Phase Equilibria, Thermodynamics and Solidified Microstructure in the Copper–Zirconium–Yttrium System
Abstract
1. Introduction
2. Experimental Procedure
3. Thermodynamic Models
3.1. Pure Elements
3.2. The Solution Phases
3.3. Intermetallic Compounds
4. Results and Discussion
4.1. Microstructure and Phase Transition Temperatures Analysis
4.1.1. Microstructure of Solidification
4.1.2. Microstructure of Annealed Alloys
4.2. Thermodynamic Assessment
5. Conclusions and Summary
- The solid solubility in the ternary system is determined. The maximum solubility of Zr in Cu6Y, Cu4Y and Cu7Y2 are about 6.61, 6.27 and 7.83 at.% Zr, respectively. The solubility of Y in Cu5Zr, Cu51Zr14 and CuZr are about 2.57, 4.45 and 3.38 at.% Y, respectively. The solubility of Cu in the hcp phase is about 2 at.%.
- The Cu–Y system and the Cu–Zr–Y system were optimized by the CALPHAD method. The calculated isothermal sections, liquidus projection and vertical section are consistent with the experimental data.
- The observed solidified microstructure agrees with the result of the Scheil solidification simulations using the thermodynamic parameters. The presently obtained thermodynamic description for the Cu–Zr–Y system can be used to guide the composition and microstructure design of Cu–Zr–Y alloys.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minneci, R.P.; Lass, E.A.; Bunn, J.R.; Choo, H.; Rawn, C.J. Copper-based alloys for structural high-heat-flux applications: A review of development, properties, and performance of Cu-rich Cu-Cr-Nb alloys. Int. Mater. Rev. 2021, 66, 394–425. [Google Scholar] [CrossRef]
- Lin, C.H. Newly-developed Cu(NbZrNx) copper-alloy films for microelectronic manufacture advancement. Mater. Trans. 2022, 63, 1080–1086. [Google Scholar] [CrossRef]
- Qin, Y.Q.; Tian, Y.; Peng, Y.Q.; Luo, L.M.; Zan, X.; Xu, Q.; Wu, Y.C. Research status and development trend of preparation technology of ceramic particle dispersion strengthened copper-matrix composites. J. Alloys Compd. 2020, 848, 156475. [Google Scholar] [CrossRef]
- Zhang, H.T.; Fu, H.D.; Zhu, S.C.; Yong, W.; Xie, J.X. Machine learning assisted composition effective design for precipitation strengthened copper alloys. Acta Mater. 2021, 215, 117118. [Google Scholar] [CrossRef]
- Wang, C.S.; Fu, H.D.; Jiang, L.; Xue, D.Z.; Xie, J.X. A property-oriented design strategy for high performance copper alloys via machine learning. NPJ Comput. Mater. 2019, 5, 87. [Google Scholar] [CrossRef]
- Yang, H.Y.; Ma, Z.C.; Lei, C.H.; Meng, L.; Fang, Y.T.; Liu, J.B.; Wang, H.T. High strength and high conductivity Cu alloys: A review. Sci. China Technol. Sci. 2020, 63, 2505–2517. [Google Scholar] [CrossRef]
- Zhang, P.C.; Shi, J.F.; Yu, Y.S.; Sun, J.C.; Li, T.J. Effect of cryorolling on microstructure and property of high strength and high conductivity Cu-0.5wt.%Cr alloy. T Nonferr. Metal. Soc. 2020, 30, 2472–2479. [Google Scholar] [CrossRef]
- Chen, J.S.; Xiao, X.P.; Guo, C.J.; Yuan, D.W.; Huang, H.; Yang, B. Regulation of primary phase in Cu-Cr-Zr alloy and its effect on nano-structure and properties. J. Alloys Compd. 2022, 926, 166836. [Google Scholar] [CrossRef]
- Peng, L.J.; Xie, H.F.; Huang, G.J.; Li, Y.F.; Yin, X.Q.; Feng, X.; Mi, X.J.; Yang, Z. The phase transformation and its effects on properties of a Cu−0.12wt% Zr alloy. Mat. Sci. Eng. A-Struct. 2015, 633, 28–34. [Google Scholar] [CrossRef]
- Du, Y.B.; Zhou, Y.J.; Song, K.X.; Huang, T.; Hui, D.; Liu, H.T.; Cheng, C.; Yang, J.Z.; Niu, L.Y.; Guo, H.W. Zr-containing precipitate evolution and its effect on the mechanical properties of Cu-Cr-Zr alloys. J. Mater. Res. Technol. 2021, 14, 1451–1458. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.B.; Huang, Z.C.; Guo, J.L.; Gong, S.; Xie, G.L.; Peng, L.J.; Li, Z. Microstructure and properties of a novel ultra-high strength, high elasticity and high plasticity Cu-20Ni-20Mn-0.3Nb-0.3Cr-0.1Zr alloy. J. Alloys Compd. 2021, 853, 157402. [Google Scholar] [CrossRef]
- Gao, L.Q.; Yang, X.; Zhang, X.F.; Zhang, Y.; Sun, H.L.; Li, N. Aging behavior and phase transformation of the Cu-0.2 wt%Zr-0.15 wt%Y alloy. Vacuum 2019, 159, 367–373. [Google Scholar] [CrossRef]
- Li, H.H.; Liu, X.; Li, Y.; Zhang, S.H.; Chen, Y.; Wang, S.W.; Liu, J.S.; Wu, J.H. Effects of rare earth Ce addition on microstructure and mechanical properties of impure copper containing Pb. Trans. Nonferrous Met. Soc. China 2020, 30, 1574–1581. [Google Scholar] [CrossRef]
- Li, H.H.; Sun, X.Q.; Zhang, S.Z.; Zhao, Q.Y.; Wang, G.Z. Application of rare-earth element Y in refining impure copper. Int. J. Min. Met. Mater. 2015, 22, 453–459. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, J.; Wang, X.; Jie, J.; Li, T. Effects of Y addition on the microstructure, properties and softening resistance of Cu-Cr alloy. J. Alloys Compd. 2022, 902, 163816. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.L.; Volinsky, A.A.; Wang, B.J.; Tian, B.H.; Chai, Z.; Liu, Y.; Song, K.X. Small Y addition effects on hot deformation behavior of copper-matrix alloys. Adv. Eng. Mater. 2017, 19, 1700197. [Google Scholar] [CrossRef]
- Liu, X.J.; Oikawa, K.; Ohnuma, I.; Kainuma, R.; Ishida, K. The use of phase diagrams and thermodynamic databases for electronic materials. JOM-US 2003, 55, 53–59. [Google Scholar] [CrossRef]
- He, X.C.; Liu, H.S. Determination of the isothermal section of the Cu–Zr–Y ternary system at 978K. J. Alloys Compd. 2009, 475, 245–251. [Google Scholar] [CrossRef]
- Myronenko, P.; Myakush, O.; Babizhetskyy, V.; Kotur, B. Phase equilibria in Y-Zr-Cu system at 870 K. Visnyk Lviv. Univ. Ser. Chem. 2011, 52, 22–26. [Google Scholar]
- Fries, S.G.; Lukas, H.L.; Konetzki, R.; Schmid-Fetzer, R. Experimental investigation and thermodynamic optimization of the Y-Cu binary system. J. Phase Equilib. 1994, 15, 606–614. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Zhang, C.; Du, Y.; Wang, J.; Li, Y. Experimental investigation and thermodynamic description of the Cu-Zr system. J. Phase Equilib. Diffus. 2017, 38, 121–134. [Google Scholar] [CrossRef]
- Bu, M.J.; Wang, P.S.; Xu, H.H.; Liu, S.H.; Sha, C.S.; Du, Y.; Pan, F.S.; Tang, A.T. Experimental investigation and thermodynamic modeling of the Zr-Y system. J. Min. Metall. B 2010, 46, 181–192. [Google Scholar] [CrossRef]
- Zeng, G.; Hu, B.; Jin, C.G.; Chen, X.J.; Liu, S.H.; Du, Y.; Hu, J.Q. Experimental investigation and thermodynamic assessment of the Ag-Cr-Y and Ag-Cu-Y ternary systems. Calphad 2022, 78, 102455. [Google Scholar] [CrossRef]
- Shi, C.Y.; Liu, Y.L.; Yang, B.B.; Jin, B.; Hu, B.; Rajkumar, V.B.; Du, Y. Phase equilibria thermodynamics and solidified microstructure in the Ag-Cr-Zr system. J. Alloys Compd. 2021, 863, 158618. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhou, P.; Liu, S.H.; Du, Y. Experimental investigation and thermodynamic description of the Cu-Cr-Zr system. Calphad 2017, 59, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, B.; Li, B.; Zhang, M.; Wang, Q.; Du, Y. Experimental investigation and CALPHAD modeling of the Cu–Cr–Si ternary system. Calphad 2021, 74, 102324. [Google Scholar] [CrossRef]
- Okamoto, H. Cu-Y (Copper-Yttrium). J. Phase Equilib. 1992, 13, 102–103. [Google Scholar] [CrossRef]
- Ellinger, F.H.; Land, C.C. On the plutonium-zirconium phase diagram. Nucl. Metall. 1971, 17, 686–698. [Google Scholar]
- Yasohama, K.; Ogasawara, T. Specific heat and superconducting properties of zirconium-molybdenum alloys. J. Phys. Soc. Jpn. 1974, 36, 1349–1355. [Google Scholar] [CrossRef]
- Forey, P.; Glimois, J.L.; Feron, J.L.; Develey, G.; BECLE, C. Cheminform abstract: Synthesis, characterization and crystal structure of copper-zirconium (Cu5Zr). Chem. Inf. 1981, 12, 10. [Google Scholar] [CrossRef]
- Bsenko, L. Crystallographic data for intermediate phases in the copper-zirconium and copper-hafnium systems. J. Less-Common Met. 1975, 40, 365–366. [Google Scholar] [CrossRef]
- Glimois, J.L.; Forey, P.; Feron, J.; Becle, C. Structural investigations of the pseudo-binary compounds nickel-copper-zirconium (Ni10-xCuxZr7). J. Less-Common Met. 1981, 78, 45–50. [Google Scholar] [CrossRef]
- Carvalho, E.M.; Harris, I.R. Constitutional and structural studies of the intermetallic phase, zirconium-copper (ZrCu). J. Mater. Sci. 1980, 15, 1224–1230. [Google Scholar] [CrossRef]
- Nevitt, M.V.; Downey, J.W. A family of intermediate phases having the Si2Mo type structure. Trans. Am. Inst. Min. Metall. Pet. Eng. 1962, 224, 195–196. [Google Scholar]
- Belyavina, N.N.; Markiv, V.Y.; Nakonechna, O.I. Reinvestigation of the Y–Cu–Ga system at 700°C. J. Alloys Compd. 2012, 541, 288–296. [Google Scholar] [CrossRef]
- Dinsdale, A.T. SGTE data for pure substances. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Redlich, O.; Kister, A.T. Algebraic representation of thermodynamic properties and the classification of solutions. J. Ind. Eng. Chem. 1948, 40, 345–348. [Google Scholar] [CrossRef]
- Liu, X.X.; Du, Y.; Liu, S.H.; Pan, Y.F. Measurement of the phase equilibria in the Al-Zr-Y system at 673 and 823 K. Calphad 2020, 68, 101726. [Google Scholar] [CrossRef]
- Bao, X.; Liu, L.; Huang, S.; Jiang, Y.; Wang, X.; Zhang, L. Phase relationships in the al-rich region of the Al-Y-Zr system. J. Min. Metall. B 2017, 53, 9–12. [Google Scholar] [CrossRef]
- Ryan, A.J.; Evans, W.J. The periodic table as a career guide: A journey to rare earths. In Periodic Table I: Historical Development and Essential Features; Mingos, D.M.P., Ed.; Springer: Berlin, Germany, 2019; Volume 181, pp. 197–224. [Google Scholar]
- Sundman, B.; Jansson, B.; Andersson, J.O. The thermo-calc databank system. Calphad 1985, 9, 153–190. [Google Scholar] [CrossRef]
- Domagala, R.F.; Rausch, J.J.; Levinson, D.W. The systems Y–Fe, Y–Ni, and Y–Cu. Trans. Am. Soc. Met 1961, 53, 137–155. [Google Scholar]
- Qi, G.; Itagaki, K.; Yazawa, A. High temperature heat content measurements of Cu-RE (RE=Y, La, Ce, Pr, Nd) binary systems. Mater. Trans. JIM 1989, 30, 273–282. [Google Scholar] [CrossRef]
- Abend, U.; Schaller, H.J. Constitution and thermodynamics of Cu-Y alloys. Ber. Bunsenges. Phys. Chem. 1997, 101, 741–748. [Google Scholar] [CrossRef]
- Massalski, T.B.; Murray, J.L.; Bennett, L.H. Binary Alloy Phase Diagrams: Cu-Y.; Barker, H., Ed.; ASM International, Materials Park: Novelty, OH, USA, 1986; Volume 1. [Google Scholar]



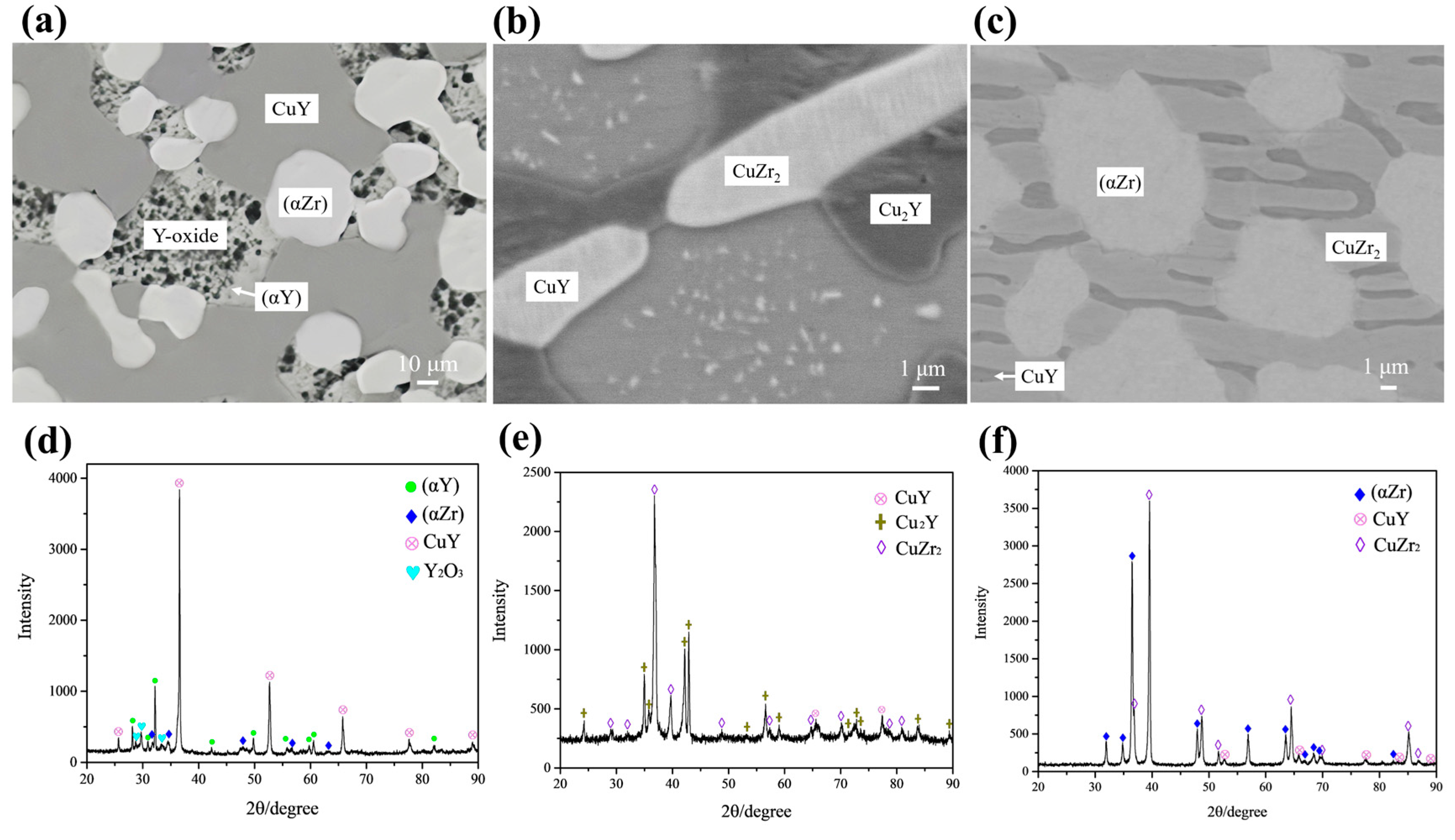
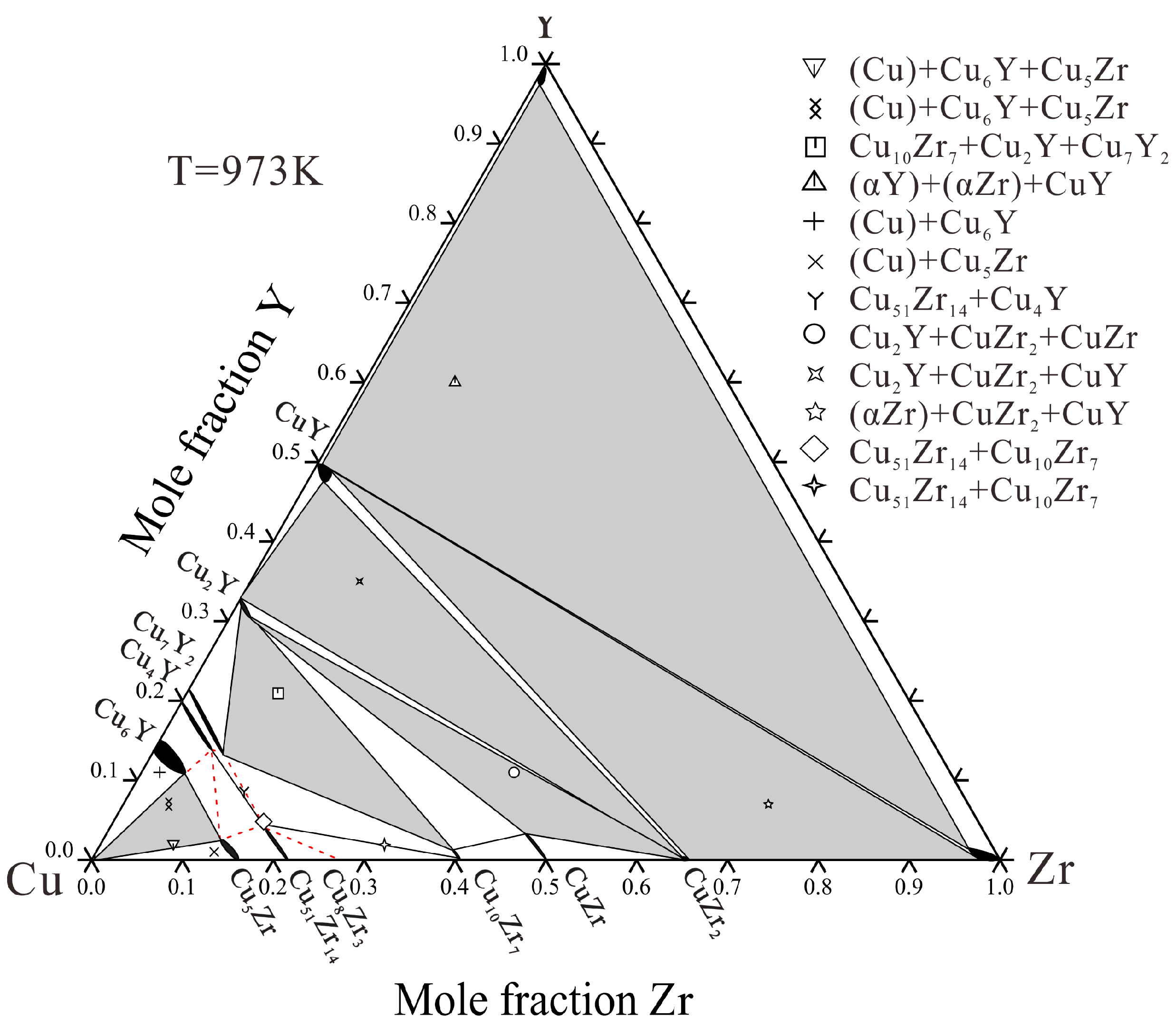

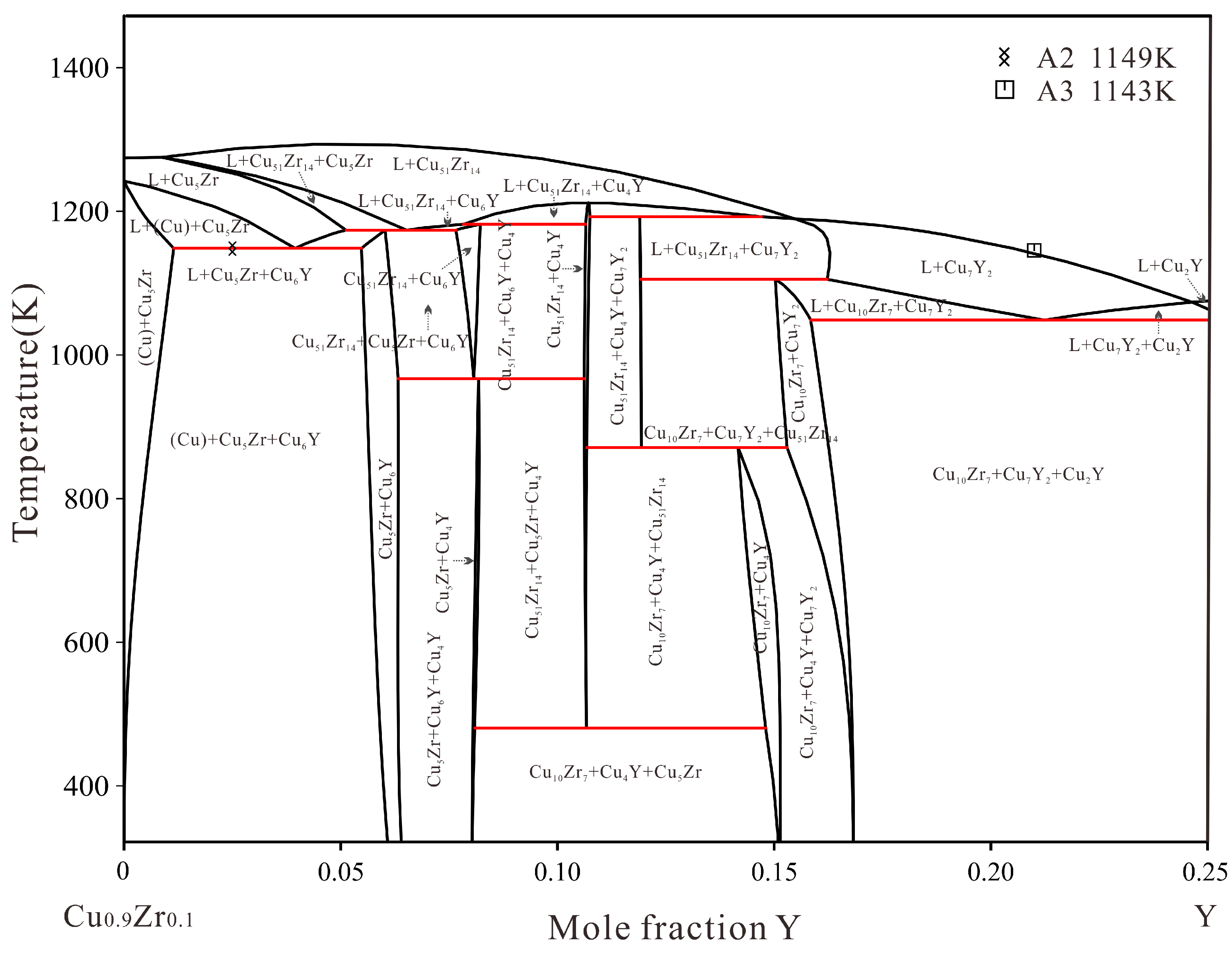
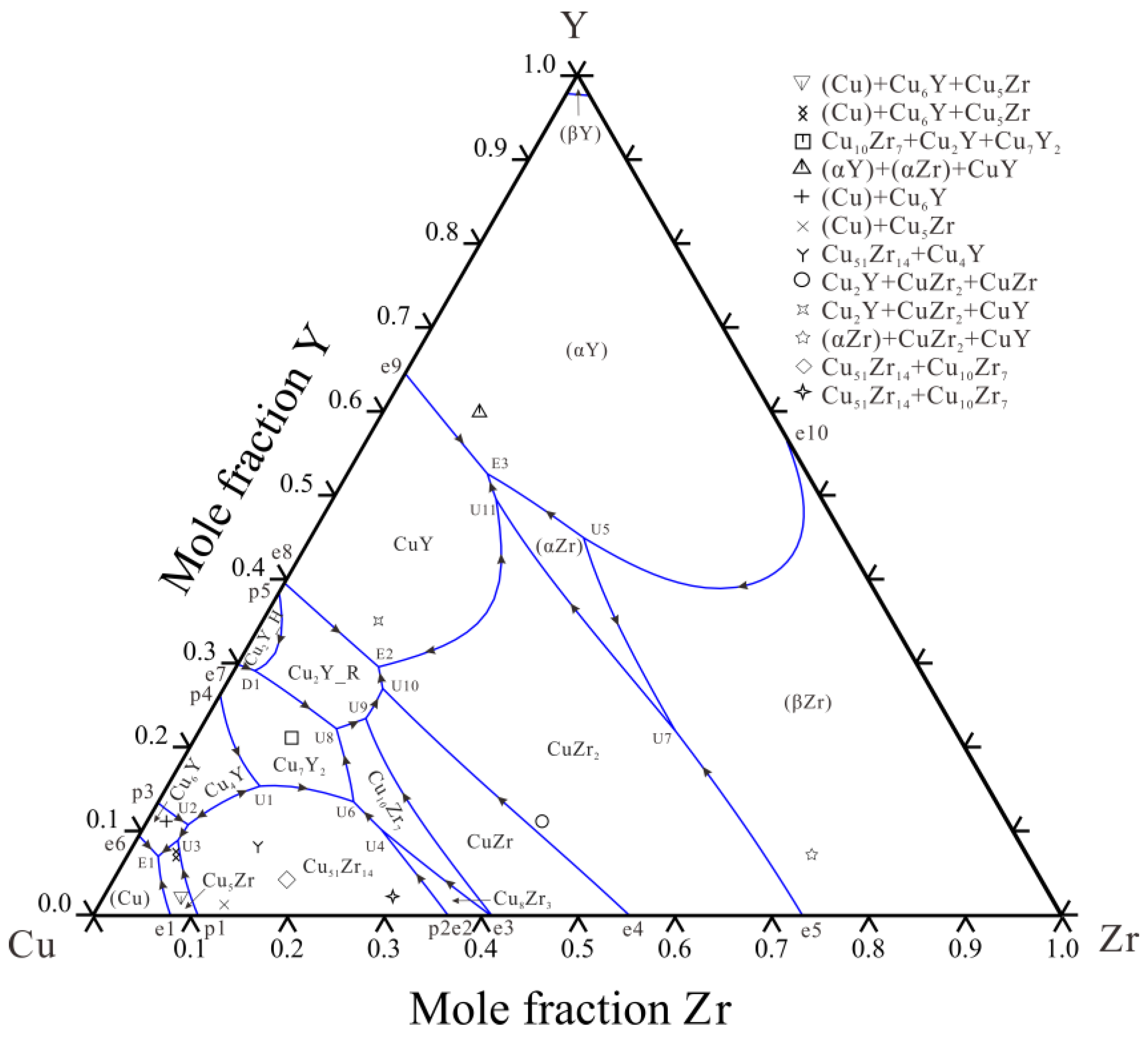

| Alloy | Alloy | Primary | Primary Phase | Phase | Equilibrium Phase | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Composition | Phase | Composition (at.%) | Solidification Paths | Equilibria | Composition (at.%) | ||||
| (at.%) | Cu | Y | Zr | Cu | Y | Zr | ||||
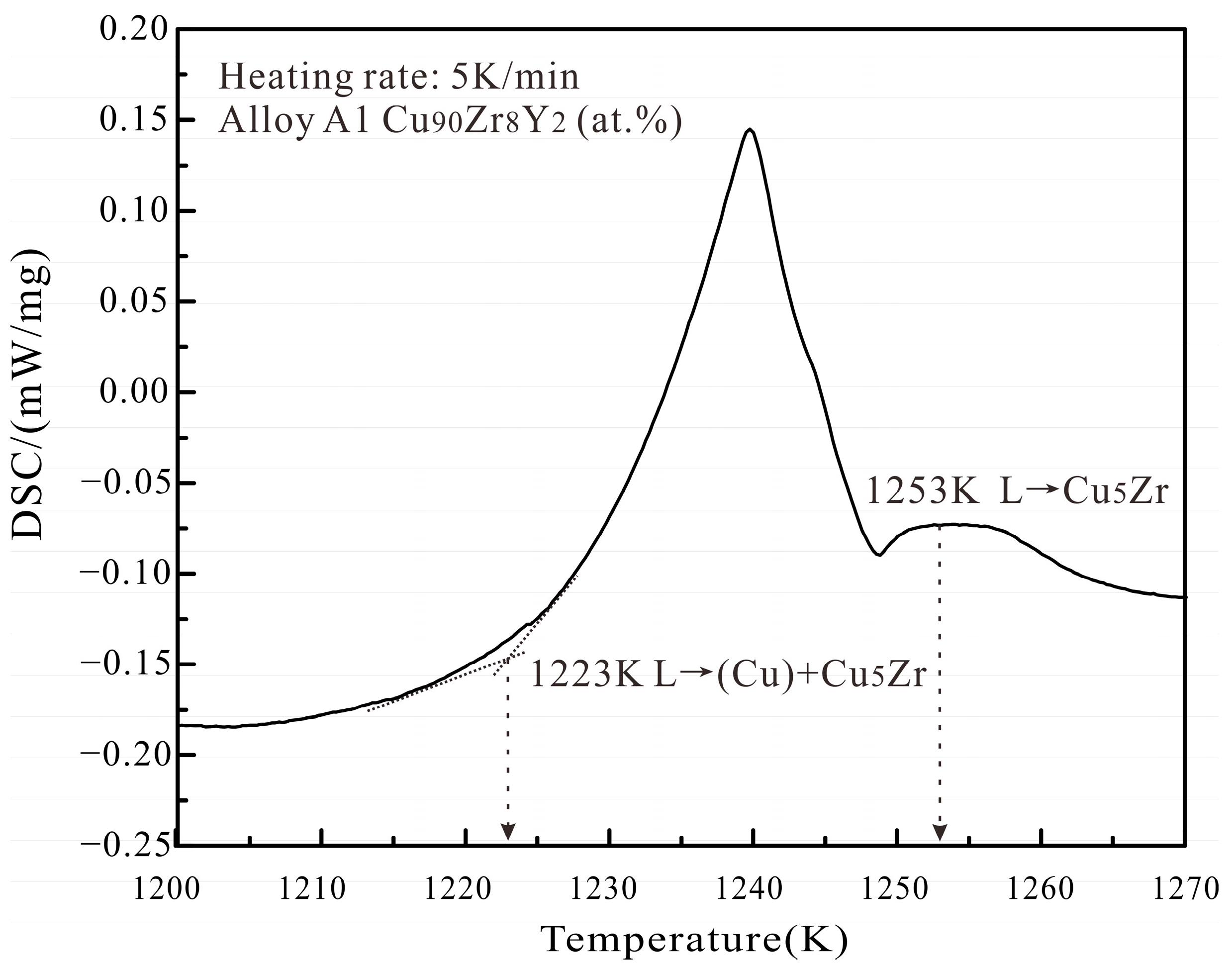
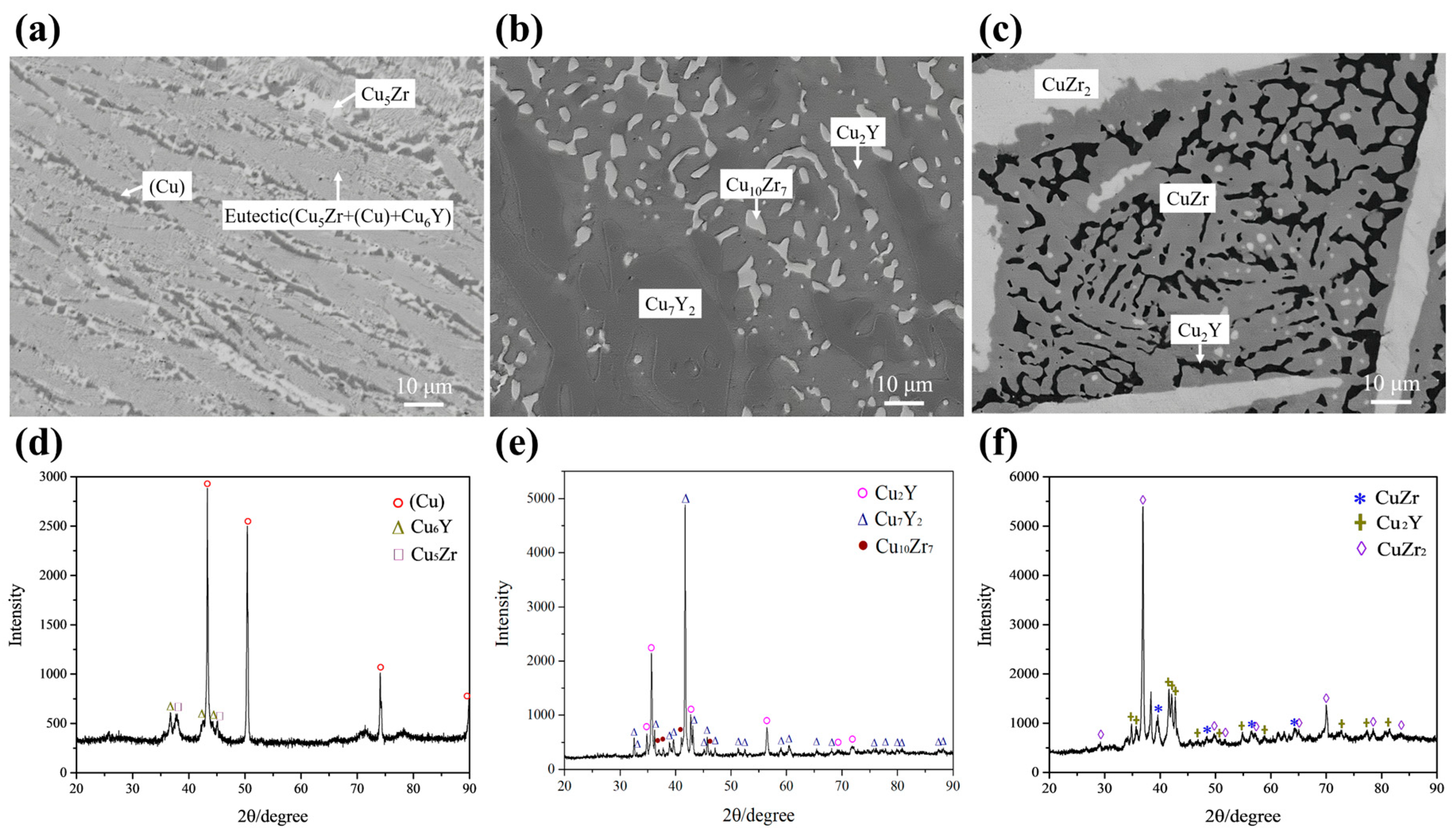
| A1 | Cu90Zr8Y2 | Cu5Zr | 83.05 | 1.05 | 15.9 | L → Cu5Zr | (Cu) | 99.95 | 0 | 0.05 |
| L → (Cu) + Cu5Zr | Cu5Zr | 83.22 | 2.03 | 14.75 | ||||||
| L → (Cu) + Cu5Zr + Cu6Y | Cu6Y | 84.69 | 8.7 | 6.61 | ||||||
| A2 | Cu88Zr5Y7 | Cu5Zr | 82.84 | 7.08 | 10.08 | L → Cu51Zr14 | (Cu) | 99.62 | 0.15 | 0.23 |
| L + Cu51Zr14 → Cu5Zr | Cu5Zr | 82.20 | 2.57 | 15.23 | ||||||
| L → (Cu) + Cu5Zr | Cu6Y | 84.26 | 9.84 | 5.9 | ||||||
| A3 | Cu69Zr10Y21 | Cu7Y2 | 77.18 | 17.00 | 5.82 | L → Cu7Y2 | Cu10Zr7 | 59.74 | 1.53 | 38.73 |
| L → Cu7Y + Cu2Y | Cu2Y | 67.59 | 31.79 | 0.62 | ||||||
| L + Cu7Y2 → Cu10Zr7 + Cu2Y | Cu7Y2 | 78.79 | 13.38 | 7.83 | ||||||
| A4 | Cu33Zr7Y60 | (αY) | 4.78 | 94.28 | 0.94 | L → (αY) | (αY) | 2.06 | 97.26 | 0.68 |
| L → (αY) + (αZr) | (αZr) | 2.81 | 1.51 | 95.68 | ||||||
| L → (αY) + (αZr) + CuY | CuY | 49.64 | 49.76 | 0.60 | ||||||
| A5 | Cu87Zr2Y11 | Cu6Y | 84.25 | 11.12 | 4.63 | L → Cu6Y | (Cu) | 98.80 | 1.19 | 0.01 |
| L → Cu6Y + (Cu) | Cu6Y | 85.34 | 11.56 | 3.1 | ||||||
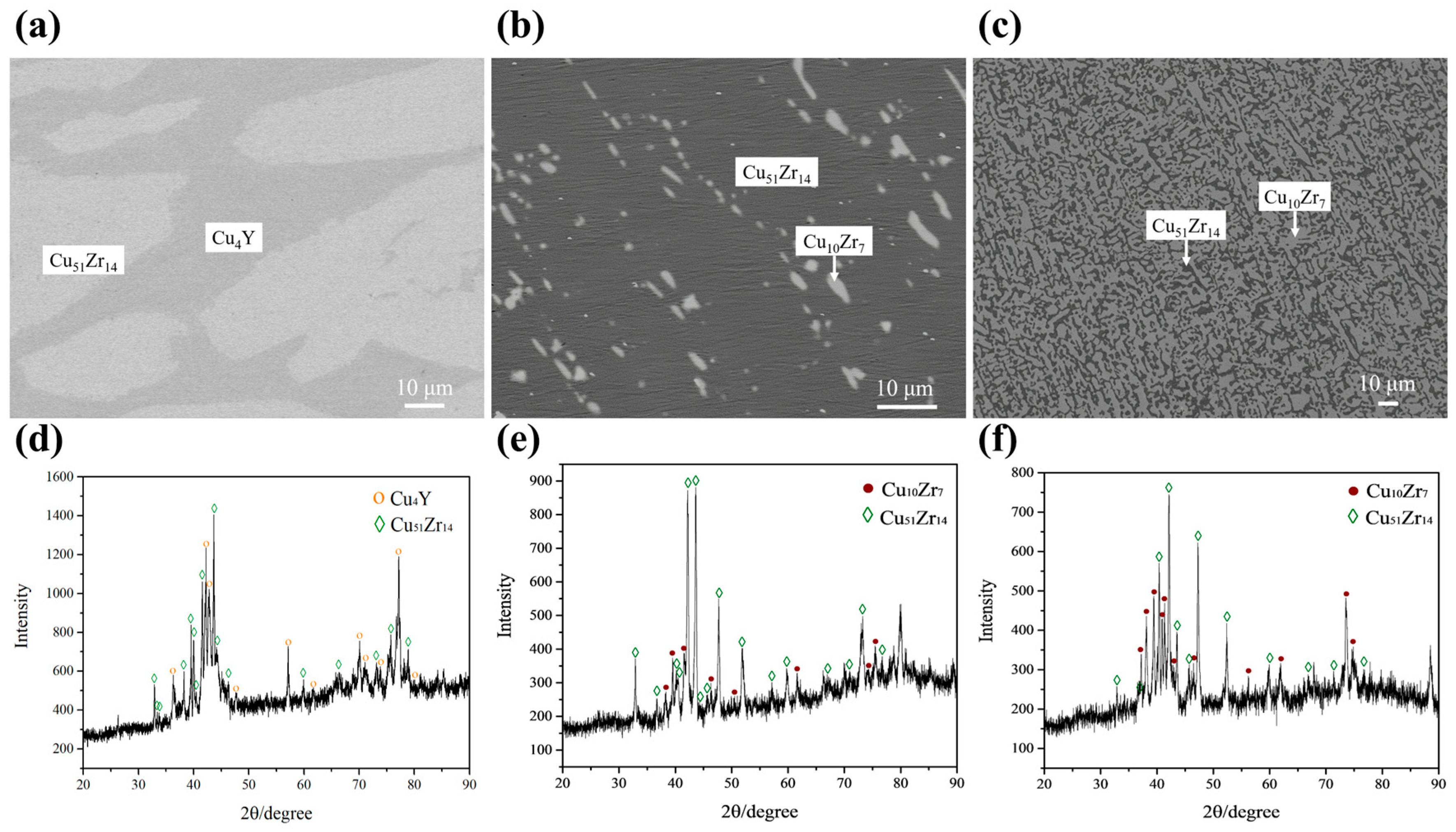
| A6 | Cu86Zr13Y1 | Cu51Zr14 | 79.46 | 0.13 | 20.41 | L → Cu51Zr14 | (Cu) | 98.98 | 0.16 | 0.86 |
| L + Cu51Zr14 → Cu5Zr | Cu5Zr | 83.07 | 1.04 | 15.89 | ||||||
| L → (Cu) + Cu5Zr | ||||||||||
| A7 | Cu79Zr13Y8 | Cu51Zr14 | 79.17 | 3.72 | 17.11 | L → Cu51Zr14 | Cu51Zr14 | 78.87 | 3.99 | 17.14 |
| L → Cu4Y | Cu4Y | 79.88 | 13.85 | 6.27 | ||||||
| A8 | Cu48Zr41Y11 | CuZr2 | 34.52 | 0.04 | 65.44 | L → CuZr2 | CuZr2 | 34.55 | 0.1 | 65.35 |
| L → CuZr + CuZr2 | CuZr | 50.54 | 3.38 | 46.08 | ||||||
| L + CuZr → Cu2Y + CuZr2 | Cu2Y | 67.38 | 30.50 | 2.12 | ||||||
| A9 | Cu53Zr12Y35 | CuY | 50.14 | 46.08 | 3.78 | L → CuY | CuY | 50.76 | 47.43 | 1.81 |
| L → CuY + Cu2Y | Cu2Y | 67.05 | 32.95 | 0 | ||||||
| L → CuY + Cu2Y + CuZr2 | CuZr2 | 34.78 | 0 | 65.22 | ||||||
| A10 | Cu22Zr71Y7 | (αZr) | 4.34 | 0 | 95.66 | L → (αZr) | (αZr) | 2.28 | 0 | 97.72 |
| L → (αZr) + CuZr2 | CuZr2 | 34.06 | 0 | 65.94 | ||||||
| L → (αZr) + CuZr2 + CuY | CuY | 49.09 | 48.78 | 2.13 | ||||||
| A11 | Cu78Zr18Y4 | Cu51Zr14 | 79.10 | 6.16 | 14.74 | L → Cu51Zr14 | Cu51Zr14 | 78.80 | 4.42 | 16.78 |
| L → Cu10Zr7 + Cu51Zr14 | Cu10Zr7 | 59.82 | 0.31 | 39.87 | ||||||
| A12 | Cu66Zr32Y2 | Cu51Zr14 | 73.39 | 1.87 | 24.73 | L → Cu51Zr14 | Cu51Zr14 | 78.23 | 4.45 | 17.32 |
| L → Cu10Zr7 + Cu51Zr14 | Cu10Zr7 | 59.66 | 0.01 | 40.33 | ||||||
| Alloy | Alloy | Phase Transition Temperatures (K) | |||
|---|---|---|---|---|---|
| No. | Composition | Liquidus | Invariant | ||
| (at.%) | Exp. | Calc. | Exp. | Calc. | |
| A1 | Cu90Zr8Y2 | 1253 | 1252 | ||
| 1223 | 1217 | ||||
| A2 | Cu88Zr5Y7 | 1225 | 1197 | 1149 | 1149 |
| A3 | Cu69Zr10Y21 | 1143 | 1141 | ||
| A4 | Cu33Zr7Y60 | 1093 | 1090 | ||
| A5 | Cu87Zr2Y11 | 1180 | 1179 | ||
| 1153 | 1151 | ||||
| A6 | Cu86Zr13Y1 | 1223 | 1217 | ||
| A7 | Cu79Zr13Y8 | 1295 | 1299 | ||
| Phases | Models | Thermodynamic Parameters |
|---|---|---|
| Liquid | (Cu,Y,Zr)1 | 89300 |
| 2000 | ||
| CuZr | (Cu)1(Zr,Y)1 | 11582 |
| Cu51Zr14 | (Cu)51(Zr,Y)14 | 896480 |
| Cu5Zr | (Cu)5(Zr,Y)1 | 64998 |
| Cu4Y | (Cu)4(Y,Zr)1 | 89500 |
| 40366 | ||
| Cu6Y | (Cu)5(Cu2,Y,Zr)1 | 90000 |
| 45447 | ||
| Cu7Y2 | (Cu)7(Y,Zr)2 | 168880 |
| 55642 | ||
| 35442 | ||
| CuY | (Cu)1(Y)1 | 44760 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, F.; Liu, Y.; Du, Y.; Shi, C.; Hu, B.; He, X. Phase Equilibria, Thermodynamics and Solidified Microstructure in the Copper–Zirconium–Yttrium System. Materials 2023, 16, 2063. https://doi.org/10.3390/ma16052063
Jing F, Liu Y, Du Y, Shi C, Hu B, He X. Phase Equilibria, Thermodynamics and Solidified Microstructure in the Copper–Zirconium–Yttrium System. Materials. 2023; 16(5):2063. https://doi.org/10.3390/ma16052063
Chicago/Turabian StyleJing, Fengting, Yuling Liu, Yong Du, Chenying Shi, Biao Hu, and Xiancong He. 2023. "Phase Equilibria, Thermodynamics and Solidified Microstructure in the Copper–Zirconium–Yttrium System" Materials 16, no. 5: 2063. https://doi.org/10.3390/ma16052063
APA StyleJing, F., Liu, Y., Du, Y., Shi, C., Hu, B., & He, X. (2023). Phase Equilibria, Thermodynamics and Solidified Microstructure in the Copper–Zirconium–Yttrium System. Materials, 16(5), 2063. https://doi.org/10.3390/ma16052063






