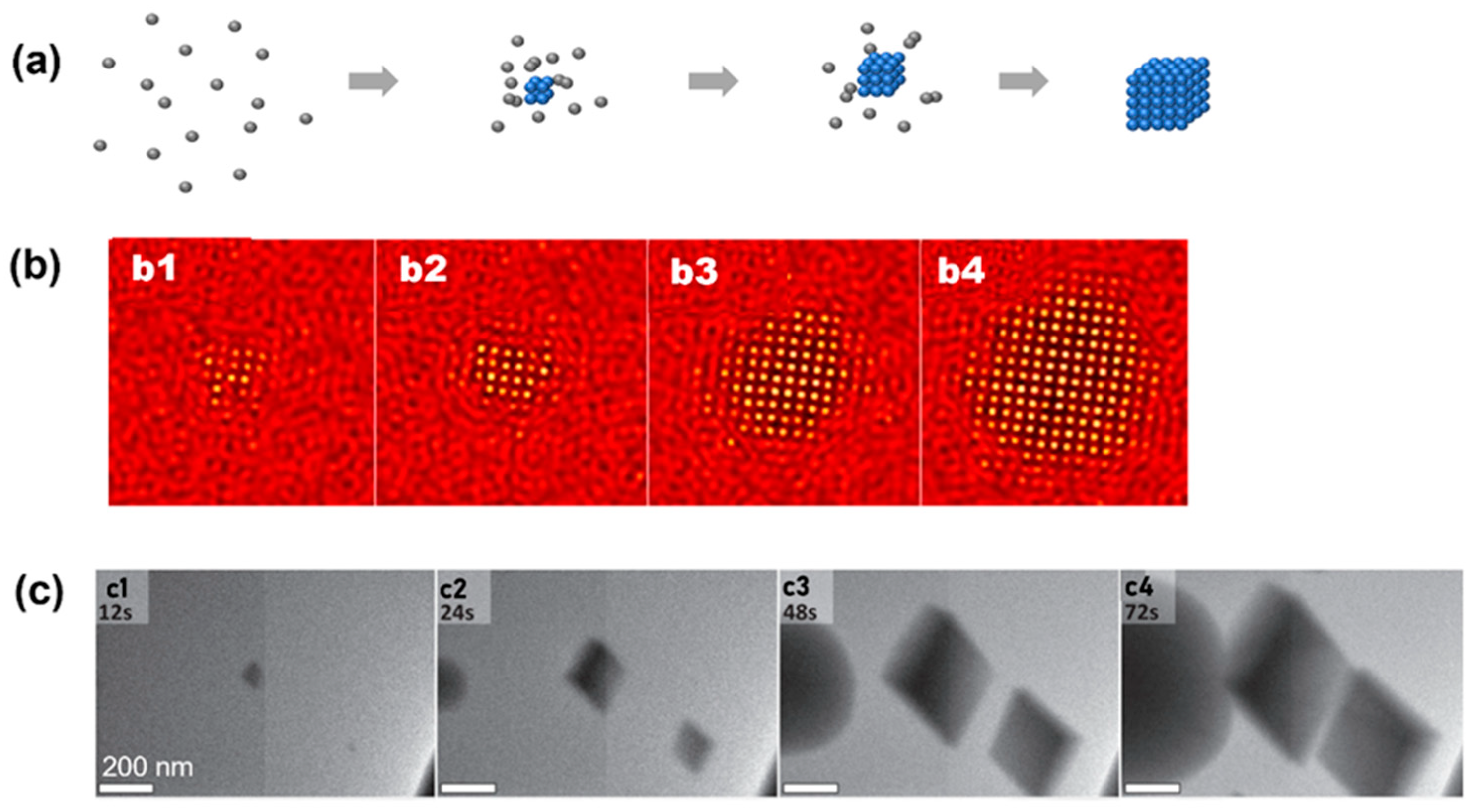
4. Observations of Nonclassical Crystallization Pathways
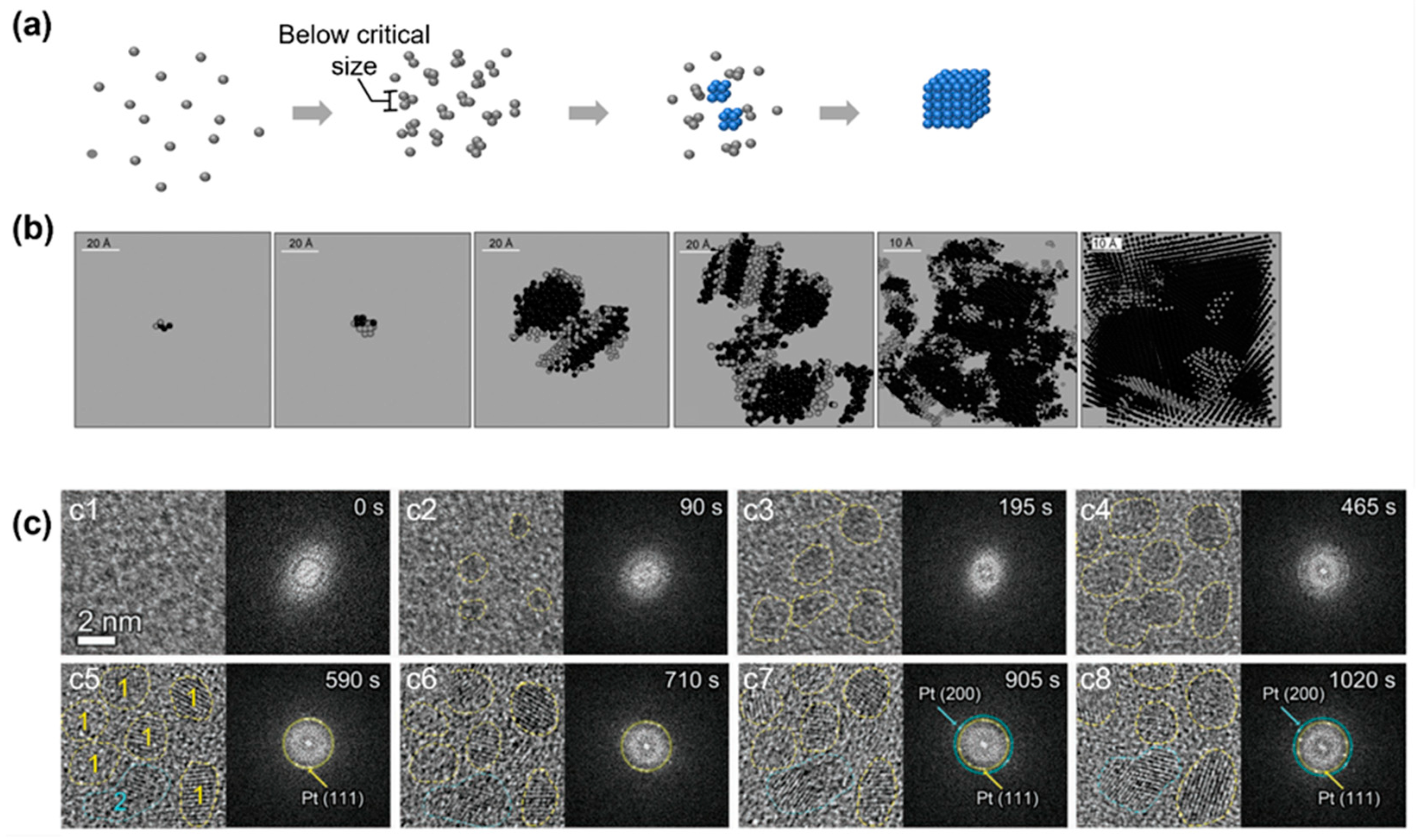
4.1. Nonclassical Crystallization Pathway 1: Amorphous Structure below Critical Nucleus Size
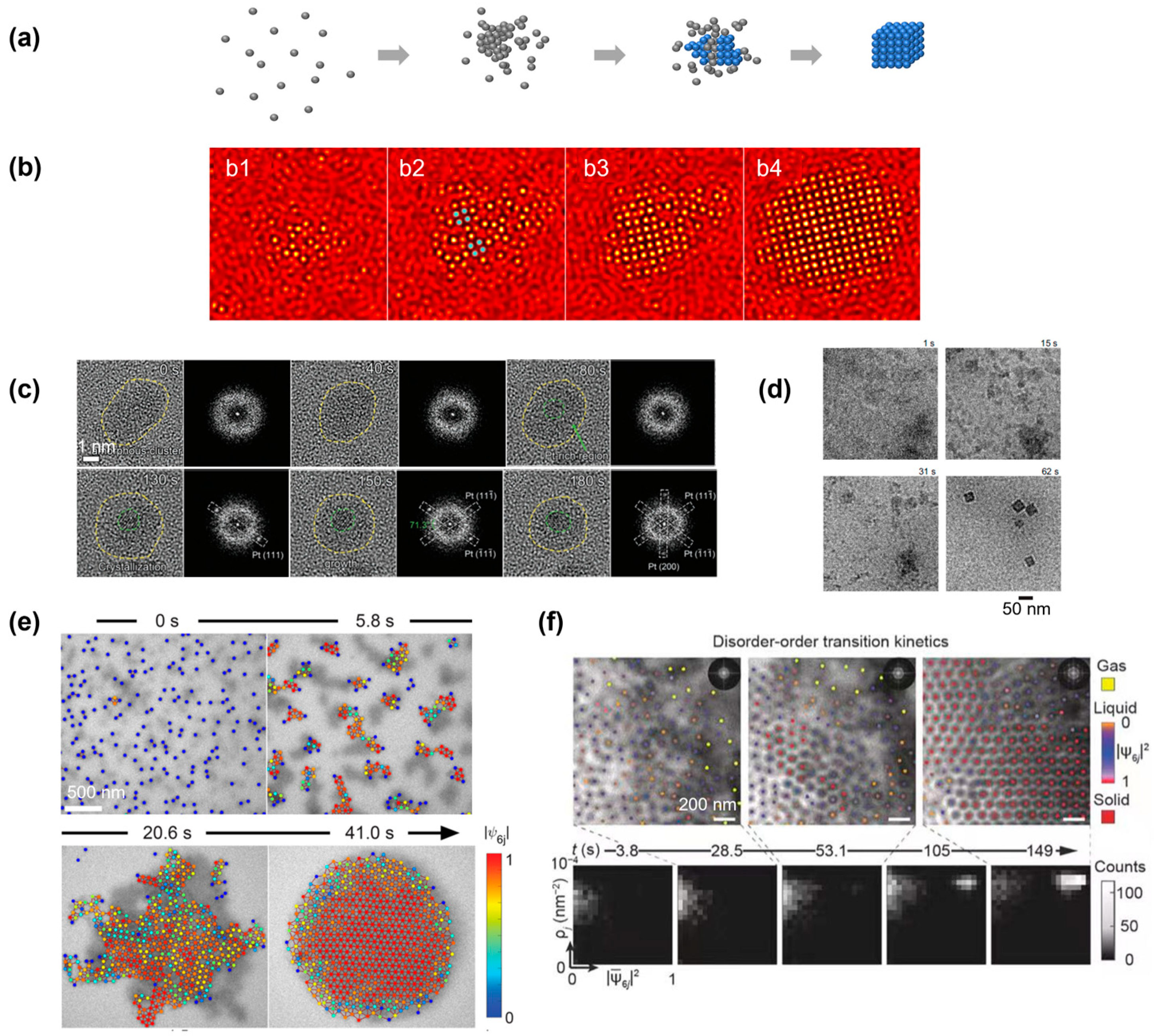
4.2. Nonclassical Crystallization Pathway 2: Nucleation of Crystalline Phase from an Amorphous Intermediate
4.3. Nonclassical Crystallization Pathway 3: Transition between Multiple Crystalline Structures before Achieving the Final Product
5. Crystal Growth Pathways after Nucleation Observed by Liquid-Phase TEM
6. Discussions
- ●
- First, the electron beam will interact with the materials, as well as the water molecules, during illumination, creating ions and radicals that affect the aqueous environment. Previous work on numerical simulation carefully illustrated all of the radiation kinetic processes when an electron beam interacts with water and predicted the temporal evolution of different species in an aqueous environment [39]. Such radiolysis has also been independently verified in the observations of nanobubble formation and growth inside liquid-phase TEM [85,86]. These radiolysis species become crucially important for the study of the crystallization of organic or biological materials, such as the assembly of peptides [87] and virus shells from proteins [88]. Although radiolysis damage was still observed during the imaging process of liquid-phase TEM, some biological samples were reported to be approximately ten times more stable than frozen-hydrated states, which are used in cryo-EM [89]. In one example, the dynamic motion of adeno-associated virus (AAV) has been achieved after optimizing the liquid thickness and electron beam dose rate, and the resolution was comparable to cryo-EM [89]. These damages can be further improved by the technical advancements of electron microscopy, especially in the imaging routine and detector efficiencies;
- ●
- Second, the intensity contrast of TEM comes from the intrinsic properties of individual elements, and it is challenging to image biological samples [90,91,92]. One of the reasons that previous studies chose materials with relatively large element numbers, such as gold and platinum, is due to the fact of their strong contrast under electron beam illumination. The contrast of materials under electron beam illumination can be quantitatively evaluated by Beer’s law, where the transmitted intensity of the electron beam decays exponentially with the increasing thickness of the materials [43]. Table 1 includes the mean free path of an electron beam in materials based on the database released by the National Institute of Standards and Technology (NIST) [93]. At typical accelerating voltages used for TEM imaging, the mean free path of noble metals is approximately a third of carbon, which is the main component of all biological materials. This challenge is not only limited to liquid-phase TEM, several contrast enhancement techniques have been developed in conventional TEM to improve the contrast of elements, including phase contrasting methods [94] and chemical staining techniques [95];
- ●
- Third, the liquid chamber for liquid-phase TEM is relatively small, and a typical thickness is several hundred nanometers, which is different from the normal experimental conditions, and the substrate plays a critical role in the crystallization kinetics [49]. The small volume of the chamber could affect the crystallization kinetics from multiple different aspects. On the one hand, it has been pointed out in both experiments and simulations that the crystallization kinetics for systems with different size limits are different [96,97]. On the other hand, the physical existence of boundaries in liquid-phase TEM will also affect the crystallization kinetics by attracting the atoms or nanoparticles to the vicinity of the membrane [70,78]. This boundary might also align the nanoparticles into a certain orientation, facilitating the self-assembly process [49]. Lastly, this macroscopically uniform chamber film might not be nanoscopically uniform in terms of surface chemistry, as pointed out by similar spatial mappings between the heterogeneous nucleation domains imaged by liquid-phase TEM and the functional group domains mapped by liquid-phase AFM [98];
- ●
- Forth, TEM collects all of the electrons transmitting through the sample, which includes all materials and beam trajectories, making it difficult to interpret the three-dimensional structural details. This challenge applies to all imaging techniques relying on the transmission of the probe through the sample, such as the conventional optical microscope. To overcome this challenge, a confocal microscope introduces a pinhole to reduce the light collected from the region out of the focal plane, and this would improve the resolution along the beam direction [99]. With such improved resolution, it is then possible to allow the scanning of the focal plane through the sample and the differentiation of the images captured at different depths. Similar techniques have also been applied to electron microscopes to capture “depth sectioning” images, and the three-dimensional reconstruction of nanostructures based on sectioning images has been also demonstrated [100]. Another strategy is to compare the contrast of experimental TEM with simulation models, as they can be quantitatively simulated after taking all the materials along the electron beam and noise origins into consideration [43]. This could potentially be used to illustrate the three-dimensional organization of materials inside the liquid-phase chamber [43,101]. For example, in a colloidal superlattice where multiple layers of nanoparticles stack together, understanding the particle locations in each layer becomes crucial to understand the two-dimensional projections captured by liquid-phase TEM. In a study of the assembly of gold nanospheres at the solid–liquid interface, it is reported that the second layer did not necessarily follow the close-packed hexagonal lattice, following the first layer [102]. Instead, it could pack into a quasicrystalline lattice if the dielectric constant of the solution could be modulated correctly. Such mechanism studies on the formation of multilayer structures offer important insights into the growth of three-dimensional materials, such as the nanoparticle substrates used in novel devices [103].
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Yoreo, J.J.; Vekilov, P.G. Principles of Crystal Nucleation and Growth. Rev. Mineral. Geochem. 2003, 54, 57–93. [Google Scholar] [CrossRef] [Green Version]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760. [Google Scholar] [CrossRef] [PubMed]
- Vekilov, P.G. Crystallization tracked atom by atom. Nature 2019, 570, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palberg, T. Crystallization kinetics of colloidal model suspensions: Recent achievements and new perspectives. J. Phys. Condens. Matter 2014, 26, 333101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Zhou, D.; Han, Y. Assembly and phase transitions of colloidal crystals. Nat. Rev. Mater. 2016, 1, 15011. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liu, C.; Chen, Q. In-situ imaging of nucleation and growth of superlattices from nanoscale colloidal nanoparticles. J. Cryst. Growth 2023, 601, 126955. [Google Scholar] [CrossRef]
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-Assembly of Colloidal Nanocrystals: From Intricate Structures to Functional Materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef]
- Nagaoka, Y.; Zhu, H.; Eggert, D.; Chen, O. Single-component quasicrystalline nanocrystal superlattices through flexible polygon tiling rule. Science 2018, 362, 1396–1400. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Lee, S.; Sun, L.; Spellings, M.; Engel, M.; Glotzer, S.C.; Mirkin, C.A. Clathrate colloidal crystals. Science 2017, 355, 931–935. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Li, J.; Lu, J.; Liu, H.; Kim, J.-Y.; Kim, A.; Yao, L.; Liu, C.; Qian, C.; Hood, Z.D.; et al. Chiral assemblies of pinwheel superlattices on substrates. Nature 2022, 612, 259–265. [Google Scholar] [CrossRef]
- Kagan, C.R.; Hyeon, T.; Kim, D.-H.; Ruiz, R.; Tung, M.C.; Wong, H.S.P. Self-assembly for electronics. MRS Bull. 2020, 45, 807–814. [Google Scholar] [CrossRef]
- Yadav, A.; Gerislioglu, B.; Ahmadivand, A.; Kaushik, A.; Cheng, G.J.; Ouyang, Z.; Wang, Q.; Yadav, V.S.; Mishra, Y.K.; Wu, Y.; et al. Controlled self-assembly of plasmon-based photonic nanocrystals for high performance photonic technologies. Nano Today 2021, 37, 101072. [Google Scholar] [CrossRef]
- Schulz, F.; Lokteva, I.; Parak, W.J.; Lehmkühler, F. Recent Notable Approaches to Study Self-Assembly of Nanoparticles with X-Ray Scattering and Electron Microscopy. Part. Part. Syst. Charact. 2021, 38, 2100087. [Google Scholar] [CrossRef]
- Stipp, A.; Schöpe, H.-J.; Palberg, T.; Eckert, T.; Biehl, R.; Bartsch, E. Optical experiments on a crystallizing hard-sphere--polymer mixture at coexistence. Phys. Rev. E 2010, 81, 051401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauter, A.; Roosen-Runge, F.; Zhang, F.; Lotze, G.; Jacobs, R.M.J.; Schreiber, F. Real-Time Observation of Nonclassical Protein Crystallization Kinetics. J. Am. Chem. Soc. 2015, 137, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Pujals, S.; Feiner-Gracia, N.; Delcanale, P.; Voets, I.; Albertazzi, L. Super-resolution microscopy as a powerful tool to study complex synthetic materials. Nat. Rev. Chem. 2019, 3, 68–84. [Google Scholar] [CrossRef] [Green Version]
- Pach, E.; Verdaguer, A. Studying Ice with Environmental Scanning Electron Microscopy. Molecules 2022, 27, 258. [Google Scholar] [CrossRef] [PubMed]
- Mirsaidov, U.; Patterson, J.P.; ZhengGuest, H. Liquid phase transmission electron microscopy for imaging of nanoscale processes in solution. MRS Bull. 2020, 45, 704–712. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Farrance, O.E.; Kailas, L. How atomic force microscopy has contributed to our understanding of polymer crystallization. Polymer 2009, 50, 4281–4292. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Yuk, J.M.; Hauwiller, M.R.; Park, J.; Dae, K.S.; Kim, J.S.; Alivisatos, A.P. Nucleation, growth, and superlattice formation of nanocrystals observed in liquid cell transmission electron microscopy. MRS Bull. 2020, 45, 713–726. [Google Scholar] [CrossRef]
- Ross, F.M. Opportunities and challenges in liquid cell electron microscopy. Science 2015, 350, aaa9886. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zeng, Z.; Kang, J.; Betzler, S.; Czarnik, C.; Zhang, X.; Ophus, C.; Yu, C.; Bustillo, K.; Pan, M.; et al. Formation of two-dimensional transition metal oxide nanosheets with nanoparticles as intermediates. Nat. Mater. 2019, 18, 970–976. [Google Scholar] [CrossRef]
- Ou, Z.; Liu, C.; Yao, L.; Chen, Q. Nanoscale Cinematography of Soft Matter System under Liquid-Phase TEM. Acc. Mater. Res. 2020, 1, 41–52. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Savitzky, B.H.; Chen, Z.; Wang, Y.; Betzler, S.; Bustillo, K.; Persson, K.; Cui, Y.; Wang, L.-W.; et al. Spatially resolved structural order in low-temperature liquid electrolyte. Sci. Adv. 2023, 9, eadc9721. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Wang, Y.H.; Zhuang, P.X.; Li, Y.T.; Wan, Q.Q.; Ma, Y.X.; Tay, F.R.; Niu, L.N. Applications of Cryogenic Electron Microscopy in Biomineralization Research. J. Dent. Res. 2022, 101, 505–514. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Y.; Yang, Y.; Kim, D.S.; Yuan, A.; Tian, X.; Ophus, C.; Sun, F.; Schmid, A.K.; Nathanson, M.; et al. Observing crystal nucleation in four dimensions using atomic electron tomography. Nature 2019, 570, 500–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, J.L.; Massa, M.V.; Dalnoki-Veress, K. Ellipsometry as a probe of crystallization kinetics in thin diblock copolymer films. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 3448–3452. [Google Scholar] [CrossRef]
- Desbois, S.; Seabrook, S.A.; Newman, J. Some practical guidelines for UV imaging in the protein crystallization laboratory. Acta Crystallogr. Sect. F 2013, 69, 201–208. [Google Scholar] [CrossRef]
- Lutsko, J.F.; Nicolis, G. Theoretical Evidence for a Dense Fluid Precursor to Crystallization. Phys. Rev. Lett. 2006, 96, 046102. [Google Scholar] [CrossRef] [Green Version]
- Wolde, P.R.t.; Frenkel, D. Enhancement of Protein Crystal Nucleation by Critical Density Fluctuations. Science 1997, 277, 1975–1978. [Google Scholar] [CrossRef] [Green Version]
- Algara-Siller, G.; Lehtinen, O.; Wang, F.C.; Nair, R.R.; Kaiser, U.; Wu, H.A.; Geim, A.K.; Grigorieva, I.V. Square ice in graphene nanocapillaries. Nature 2015, 519, 443–445. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chen, J.; Cox, S.J.; Liu, L.; Sosso, G.C.; Li, N.; Gao, P.; Michaelides, A.; Wang, E.; Bai, X. Microscopic Kinetics Pathway of Salt Crystallization in Graphene Nanocapillaries. Phys. Rev. Lett. 2021, 126, 136001. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chee, S.W.; Raj, S.; Sawczyk, M.; Král, P.; Mirsaidov, U. Three-step nucleation of metal–organic framework nanocrystals. Proc. Natl. Acad. Sci. USA 2021, 118, e2008880118. [Google Scholar] [CrossRef]
- Nielsen, M.H.; Aloni, S.; De Yoreo, J.J. In Situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways. Science 2014, 345, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, J.; Li, J.; Wang, Z.; Tang, S. Kinetic Pathways and Mechanisms of Two-Step Nucleation in Crystallization. J. Phys. Chem. Lett. 2016, 7, 5008–5014. [Google Scholar] [CrossRef] [PubMed]
- Sutter, E.; Sutter, P.; Tkachenko, A.V.; Krahne, R.; de Graaf, J.; Arciniegas, M.; Manna, L. In Situ microscopy of the self-assembly of branched nanocrystals in solution. Nat. Commun. 2016, 7, 11213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Guo, X.; Sun, M.; Qu, A.; Hao, C.; Wu, X.; Guo, J.; Xu, C.; Kuang, H.; Xu, L. Self-limiting self-assembly of supraparticles for potential biological applications. Nanoscale 2021, 13, 2302–2311. [Google Scholar] [CrossRef] [PubMed]
- Crocker, J.C.; Grier, D.G. Methods of Digital Video Microscopy for Colloidal Studies. J. Colloid Interface Sci. 1996, 179, 298–310. [Google Scholar] [CrossRef] [Green Version]
- Schneider, N.M.; Norton, M.M.; Mendel, B.J.; Grogan, J.M.; Ross, F.M.; Bau, H.H. Electron-Water Interactions and Implications for Liquid Cell Electron Microscopy. J. Phys. Chem. C 2014, 118, 22373–22382. [Google Scholar] [CrossRef]
- Kim, J.; Jones, M.R.; Ou, Z.; Chen, Q. In Situ Electron Microscopy Imaging and Quantitative Structural Modulation of Nanoparticle Superlattices. ACS Nano 2016, 10, 9801–9808. [Google Scholar] [CrossRef]
- Laramy, C.R.; Brown, K.A.; O’Brien, M.N.; Mirkin, C.A. High-Throughput, Algorithmic Determination of Nanoparticle Structure from Electron Microscopy Images. ACS Nano 2015, 9, 12488–12495. [Google Scholar] [CrossRef] [PubMed]
- Falk, T.; Mai, D.; Bensch, R.; Çiçek, Ö.; Abdulkadir, A.; Marrakchi, Y.; Böhm, A.; Deubner, J.; Jäckel, Z.; Seiwald, K.; et al. U-Net: Deep learning for cell counting, detection, and morphometry. Nat. Methods 2019, 16, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Ou, Z.; Luo, B.; Xu, C.; Chen, Q. Machine Learning to Reveal Nanoparticle Dynamics from Liquid-Phase TEM Videos. ACS Cent. Sci. 2020, 6, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Jones, M.R.; Frechette, L.B.; Chen, Q.; Powers, A.S.; Ercius, P.; Dunn, G.; Rotskoff, G.M.; Nguyen, S.C.; Adiga, V.P.; et al. Single-particle mapping of nonequilibrium nanocrystal transformations. Science 2016, 354, 874–877. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Leonardi, A.; Chen, J.; Cao, M.; Li, N.; Su, D.; Zhang, Q.; Engel, M.; Ye, X. Imaging the kinetics of anisotropic dissolution of bimetallic core–shell nanocubes using graphene liquid cells. Nat. Commun. 2020, 11, 3041. [Google Scholar] [CrossRef]
- Liao, H.-G.; Cui, L.; Whitelam, S.; Zheng, H. Real-Time Imaging of Pt3Fe Nanorod Growth in Solution. Science 2012, 336, 1011–1014. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.-G.; Zherebetskyy, D.; Xin, H.; Czarnik, C.; Ercius, P.; Elmlund, H.; Pan, M.; Wang, L.-W.; Zheng, H. Facet development during platinum nanocube growth. Science 2014, 345, 916–919. [Google Scholar] [CrossRef]
- Lotito, V.; Zambelli, T. Pattern detection in colloidal assembly: A mosaic of analysis techniques. Adv. Colloid Interface Sci. 2020, 284, 102252. [Google Scholar] [CrossRef]
- Ou, Z.; Wang, Z.; Luo, B.; Luijten, E.; Chen, Q. Kinetic pathways of crystallization at the nanoscale. Nat. Mater. 2020, 19, 450–455. [Google Scholar] [CrossRef]
- Chen, Q.; Cho, H.; Manthiram, K.; Yoshida, M.; Ye, X.; Alivisatos, A.P. Interaction Potentials of Anisotropic Nanocrystals from the Trajectory Sampling of Particle Motion using in Situ Liquid Phase Transmission Electron Microscopy. ACS Cent. Sci. 2015, 1, 33–39. [Google Scholar] [CrossRef]
- Anwar, J.; Boateng, P.K. Computer Simulation of Crystallization from Solution. J. Am. Chem. Soc. 1998, 120, 9600–9604. [Google Scholar] [CrossRef]
- Tan, P.; Xu, N.; Xu, L. Visualizing kinetic pathways of homogeneous nucleation in colloidal crystallization. Nat. Phys. 2014, 10, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Zhao, N.; Hou, Z. The effect of hydrodynamic interactions on nanoparticle diffusion in polymer solutions: A multiparticle collision dynamics study. Soft Matter 2017, 13, 8625–8635. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, H.; Hou, Z. Effects of hydrodynamic interactions on the crystallization of passive and active colloidal systems. Soft Matter 2015, 11, 5712–5718. [Google Scholar] [CrossRef] [PubMed]
- Tateno, M.; Yanagishima, T.; Russo, J.; Tanaka, H. Influence of Hydrodynamic Interactions on Colloidal Crystallization. Phys. Rev. Lett. 2019, 123, 258002. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.; Tanaka, H. The microscopic pathway to crystallization in supercooled liquids. Sci. Rep. 2012, 2, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, T.; Tanaka, H. Formation of a crystal nucleus from liquid. Proc. Natl. Acad. Sci. USA 2010, 107, 14036–14041. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, A.L. Machine learning and data science in soft materials engineering. J. Phys. Condens. Matter 2018, 30, 043002. [Google Scholar] [CrossRef]
- Sharp, T.A.; Thomas, S.L.; Cubuk, E.D.; Schoenholz, S.S.; Srolovitz, D.J.; Liu, A.J. Machine learning determination of atomic dynamics at grain boundaries. Proc. Natl. Acad. Sci. USA 2018, 115, 10943–10947. [Google Scholar] [CrossRef] [Green Version]
- Tah, I.; Ridout, S.A.; Liu, A.J. Fragility in glassy liquids: A structural approach based on machine learning. J. Chem. Phys. 2022, 157, 124501. [Google Scholar] [CrossRef]
- Li, J.; Deepak, F.L. In Situ Kinetic Observations on Crystal Nucleation and Growth. Chem. Rev. 2022, 122, 16911–16982. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Peters, B. Solute Precipitate Nucleation: A Review of Theory and Simulation Advances. In Advances in Chemical Physics: Volume 155; Wiley-Blackwell: Hoboken, NJ, USA, 2014; pp. 97–160. [Google Scholar]
- Lee, B.-S.; Burr, G.W.; Shelby, R.M.; Raoux, S.; Rettner, C.T.; Bogle, S.N.; Darmawikarta, K.; Bishop, S.G.; Abelson, J.R. Observation of the Role of Subcritical Nuclei in Crystallization of a Glassy Solid. Science 2009, 326, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Chen, J.; Chang, F.; Zeng, Z.; He, T.; Jie, W. Simulation of molecular dynamics of silver subcritical nuclei and crystal clusters during solidification. Sci. China Technol. Sci. 2010, 53, 3203–3208. [Google Scholar] [CrossRef]
- Ye, H.; Yang, F.; Sun, Y.; Wang, R. Atom-Resolved Investigation on Dynamic Nucleation and Growth of Platinum Nanocrystals. Small Methods 2022, 6, 2200171. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Biskupek, J.; Stoppiello, C.T.; McSweeney, R.L.; Chamberlain, T.W.; Liu, Z.; Suenaga, K.; Skowron, S.T.; Besley, E.; Khlobystov, A.N.; et al. Atomic mechanism of metal crystal nucleus formation in a single-walled carbon nanotube. Nat. Chem. 2020, 12, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.R.; Dinsmore, A.D. Experimental Evidence for Two-Step Nucleation in Colloidal Crystallization. Phys. Rev. Lett. 2009, 102, 198302. [Google Scholar] [CrossRef] [PubMed]
- Kashchiev, D.; Vekilov, P.G.; Kolomeisky, A.B. Kinetics of two-step nucleation of crystals. J. Chem. Phys. 2005, 122, 244706. [Google Scholar] [CrossRef]
- Loh, N.D.; Sen, S.; Bosman, M.; Tan, S.F.; Zhong, J.; Nijhuis, C.A.; Král, P.; Matsudaira, P.; Mirsaidov, U. Multistep nucleation of nanocrystals in aqueous solution. Nat. Chem. 2017, 9, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Allen, V.R.; Chen, J.; Wang, Y.; Ye, X. Multistep Crystallization of Dynamic Nanoparticle Superlattices in Nonaqueous Solutions. J. Am. Chem. Soc. 2022, 144, 14915–14922. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, F.; Wang, Z.; Alsayed, A.M.; Zhang, Z.; Yodh, A.G.; Han, Y. Two-step nucleation mechanism in solid–solid phase transitions. Nat. Mater. 2015, 14, 101–108. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, Z.; Tang, S.; Tong, H.; Yanagishima, T.; Tanaka, H.; Tan, P. Morphology selection kinetics of crystallization in a sphere. Nat. Phys. 2021, 17, 121–127. [Google Scholar] [CrossRef]
- Sun, M.; Cheng, Z.; Chen, W.; Jones, M. Understanding Symmetry Breaking at the Single-Particle Level via the Growth of Tetrahedron-Shaped Nanocrystals from Higher-Symmetry Precursors. ACS Nano 2021, 15, 15953–15961. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Yao, L.; An, H.; Shen, B.; Chen, Q. Imaging how thermal capillary waves and anisotropic interfacial stiffness shape nanoparticle supracrystals. Nat. Commun. 2020, 11, 4555. [Google Scholar] [CrossRef]
- Zhu, C.; Liang, S.; Song, E.; Zhou, Y.; Wang, W.; Shan, F.; Shi, Y.; Hao, C.; Yin, K.; Zhang, T.; et al. In-situ liquid cell transmission electron microscopy investigation on oriented attachment of gold nanoparticles. Nat. Commun. 2018, 9, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Ou, Z.; Guo, F.; Luo, B.; Chen, W.; Qi, L.; Chen, Q. “Colloid–Atom Duality” in the Assembly Dynamics of Concave Gold Nanoarrows. J. Am. Chem. Soc. 2020, 142, 11669–11673. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, B.B.V.; van der Sluijs, M.M.; Soligno, G.; Vanmaekelbergh, D. Oriented Attachment: From Natural Crystal Growth to a Materials Engineering Tool. Acc. Chem. Res. 2021, 54, 787–797. [Google Scholar] [CrossRef]
- Kim, J.; Ou, Z.; Jones, M.R.; Song, X.; Chen, Q. Imaging the polymerization of multivalent nanoparticles in solution. Nat. Commun. 2017, 8, 761. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Sushko, M.L.; Loring, J.S.; Legg, B.A.; Song, M.; Soltis, J.A.; Huang, X.; Rosso, K.M.; De Yoreo, J.J. Self-similar mesocrystals form via interface-driven nucleation and assembly. Nature 2021, 590, 416–422. [Google Scholar] [CrossRef]
- Silvera Batista, C.A.; Larson, R.G.; Kotov, N.A. Nonadditivity of nanoparticle interactions. Science 2015, 350, 1242477. [Google Scholar] [CrossRef] [Green Version]
- Anwar, J.; Zahn, D. Uncovering Molecular Processes in Crystal Nucleation and Growth by Using Molecular Simulation. Angew. Chem. Int. Ed. 2011, 50, 1996–2013. [Google Scholar] [CrossRef]
- Jeon, S.; Heo, T.; Hwang, S.-Y.; Ciston, J.; Bustillo, K.C.; Reed, B.W.; Ham, J.; Kang, S.; Kim, S.; Lim, J.; et al. Reversible disorder-order transitions in atomic crystal nucleation. Science 2021, 371, 498–503. [Google Scholar] [CrossRef]
- Yao, L.; An, H.; Zhou, S.; Kim, A.; Luijten, E.; Chen, Q. Seeking regularity from irregularity: Unveiling the synthesis–nanomorphology relationships of heterogeneous nanomaterials using unsupervised machine learning. Nanoscale 2022, 14, 16479–16489. [Google Scholar] [CrossRef]
- Zhu, G.; Reiner, H.; Cölfen, H.; De Yoreo, J.J. Addressing some of the technical challenges associated with liquid phase S/TEM studies of particle nucleation, growth and assembly. Micron 2019, 118, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, Q.; Shin, D.; Park, J.; Weitz, D.A.; Jhe, W. Initial growth dynamics of 10 nm nanobubbles in the graphene liquid cell. Appl. Nanosci. 2021, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.; Kang, S.; Kim, B.H.; Lim, K.; Jeon, S.; Shim, S.; Lee, W.C.; Park, J. Nanobubble Dynamics in Aqueous Surfactant Solutions Studied by Liquid-Phase Transmission Electron Microscopy. Engineering 2021, 7, 630–635. [Google Scholar] [CrossRef]
- Touve, M.A.; Carlini, A.S.; Gianneschi, N.C. Self-assembling peptides imaged by correlated liquid cell transmission electron microscopy and MALDI-imaging mass spectrometry. Nat. Commun. 2019, 10, 4837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luque, D.; Castón, J.R. Cryo-electron microscopy for the study of virus assembly. Nat. Chem. Biol. 2020, 16, 231–239. [Google Scholar] [CrossRef]
- Jonaid, G.; Dearnaley, W.J.; Casasanta, M.A.; Kaylor, L.; Berry, S.; Dukes, M.J.; Spilman, M.S.; Gray, J.L.; Kelly, D.F. High-Resolution Imaging of Human Viruses in Liquid Droplets. Adv. Mater. 2021, 33, 2103221. [Google Scholar] [CrossRef]
- Smith, J.W.; Chen, Q. Liquid-phase electron microscopy imaging of cellular and biomolecular systems. J. Mater. Chem. B 2020, 8, 8490–8506. [Google Scholar] [CrossRef]
- Wu, H.; Friedrich, H.; Patterson, J.P.; Sommerdijk, N.A.J.M.; de Jonge, N. Liquid-Phase Electron Microscopy for Soft Matter Science and Biology. Adv. Mater. 2020, 32, 2001582. [Google Scholar] [CrossRef]
- Peckys, D.B.; Macías-Sánchez, E.; de Jonge, N. Liquid phase electron microscopy of biological specimens. MRS Bull. 2020, 45, 754–760. [Google Scholar] [CrossRef]
- Jablonski, A.; Powell, C. NIST Electron Inelastic-Mean-Free-Path Database; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2010.
- Nagayama, K.; Danev, R. Phase contrast electron microscopy: Development of thin-film phase plates and biological applications. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 2153–2162. [Google Scholar] [CrossRef] [Green Version]
- Debora Ferreira, B.-V.; Ortrud Monika, B. Negative and Positive Staining in Transmission Electron Microscopy for Virus Diagnosis. In Microbiology in Agriculture and Human Health; Mohammad Manjur, S., Ed.; IntechOpen: Rijeka, Croatia, 2015; Chapter 3. [Google Scholar]
- Visscher, P.B. Finite size effects in the simulation of crystallization. J. Chem. Phys. 1994, 101, 6091–6095. [Google Scholar] [CrossRef]
- Očenášek, J.; Novák, P.; Agbo, S. Finite-thickness effect on crystallization kinetics in thin films and its adaptation in the Johnson–Mehl–Avrami–Kolmogorov model. J. Appl. Phys. 2014, 115, 043505. [Google Scholar] [CrossRef]
- Wang, M.; Dissanayake, T.U.; Park, C.; Gaskell, K.; Woehl, T.J. Nanoscale Mapping of Nonuniform Heterogeneous Nucleation Kinetics Mediated by Surface Chemistry. J. Am. Chem. Soc. 2019, 141, 13516–13524. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.D. Confocal Microscopy: Principles and Modern Practices. Curr. Protoc. Cytom. 2020, 92, e68. [Google Scholar] [CrossRef]
- Mitsuishi, K.; Takeguchi, M. Scanning Confocal Electron Microscopy. In Scanning Transmission Electron Microscopy of Nanomaterials; World Scientific: Singapore, 2014; pp. 345–382. [Google Scholar]
- Smith, J.W.; Carnevale, L.N.; Das, A.; Chen, Q. Electron Videography of a Lipid–Protein Tango. bioRxiv 2022. [Google Scholar] [CrossRef]
- Cepeda-Perez, E.; Doblas, D.; Kraus, T.; de Jonge, N. Electron microscopy of nanoparticle superlattice formation at a solid-liquid interface in nonpolar liquids. Sci. Adv. 2020, 6, eaba1404. [Google Scholar] [CrossRef]
- Kagan, C.R.; Lifshitz, E.; Sargent, E.H.; Talapin, D.V. Building devices from colloidal quantum dots. Science 2016, 353, aac5523. [Google Scholar] [CrossRef]
- Botifoll, M.; Pinto-Huguet, I.; Arbiol, J. Machine learning in electron microscopy for advanced nanocharacterization: Current developments, available tools and future outlook. Nanoscale Horiz. 2022, 7, 1427–1477. [Google Scholar] [CrossRef]
- Saghi, Z.; Benning, M.; Leary, R.; Macias-Montero, M.; Borras, A.; Midgley, P.A. Reduced-dose and high-speed acquisition strategies for multi-dimensional electron microscopy. Adv. Struct. Chem. Imaging 2015, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Grau-Carbonell, A.; Hagemans, F.; Bransen, M.; Elbers, N.A.; van Dijk-Moes, R.J.A.; Sadighikia, S.; Welling, T.A.J.; van Blaaderen, A.; van Huis, M.A. In Situ single particle characterization of the themoresponsive and co-nonsolvent behavior of PNIPAM microgels and silica@PNIPAM core-shell colloids. J. Colloid Interface Sci. 2023, 635, 552–561. [Google Scholar] [CrossRef] [PubMed]

| Element | Element Number | Energy (eV) | Mean Free Path (nm) |
|---|---|---|---|
| Carbon | 6 | 1000 | 3.270 |
| 2000 | 5.297 | ||
| Phosphorous | 15 | 1000 | 2.481 |
| 2000 | 4.329 | ||
| Sulfur | 16 | 1000 | 2.399 |
| 2000 | 4.175 | ||
| Silver | 47 | 1000 | 1.125 |
| 2000 | 1.827 | ||
| 3000 | 2.425 | ||
| Platinum | 78 | 1000 | 0.972 |
| 2000 | 1.382 | ||
| Gold | 79 | 1000 | 1.378 |
| 2000 | 2.340 | ||
| 3000 | 3.204 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Ou, Z. Direct Imaging of the Kinetic Crystallization Pathway: Simulation and Liquid-Phase Transmission Electron Microscopy Observations. Materials 2023, 16, 2026. https://doi.org/10.3390/ma16052026
Xu Z, Ou Z. Direct Imaging of the Kinetic Crystallization Pathway: Simulation and Liquid-Phase Transmission Electron Microscopy Observations. Materials. 2023; 16(5):2026. https://doi.org/10.3390/ma16052026
Chicago/Turabian StyleXu, Zhangying, and Zihao Ou. 2023. "Direct Imaging of the Kinetic Crystallization Pathway: Simulation and Liquid-Phase Transmission Electron Microscopy Observations" Materials 16, no. 5: 2026. https://doi.org/10.3390/ma16052026
APA StyleXu, Z., & Ou, Z. (2023). Direct Imaging of the Kinetic Crystallization Pathway: Simulation and Liquid-Phase Transmission Electron Microscopy Observations. Materials, 16(5), 2026. https://doi.org/10.3390/ma16052026







