Enhancing Removal of Pollutants by Combining Photocatalysis and Photo-Fenton Using Co, Fe-Doped Titanate Nanowires
Abstract
:1. Introduction
2. Materials and Methods
2.1. M-Containing Precursors (M = Fe, Co) Synthesis
2.2. Pristine and Doped TNW Particles Synthesis
2.3. Photocatalytic Degradation Studies
2.4. Hydroxyl Radical (•OH) Photocatalytic Production
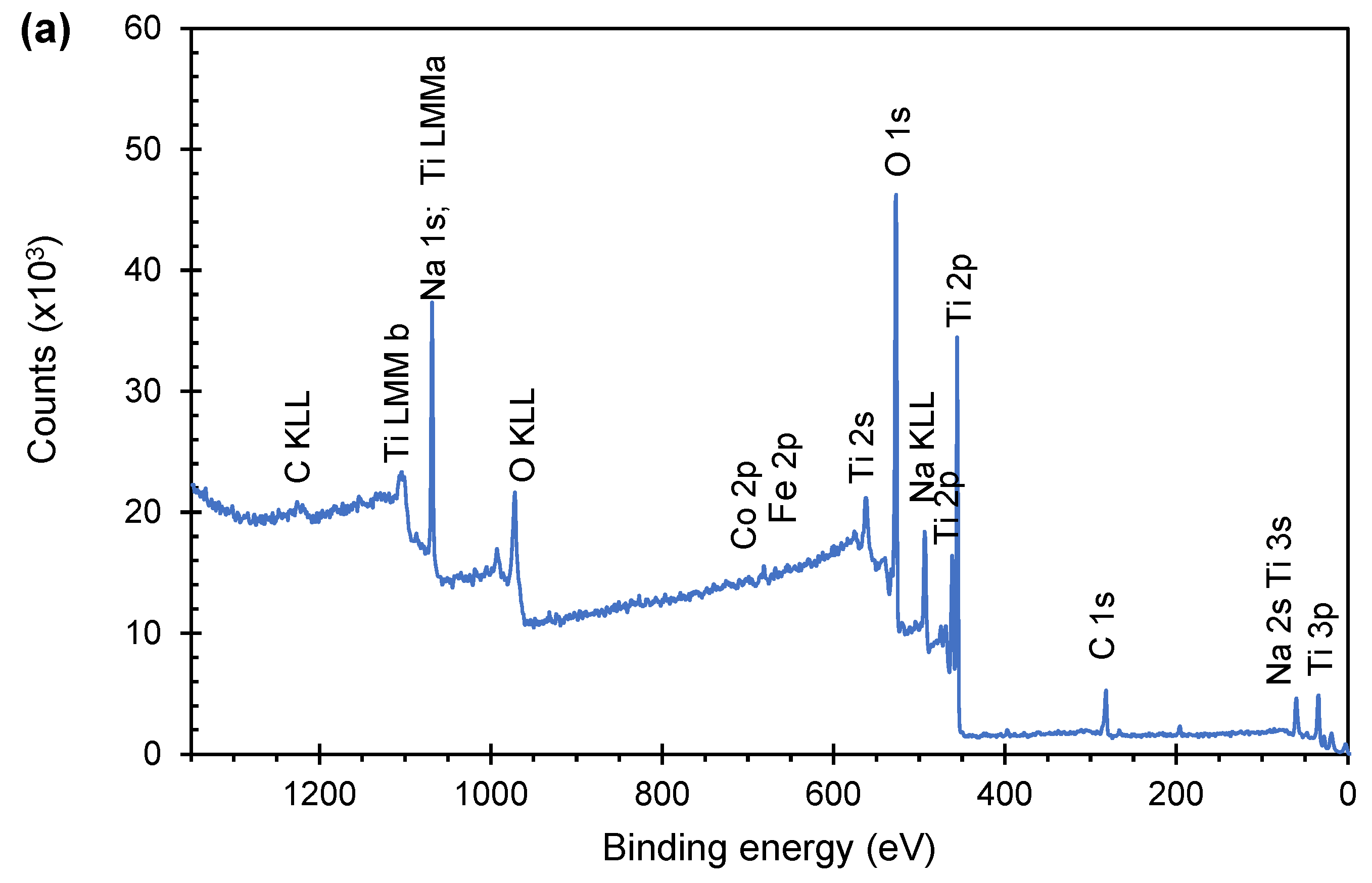
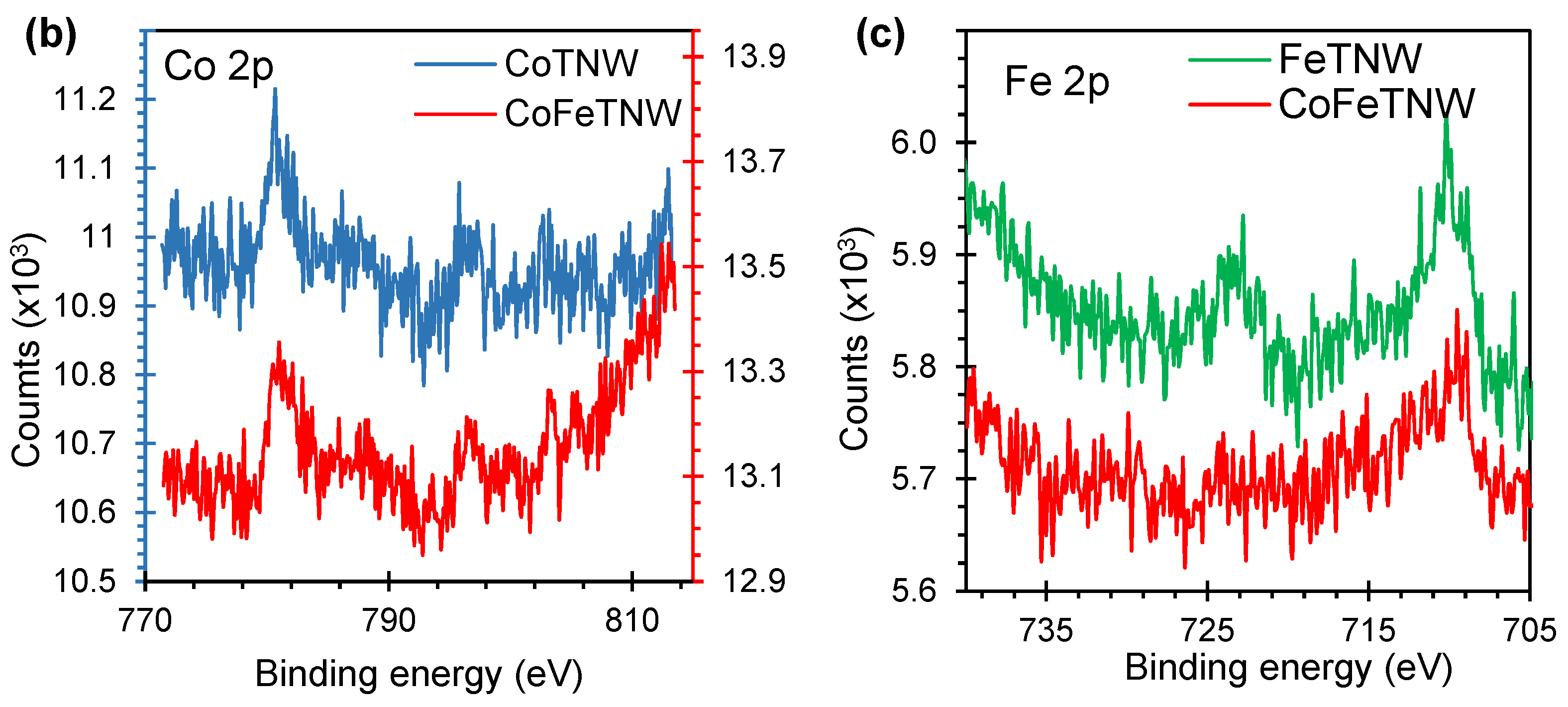
2.5. Characterization
3. Results and Discussion
3.1. Structural and Morphological Characterization
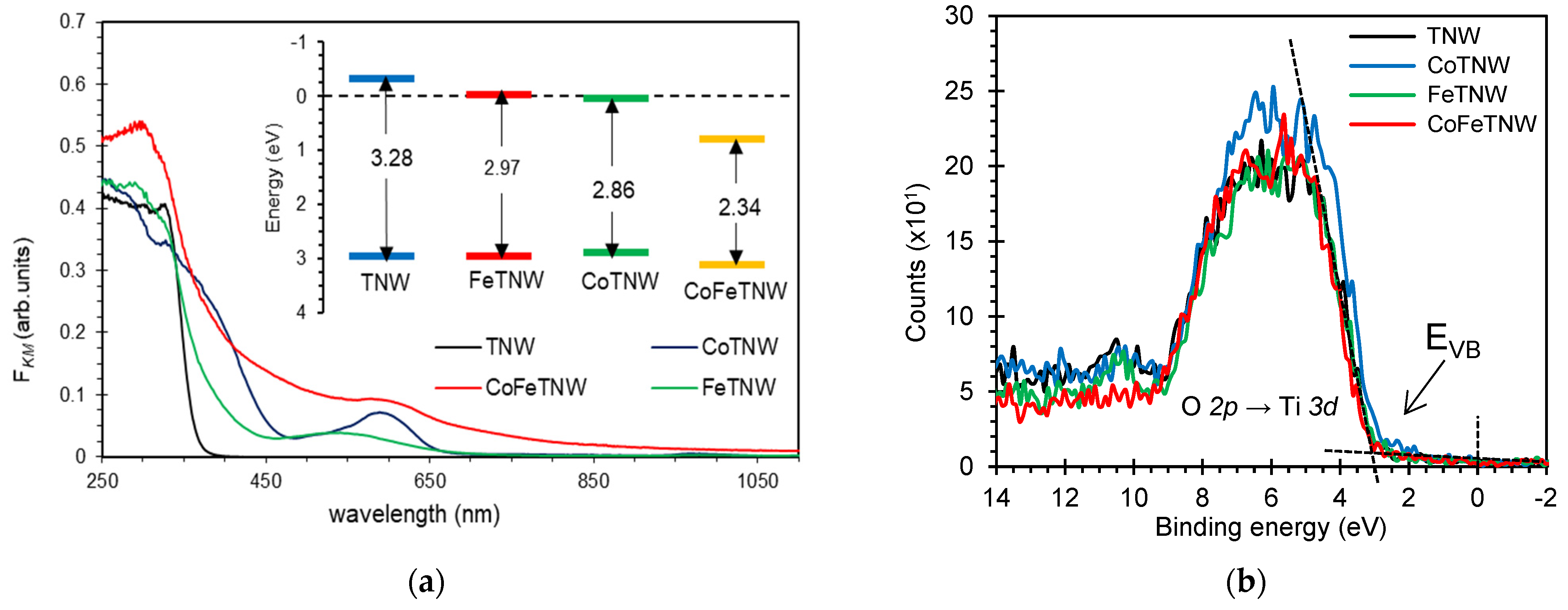
3.2. Optical Characterization
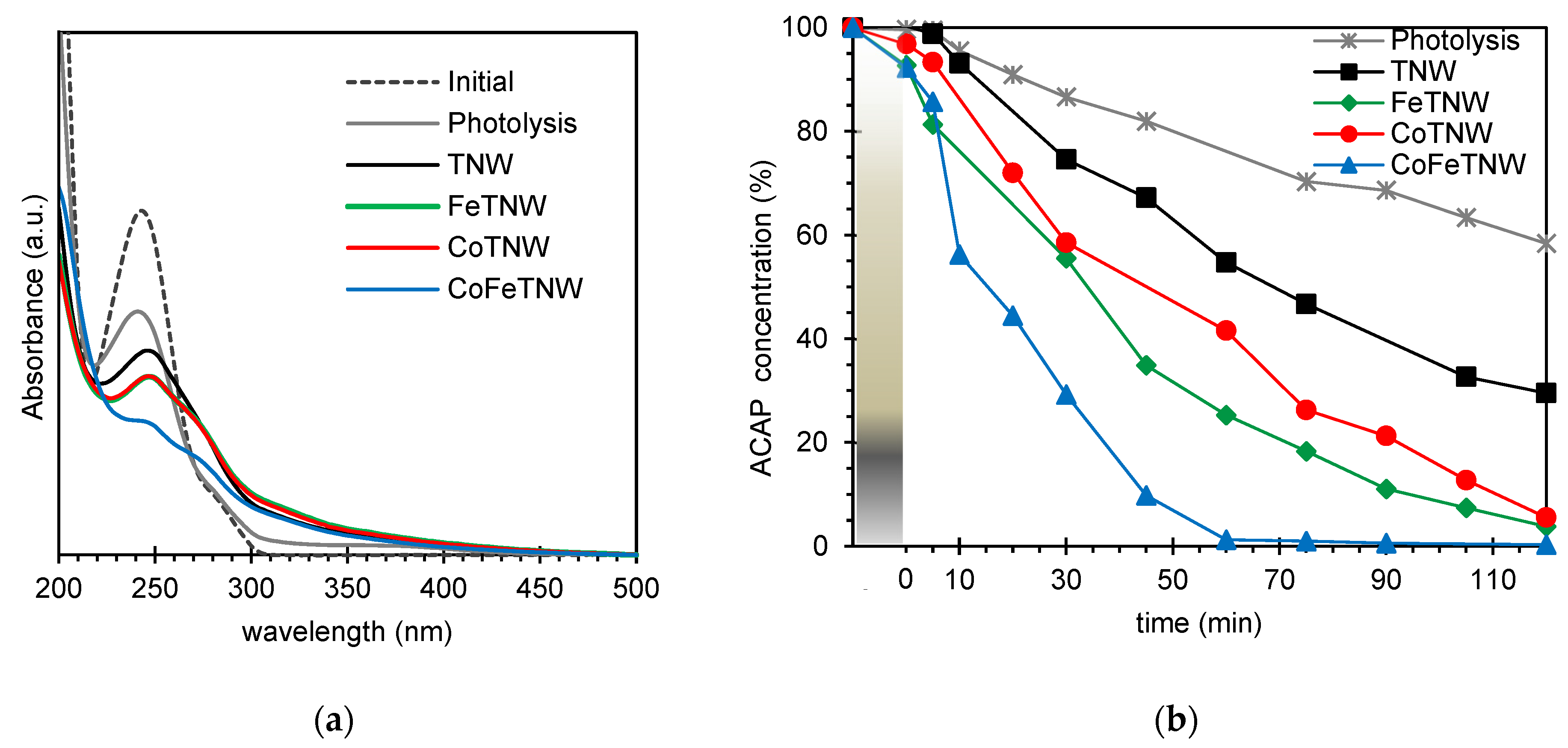
3.3. Photocatalytic Performance
3.4. ACAP and CAF Mixture Photocatalytic Removal
3.5. Reusability and Stability
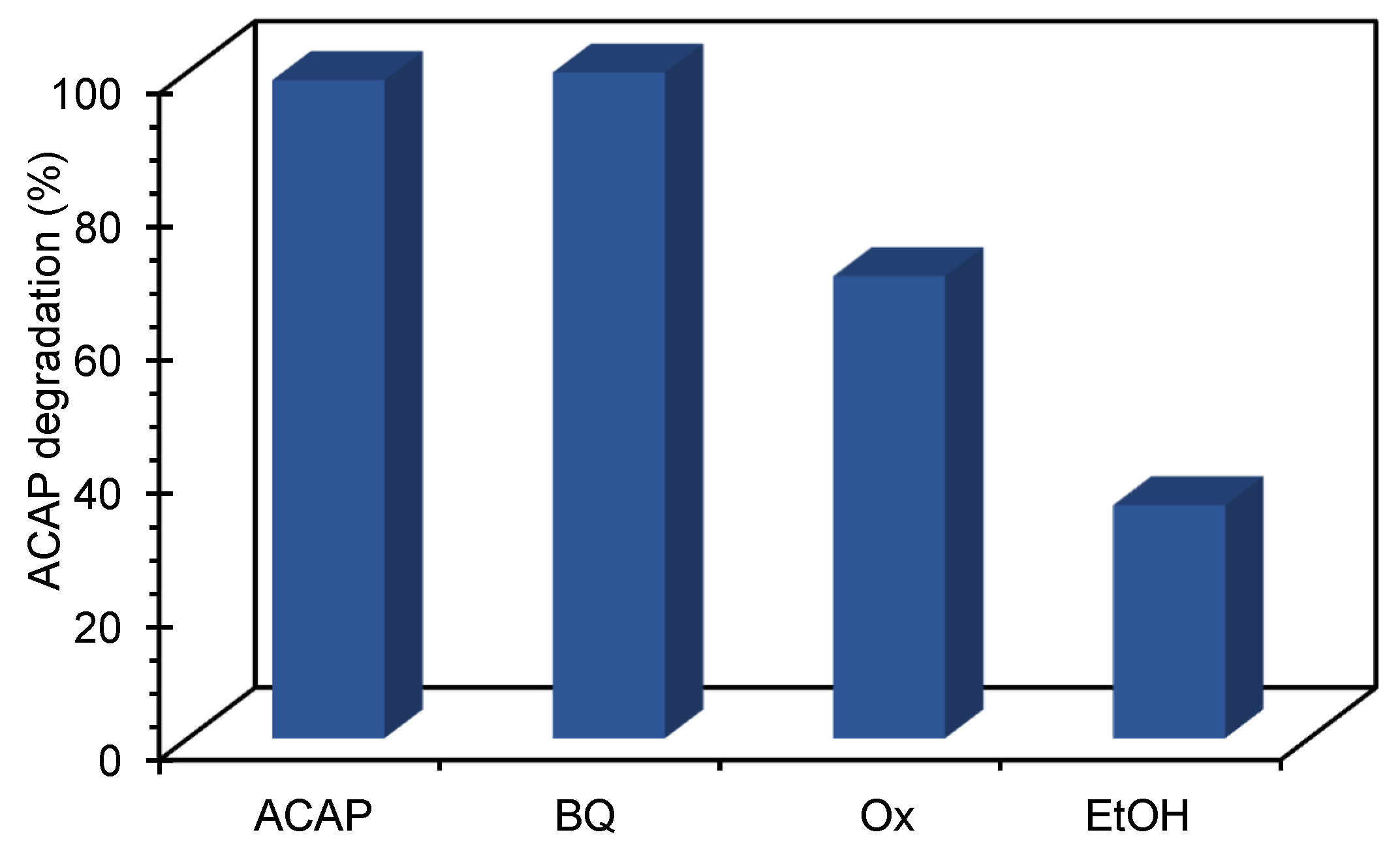
3.6. Scavenger’s Study
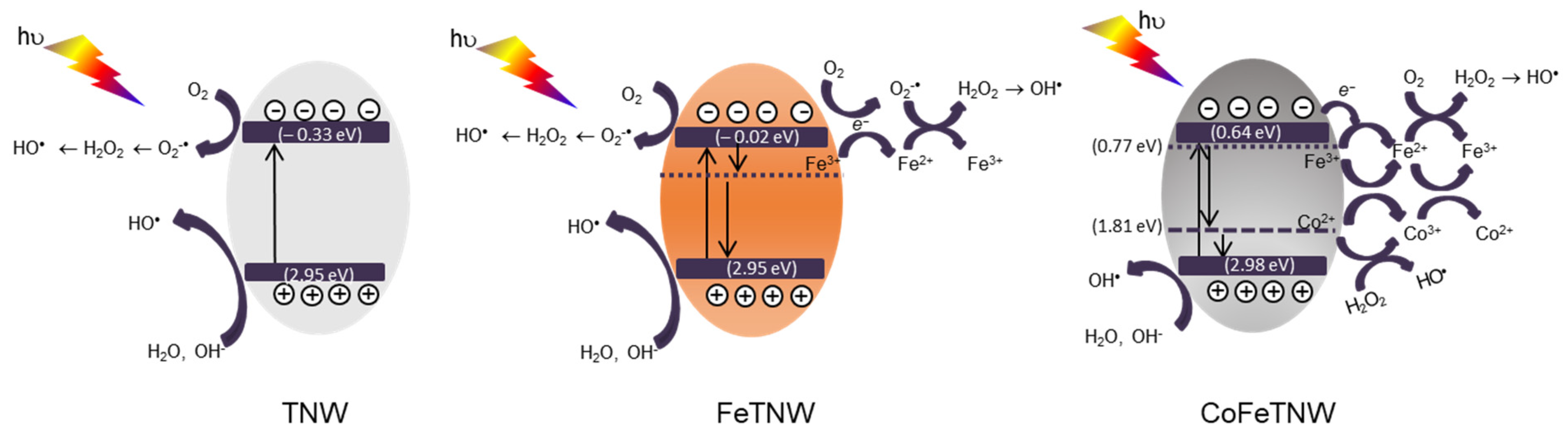
3.7. Photo-Activation Mechanism Proposal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dębska, K.; Rutkowska, B.; Szulc, W. The influence of a dam reservoir on water quality in a small lowland river. Environ. Monit. Assess. 2021, 193, 123. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Pei, Y.; Zheng, H.; Zhao, Y.; Shu, L.; Zhang, H. Twenty years of China’s water pollution control: Experiences and challenges. Chemosphere 2022, 295, 133875. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ngo, H.; Guo, W.; Chang, S.; Nguyen, D.; Liu, Y.; Wei, Q.; Wie, D. A critical review on antibiotics and hormones in swine wastewater: Water pollution problems and control approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Rauf, M.; Ashraf, S. Fundamental principles, and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Oturan, M.; Aaron, J.-J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- Ling, S.; Wang, S.; Peng, Y. Oxidative degradation of dyes in water using Co2+/H2O2 and Co2+/peroxymonosulfate. J. Hazard. Mater. 2010, 178, 359–385. [Google Scholar] [CrossRef]
- Bokare, A.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; Jery, A.; Khezami, L.; Assadi, A. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Alves, D.; Prata, J.; Silvestre, A.; Monteiro, O. Novel C-dots/titanate nanotubular hybrid materials with enhanced optical and photocatalytic properties. J. Alloys Compd. 2023, 936, 168143. [Google Scholar] [CrossRef]
- Ylhäinen, E.; Nunes, M.; Silvestre, A.; Monteiro, O. Synthesis of titanate nanostructures using amorphous precursor material and their adsorption/photocatalytic properties. J. Mater. Sci. 2012, 47, 4305–4312. [Google Scholar] [CrossRef]
- Bem, V.; Neves, M.; Nunes, M.; Silvestre, A.; Monteiro, O. Influence of the sodium/proton replacement on the structural, morphological, and photocatalytic properties of titanate nanotubes. J. Photochem. Photobiol. A 2012, 232, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Barrocas, B.; Neves, M.; Oliveira, M.C.; Monteiro, O. Enhanced photocatalytic degradation of psychoactive substances using amine-modified elongated titanate nanostructures. Environ. Sci. Nano 2018, 5, 350–361. [Google Scholar] [CrossRef]
- Silva, T.; Diniz, J.; Paixão, L.; Vieira, B.; Barrocas, B.; Nunes, C.; Monteiro, O. Novel titanate nanotubes-cyanocobalamin materials: Synthesis and enhanced photocatalytic properties for pollutants removal. Solid State Sci. 2017, 63, 30–41. [Google Scholar] [CrossRef]
- Barrocas, B.; Entradas, T.; Nunes, C.; Monteiro, O. Titanate nanofibers sensitized with ZnS and Ag2S nanoparticles as novel photocatalysts for phenol removal. Appl. Catal. B 2017, 218, 709–720. [Google Scholar] [CrossRef]
- Naudin, G.; Entradas, T.; Barrocas, B.; Monteiro, O. Titanate Nanorods Modified with Nanocrystalline ZnS Particles and Their Photocatalytic Activity on Pollutant Removal. J. Mater. Sci. Technol. 2016, 32, 1122–1128. [Google Scholar] [CrossRef]
- Barrocas, B.; Nunes, C.; Carvalho, M.; Monteiro, O. Titanate nanotubes sensitized with silver nanoparticles: Synthesis, characterization and in-situ pollutants photodegradation. Appl. Surf. Sci. 2016, 385, 18–27. [Google Scholar] [CrossRef]
- Valadez-Renteria, E.; Oliva, J.; Navarro-Garcia, N.; Rodriguez-Gonzalez, V. Novel sustainable composites made of car’s waste and sodium titanate for the efficient photocatalytic removal of the bromophenol blue dye: Study under solar and UV–Vis light. Environ. Sci. Pollut. Res. 2022, 29, 76752–76765. [Google Scholar] [CrossRef]
- Barrocas, B.; Silvestre, A.; Rolo, A.; Monteiro, O. The effect of ionic Co presence on the structural, optical and photocatalytic properties of modified cobalt-titanate nanotubes. Phys. Chem. Chem. Phys. 2016, 18, 18081–18093. [Google Scholar] [CrossRef]
- Barrocas, B.T.; Oliveira, M.C.; Nogueira, H.I.; Fateixa, S.; Monteiro, O.C. Monteiro Ruthenium-Modified Titanate Nanowires for the Photocatalytic Oxidative Removal of Organic Pollutants from Water. ACS Appl. Nano Mater. 2019, 2, 1341–1349. [Google Scholar] [CrossRef]
- Barrocas, B.; Oliveira, M.C.; Nogueira, H.; Fateixa, S.; Monteiro, O. A comparative study on emergent pollutants photo-assisted degradation using ruthenium modified titanate nanotubes and nanowires as catalysts. J. Environ. Sci. 2020, 92, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Zhang, J.; Chen, F. Photocatalytic performance of N-doped TiO2 adsorbed with Fe3+ ions under visible light by a redox treatment. J. Phys. Chem. C 2009, 113, 12848–12853. [Google Scholar] [CrossRef]
- Duan, L.; Jiang, N.; Lu, N.; Shang, K.; Li, J.; Wu, Y. Synergetic effect of TiO2 and Fe3+ as co-catalysts for enhanced phenol degradation in pulsed discharge system. Appl. Catal. B 2018, 221, 521–529. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, X.; Miyauchi, M.; Hashimoto, K. Energy-level matching of Fe (III) ions grafted at surface and doped in bulk for efficient visible-light photocatalysts. J. Am. Chem. Soc. 2013, 135, 10064–10072. [Google Scholar] [CrossRef] [PubMed]
- Barrocas, B.; Chiavassa, L.; Oliveira, M.C.; Monteiro, O. Impact of Fe, Mn co-doping in titanate nanowires photocatalytic performance for emergent organic pollutants removal. Chemosphere 2020, 250, 126240. [Google Scholar] [CrossRef]
- Mahmoud, M.; Ahmed, E.; Farghali, A.A.; Zakim, A.; Barakat, N. Synthesis of Fe/Co-doped titanate nanotube as redox catalyst for photoninduced water splitting. Mater. Chem. Phys. 2018, 217, 125–132. [Google Scholar]
- Nunes, M.; Monteiro, O.; Castro, A.; Vasconcelos, D.; Silvestre, A. A new chemical route to synthesise TM-doped (TM = Co, Fe) TiO2 nanoparticle. Eur. J. Inorg. Chem. 2008, 6, 961–965. [Google Scholar] [CrossRef]
- Franco, A.; Neves, M.; Carrott, M.; Mendonça, M.; Pereira, M.I.; Monteiro, O. Photocatalytic decolorization of methylene blue in the presence of TiO2/ZnS nanocomposites. J. Hazard. Mater. 2009, 161, 545–550. [Google Scholar] [CrossRef]
- Ishibashi, K.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
- Sousa, S.; Cardoso, J.; Monteiro, O. Improved performance of titanate nanostructures for manganese adsorption and posterior pollutants photocatalytic degradation. J. Photochem. Photobiol. A 2019, 378, 9–16. [Google Scholar] [CrossRef]
- Bavkin, F. RSC Nanosience & Nanotechnology Series; RSC Publishing: Cambridge, UK, 2010. [Google Scholar]
- Dong, F.; Zhao, W.; Wu, Z. Characterization and photocatalytic activities of C, N and S co-doped TiO2 with 1D nanostructure prepared by the nano-confinement effect. Nanotechnology 2008, 19, 365607–365617. [Google Scholar] [CrossRef] [PubMed]
- Marques, T.; Ferreira, O.; Costa, J.; Fujisawa, K.; Terrones, M.; Viana, B. Study of the growth of CeO2 nanoparticles onto titanate nanotubes. J. Phys. Chem. Solids 2015, 87, 213–220. [Google Scholar] [CrossRef]
- Kielland, J. Individual activity coefficients of ions in aqueous solutions. J. Am. Chem. Soc. 1937, 59, 1675–1678. [Google Scholar] [CrossRef]
- Bavykin, D.; Walsh, F. Titanate and Titania Nanotubes: Synthesis, Properties and Applications; RSC Publishing: Cambridge, UK, 2010. [Google Scholar]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania nanotubes prepared by chemical processing. Adv. Mater 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Morgado, E.; Marinkovic, B.; Jardim, P.; Abreu, M.; Rizzo, F. Characterization and thermal stability of cobalt-modified 1-D nanostructured trititanates. J. Solid State Chem. 2009, 182, 172–181. [Google Scholar] [CrossRef]
- Finetti, P.; Sedona, F.; Rizzi, G.; Mick, U.; Sutara, F.; Svec, M.; Matolin, V.; Schierbaum, K.; Granozzi, G. Core and valence band photoemission spectroscopy of well-ordered ultrathin TiOx films on Pt(111). J. Phys. Chem. C 2007, 111, 869–876. [Google Scholar] [CrossRef]
- Rempel, A.; Kozlova, E.; Gorbunova, T.; Cherepanova, S.; Gerasimov, E.; Kozhevnikova, N.; Valeeva, A.; Korovin, E.; Kaichev, V.; Shchipunov, Y. Synthesis and solar light catalytic properties of titania–cadmium sulfide hybrid nanostructures. Catal. Commun. 2015, 68, 61–66. [Google Scholar] [CrossRef]
- Pham, T.-D.; Lee, B.-K. Feasibility of silver doped TiO2/glass fiber photocatalyst under visible irradiation as an indoor air germicide. Int. J. Environ. Res. Public Health 2014, 11, 3271–3288. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Li, R.; Zhao, S.; Xing, Y. Microwave-assisted preparation of flower-like cobalt phosphate and its application as a new heterogeneous Fenton–like catalyst. Appl. Surf. Sci. 2017, 396, 1393–1402. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, S.; Wang, Z.; Cui, W.; Zhang, H.; Yang, L.; Zhang, Y.; Li, P. Superior lithium adsorption and required magnetic separation behavior of iron-doped lithium ion-sieves. Chem. Eng. J. 2018, 332, 160–168. [Google Scholar] [CrossRef]
- Mullet, M.; Khare, V.; Ruby, C. XPS study of Fe(II)-Fe(III) (oxy)hydroxycarbonate green rust compounds. Surf. Interface Anal. 2010, 40, 323–328. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Kortuem, G. Reflectance Spectroscopy: Principles, Methods, and Applications; Springer: New York, NY, USA, 1969. [Google Scholar]
- Cox, P.A. Transition Metal Oxides: An Introduction to Their Electronic Structure and Properties; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Morgado, E.; Marinkovic, B.; Jardim, P.; Abreu, M.; Rocha, M.; Bargiela, P. Studies on Fe-modified nanostructured trititanates. Chem. Mater. 2011, 126, 118–127. [Google Scholar] [CrossRef]
- Garg, A.; Singh, A.; Sangal, V.; Bajpai, P.; Garg, N. Synthesis, characterization and anticancer activities of metal ions Fe and Cu doped and co-doped TiO2. New J. Chem. 2017, 41, 9931–9937. [Google Scholar] [CrossRef]
- Nguyen-Phan, T.-D.; Luo, S.; Vovchok, D.; Llorca, J.; Sallis, S.; Kattel, S.; Xu, W.; Piper, L.; Polyansky, D.; Senanayake, S.; et al. Three-dimensional ruthenium-doped TiO2 sea urchins for enhanced visible-light-responsive H2 production. Phys. Chem. Chem. Phys. 2016, 18, 15972–15979. [Google Scholar] [CrossRef]
- Pozdnyakov, I.; Zhang, X.; Maksimova, T.; Yanshole, V.; Wu, F.; Grivin, V.P.; Plyusnin, V. Wavelength-dependent photochemistry of acetaminophen in aqueous solutions. Phys. Chem. Chem. Phys. 2014, 274, 117–123. [Google Scholar] [CrossRef]
- Fernandes, T.; Mendo, S.; Ferreira, L.; Neng, N.; Oliveira, M.; Gil, A.; Carvalho, M.; Monteiro, O.; Nogueira, J.; Calhorda, M.J. Photocatalytic degradation of acetaminophen and caffeine using magnetite–hematite combined nanoparticles: Kinetics and mechanisms. Environ. Sci. Pollut. Res. 2021, 28, 17228–17243. [Google Scholar] [CrossRef]
- Carneiro, J.; Azevedo, S.; Fernandes, F.; Freitas, E.; Pereira, M.; Tavares, C.; Lanceros-Méndez, S.; Teixeira, V. Synthesis of iron-doped TiO2 nanoparticles by ball-milling process: The influence of process parameters on the structural, optical, magnetic, and photocatalytic properties. J. Mater. Sci. 2014, 49, 7476–7488. [Google Scholar] [CrossRef]
- Li, J.; Ren, D.; Wu, Z.; Huang, C.; Yang, H.; Chen, Y.; Yu, H. Visible-light-mediated antifungal bamboo based on Fe-doped TiO2 thin films. RSC Adv. 2017, 7, 55131–55140. [Google Scholar] [CrossRef] [Green Version]
- Teranishi, M.; Naya, S.; Tada, H. In situ liquid phase synthesis of hydrogen peroxide from molecular oxygen using gold nanoparticle-loaded titanium (IV) dioxide photocatalyst. J. Am. Chem. Soc. 2010, 132, 7850–7851. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Zhang, Y.; Wang, Y.; Sun, S.-P.; Wu, W.; Wu, Z. Nanostructured semiconductor supported iron catalysts for heterogeneous photo-Fenton oxidation: A review. J. Mater. Chem. A 2020, 8, 15513–15546. [Google Scholar] [CrossRef]



| Sample ID | Fe/Ti (%) | Co/Ti (%) | AB.E.T. (m2 g−1) |
|---|---|---|---|
| TNW | - | - | 238.10 |
| FeTNW | 0.14 | - | 124.74 |
| CoTNW | - | 1.6 | 237.52 |
| CoFeTNW | 0.15 | 1.7 | 180.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrocas, B.T.; Osawa, R.; Oliveira, M.C.; Monteiro, O.C. Enhancing Removal of Pollutants by Combining Photocatalysis and Photo-Fenton Using Co, Fe-Doped Titanate Nanowires. Materials 2023, 16, 2051. https://doi.org/10.3390/ma16052051
Barrocas BT, Osawa R, Oliveira MC, Monteiro OC. Enhancing Removal of Pollutants by Combining Photocatalysis and Photo-Fenton Using Co, Fe-Doped Titanate Nanowires. Materials. 2023; 16(5):2051. https://doi.org/10.3390/ma16052051
Chicago/Turabian StyleBarrocas, B. T., R. Osawa, M. Conceição Oliveira, and O. C. Monteiro. 2023. "Enhancing Removal of Pollutants by Combining Photocatalysis and Photo-Fenton Using Co, Fe-Doped Titanate Nanowires" Materials 16, no. 5: 2051. https://doi.org/10.3390/ma16052051






