Review of Recent Advances in Gas-Assisted Focused Ion Beam Time-of-Flight Secondary Ion Mass Spectrometry (FIB-TOF-SIMS)
Abstract
:1. Introduction
1.1. Principles of TOF-SIMS
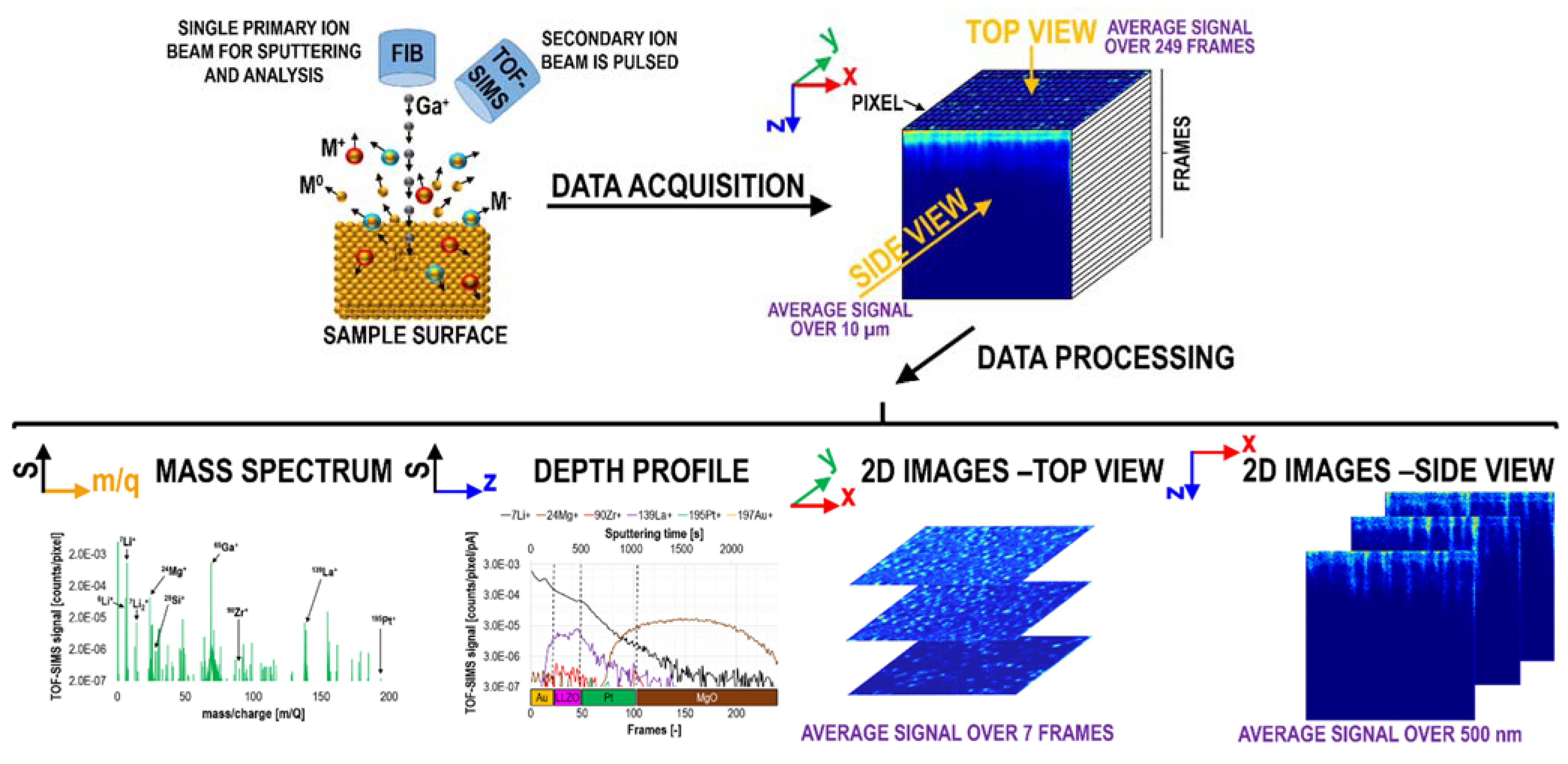
1.2. Data Acquisition, Processing, and Representation
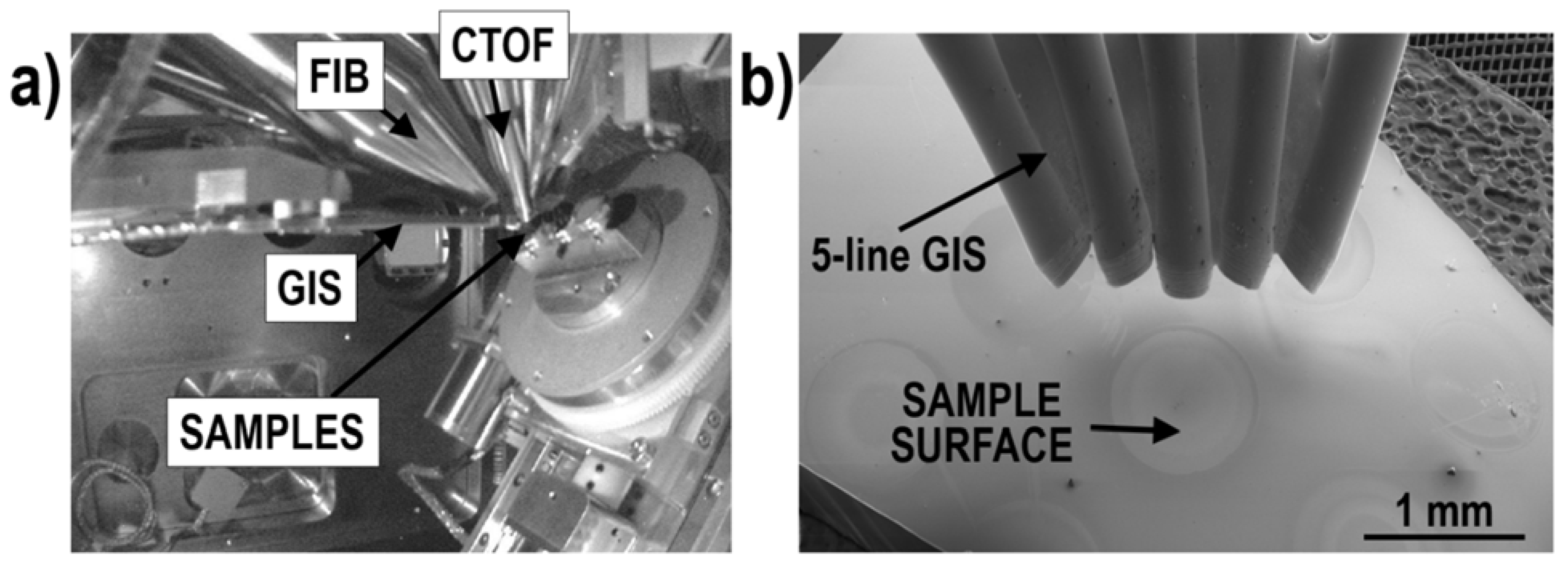
1.3. Focused Ion Beam Time-of-Flight Secondary Ion Mass Spectrometry (FIB-TOF-SIMS)
| CTOF and HTOF (from TOFWERK) | TOF.SIMS5 (from IONTOF) | |
|---|---|---|
| Type of detector | Add-on to FIB-SEM instruments (a) | Dedicated standalone detector |
| Primary ion beam species | Ga+ (potentially also Ne, He, Xe, O, and N) [6] | Ga, Bin, O2, Cs, Ar, Xe, SF5, C60 |
| Number of available primary ion beams | 1 (used for sputtering and analysis) | 2 (one primary ion beam for sputtering and the other for analysis) |
| Type of primary ion beam | Continuous | Pulsed |
| Vacuum | HV (b) | UHV |
| Lateral resolution | <50 nm (c) | <60 nm (d) |
| Depth resolution | <10 nm | <1 nm |
| Mass resolving power | 700–1100 Th/Th for CTOF 3000–7000 Th/Th for HTOF | >10,000 Th/Th |
| Mass resolution | fixed, cannot be easily modified | can be increased by decreasing the pulse duration [52,53,54] |
| Detection limit | ppm | ppm-ppb |
| Ion extraction optics | optimized to fit into existing FIB/SEM systems | maximized |
| Standard gases available for ion yield enhancement | XeF2 and H2O | O2 flooding |
1.4. Gas Injection System (GIS)
2. Advances in Gas-Assisted FIB-TOF-SIMS
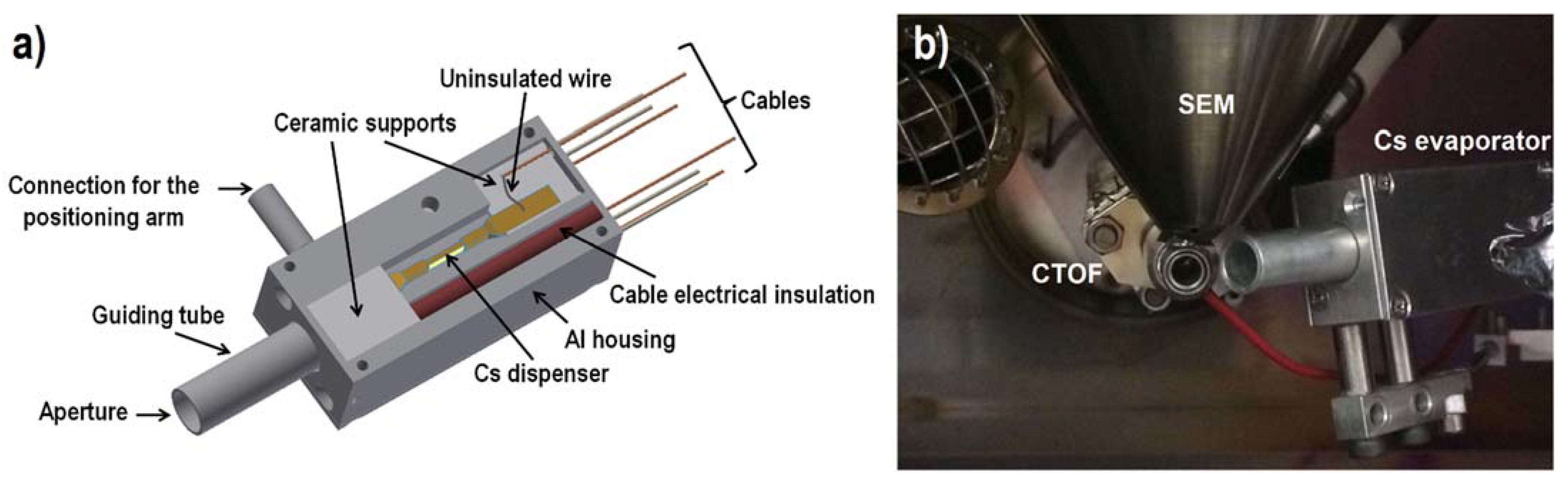
2.1. Enhancement of Negative Secondary Ions with a Home-Built CS Evaporator
2.2. Enhancement of Positive Secondary Ions with H2O and XeF2
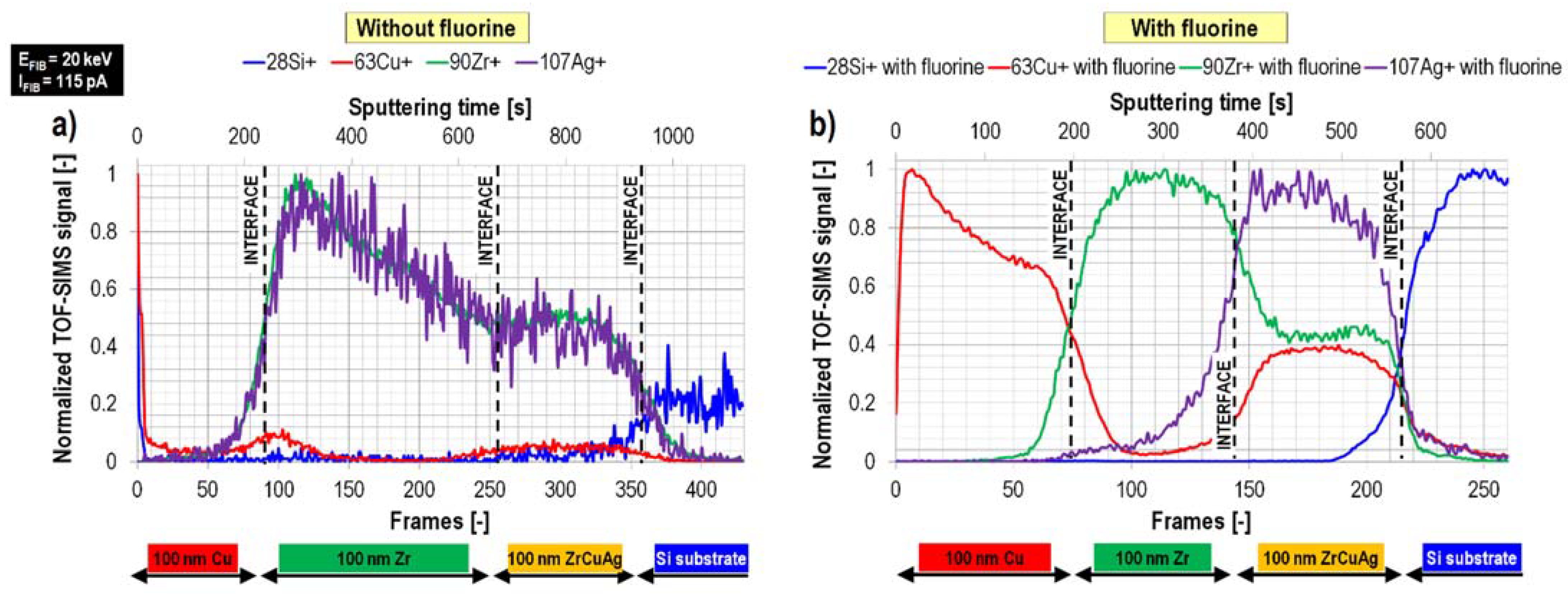
2.3. Fluorine Gas-Induced Enhancement of Spatial Resolution and Separation of Mass Interference
2.4. Fluorine Gas-Induced Inversion of Charge Polarity from Negative to Positive
2.5. Advantages and Disadvantages of Using Fluorine during FIB-TOF-SIMS
2.6. Applications of Gas-Assisted FIB-TOF-SIMS
2.6.1. Thin Films
2.6.2. Li-Ion Solid State Batteries
2.6.3. Complex Alloys
3. Prospective and Future Directions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Benninghoven, A.; Rudenauer, F.G.; Werner, H.W. Secondary Ion Mass Spectrometry–Basic Concepts, Instrumental Aspects, Applications and Trends. Surf. Interface Anal. 1987, 10, 435. [Google Scholar] [CrossRef]
- Van de Heide, P. Secondary Ion Mass Spectrometry: An Introduction to Principles and Practices; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Vickermann, J.C.; Briggs, D. ToF-SIMS: Materials Analysis by Mass Spectrometry, 2nd ed.; IM Publications LLP: West Sussex, UK, 2013; ISBN 1906715173. [Google Scholar]
- Priebe, A.; Bleuet, P.; Goret, G.; Laurencin, J.; Montinaro, D.; Barnes, J.-P. State-of-the-Art Three-Dimensional Chemical Characterization of Solid Oxide Fuel Cell Using Focused Ion Beam Time-of-Flight Secondary Ion Mass Spectrometry Tomography. Microsc. Microanal. 2016, 22, 1261–1269. [Google Scholar] [CrossRef]
- Priebe, A.; Pethö, L.; Michler, J. Fluorine Gas Coinjection as a Solution for Enhancing Spatial Resolution of Time-of-Flight Secondary Ion Mass Spectrometry and Separating Mass Interference. Anal. Chem. 2020, 92, 2121–2129. [Google Scholar] [CrossRef]
- Priebe, A.; Huszar, E.; Nowicki, M.; Pethö, L.; Michler, J. Mechanisms of Fluorine-Induced Separation of Mass Interference during TOF-SIMS Analysis. Anal. Chem. 2021, 93, 10261–10271. [Google Scholar] [CrossRef]
- Stephan, T. TOF-SIMS in Cosmochemistry. Planet. Space Sci. 2001, 49, 859–906. [Google Scholar] [CrossRef]
- Benninghoven, A.; Colton, R.J.; Simons, D.S.; Werner, H.W. Secondary Ion Mass Spectrometry SIMS V; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Tokyo, Japan, 1986; ISBN 978-3-642-82724-2. [Google Scholar]
- Benninghoven, A. Ion Formation from Organic Solids; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Tokyo, Japan, 1983; ISBN 978-3-642-87148-1. [Google Scholar]
- Kollmer, F. Cluster Primary Ion Bombardment of Organic Materials. Appl. Surf. Sci. 2004, 231–232, 153–158. [Google Scholar] [CrossRef]
- Weibel, D.; Wong, S.; Lockyer, N.; Blenkinsopp, P.; Hill, R.; Vickerman, J.C. A C60 primary Ion Beam System for Time of Flight Secondary Ion Mass Spectrometry: Its Development and Secondary Ion Yield Characteristics. Anal. Chem. 2003, 75, 1754–1764. [Google Scholar] [CrossRef] [PubMed]
- Pillatsch, L.; Wirtz, T. SIMS Using O-, F-, Cl-, Br- and I-Primary Ion Bombardment. Surf. Interface Anal. 2012, 44, 1370–1372. [Google Scholar] [CrossRef]
- Deline, V.R.; Katz, W.; Evans, C.A.; Williams, P. Mechanism of the SIMS Matrix Effect. Appl. Phys. Lett. 1978, 33, 832–835. [Google Scholar] [CrossRef]
- Priebe, A.; Xie, T.; Bürki, G.; Pethö, L.; Michler, J. Matrix Effect in TOF-SIMS Analysis of Two-Element Inorganic Thin Films. J. Anal. At. Spectrom. 2020, 35, 1156–1166. [Google Scholar] [CrossRef]
- Deline, V.R.; Evans, C.A.; Williams, P. A Unified Explanation for Secondary Ion Yields. Appl. Phys. Lett. 1978, 33, 578–580. [Google Scholar] [CrossRef]
- Wittmaack, K. Comment on “A Unified Explanation for Secondary-Ion Yields and “Mechanism of the SIMS Matrix Effect”. J. Appl. Phys. 1981, 52, 527–529. [Google Scholar] [CrossRef]
- Williams, P.; Deline, V.R.; Evans, C.A.; Katz, W. Reply to “Comment on ‘A Unified Explanation for Secondary Ion Yields’ and ‘Mechanism of the SIMS Matrix Effect’”. J. Appl. Phys. 1981, 52, 530–532. [Google Scholar] [CrossRef]
- Storms, H.A.; Brown, K.F.; Stein, J.D. Evaluation of a Cesium Positive Ion Source for Secondary Ion Mass Spectrometry. Anal. Chem. 1977, 49, 2023–2030. [Google Scholar] [CrossRef]
- Giannuzzi, L.A.; Stevie, F.A. A Review of Focused Ion Beam Milling Techniques for TEM Specimen Preparation. Micron 1999, 30, 197–204. [Google Scholar] [CrossRef]
- Priebe, A.; Jean-Paul, B.; Edwards, T.E.J.; Huszár, E.; Pethö, L.; Michler, J. Elemental Characterization of Al Nanoparticles Buried under a Cu Thin Film—TOF-SIMS vs. STEM/EDX. Anal. Chem. 2020, 92, 12518–12527. [Google Scholar] [CrossRef]
- Priebe, A.; Utke, I.; Pethö, L.; Michler, J. Application of a Gas-Injection System during the FIB-TOF-SIMS Analysis—Influence of Water Vapor and Fluorine Gas on Secondary Ion Signals and Sputtering Rates. Anal. Chem. 2019, 91, 11712–11722. [Google Scholar] [CrossRef] [PubMed]
- Priebe, A.; Sastre, J.; Futscher, M.H.; Jurczyk, J.; Puydinger Dos Santos, M.V.; Romanyuk, Y.E.; Michler, J. Detection of Au+ Ions During Fluorine Gas-Assisted Time-of-Flight Secondary Ion Mass Spectrometry (TOF-SIMS) for the Complete Elemental Characterization of Microbatteries. ACS Appl. Mater. Interfaces 2021, 13, 41262–41274. [Google Scholar] [CrossRef]
- Priebe, A.; Michler, J. Application of a Novel Compact Cs Evaporator Prototype for Enhancing Negative Ion Yields during FIB-TOF-SIMS Analysis in High Vacuum. Ultramicroscopy 2019, 196, 10–17. [Google Scholar] [CrossRef]
- Priebe, A.; Pethö, L.; Huszár, E.; Xie, T.; Utke, I.; Michler, J. High Sensitivity of Fluorine Gas-Assisted FIB-TOF-SIMS for Chemical Characterization of Buried Sublayers in Thin Films. ACS Appl. Mater. Interfaces 2021, 13, 15890–15900. [Google Scholar] [CrossRef]
- Marseilhan, D.; Barnes, J.P.; Fillot, F.; Hartmann, J.M.; Holliger, P. Quantification of SiGe Layer Composition Using MCs+ and MCs2 Secondary Ions in ToF-SIMS and Magnetic SIMS. Appl. Surf. Sci. 2008, 255, 1412–1414. [Google Scholar] [CrossRef]
- Py, M.; Barnes, J.P.; Hartmann, J.M. Quantification of Germanium in Si1-XGex Alloys by Negative Mode ToF-SIMS: The Interest of the Full Spectrum Method. Surf. Interface Anal. 2011, 43, 539–542. [Google Scholar] [CrossRef]
- Inoue, M.; Murase, A. Reduction of Matrix Effects in TOF-SIMS Analysis by Metal-Assisted SIMS (MetA-SIMS). Surf. Interface Anal. 2005, 37, 1111–1114. [Google Scholar] [CrossRef]
- Alnajeebi, A.M.; Sheraz (née Rabbani), S.; Vickerman, J.C.; Lockyer, N.P. SIMS Matrix Effects of Biological Molecules under Cluster Ion Beam Bombardment. In Proceedings of the Eighth Saudi Students Conference in the UK; Alford, N., Frechet, J., Eds.; Imperial Collegue Press: London, UK, 2006; pp. 437–444, ISBN 9781783269143. [Google Scholar]
- Tyler, B.J.; Rayal, G.; Castner, D.G. Multivariate Analysis Strategies for Processing ToF-SIMS Images of Biomaterials. Biomaterials 2007, 28, 2412–2423. [Google Scholar] [CrossRef] [Green Version]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95442-4. [Google Scholar]
- Graham, D.J.; Castner, D.G. Multivariate Analysis of ToF-SIMS Data from Multicomponent Systems: The Why, When, and How. Biointerphases 2012, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Cumpson, P.J.; Sano, N.; Fletcher, I.W.; Portoles, J.F.; Bravo-Sanchez, M.; Barlow, A.J. Multivariate Analysis of Extremely Large ToFSIMS Imaging Datasets by a Rapid PCA Method. Surf. Interface Anal. 2015, 47, 986–993. [Google Scholar] [CrossRef] [Green Version]
- Rangarajan, S.; Tyler, B.J. Topography in Secondary Ion Mass Spectroscopy Images. J. Vac. Sci. Technol. A Vac. Surf. Film. 2006, 24, 1730–1736. [Google Scholar] [CrossRef]
- Lee, J.L.S.; Gilmore, I.S.; Fletcher, I.W.; Seah, M.P. Topography and Field Effects in the Quantitative Analysis of Conductive Surfaces Using ToF-SIMS. Appl. Surf. Sci. 2008, 255, 1560–1563. [Google Scholar] [CrossRef]
- Lee, J.L.S.; Gilmore, I.S.; Seah, M.P.; Levick, A.P.; Shard, A.G. Topography and Field Effects in Secondary Ion Mass Spectrometry Part II: Insulating Samples. Surf. Interface Anal. 2012, 44, 238–245. [Google Scholar] [CrossRef]
- Priebe, A.; Audoit, G.; Barnes, J.P. A Novel PFIB Sample Preparation Protocol for Correlative 3D X-Ray CNT and FIB-TOF-SIMS Tomography. Ultramicroscopy 2017, 173, 10–13. [Google Scholar] [CrossRef]
- Iida, S.; Chang, H.-Y.; Fisher, G.L.; Miyayama, T.; Tanaka, I. Wider Vision Capability Provided by a Curved Surface Sample Holder for TOF-SIMS Imaging. J. Vac. Sci. Technol. B 2020, 38, 034013. [Google Scholar] [CrossRef]
- Koch, S.; Ziegler, G.; Hutter, H. ToF-SIMS Measurements with Topographic Information in Combined Images. Anal. Bioanal. Chem. 2013, 405, 7161–7167. [Google Scholar] [CrossRef]
- Wucher, A.; Cheng, J.; Winograd, N. Protocols for Three-Dimensional Molecular Imaging Using Mass Spectrometry. Anal. Chem. 2007, 79, 5529–5539. [Google Scholar] [CrossRef]
- Fletcher, J.S.; Henderson, A.; Biddulph, G.X.; Vaidyanathan, S.; Lockyer, N.P.; Vickerman, J.C. Uncovering New Challenges in Bio-Analysis with ToF-SIMS. Appl. Surf. Sci. 2008, 255, 1264–1270. [Google Scholar] [CrossRef]
- Ziegler, G.; Hutter, H. Correction of Topographic Artefacts of ToF-SIMS Element Distributions. Surf. Interface Anal. 2013, 45, 457–460. [Google Scholar] [CrossRef]
- Terlier, T.; Lee, J.; Lee, K.; Lee, Y. Improvement of the Correlative AFM and ToF-SIMS Approach Using an Empirical Sputter Model for 3D Chemical Characterization. Anal. Chem. 2018, 90, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Pillatsch, L.; Kalácska, S.; Maeder, X.; Michler, J. In Situ Atomic Force Microscopy Depth-Corrected Three-Dimensional Focused Ion Beam Based Time-of-Flight Secondary Ion Mass Spectroscopy: Spatial Resolution, Surface Roughness, Oxidation. Microsc. Microanal. 2020, 27, 65–73. [Google Scholar] [CrossRef]
- Moreno, M.A.; Mouton, I.; Chevalier, N.; Barnes, J.-P.; Bassani, F.; Gautier, B. Combined ToF-SIMS and AFM Protocol for Accurate 3D Chemical Analysis and Data Visualization. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2018, 36, 03F122. [Google Scholar] [CrossRef]
- Priebe, A.; Aribia, A.; Sastre, J.; Romanyuk, Y.E.; Michler, J. 3D High-Resolution Chemical Characterization of Sputtered Li-Rich NMC811 Thin Films Using TOF-SIMS. Anal. Chem. 2023, 95, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Whitby, J.A.; Östlund, F.; Horvath, P.; Gabureac, M.; Riesterer, J.L.; Utke, I.; Hohl, M.; Sedláček, L.; Jiruše, J.; Friedli, V.; et al. High Spatial Resolution Time-of-Flight Secondary Ion Mass Spectrometry for the Masses: A Novel Orthogonal ToF FIB-SIMS Instrument with in Situ AFM. Adv. Mater. Sci. Eng. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Alberts, D.; von Werra, L.; Oestlund, F.; Rohner, U.; Hohl, M.; Michler, J.; Whitby, J.A. Design and Performance of Two Orthogonal Extraction Time-of-Flight Secondary Ion Mass Spectrometers for Focused Ion Beam Instruments. Instrum. Sci. Technol. 2014, 42, 432–445. [Google Scholar] [CrossRef]
- Pillatsch, L.; Östlund, F.; Michler, J. FIBSIMS: A Review of Secondary Ion Mass Spectrometry for Analytical Dual Beam Focussed Ion Beam Instruments. Prog. Cryst. Growth Charact. Mater. 2019, 65, 1–19. [Google Scholar] [CrossRef]
- Guilhaus, M.; Selby, D.; Mlynski, V. Orthogonal Acceleration Time-of-Flight Mass Spectrometry. Mass Spectrom. Rev. 2000, 19, 65–107. [Google Scholar] [CrossRef]
- Priebe, A.; Barnes, J.-P.; Edwards, T.E.J.; Pethö, L.; Balogh, I.; Michler, J. 3D Imaging of Nanoparticles in an Inorganic Matrix Using TOF-SIMS Validated with STEM and EDX. Anal. Chem. 2019, 91, 11834–11839. [Google Scholar] [CrossRef] [PubMed]
- Audinot, J.N.; Philipp, P.; De Castro, O.; Biesemeier, A.; Hoang, Q.H.; Wirtz, T. Highest Resolution Chemical Imaging Based on Secondary Ion Mass Spectrometry Performed on the Helium Ion Microscope. Reports Prog. Phys. 2021, 84, 105901. [Google Scholar] [CrossRef]
- Sodhi, R.N.S. Time-of-Flight Secondary Ion Mass Spectrometry (TOF-SIMS):—Versatility in Chemical and Imaging Surface Analysis. Analyst 2004, 129, 483–487. [Google Scholar] [CrossRef]
- Hagenhoff, B. High Resolution Surface Analysis by TOF-SIMS. Mikrochim. Acta 2000, 132, 259–271. [Google Scholar] [CrossRef]
- Kubicek, M.; Holzlechner, G.; Opitz, A.K.; Larisegger, S.; Hutter, H.; Fleig, J. A Novel ToF-SIMS Operation Mode for Sub 100 Nm Lateral Resolution: Application and Performance. Appl. Surf. Sci. 2014, 289, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almoric, J.; Fraczkiewicz, A.; Durand, M.; Seret, A.; Nicolay, A.; Houel, A.; Delobbe, A.; Berbezier, I.; Bozzolo, N. Development of an O-TOFSIMS Integrated on a UHV FIB/SEM Workstation: Challenges and Innovative Approaches for in Depth Correlated Nano-Analyzes and High-Resolution Imaging. In Proceedings of the Abstract book of 4th EuFN and FIT4NANO Joint Workshop/Meeting, Viena, Austria, 27–30 September 2021; pp. 98–99. [Google Scholar]
- Fleck, R.A.; Humbel, B.M. Biological Field Emission Scanning Electron Microscopy; John Wiley & Sons, Inc.: West Sussex, UK, 2019; ISBN 978-1-118-65406-4. [Google Scholar]
- Kollmer, F.; Paul, W.; Krehl, M.; Niehuis, E. Ultra High Spatial Resolution SIMS with Cluster Ions—Approaching the Physical Limits. Surf. Interface Anal. 2013, 45, 312–314. [Google Scholar] [CrossRef]
- Available online: https://www.iontof.com/tof-sims-secondary-ion-mass-spectrometry.html (accessed on 1 September 2022).
- Wieczerzak, K.; Priebe, A.; Utke, I.; Michler, J. Practical Aspects of Focused Ion Beam Time-of-Flight Secondary Ion Mass Spectrometry Analysis Enhanced by Fluorine Gas Coinjection. Chem. Mater. 2021, 33, 1581–1593. [Google Scholar] [CrossRef]
- Xu, X.; Hao, M.; Chen, J.; He, W.; Li, G.; Li, K.; Jiao, C.; Zhong, X.L.; Moore, K.L.; Burnett, T.L.; et al. Role of Constituent Intermetallic Phases and Precipitates in Initiation and Propagation of Intergranular Corrosion of an Al-Li-Cu-Mg Alloy. Corros. Sci. 2022, 201, 110294. [Google Scholar] [CrossRef]
- Xu, X.; Jiao, C.; Li, K.; Hao, M.; Moore, K.L.; Burnett, T.L.; Zhou, X. Application of High-Spatial-Resolution Secondary Ion Mass Spectrometry for Nanoscale Chemical Mapping of Lithium in an Al-Li Alloy. Mater. Charact. 2021, 181, 111442. [Google Scholar] [CrossRef]
- Vida, Á.; Lábár, J.; Dankházi, Z.; Maksa, Z.; Molnár, D.; Varga, L.K.; Kalácska, S.; Windisch, M.; Huhn, G.; Chinh, N.Q. A Sequence of Phase Transformations and Phases in Nicofecrga High Entropy Alloy. Materials 2021, 14, 1076. [Google Scholar] [CrossRef]
- Tan, L.; He, G.; Liu, F.; Li, Y.; Jiang, L. Effects of Temperature and Pressure of Hot Isostatic Pressing on the Grain Structure of Powder Metallurgy Superalloy. Materials 2018, 11, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.; Li, Y.; Liu, C.; Yang, C.; Ding, H.; Huang, L.; Liu, F.; Qin, Z.; Jiang, L. The Evolution History of Superalloy Powders during Hot Consolidation and Plastic Deformation. Mater. Charact. 2018, 140, 30–38. [Google Scholar] [CrossRef]
- Almoric, J.; Durand, M.; Seret, A.; Nicolaÿ, A.; Houel, A.; Berbezier, I.; Bozzolo, N. Implementation of Nanoscale SIMS Analyses: Application to Ni-based Superalloys. Phys. Status Solidi A 2021, 219, 2100414. [Google Scholar] [CrossRef]
- Priebe, A.; Xie, T.; Pethö, L.; Michler, J. Potential of Gas-Assisted Time-of-Flight Secondary Ion Mass Spectrometry for Improving Elemental Characterization of Complex Metal-Based Systems. J. Anal. At. Spectrom. 2020, 35, 2997–3006. [Google Scholar] [CrossRef]
- Stachowiak, G.B.; Salasi, M.; Rickard, W.D.A.; Stachowiak, G.W. The Effects of Particle Angularity on Low-Stress Three-Body Abrasion-Corrosion of 316L Stainless Steel. Corros. Sci. 2016, 111, 690–702. [Google Scholar] [CrossRef]
- Montecinos, S.; Klímek, P.; Sláma, M.; Suarez, S.; Simison, S. Corrosion Behavior of a β CuAlBe Shape Memory Alloy Containing Stress Induced Martensite. Appl. Surf. Sci. 2019, 466, 165–170. [Google Scholar] [CrossRef]
- Zijlstra, G.; Šamořil, T.; Tesařová, H.; Ocelík, V.; De Hosson, J.T.M. Depth Profile Analysis of Thin Oxide Layers on Polycrystalline Fe-Cr. Microsc. Microanal. 2020, 26, 112–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Huang, Y.; Feyerabend, F.; Blawert, C.; Gan, W.; Maawad, E.; You, S.; Gavras, S.; Scharnagl, N.; Bode, J.; et al. Influence of the Amount of Intermetallics on the Degradation of Mg-Nd Alloys under Physiological Conditions. Acta Biomater. 2021, 121, 695–712. [Google Scholar] [CrossRef]
- Torrisi, A.; Horák, P.; Vacík, J.; Lavrentiev, V.; Cannavò, A.; Ceccio, G.; Vaniš, J.; Yatskiv, R.; Grym, J. Synthesis of Cu–Ti Thin Film Multilayers on Silicon Substrates. Bull. Mater. Sci. 2021, 44, 50. [Google Scholar] [CrossRef]
- Avancini, E.; Keller, D.; Carron, R.; Arroyo-Rojas Dasilva, Y.; Erni, R.; Priebe, A.; Di Napoli, S.; Carrisi, M.; Sozzi, G.; Menozzi, R.; et al. Voids and Compositional Inhomogeneities in Cu(In,Ga)Se2 Thin Films: Evolution during Growth and Impact on Solar Cell Performance. Sci. Technol. Adv. Mater. 2018, 19, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Tepliakova, M.M.; Mikheeva, A.N.; Somov, P.A.; Statnik, E.S.; Korsunsky, A.M.; Stevenson, K.J. Combination of Metal Oxide and Polytriarylamine: A Design Principle to Improve the Stability of Perovskite Solar Cells. Energies 2021, 14, 5115. [Google Scholar] [CrossRef]
- Tennyson, E.M.; Abdi-Jalebi, M.; Ji, K.; Garrett, J.L.; Gong, C.; Pawlicki, A.A.; Ovchinnikova, O.S.; Munday, J.N.; Stranks, S.D.; Leite, M.S. Correlated Electrical and Chemical Nanoscale Properties in Potassium-Passivated, Triple-Cation Perovskite Solar Cells. Adv. Mater. Interfaces 2020, 7, 2000515. [Google Scholar] [CrossRef]
- Pham, H.T.; Duong, T.; Rickard, W.D.A.; Kremer, F.; Weber, K.J.; Wong-Leung, J. Understanding the Chemical and Structural Properties of Multiple-Cation Mixed Halide Perovskite. J. Phys. Chem. C 2019, 123, 26718–26726. [Google Scholar] [CrossRef]
- Yao, X.; Šamořil, T.; Dluhoš, J.; Watts, J.F.; Du, Z.; Song, B.; Silva, S.R.P.; Sui, T.; Zhao, Y. Degradation Diagnostics from the Subsurface of Lithium-Ion Battery Electrodes. Energy Environ. Mater. 2022, 5, 662–669. [Google Scholar] [CrossRef]
- Paolella, A.; Turner, S.; Bertoni, G.; Hovington, P.; Flacau, R.; Boyer, C.; Feng, Z.; Colombo, M.; Marras, S.; Prato, M.; et al. Accelerated Removal of Fe-Antisite Defects While Nanosizing Hydrothermal LiFePO4 with Ca2+. Nano Lett. 2016, 16, 2692–2697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Zhao, C.Z.; Zhang, R.; Yuan, H.; Hou, L.P.; Fu, Z.H.; Chen, X.; Huang, J.Q.; Zhang, Q. The Carrier Transition from Li Atoms to Li Vacancies in Solid-State Lithium Alloy Anodes. Sci. Adv. 2021, 7, eabi5520. [Google Scholar] [CrossRef]
- Dermenci, K.B.; Tesařová, H.; Šamořil, T.; Turan, S. Comparison of Xenon and Gallium Sources on the Detection and Mapping of Lithium in Li-Containing Materials by Using ToF-SIMS Combined FIB-SEM. J. Microsc. 2020, 277, 42–48. [Google Scholar] [CrossRef]
- Miller, D.J.; Zapotok, D.; Hiller, J.; Juracka, Z.; Schmidt, U.; Ezell, T.; Senabulya, N.; Kerns, B.; Fonseca Rodrigues, M.-T.; Maroni, V.A.; et al. Exploring Heterogeneity in Li Battery Electrodes Using FIB-SEM Integrated with Raman and TOF-SIMS. Microsc. Microanal. 2019, 25, 862–863. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Liu, N.; Chen, L.; Su, Y.; Tan, G.; Bao, L.; Zhang, Q.; Lu, Y.; Wang, J.; Chen, S.; et al. Improving the Reversibility of the H2-H3 Phase Transitions for Layered Ni-Rich Oxide Cathode towards Retarded Structural Transition and Enhanced Cycle Stability. Nano Energy 2019, 59, 50–57. [Google Scholar] [CrossRef]
- Yang, C.; Xie, H.; Ping, W.; Fu, K.; Liu, B.; Rao, J.; Dai, J.; Wang, C.; Pastel, G.; Hu, L. An Electron/Ion Dual-Conductive Alloy Framework for High-Rate and High-Capacity Solid-State Lithium-Metal Batteries. Adv. Mater. 2019, 31, e1804815. [Google Scholar] [CrossRef]
- Miller, D.J.; Zapotok, D.; Anzalone, P.; Šamořil, T.; Hladík, L.; Tesařová, H.; Yao, K.P.C.; Maroni, V.A.; Abraham, D.P. Exploring Li Distribution in Li-Ion Batteries with FIB-SEM and TOF-SIMS Dean. Microsc. Microanal. 2018, 24, 370–371. [Google Scholar] [CrossRef] [Green Version]
- Tian, R.; Feng, X.; Duan, H.; Zhang, P.; Li, H.; Liu, H.; Gao, L. Low-Weight 3D Al2O3 Network as an Artificial Layer to Stabilize Lithium Deposition. ChemSusChem 2018, 11, 3243–3252. [Google Scholar] [CrossRef] [PubMed]
- Bessette, S.; Hovington, P.; Kim, C.; Gauvin, R.; Demers, H.; Zaghib, K. Development of Quantitative Techniques with Time-of-Flight Secondary Ion Mass Spectrometry (TOF-SIMS) for Li Characterization in High Energy Batteries. Microsc. Microanal. 2017, 23, 2088–2089. [Google Scholar] [CrossRef] [Green Version]
- Sastre, J.; Lin, T.-Y.; Filippin, A.N.; Priebe, A.; Avancini, E.; Michler, J.; Tiwari, A.N.; Romanyuk, Y.E.; Buecheler, S. Aluminum-Assisted Densification of Cosputtered Lithium Garnet Electrolyte Films for Solid-State Batteries. ACS Appl. Energy Mater. 2019, 2, 8511–8524. [Google Scholar] [CrossRef] [Green Version]
- Sastre, J.; Priebe, A.; Döbeli, M.; Michler, J.; Tiwari, A.N.; Romanyuk, Y.E. Lithium Garnet Li7La3Zr2O12 Electrolyte for All-Solid-State Batteries: Closing the Gap between Bulk and Thin Film Li-Ion Conductivities. Adv. Mater. Interfaces 2020, 7, 2000425. [Google Scholar] [CrossRef]
- Sastre, J.; Futscher, M.H.; Pompizi, L.; Aribia, A.; Priebe, A.; Overbeck, J.; Stiefel, M.; Tiwari, A.N.; Romanyuk, Y.E. Blocking Lithium Dendrite Growth in Solid-State Batteries with an Ultrathin Amorphous Li-La-Zr-O Solid Electrolyte. Commun. Mater. 2021, 2, 76. [Google Scholar] [CrossRef]
- Dubey, R.; Sastre, J.; Cancellieri, C.; Okur, F.; Forster, A.; Pompizii, L.; Priebe, A.; Romanyuk, Y.E.; Jeurgens, L.P.H.; Kovalenko, M.V.; et al. Building a Better Li-Garnet Solid Electrolyte/Metallic Li Interface with Antimony. Adv. Energy Mater. 2021, 11, 2102086. [Google Scholar] [CrossRef]
- Jiao, C.; Pillatsch, L.; Mulders, J.; Wall, D. Three-Dimensional Time-of-Flight Secondary Ion Mass Spectrometry and DualBeam FIB/SEM Imaging of Lithium-Ion Battery Cathode. Microsc. Microanal. 2019, 25, 876–877. [Google Scholar] [CrossRef] [Green Version]
- Bessette, S.; Paolella, A.; Kim, C.; Zhu, W.; Hovington, P.; Gauvin, R.; Zaghib, K. Nanoscale Lithium Quantification in LiXNiyCowMnZO2 as Cathode for Rechargeable Batteries. Sci. Rep. 2018, 8, 17575. [Google Scholar] [CrossRef] [Green Version]
- Aribia, A.; Sastre, J.; Chen, X.; Rumpel, M.; Priebe, A.; Döbeli, M.; Gerstl, S.; Tiwari, A.N.; Romanyuk, Y.E. Unlocking Stable Multi-Electron Cycling in NMC811 Thin-Films between 1.5–4.7 V. Adv. Energy Mater. 2022, 12, 2201750. [Google Scholar] [CrossRef]
- Sui, T.; Song, B.; Dluhos, J.; Lu, L.; Korsunsky, A.M. Nanoscale Chemical Mapping of Li-Ion Battery Cathode Material by FIB-SEM and TOF-SIMS Multi-Modal Microscopy. Nano Energy 2015, 17, 254–260. [Google Scholar] [CrossRef]
- Belanger, R.L.; Commarieu, B.; Paolella, A.; Daigle, J.C.; Bessette, S.; Vijh, A.; Claverie, J.P.; Zaghib, K. Diffusion Control of Organic Cathode Materials in Lithium Metal Battery. Sci. Rep. 2019, 9, 1213. [Google Scholar] [CrossRef] [Green Version]
- Bärtsch, M.; Sarnowska, M.; Krysiak, O.; Willa, C.; Huber, C.; Pillatsch, L.; Reinhard, S.; Niederberger, M. Multicomposite Nanostructured Hematite-Titania Photoanodes with Improved Oxygen Evolution: The Role of the Oxygen Evolution Catalyst. ACS Omega 2017, 2, 4531–4539. [Google Scholar] [CrossRef] [Green Version]
- Stevie, F.A.; Sedlacek, L.; Babor, P.; Jiruse, J.; Principe, E.; Klosova, K. FIB-SIMS Quantification Using TOF-SIMS with Ar and Xe Plasma Sources. Surf. Interface Anal. 2014, 46, 285–287. [Google Scholar] [CrossRef]
- Mosse, I.S.; Sodisetti, V.R.; Coleman, C.; Ncube, S.; de Sousa, A.S.; Erasmus, R.M.; Flahaut, E.; Blon, T.; Lassagne, B.; Šamořil, T.; et al. Tuning Magnetic Properties of a Carbon Nanotube-Lanthanide Hybrid Molecular Complex through Controlled Functionalization. Molecules 2021, 26, 563. [Google Scholar] [CrossRef]
- Kvitek, O.; Havelka, V.; Vesely, M.; Reznickova, A.; Svorcik, V. Preparation of Alloyed and “Core-Shell” Au/Ag Bimetallic Nanostructures on Glass Substrate by Solid State Dewetting. J. Alloys Compd. 2020, 829, 154627. [Google Scholar] [CrossRef]
- Torrisi, A.; Horák, P.; Vacík, J.; Cannavò, A.; Ceccio, G.; Vaniš, J.; Yatskiv, R.; Grym, J. Multilayered Cu–Ti Deposition on Silicon Substrates for Chemiresistor Applications. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 932–935. [Google Scholar] [CrossRef]
- Sedláček, L.; Kološová, J.; Jiruše, J.; Stevie, F.A. Insulator Analysis Using Combined FIB-SEM Instrument with TOF-SIMS. Microsc. Microanal. 2014, 20, 306–307. [Google Scholar] [CrossRef] [Green Version]
- Rickard, W.D.A.; Reddy, S.M.; Saxey, D.W.; Fourgerouse, D.; van Riessen, A. Correlative Analysis Using FIB-ToF-SIMS and Atom Probe Tomography on Geological Materials. Microsc. Microanal. 2016, 22, 684–685. [Google Scholar] [CrossRef] [Green Version]
- Timms, N.E.; Kirkland, C.L.; Cavosie, A.J.; Rae, A.S.P.; Rickard, W.D.A.; Evans, N.J.; Erickson, T.M.; Wittmann, A.; Ferrière, L.; Collins, G.S.; et al. Shocked Titanite Records Chicxulub Hydrothermal Alteration and Impact Age. Geochim. Cosmochim. Acta 2020, 281, 12–30. [Google Scholar] [CrossRef]
- Peterman, E.M.; Reddy, S.M.; Saxey, D.W.; Fougerouse, D.; Snoeyenbos, D.R.; Rickard, W.D.A. Nanoscale Processes of Trace Element Mobility in Metamorphosed Zircon. Contrib. Mineral. Petrol. 2019, 174, 92. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Merigot, K.; Rickard, W.D.A.; Evans, N.J.; McDonald, B.J.; Catovic, E.; Spitalny, P. Assessment of a Spodumene Ore by Advanced Analytical and Mass Spectrometry Techniques to Determine Its Amenability to Processing for the Extraction of Lithium. Miner. Eng. 2018, 119, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Aylmore, M.G.; Merigot, K.; Quadir, Z.; Rickard, W.D.A.; Evans, N.J.; McDonald, B.J.; Catovic, E.; Spitalny, P. Applications of Advanced Analytical and Mass Spectrometry Techniques to the Characterisation of Micaceous Lithium-Bearing Ores. Miner. Eng. 2018, 116, 182–195. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Reddy, S.M.; Saxey, D.W.; Fougerouse, D.; Timms, N.E.; Daly, L.; Peterman, E.; Cavosie, A.J.; Jourdan, F. Novel Applications of FIB-SEM-Based ToF-SIMS in Atom Probe Tomography Workflows. Microsc. Microanal. 2020, 26, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, S.D.; Reddy, S.M.; Saxey, D.W.; Rickard, W.D.A.; Fougerouse, D.; Quadir, Z.; Johnson, T.E. Nanoscale Constraints on the Shock-Induced Transformation of Zircon to Reidite. Chem. Geol. 2019, 507, 85–95. [Google Scholar] [CrossRef]
- Plet, C.; Grice, K.; Pagès, A.; Verrall, M.; Coolen, M.J.L.; Ruebsam, W.; Rickard, W.D.A.; Schwark, L. Palaeobiology of Red and White Blood Cell-like Structures, Collagen and Cholesterol in an Ichthyosaur Bone. Sci. Rep. 2017, 7, 13776. [Google Scholar] [CrossRef] [Green Version]
- Tacchetto, T.; Reddy, S.M.; Bartoli, O.; Rickard, W.D.A.; Fougerouse, D.; Saxey, D.W.; Quadir, Z.; Clark, C. Pre-Nucleation Geochemical Heterogeneity within Glassy Anatectic Inclusions and the Role of Water in Glass Preservation. Contrib. Mineral. Petrol. 2021, 176, 70. [Google Scholar] [CrossRef]
- Cavosie, A.J.; Rickard, W.D.A.; Evans, N.J.; Rankenburg, K.; Roberts, M.; Macris, C.A.; Koeberl, C. Origin of SS-Cristobalite in Libyan Desert Glass—The Hottest Naturally Occurring Silica Polymorph? Am. Mineral. 2021, 107, 1325–1340. [Google Scholar] [CrossRef]
- Jonas Barthel, H.; Fougerouse, D.; Geisler, T.; Rust, J. Fluoridation of a Lizard Bone Embedded in Dominican Amber Suggests Open-System Behavior. PLoS ONE 2020, 15, e0228843. [Google Scholar] [CrossRef] [Green Version]
- Aylmore, M.G. Assessment of Lithium Pegmatite Ore Bodies to Determine Their Amenability to Processing for the Extraction of Lithium. Extr. Miner. Met. Mater. Ser. 2018, 2261–2279. [Google Scholar] [CrossRef]
- Spinks, S.C.; Pearce, M.A.; Liu, W.; Kunzmann, M.; Ryan, C.G.; Moorhead, G.F.; Kirkham, R.; Blaikie, T.; Sheldon, H.A.; Schaubs, P.M.; et al. Carbonate Replacement as the Principal Ore Formation Process in the Proterozoic Mcarthur River (Hyc) Sediment-Hosted Zn-Pb Deposit, Australia. Econ. Geol. 2021, 116, 693–718. [Google Scholar] [CrossRef]
- Klímek, P.; Wimmer, R.; Meinlschmidt, P. Tof-Sims Molecular Imaging and Properties of Pmdi-Bonded Particleboards Made from Cup-Plant and Wood. Appl. Sci. 2021, 11, 1604. [Google Scholar] [CrossRef]
- Liu, D.; Yuan, P.; Tian, Q.; Liu, H.; Deng, L.; Song, Y.; Zhou, J.; Losic, D.; Zhou, J.; Song, H.; et al. Lake Sedimentary Biogenic Silica from Diatoms Constitutes a Significant Global Sink for Aluminium. Nat. Commun. 2019, 10, 4829. [Google Scholar] [CrossRef] [Green Version]
- Thrush, S.J.; Comfort, A.S.; Dusenbury, J.S.; Nautiyal, P.; Elinski, M.B.; Carpick, R.W.; Demas, N.G.; Gould, B.J.; Han, X.; Wang, X.; et al. Growth and Morphology of Thermally Assisted Sinterable Zirconia Nanoparticle Tribofilm. Tribol. Int. 2022, 175, 107820. [Google Scholar] [CrossRef]
- Lorinčík, J.; Kašík, I.; Vaniš, J.; Sedláček, L.; Dluhoš, J. Imaging of Dopant Distribution in Optical Fibers with an Orthogonal TOF SIMS. Surf. Interface Anal. 2014, 46, 238–240. [Google Scholar] [CrossRef]
- Antonatos, N.; Sedmidubsky, D.; Levinsky, P. Black Phosphorus: Fundamental Properties and Influence of Impurities Induced by Its Synthesis. ACS Appl. Mater. Interfaces 2022, 14, 34867–34874. [Google Scholar] [CrossRef]
- Osticioli, I.; Lavacchi, A.; Capozzoli, L.; Berretti, E.; Gallo, L.; Berberich, C.; Agresti, J.; Siano, S. Novel Insights on the Study of a Fifteenth-Century Oro Di Metà/Zwischgold Gilding by Means of Ion and Electron Microscopy: Characterization of the Stratigraphy Avoiding Cross-Sections Preparation. J. Cult. Herit. 2020, 44, 297–306. [Google Scholar] [CrossRef]
- Yatskiv, R.; Tiagulskyi, S.; Grym, J.; Vaniš, J.; Bašinová, N.; Horak, P.; Torrisi, A.; Ceccio, G.; Vacik, J.; Vrňata, M. Optical and Electrical Characterization of CuO/ZnO Heterojunctions. Thin Solid Films 2020, 693, 137656. [Google Scholar] [CrossRef]
- Drdlikova, K.; Klement, R.; Drdlik, D.; Galusek, D.; Maca, K. Processing and Properties of Luminescent Cr3+ Doped Transparent Alumina Ceramics. J. Eur. Ceram. Soc. 2020, 40, 2573–2580. [Google Scholar] [CrossRef]
- Drdlikova, K.; Maca, K.; Slama, M.; Drdlik, D. Transparent Alumina Ceramics: Effect of Standard and Plasma Generated Stabilizing Approaches in Colloidal Processing. J. Am. Ceram. Soc. 2019, 102, 7137–7144. [Google Scholar] [CrossRef]
- Chen, K.; Li, N.; Ai, N.; Li, M.; Cheng, Y.; Rickard, W.D.A.; Li, J.; Jiang, S.P. Direct Application of Cobaltite-Based Perovskite Cathodes on the Yttria-Stabilized Zirconia Electrolyte for Intermediate Temperature Solid Oxide Fuel Cells. J. Mater. Chem. A 2016, 4, 17678–17685. [Google Scholar] [CrossRef] [Green Version]
- Yue, Z.; Jiang, L.; Ai, N.; Guan, C.; Jiang, S.P.; Sun, X.; Rickard, W.D.A.; Wang, X.; Shao, Y.; Chen, K. Facile Co-Synthesis and Utilization of Ultrafine and Highly Active PrBa0.8Ca0.2Co2O5+δ-Gd0.2Ce0.8O1.9 Composite Cathodes for Solid Oxide Fuel Cells. Electrochim. Acta 2022, 403, 139673. [Google Scholar] [CrossRef]
- He, Z.; Ai, N.; He, S.; Jiang, S.P.; Zhang, L.; Rickard, W.D.A.; Tang, D.; Chen, K. Positive Effect of Incorporating Er0.4Bi1.6O3 on the Performance and Stability of La2NiO4+δ Cathode. J. Electrochem. Soc. 2019, 166, F796–F804. [Google Scholar] [CrossRef]
- Chen, K.; He, S.; Li, N.; Cheng, Y.; Ai, N.; Chen, M.; Rickard, W.D.A.; Zhang, T.; Jiang, S.P. Nb and Pd Co-Doped La0.57Sr0.38Co0.19Fe0.665Nb0.095Pd0.05O3-Δ as a Stable, High Performance Electrode for Barrier-Layer-Free Y2O3-ZrO2 Electrolyte of Solid Oxide Fuel Cells. J. Power Sources 2018, 378, 433–442. [Google Scholar] [CrossRef]
- Jurczyk, J.; Pillatsch, L.; Berger, L.; Priebe, A.; Madajska, K.; Michler, J.; Utke, I.; Kapusta, C.; Szyma, I.B. In Situ Time-of-Flight Mass Spectrometry of Ionic Fragments Induced by Focused Electron Beam Irradiation: Investigation of Electron Driven Surface Chemistry inside an SEM under High Vacuum. Nanomaterials 2022, 12, 2710. [Google Scholar] [CrossRef] [PubMed]
- Utke, I.; Moshkalev, S.; Russell, P. Nanofabrication Using Focused Ion and Electron Beams: Principles and Applications; Utke, I., Moshkalev, S., Russell, P., Eds.; Oxford University Press: Oxford, UK, 2012; ISBN 9780199734214. [Google Scholar]
- Wang, Z.M. FIB Nanostructures; Springer: Heidelberg, Germnay, 2013; ISBN 978-3-319-02874-3. [Google Scholar]
- Volkert, C.A.; Minor, A.M. Focused Ion Beam Micromachining. MRS Bull. 2007, 32, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Giannuzzi, L.A.; Stevie, F.A. Introduction to Focused Ion Beams Instrumentation, Theory, Techniques and Practice; Springer: New York, NY, USA, 2005. [Google Scholar]
- Castillo, R.C. Functional Nanostructures Fabricated by Focused Electron/Ion Beam Induced Deposition; Springer: Cham, Switzerland, 2014; ISBN 978-3-319-02081-5. [Google Scholar]
- Bleuet, P.; Audoit, G.; Barnes, J.; Bertheau, J.; Dabin, Y.; Dansas, H.; Fabbri, J. Specifications for Hard Condensed Matter Specimens for Three-Dimensional High-Resolution Tomographies. Microsc. Microanal. 2013, 19, 726–739. [Google Scholar] [CrossRef]
- Priebe, A.; Goret, G.; Bleuet, P.; Audoit, G.; Laurencin, J.; Barnes, J.P. 3D Correlative Morphological and Elemental Characterization of Materials at the Deep Submicrometre Scale. J. Microsc. 2016, 264, 247–251. [Google Scholar] [CrossRef]
- Mayer, J.; Giannuzzi, L.A.; Kamino, T.; Michael, J. TEM Sample Preparation and Damage. MRS Bull. 2007, 32, 400–407. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.K.; Kenik, E.A. Atom Probe Tomography: A Technique for Nanoscale Characterization. Microsc. Microanal. 2004, 10, 336–341. [Google Scholar] [CrossRef]
- Miller, M.K.; Russell, K.F.; Thompson, K.; Alvis, R.; Larson, D.J. Review of Atom Probe FIB-Based Specimen Preparation Methods. Microsc. Microanal. 2007, 13, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Cleaver, J.R.A.; Ahmed, H. Characteristics of Gas-Assisted Focused Ion Beam Etching. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. Process. Meas. Phenom. 1993, 11, 234–241. [Google Scholar] [CrossRef]
- Casey , J.D., Jr.; Doyle, A.F.; Lee, R.G.; Stewart, D.K.; Zimmermann, H. Gas-Assisted Etching with Focused Ion Beam Technology. Microelectron. Eng. 1994, 24, 43–50. [Google Scholar] [CrossRef]
- Kwakman, L.; Franz, G.; Taklo, M.M.V.; Klumpp, A.; Ramm, P. Characterization and Failure Analysis of 3D Integrated Systems Using a Novel Plasma-FIB System. AIP Conf. Proc. 2011, 1395, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Tomus, D.; Ng, H.P. In Situ Lift-out Dedicated Techniques Using FIB-SEM System for TEM Specimen Preparation. Micron 2013, 44, 115–119. [Google Scholar] [CrossRef]
- Ivan, S.; Pillatsch, L.; Utke, I. Direct–Write Milling and Deposition with Noble Gases. In Helium Ion Microscopy; Hlawacek, G., Gölzhäuser, A., Eds.; Springer: Cham, Switzerland, 2016; pp. 355–393. ISBN 978-3-319-41988-6. [Google Scholar]
- Melngailis, J. Focused Ion Beam Technology and Applications. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2002, 5, 469. [Google Scholar] [CrossRef]
- Utke, I.; Hoffmann, P.; Melngailis, J. Gas-Assisted Focused Electron Beam and Ion Beam Processing and Fabrication. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2008, 26, 1197. [Google Scholar] [CrossRef] [Green Version]
- Gierak, J. Focused Ion Beam Technology and Ultimate Applications. Semicond. Sci. Technol. 2009, 24, 043001. [Google Scholar] [CrossRef]
- Toth, M.; Lobo, C.; Friedli, V.; Szkudlarek, A.; Utke, I. Continuum Models of Focused Electron Beam Induced Processing. Beilstein J. Nanotechnol. 2015, 6, 1518–1540. [Google Scholar] [CrossRef] [PubMed]
- Friedli, V.; Wanzenböck, H.D.; Utke, I. Gas Injection Systems for FEB and FIB Processing: Theory and Experiment. In Nanofabrication Using Focused Ion and Electron Beams: Principles and Applications; Utke, I., Moshkalev, S., Russel, P., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 126–183. [Google Scholar]
- Friedli, V.; Utke, I. Optimized Molecule Supply from Nozzle-Based Gas Injection Systems for Focused Electron- and Ion-Beam Induced Deposition and Etching: Simulation and Experiment. J. Phys. D. Appl. Phys. 2009, 42, 125305. [Google Scholar] [CrossRef]
- Verma, N.K.; Khanna, S.K.; Kapila, B. Comprehensive Chemistry Volume I; Laxmi Publications: Delhi, India, 2008. [Google Scholar]
- Sharma, B.K. Objective Pre Engineering Chemistry; Krishna Prakashan: Meerut, India, 2015; ISBN 978-8187224457. [Google Scholar]
- Graham, W.G. Properties of Alkali Metals Adsorbed onto Metal Surfaces. In Proceedings of the Second International Symposium on the Production and Neutralization of Negative Hydrogen Ions and Beams, Upton, NY, USA, 6 October 1980; p. 126. [Google Scholar]
- Ishikawa, J. Negative Ion Sources. In The Physics and Technology of Ion Sources; Brown, I.G., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 285–310. ISBN 3527404104. [Google Scholar]
- Kiejna, A.; Wojciechowski, K.F. Metal Surface Electron Physics. Met. Surf. Electron Phys. 1996, 3–18. [Google Scholar] [CrossRef]
- Andreas Mandelis, C.C. Physics, Chemistry and Technology of Solid State Gas Sensor Devices; John Wiley & Sons, Inc.: New York, NY, USA, 1993; ISBN 978-0-471-55885-9. [Google Scholar]
- Philipp, P.; Wirtz, T.; Migeon, H.N.; Scherrer, H. Electron Work Function Decrease in SIMS Analysis Induced by Neutral Cesium Deposition. Int. J. Mass Spectrom. 2007, 264, 70–83. [Google Scholar] [CrossRef]
- Bernheim, M.; Le Bourse, F. On the Velocity Dependence for Negative Ionization of Atoms Sputtered from Cesiated Surfaces: An Experimental Study. Nucl. Inst. Methods Phys. Res. B 1987, 27, 94–103. [Google Scholar] [CrossRef]
- Bernheim, M.; Slodzian, G. Caesium Flooding on Metal Surfaces and Sputtered Negative Ion Yields. J. Phys. Lett. 1977, 38, 325–328. [Google Scholar] [CrossRef]
- Krohn, V.E. Emission of Negative Ions from Metal Surfaces Bombarded by Positive Cesium Ions. J. Appl. Phys. 1962, 33, 3523–3525. [Google Scholar] [CrossRef]
- Wirtz, T.; Migeon, H.N. Work Function Shifts and Variations of Ionization Probabilities Occurring during SIMS Analyses Using an in Situ Deposition of Cs0. Surf. Sci. 2004, 561, 200–207. [Google Scholar] [CrossRef]
- Wittmaack, K. Unravelling the Secrets of Cs Controlled Secondary Ion Formation: Evidence of the Dominance of Site Specific Surface Chemistry, Alloying and Ionic Bonding. Surf. Sci. Rep. 2013, 68, 108–230. [Google Scholar] [CrossRef]
- Brison, J.; Houssiau, L. Study of Ionization Processes during TOF-SIMS Analysis by Co-Sputtering Cesium and Xenon. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2007, 259, 984–988. [Google Scholar] [CrossRef]
- Brison, J.; Vitchev, R.G.; Houssiau, L. Cesium/Xenon Co-Sputtering at Different Energies during ToF-SIMS Depth Profiling. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2008, 266, 5159–5165. [Google Scholar] [CrossRef]
- Philipp, P.; Wirtz, T.; Migeon, H.N.; Scherrer, H. SIMS Analysis with Neutral Cesium Deposition: Negative Secondary Ion Sensitivity Increase and Quantification Aspects. Int. J. Mass Spectrom. 2006, 253, 71–78. [Google Scholar] [CrossRef]
- Iltgen, K.; Bendel, C.; Benninghoven, A.; Niehuis, E. Optimized Time-of-Flight Secondary Ion Mass Spectroscopy Depth Profiling with a Dual Beam Technique. J. Vac. Sci. Technol. A Vac. Surf. Film. 1997, 15, 460–464. [Google Scholar] [CrossRef]
- Grehl, T.; Mollers, R.; Niehuis, E. Low Energy Dual Beam Depth Profiling: Infuence of Sputter and Analysis Beam Parameters on Profile Performance Using TOF-SIMS. Appl. Surf. Sci. 2003, 203–204, 277–280. [Google Scholar] [CrossRef]
- Cramer, H.G.; Grehl, T.; Kollmer, F.; Moellers, R.; Niehuis, E.; Rading, D. Depth Profiling of Organic Materials Using Improved Ion Beam Conditions. Appl. Surf. Sci. 2008, 255, 966–969. [Google Scholar] [CrossRef]
- Ghumman, C.A.A.; Moutinho, A.M.C.; Santos, A.; Teodoro, O.M.N.D.; Tolstogouzov, A. An Upgraded TOF-SIMS VG Ionex IX23LS: Study on the Negative Secondary Ion Emission of III-V Compound Semiconductors with Prior Neutral Cesium Deposition. Appl. Surf. Sci. 2012, 258, 2490–2497. [Google Scholar] [CrossRef]
- Wirtz, T.; Migeon, H.N. In Situ Deposition of Neutral Cs for Secondary Ion Mass Spectrometry. Appl. Surf. Sci. 2004, 222, 186–197. [Google Scholar] [CrossRef]
- Wirtz, T.; Migeon, H.N. Development of a Column Delivering a Collimated Stream of Cs0 for SIMS Purposes. Appl. Surf. Sci. 2004, 231–232, 940–944. [Google Scholar] [CrossRef]
- Philipp, P.; Wirtz, T.; Migeon, H.N.; Scherrer, H. Important Increase of Negative Secondary Ion Sensitivity during SIMS Analysis by Neutral Cesium Deposition. Appl. Surf. Sci. 2006, 252, 7205–7207. [Google Scholar] [CrossRef]
- Succi, M.; Canino, R.; Ferrario, B. Atomic Absorption Evaporation Flow Rate Measurements of Alkali Metal Dispensers. Vacuum 1985, 35, 579–582. [Google Scholar] [CrossRef]
- Peter, A.; de Paula, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2014; ISBN -13. [Google Scholar]
- Janek, J.; Zeier, W.G. A Solid Future for Battery Development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Dudney, N.J. Solid-State Thin-Film Rechargeable Batteries. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2005, 116, 245–249. [Google Scholar] [CrossRef]
- Whitehead, A.H.; Schreiber, M. Current Collectors for Positive Electrodes of Lithium-Based Batteries. J. Electrochem. Soc. 2005, 152, A2105. [Google Scholar] [CrossRef]
- Roberts, M.; Johns, P.; Owen, J.; Brandell, D.; Edstrom, K.; El Enany, G.; Guery, C.; Golodnitsky, D.; Lacey, M.; Lecoeur, C.; et al. 3D Lithium Ion Batteries—From Fundamentals to Fabrication. J. Mater. Chem. 2011, 21, 9876–9890. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D.S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S.; et al. High-Energy Long-Cycling All-Solid-State Lithium Metal Batteries Enabled by Silver–Carbon Composite Anodes. Nat. Energy 2020, 5, 299–308. [Google Scholar] [CrossRef]
- Senoner, M.; Maaßdorf, A.; Rooch, H.; Österle, W.; Malcher, M.; Schmidt, M.; Kollmer, F.; Paul, D.; Hodoroaba, V.D.; Rades, S.; et al. Lateral Resolution of Nanoscaled Images Delivered by Surface-Analytical Instruments: Application of the BAM-L200 Certified Reference Material and Related ISO Standards. Anal. Bioanal. Chem. 2015, 407, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Aswal, D.K. Recent Advances in Thin Films, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 978-9811561153. [Google Scholar]
- Goswami, A. Thin Film Fundamentals; New Age Publications: New Dehli, India, 1996; ISBN 978-8122408584. [Google Scholar]
- Martin, P.M. Handbook of Deposition Technologies for Films and Coatings: Science, Applications and Technology, 3rd ed.; Elsevier: Oxford, UK, 2010; ISBN 9780815520313. [Google Scholar]
- Xie, T.; Edwards, T.E.J.; Della Ventura, N.; Casari, D.; Huszár, E.; Fu, L.; Zhou, L.; Maeder, X.; Schwierdrzik, J.; Utke, I.; et al. Synthesis of Model Al-Al2O3 Multilayer Systems with Monolayer Oxide Thickness Control by Circumventing Native Oxidation. Thin Solid Films 2020, 711, 138287. [Google Scholar] [CrossRef]
- Zeng, X.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of Lithium Battery Technologies for Electric Vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Andre, D.; Kim, S.J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future Generations of Cathode Materials: An Automotive Industry Perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.H.S.; Banerjee, A.; Chen, Z.; Meng, Y.S. From Nanoscale Interface Characterization to Sustainable Energy Storage Using All-Solid-State Batteries. Nat. Nanotechnol. 2020, 15, 170–180. [Google Scholar] [CrossRef]
- Lewis, J.A.; Tippens, J.; Javier, F.; Cortes, Q.; Mcdowell, M.T. Chemo-Mechanical Challenges in Solid-State Batteries. Trends Chem. 2019, 1, 845–857. [Google Scholar] [CrossRef]
- Xu, L.; Tang, S.; Cheng, Y.; Wang, K.; Liang, J.; Liu, C.; Cao, Y.C.; Wei, F.; Mai, L. Interfaces in Solid-State Lithium Batteries. Joule 2018, 2, 1991–2015. [Google Scholar] [CrossRef] [Green Version]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on Solid Electrolytes for All-Solid-State Lithium-Ion Batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Huang, R.; Ikuhara, Y. STEM Characterization for Lithium-Ion Battery Cathode Materials. Curr. Opin. Solid State Mater. Sci. 2012, 16, 31–38. [Google Scholar] [CrossRef]
- Massonnet, P.; Heeren, R.M.A. A Concise Tutorial Review of TOF-SIMS Based Molecular and Cellular Imaging. J. Anal. At. Spectrom. 2019, 34, 2217–2228. [Google Scholar] [CrossRef]
- Gao, Y.; Marie, Y.; Saldi, F.; Migeon, H.N. On the SIMS Depth Profiling Analysis: Reduction of Matrix Effect. Int. J. Mass Spectrom. Ion Process. 1995, 143, 11–18. [Google Scholar] [CrossRef]
- Brison, J.; Conard, T.; Vandervorst, W.; Houssiau, L. Cesium/Xenon Dual Beam Depth Profiling with TOF-SIMS: Measurement and Modeling of M+, MCs+, and M2Cs2+ Yields. Appl. Surf. Sci. 2004, 231–232, 749–753. [Google Scholar] [CrossRef]
- Kaufmann, K.; Wahl, S.; Meyer, S.; Hagendorf, C. Quantitative Elemental Analysis of Photovoltaic Cu(In,Ga)Se2 Thin Films Using MCs+ Clusters. Surf. Interface Anal. 2013, 45, 434–436. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.H.; Lee, K.-B.; Min, B.K.; Lee, Y. Improved Quantitative Analysis of Cu(In,Ga)Se2 Thin Films Using MCs+-SIMS Depth Profiling. Appl. Phys. A Mater. Sci. Process. 2014, 115, 1355–1364. [Google Scholar] [CrossRef]

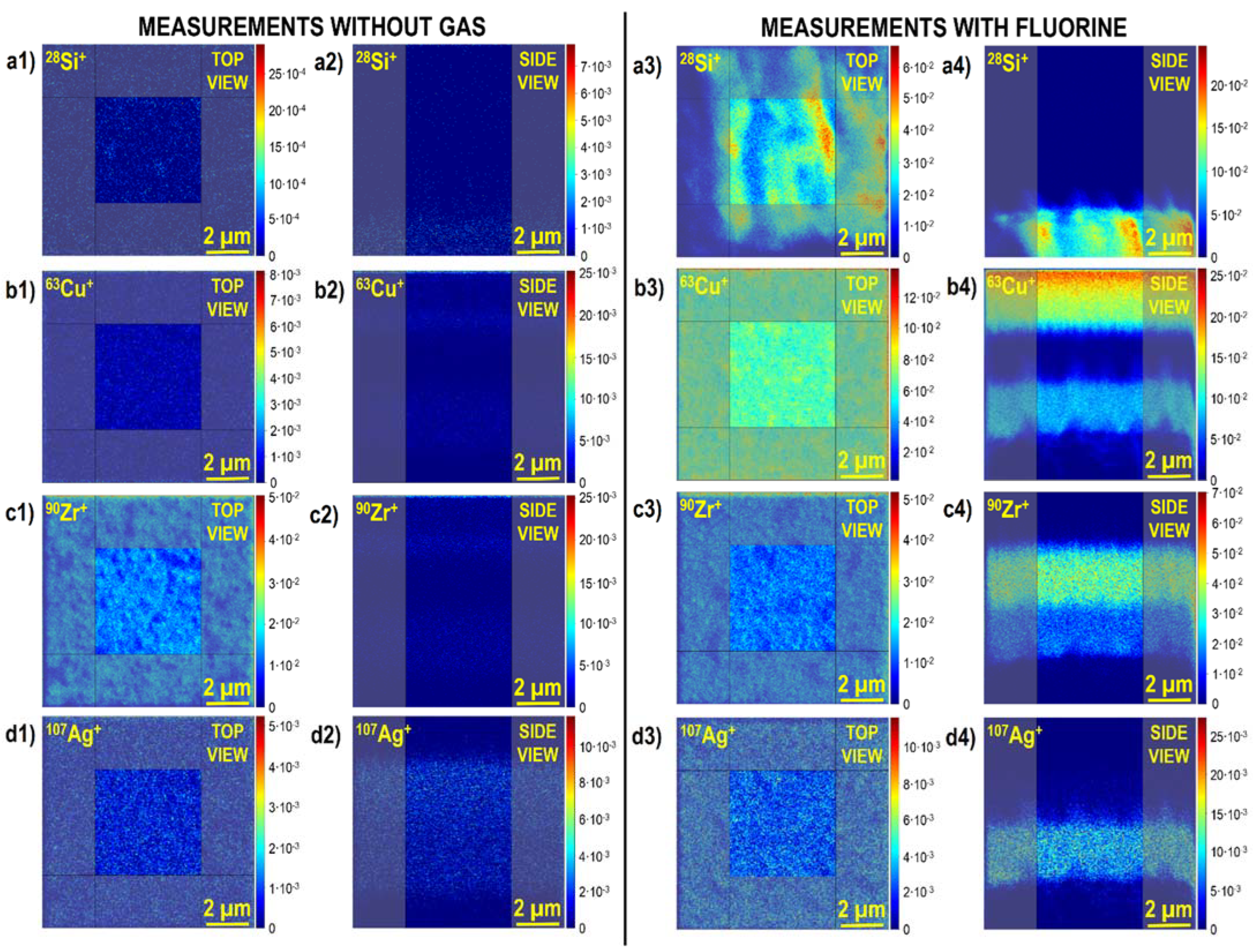
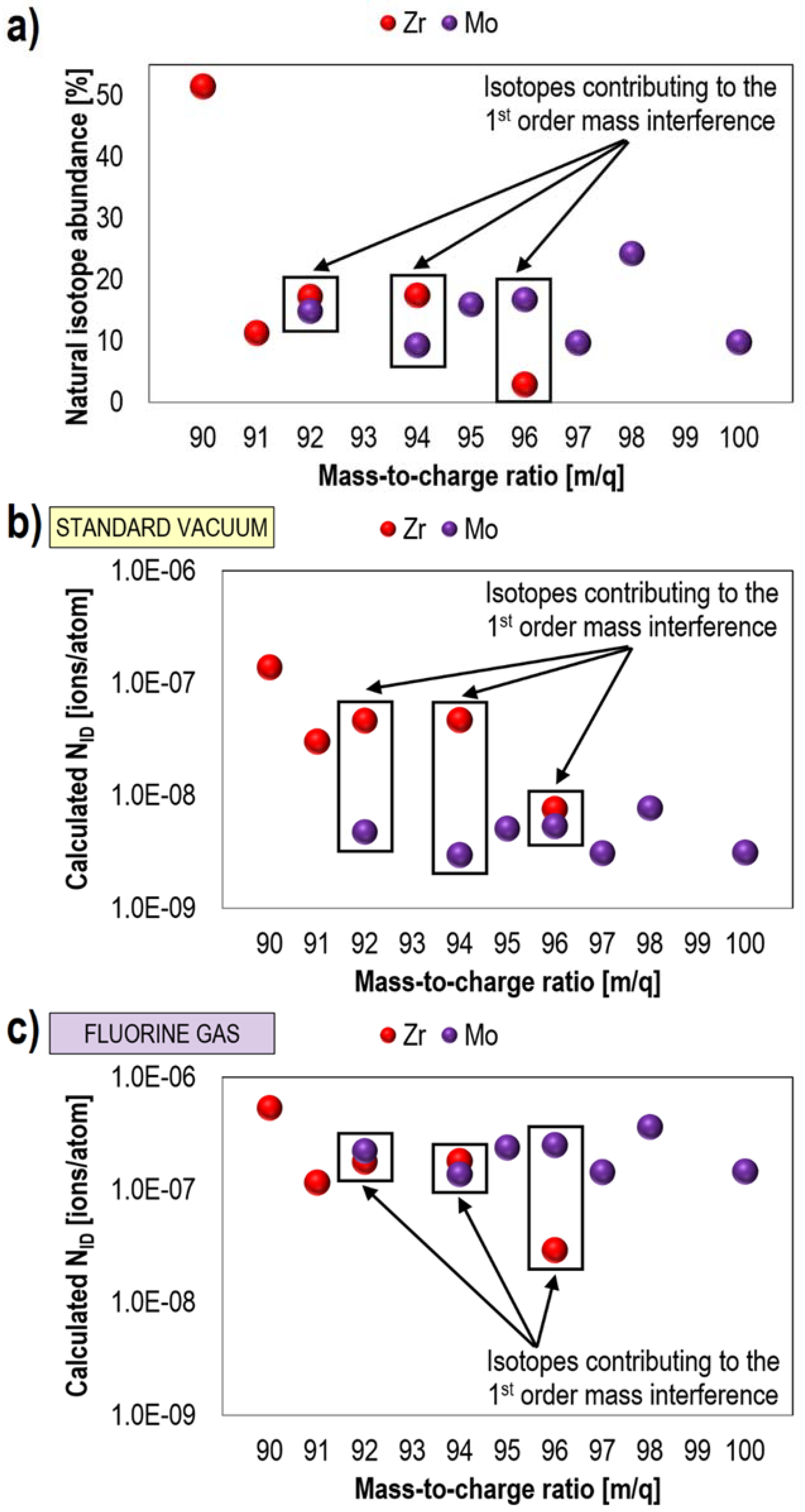
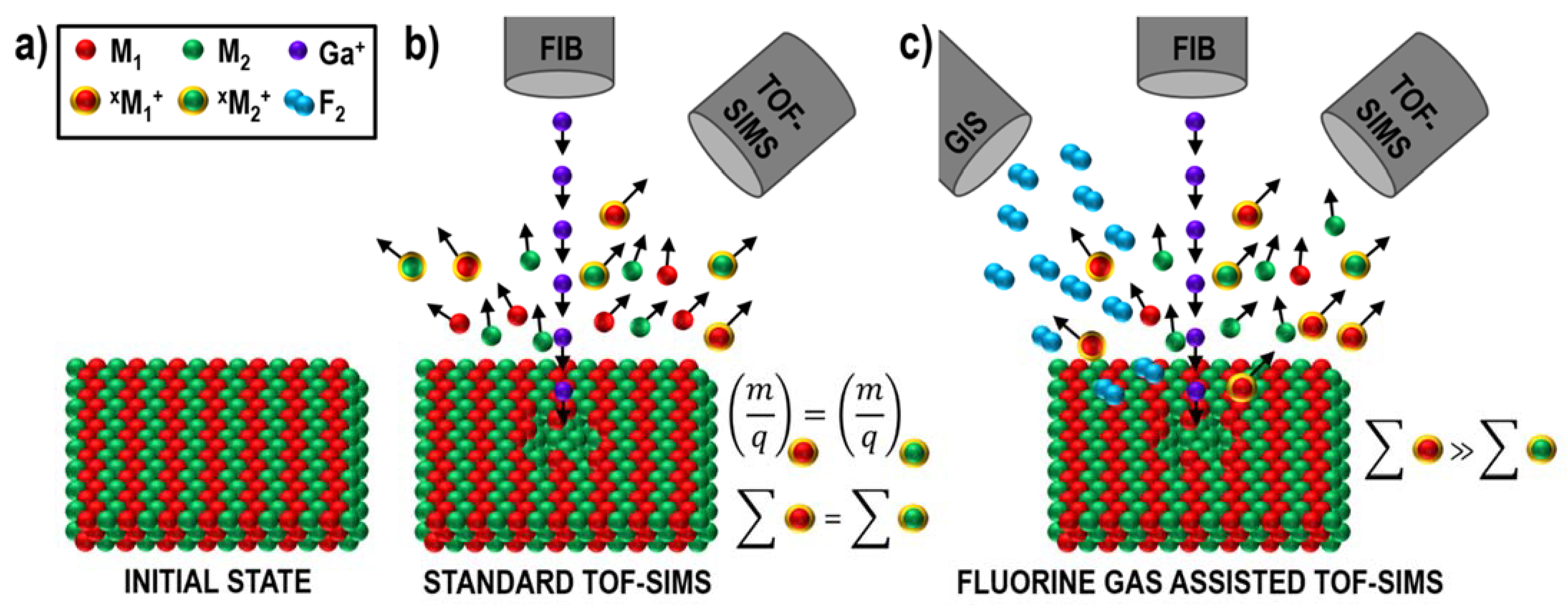

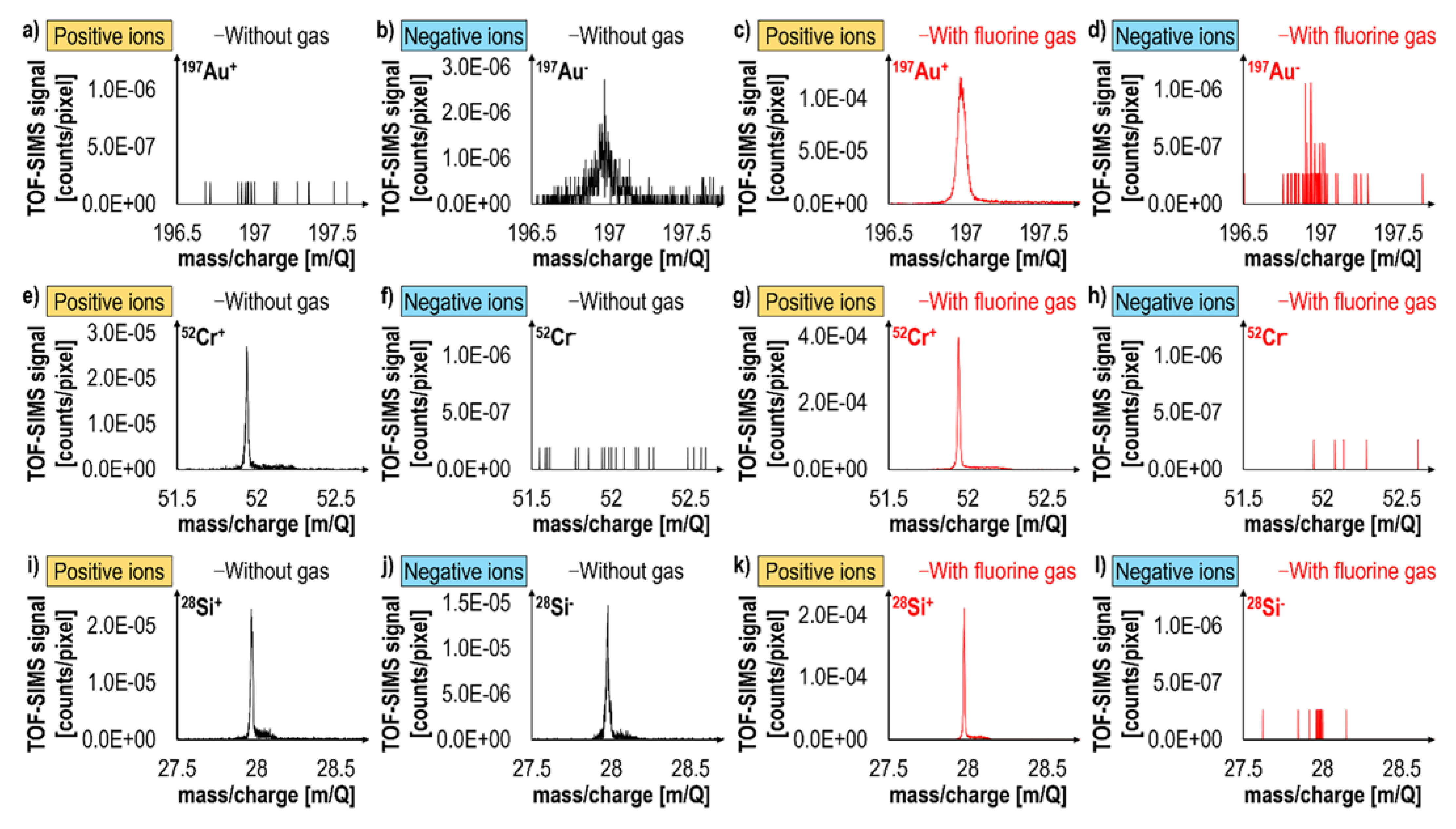

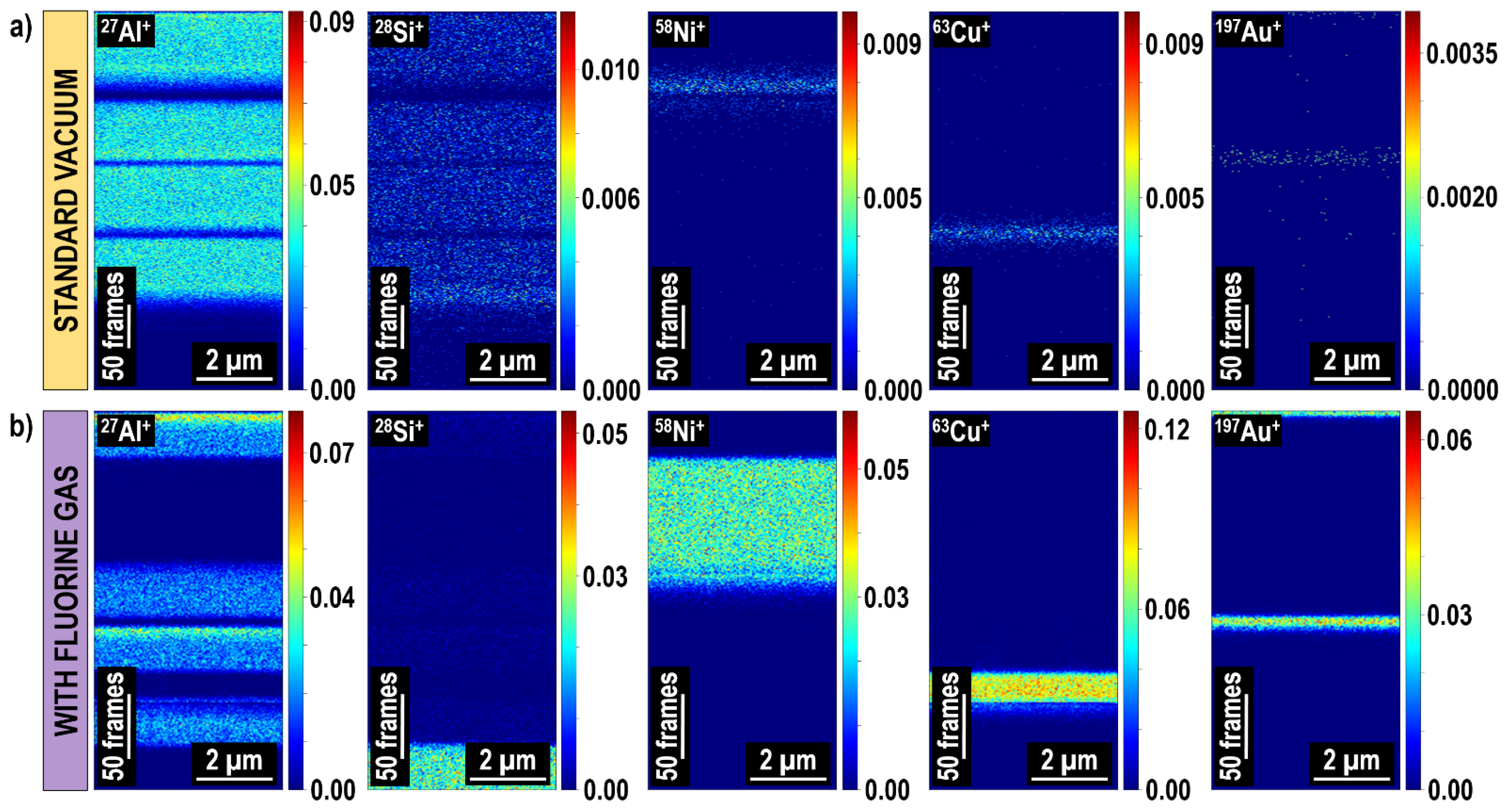
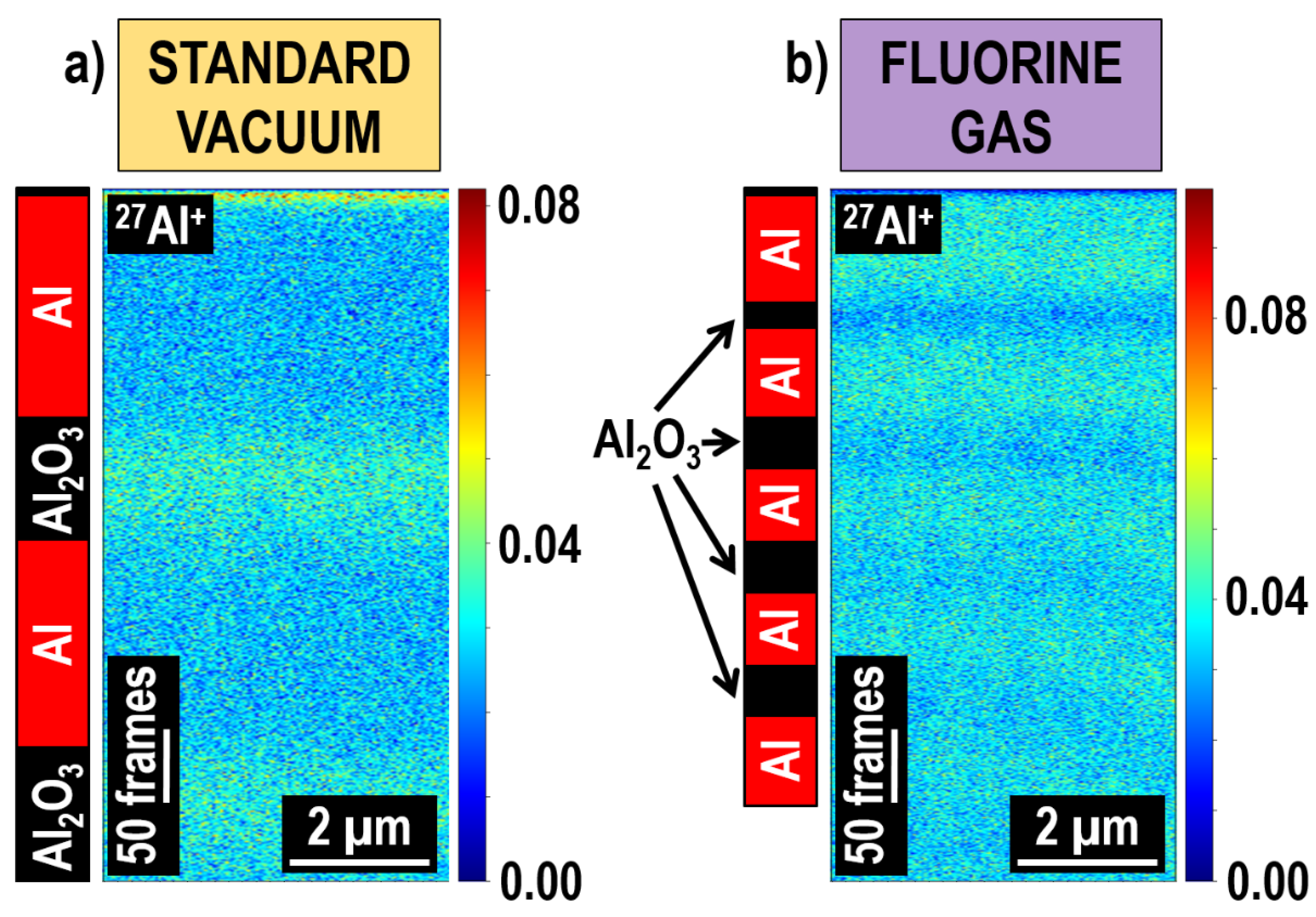
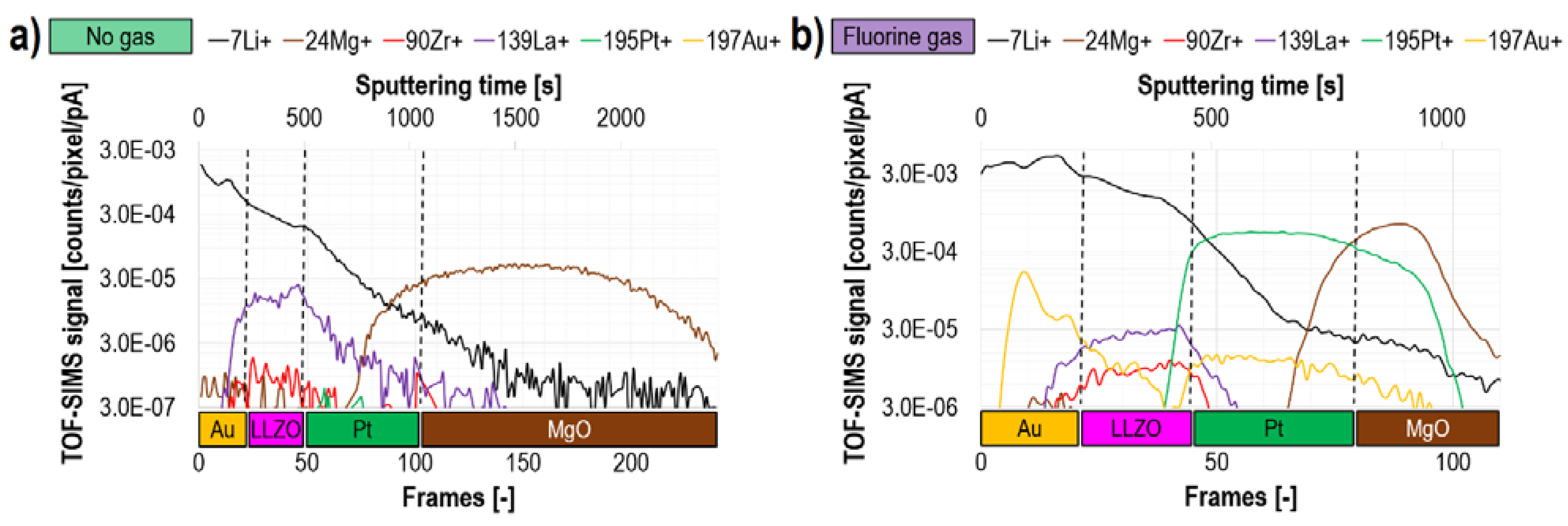

| Advantages | Disadvantages |
|---|---|
|
|
| Element | GAETOF-SIMS,water_vapor [-] | GAETOF-SIMS,fluorine [-] | GAESP,water_vapor [-] | GAESP,fluorine [-] |
|---|---|---|---|---|
| 7Li+ (in Au/Li7La3Zr2O12/Pt/MgO/Si) | (8.9 ± 0.3) (b) [22] (10.9 ± 0.5) (c) [22] | |||
| 24Mg+ (in Au/Li7La3Zr2O12/Pt/MgO/Si) | (5.4 ± 0.2) (b) [22] (8.4 ± 0.5) (c) [22] | |||
| 27Al+ (in Zr Al) | (2.7 ± 0.2) (a) [66] | (2.6 ± 0.3) (a) [66] | (0.54 ± 0.03) a) [66] | (1.9 ± 0.1) (a) [66] |
| 28Si+ (in Au/Cr/SiO2/Si) | (5± 3) (b) [66] (11 ±6) (c) [66] | |||
| 28Si+ (in ZrSi) | (3.4 ± 0.7) (a) [66] | (3.8 ± 0.8) (a) [66] | (3.8 ± 0.8) (a) [66] | (3.8 ± 0.8) (a) [66] |
| 28Si- (in Au/Cr/SiO2/Si) | (0.0062 ± 0.0003) (b) [22] (0.018 ± 0.002) (c) [22] | |||
| 52Cr+ (in Au/Cr/SiO2/Si) | 14 ± 2) (b) [22] (15 ±2) (c) [22] | |||
| 52Cr- (in Au/Cr/SiO2/Si) | (0.31 ± 0.02) (b) [22] (1.37 ± 0.09) (c) [22] | |||
| 63Cu+ | (10 ± 2) (a) [21] | (510 ± 80) (a) [21] | (0.9 ± 0.2) (a) [21] | (0.6 ± 0.2) (a) [21] |
| 63Cu+ (in ZrCu) | (15.1 ± 0.3) (a) [66] | (176 ± 15) (a) [66] | (0.69 ± 0.02) (a) [66] | (1.89 ± 0.08) (a) [66] |
| 90Zr+ | (0.18 ± 0.08) (a) [21] | (0.73 ± 0.03) (a) [21] | (6 ± 2) (a) [21] | (2.3 ± 0.2) (a) [21] |
| 90Zr+ (in ZrAl) | (2.21 ± 0.06) (a) [66] | (1.18 ± 0.06) (a) [66] | (0.54 ± 0.03) (a) [66] | (1.9 ± 0.1) (a) [66] |
| 90Zr+ (in ZrSi) | (1.29 ± 0.07) (a) [66] | (5.34 ± 0.05) (a) [66] | (0.75 ± 0.04) (a) [66] | (1.77 ± 0.08) (a) [66] |
| 90Zr+ (in ZrCu) | (3.1 ± 0.2) (a) [66] | (0.81 ± 0.05) (a) [66] | (0.69 ± 0.02) (a) [66] | (1.89 ± 0.08) (a) [66] |
| 90Zr+ (in Au/Li7La3Zr2O12/Pt/MgO/Si) | (12.4 ± 0.3) (b) [22] (20.7 ± 0.5) (c) [22] | |||
| 107Ag+ | (6.7 ± 0.8) (a) [21] | (350 ± 20) (a) [21] | (1.2 ± 0.2) (a) [21] | (2.1 ± 0.3) (a) [21] |
| 184W+ | (2.1 ± 0.3) (a) [21] | (0.170 ± 0.008) (a) [21] | (1.0 ± 0.1) (a) [21] | (2.3 ± 0.3) (a) [21] |
| 139La+ (in Au/Li7La3Zr2O12/Pt/MgO/Si) | (2.42 ± 0.06) (b) [22] (2.71 ± 0.07) (c) [22] | |||
| 195Pt+ (in Au/Li7La3Zr2O12/Pt/MgO/Si) | (3400 ± 80) (b) [22] (2150 ± 50) (c) [22] | |||
| 197Au+ (in Au/Cr/SiO2/Si) | (1990 ± 70) (b) [22] (630 ± 20) (c) [22] | |||
| 197Au- (in Au/Cr/SiO2/Si) | (0.116 ± 0.005) (b) [22] (0.39 ± 0.01) (c) [22] | |||
| 197Au+ (in Au/Li7La3Zr2O12/Pt/MgO/Si) | (730 ± 20) (b) [22] (257 ± 7) (c) [22] |
| Advantages | Disadvantages |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priebe, A.; Michler, J. Review of Recent Advances in Gas-Assisted Focused Ion Beam Time-of-Flight Secondary Ion Mass Spectrometry (FIB-TOF-SIMS). Materials 2023, 16, 2090. https://doi.org/10.3390/ma16052090
Priebe A, Michler J. Review of Recent Advances in Gas-Assisted Focused Ion Beam Time-of-Flight Secondary Ion Mass Spectrometry (FIB-TOF-SIMS). Materials. 2023; 16(5):2090. https://doi.org/10.3390/ma16052090
Chicago/Turabian StylePriebe, Agnieszka, and Johann Michler. 2023. "Review of Recent Advances in Gas-Assisted Focused Ion Beam Time-of-Flight Secondary Ion Mass Spectrometry (FIB-TOF-SIMS)" Materials 16, no. 5: 2090. https://doi.org/10.3390/ma16052090
APA StylePriebe, A., & Michler, J. (2023). Review of Recent Advances in Gas-Assisted Focused Ion Beam Time-of-Flight Secondary Ion Mass Spectrometry (FIB-TOF-SIMS). Materials, 16(5), 2090. https://doi.org/10.3390/ma16052090






