Wearable Continuous Blood Pressure Monitoring Devices Based on Pulse Wave Transit Time and Pulse Arrival Time: A Review
Abstract
:1. Introduction

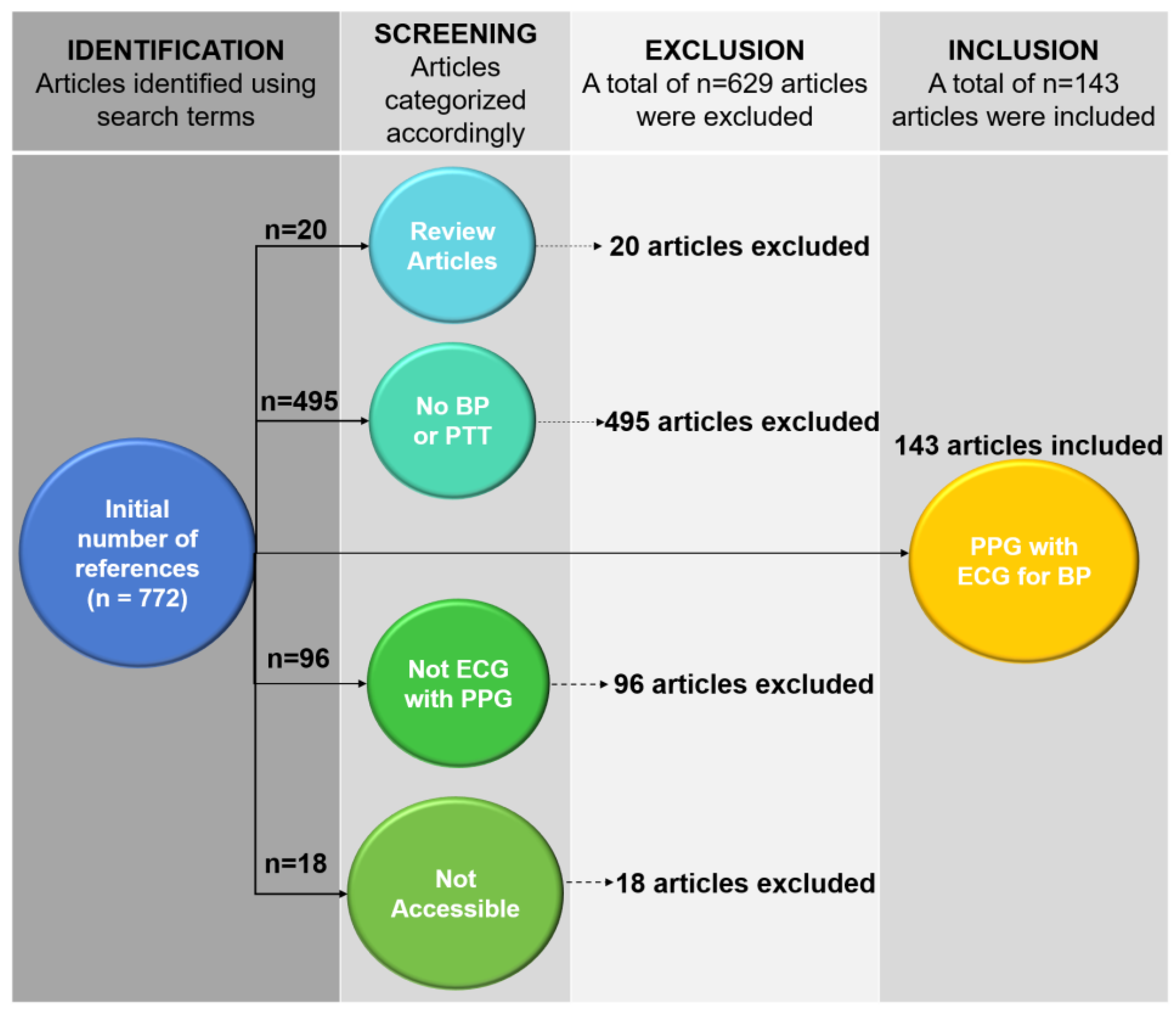
2. PTT-Based Devices for Continuous BP Monitoring
2.1. Definition of Pulse Transit Time (PTT) and Pulse Arrival Time (PAT)
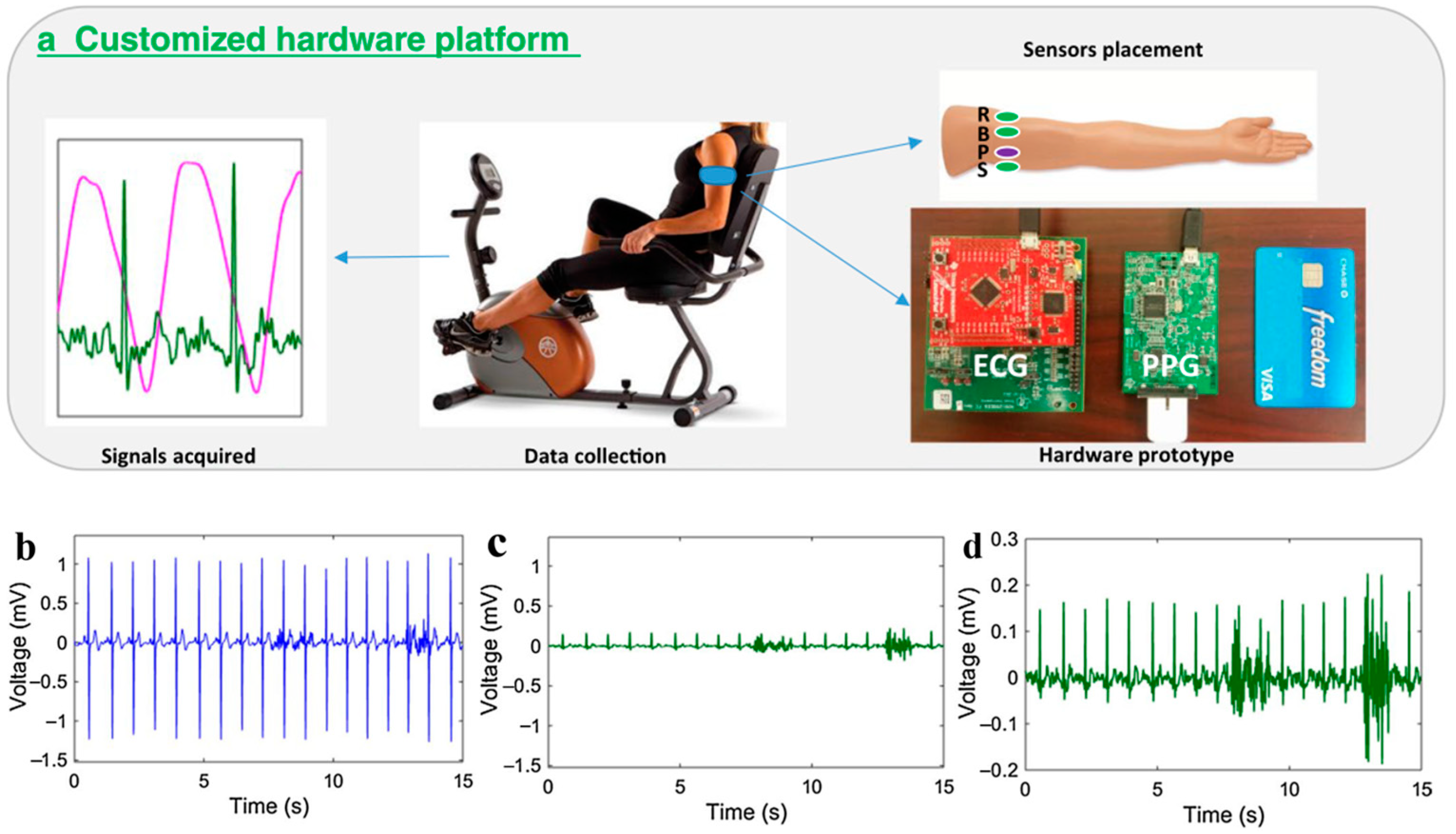
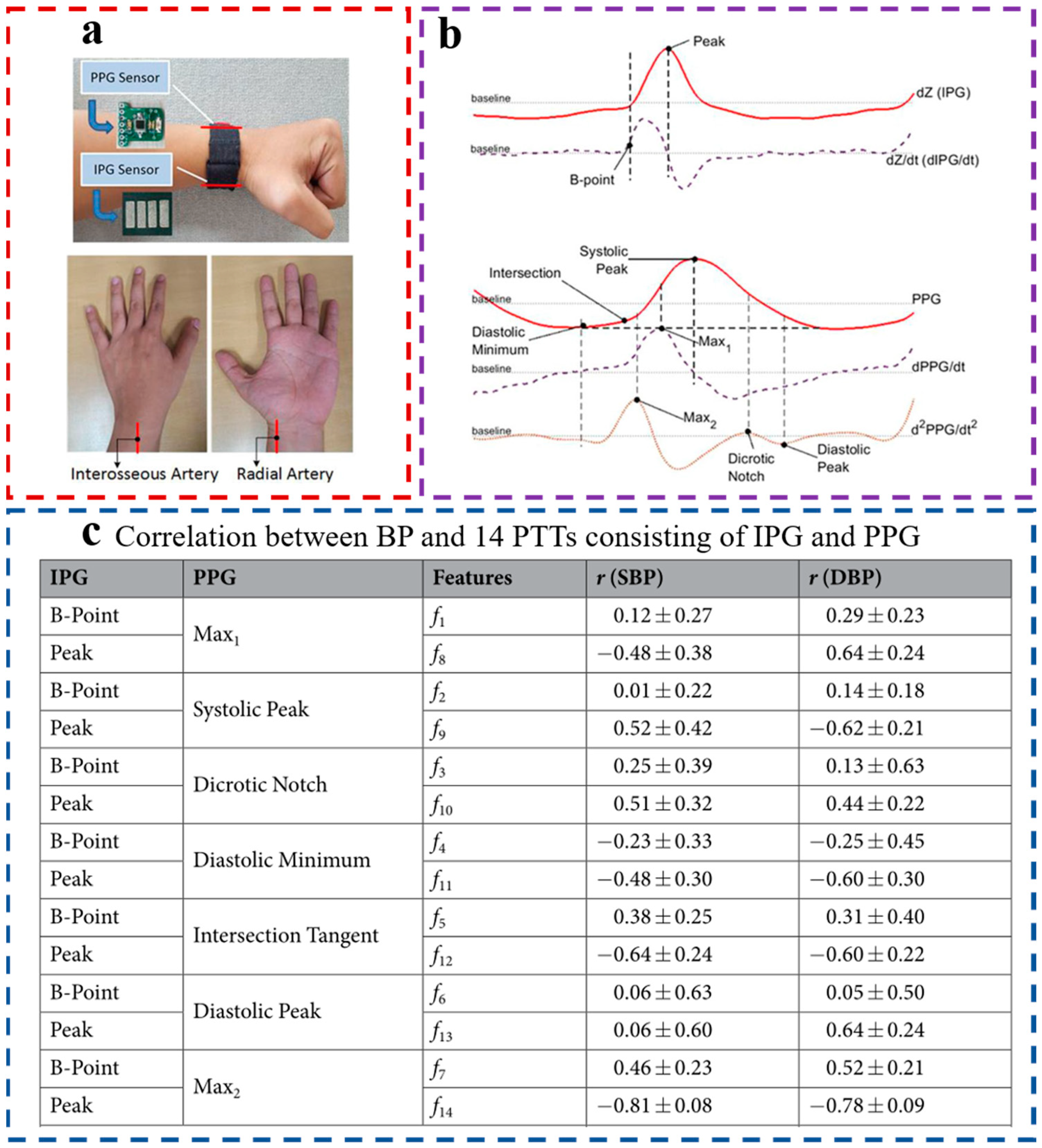
2.2. Acquisition of PTT/PAT
2.3. PPG Sensors
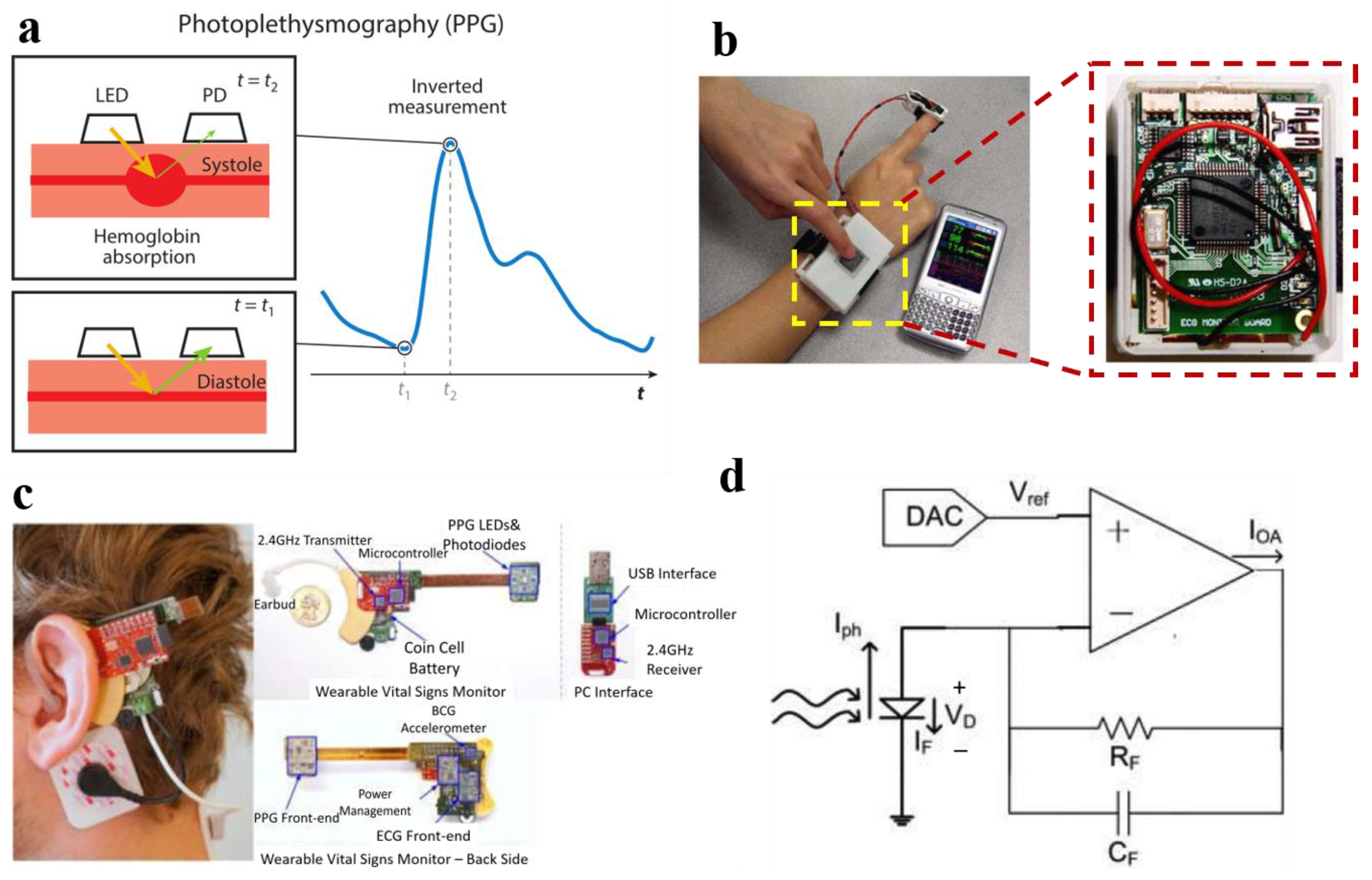
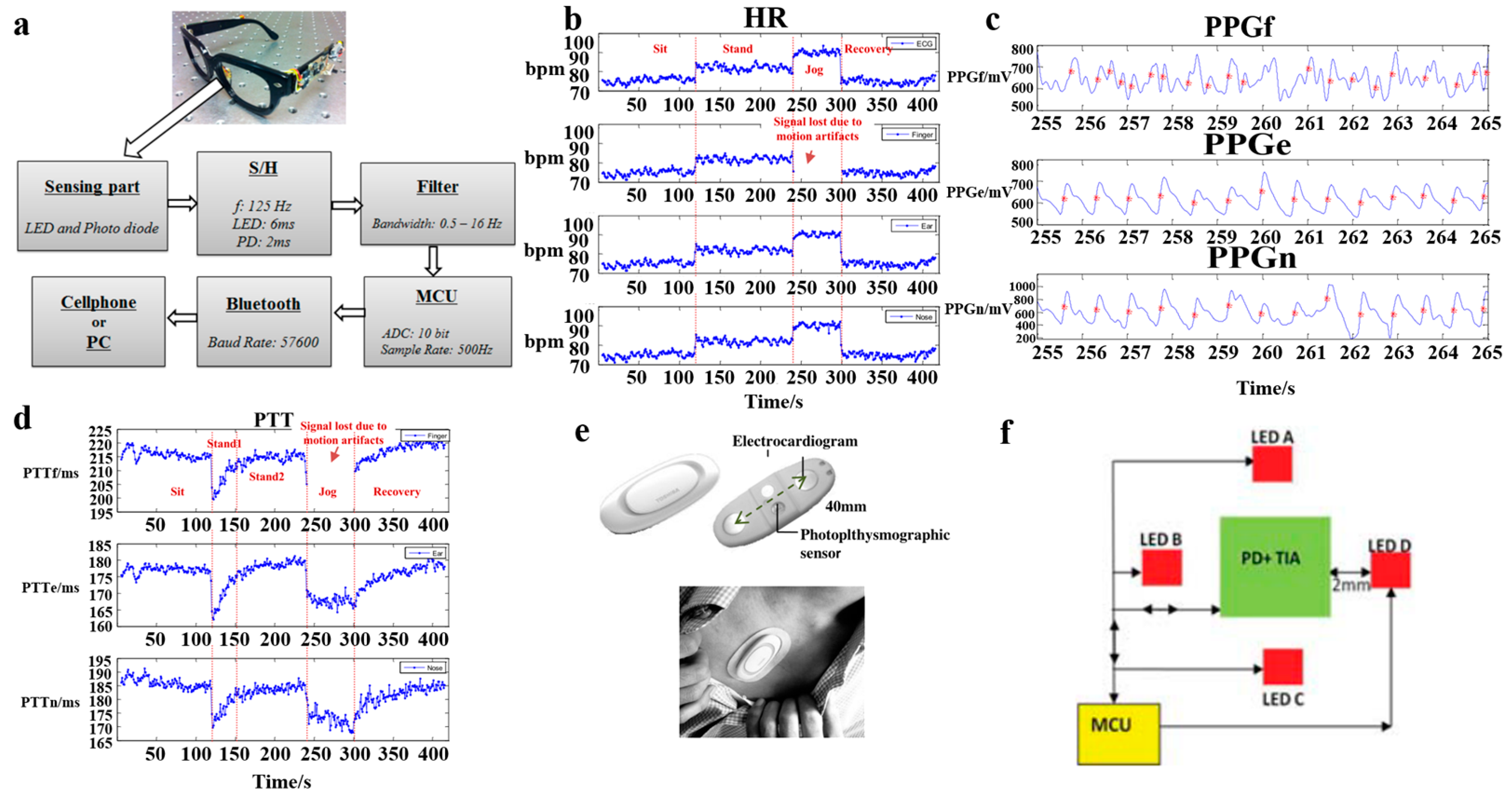
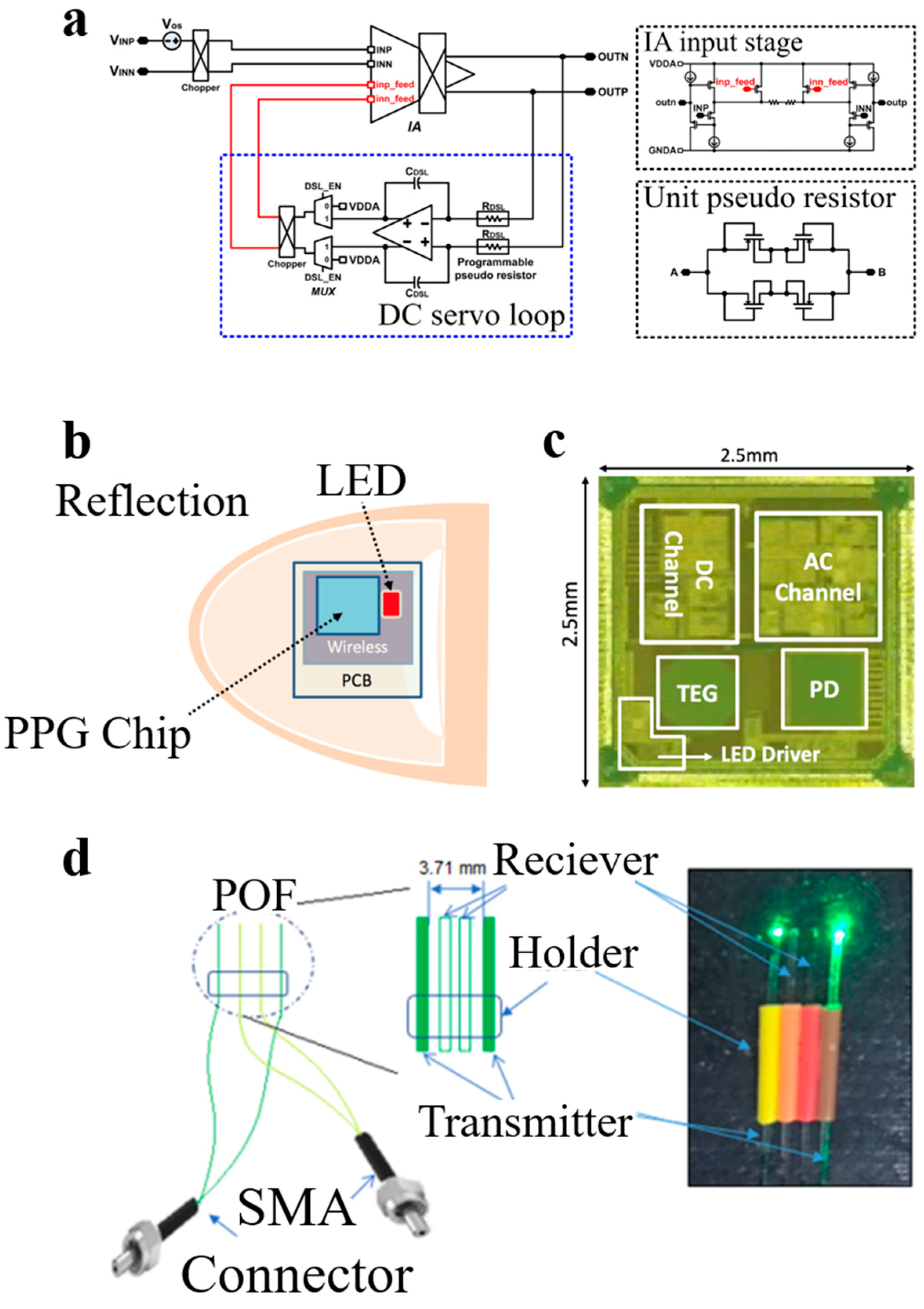
2.4. ECG Sensors
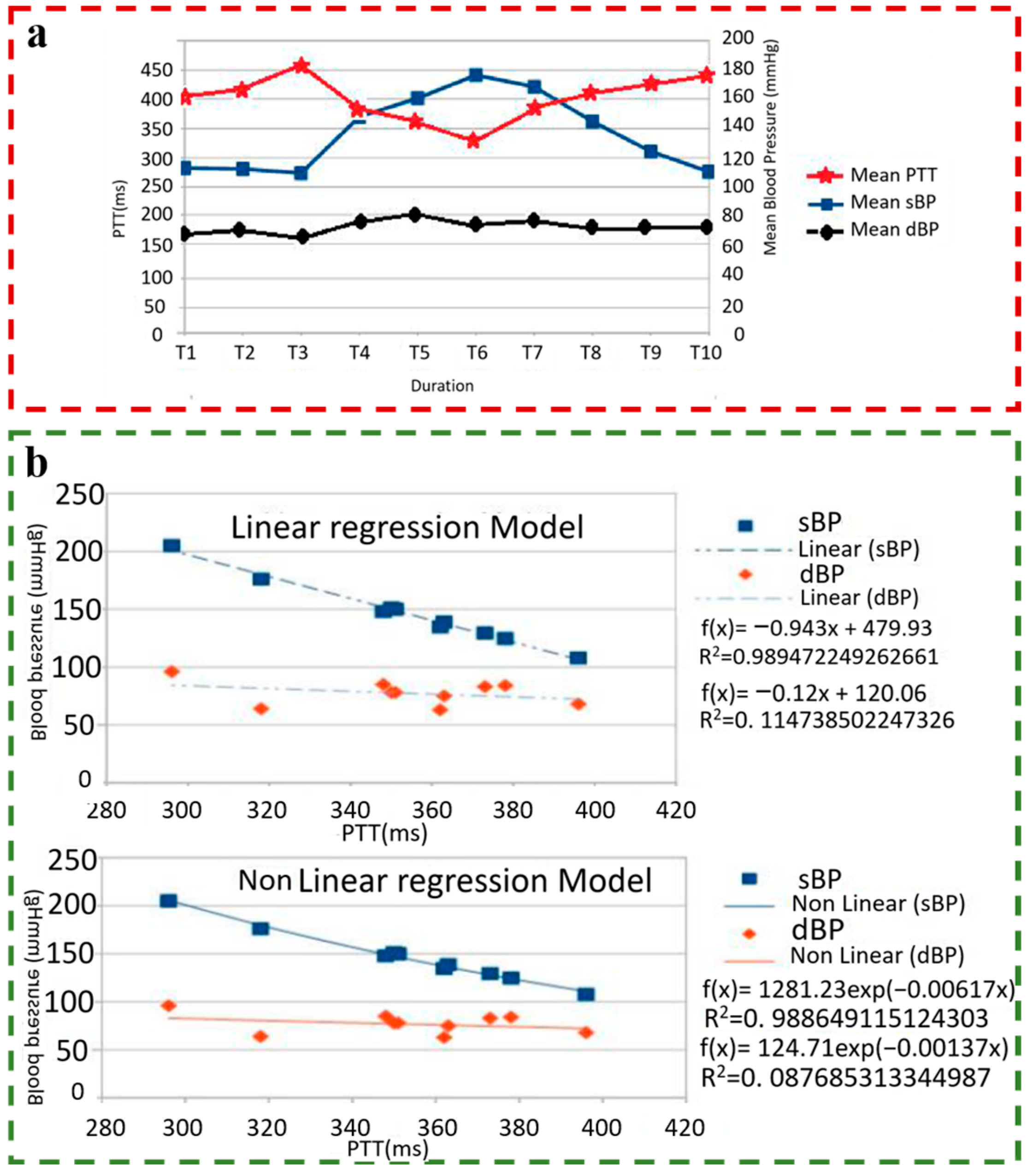
2.5. PTT-Based BP Estimation Model
3. Discussion and Outlook
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.; Chen, Z.; Ward, R.; Elgendi, M. Hypertension Assessment via ECG and PPG Signals: An Evaluation Using MIMIC Database. Diagnostics 2018, 8, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 9 December 2020).
- Krokstad, S. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2022, 399, 520. [Google Scholar]
- Lou, Y.; Ma, W.J.; Wang, Z.J.; Yang, N.; Sun, Y.J.; Liu, Y.L.; Lei, R.B.; Zhao, J.X.; Luo, X.F.; Wang, L.; et al. Writing protocols for the Chinese clinical practice guidelines of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi 2022, 50, 671–675. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Global Brief on Hypertension, Tech. Rep.; WHO: Geneva, Switzerland, 2013; pp. 1–40. Available online: http://apps.who.int/iris/bitstream/10665/79059/1/WHO_DCO_WHD_2013.2_eng.pdf?ua=1 (accessed on 25 June 2013).
- Tegtmeyer, K.; Brady, G.; Lai, S.; Hodo, R.; Braner, D. Placement of an arterial line. N. Engl. J. Med. 2006, 354, e13. [Google Scholar] [CrossRef]
- Figini, V.; Galici, S.; Russo, D.; Centonze, I.; Visintin, M.; Pagana, G. Improving Cuff-Less Continuous Blood Pressure Estimation with Linear Regression Analysis. Electronics 2022, 11, 442. [Google Scholar] [CrossRef]
- He, J.; Ou, J.; He, A.; Shu, L.; Liu, T.; Qu, R.; Xu, X.; Chen, Z.; Yan, Y. A new approach for daily life Blood-Pressure estimation using smart watch. Biomed. Signal Process. Control 2022, 75, 103616. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, Y.-T. Pulse transit time technique for cuffless unobtrusive blood pressure measurement: From theory to algorithm. Biomed. Eng. Lett. 2019, 9, 37–52. [Google Scholar] [CrossRef]
- Paviglianiti, A.; Randazzo, V.; Villata, S.; Cirrincione, G.; Pasero, E. A Comparison of Deep Learning Techniques for Arterial Blood Pressure Prediction. Cogn. Comput. 2022, 14, 1689–1710. [Google Scholar] [CrossRef]
- Scardulla, F.; D’Acquisto, L.; Colombarini, R.; Hu, S.; Pasta, S.; Bellavia, D. A Study on the Effect of Contact Pressure during Physical Activity on Photoplethysmographic Heart Rate Measurements. Sensors 2020, 20, 5052. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H.; et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2018, 2, 687–695. [Google Scholar] [CrossRef]
- Liu, Z.-D.; Liu, J.-K.; Wen, B.; He, Q.-Y.; Li, Y.; Miao, F. Cuffless Blood Pressure Estimation Using Pressure Pulse Wave Signals. Sensors 2018, 18, 4227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kireev, D.; Sel, K.; Ibrahim, B.; Kumar, N.; Akbari, A.; Jafari, R.; Akinwande, D. Continuous cuffless monitoring of arterial blood pressure via graphene bioimpedance tattoos. Nat. Nanotechnol. 2022, 17, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qi, B.; Lin, M.; Zhang, Z.; Makihata, M.; Liu, B.; Zhou, S.; Huang, Y.H.; Hu, H.; Gu, Y.; et al. Continuous monitoring of deep-tissue haemodynamics with stretchable ultrasonic phased arrays. Nat. Biomed. Eng. 2021, 5, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Xiang, W.; Atkinson, I. A computationally efficient CNN-LSTM neural network for estimation of blood pressure from features of electrocardiogram and photoplethysmogram waveforms. Knowl.-Based Syst. 2022, 250, 109151. [Google Scholar] [CrossRef]
- HUAWEI. HUAWEI WATCH D. 2022. Available online: https://consumer.huawei.com/cn/wearables/watch-d/ (accessed on 23 December 2021).
- Samsung. Samsung Galaxy Watch5. 2022. Available online: https://www.samsung.com/cn/watches/galaxy-watch/galaxy-watch5-44mm-sapphire-lte-sm-r9150zbachc/ (accessed on 22 August 2022).
- Wang, P.; Pang, Y.; Wu, B.; Liu, P. Design of non-invasive continuous blood pressure monitoring headband. J. Chongqing Univ. Posts Telecommun. 2015, 27, 250–254. [Google Scholar] [CrossRef]
- Barba, G.; Casullo, C.; Dello Russo, M.; Russo, P.; Nappo, A.; Lauria, F.; Siani, A. Gender-related differences in the relationships between blood pressure, age, and body size in prepubertal children. Am. J. Hypertens. 2008, 21, 1007–1010. [Google Scholar] [CrossRef] [Green Version]
- Barnett, S.R.; Morin, R.J.; Kiely, D.K.; Gagnon, M.; Azhar, G.; Knight, E.L.; Nelson, J.C.; Lipsitz, L.A. Effects of age and gender on autonomic control of blood pressure dynamics. Hypertension 1999, 33, 1195–1200. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Miao, F.; An, Q.; Liu, Z.; Chen, C.; Li, Y. Wearable Multimodal Vital Sign Monitoring Sensor With Fully Integrated Analog Front End. IEEE Sens. J. 2022, 22, 13462–13471. [Google Scholar] [CrossRef]
- Fine, J.; Branan, K.L.; Rodriguez, A.J.; Boonya-ananta, T.; Ajmal; Ramella-Roman, J.C.; McShane, M.J.; Cote, G.L. Sources of Inaccuracy in Photoplethysmography for Continuous Cardiovascular Monitoring. Biosensors 2021, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Ashisha, G.R.; Anitha Mary, X. Advances in Photoplethysmogram and Electrocardiogram Signal Analysis for Wearable Applications. In Intelligence in Big Data Technologies-Beyond the Hype: Proceedings of ICBDCC 2019. Advances in Intelligent Systems and Computing; Peter, J.D., Fernandes, S.L., Alavi, A.H., Eds.; Springer: Singapore, 2021; pp. 527–534. [Google Scholar] [CrossRef]
- Welykholowa, K.; Hosanee, M.; Chan, G.; Cooper, R.; Kyriacou, P.A.; Zheng, D.C.; Allen, J.; Abbott, D.; Menon, C.; Lovell, N.H.; et al. Multimodal Photoplethysmography-Based Approaches for Improved Detection of Hypertension. J. Clin. Med. 2020, 9, 1203. [Google Scholar] [CrossRef]
- Sharma, M.; Rajput, J.S.; Tan, R.S.; Acharya, U.R. Automated Detection of Hypertension Using Physiological Signals: A Review. Int. J. Environ. Res. Public Health 2021, 18, 5838. [Google Scholar] [CrossRef] [PubMed]
- Mukkamala, R.; Stergiou, G.S.; Avolio, A.P. Cuffless Blood Pressure Measurement. Annu. Rev. Biomed. Eng. 2022, 24, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Barvik, D.; Cerny, M.; Penhaker, M.; Noury, N. Noninvasive Continuous Blood Pressure Estimation From Pulse Transit Time: A Review of the Calibration Models. IEEE Rev. Biomed. Eng. 2022, 15, 138–151. [Google Scholar] [CrossRef]
- Dries, D.J. The ICU book, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Volume 27, p. 455. [Google Scholar]
- Perloff, D.; Grim, C.; Flack, J.; Frohlich, E.D.; Hill, M.; McDonald, M.; Morgenstern, B.Z. Human Blood-Pressure Determination by Sphygmomanometry. Circulation 1993, 88, 2460–2470. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Qian, Z.; Yabuki, R.; Du, B.; Kino, H.; Fukushima, T.; Kiyoyama, K.; Tanaka, T. Continuous Peripheral Blood Pressure Measurement with ECG and PPG Signals at Fingertips. In Proceedings of the IEEE Biomedical Circuits and Systems Conference (BioCAS)—Advanced Systems for Enhancing Human Health, Cleveland, OH, USA, 17–19 October 2018; pp. 471–474. [Google Scholar]
- Ding, X.R.; Liu, J.; Dai, W.X.; Carvalho, P.; Magjarevic, R.; Zhang, Y.T. An Attempt to Define the Pulse Transit Time. In Proceedings of the International Conference on Biomedical and Health Informatics (ICBHI), Haikou, China, 8–10 October 2015; pp. 219–221. [Google Scholar]
- Han, Y.; Lin, W.C.; Huang, S.C.; Tsai, C.L.; Lin, K.P. Comparison of Heart Rate Variability and Pulse Rate Variability of Respiratory Control. In Proceedings of the International Conference on Biomedical and Health Informatics (ICBHI), Haikou, China, 8–10 October 2015; pp. 193–197. [Google Scholar]
- Sola, J.; Proenca, M.; Ferrario, D.; Porchet, J.A.; Falhi, A.; Grossenbacher, O.; Allemann, Y.; Rimoldi, S.F.; Sartori, C. Noninvasive and Nonocclusive Blood Pressure Estimation Via a Chest Sensor. IEEE Trans. Biomed. Eng. 2013, 60, 3505–3513. [Google Scholar] [CrossRef]
- Smith, R.P.; Argod, J.; Pepin, J.L.; Levy, P.A. Pulse transit time: An appraisal of potential clinical applications. Thorax 1999, 54, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Karunanithi, M. Monitoring technology for the elderly patient. Expert Rev. Med. Devices 2007, 4, 267–277. [Google Scholar] [CrossRef]
- Gribbin, B.; Steptoe, A.; Sleight, P. Pulse-Wave Velocity as a Measure of Blood-Pressure Change. Psychophysiology 1976, 13, 86–90. [Google Scholar] [CrossRef]
- Obrist, P.A.; Light, K.C.; McCubbin, J.A.; Hutcheson, J.S.; Hoffer, J.L. Pulse Transit-Time—Relationship to Blood-Pressure and Myocardial Performance. Psychophysiology 1979, 16, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Ahlstrom, C.; Johansson, A.; Uhlin, F.; Lanne, T.; Ask, P. Noninvasive investigation of blood pressure changes using the pulse wave transit time: A novel approach in the monitoring of hemodialysis patients. J. Artif. Organs Off. J. Jpn. Soc. Artif. Organs 2005, 8, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, E.; Davidson, S.; Harford, M.; Jorge, J.; Watkinson, P.; Young, D.; Tarassenko, L.; Villarroel, M. Pulse arrival time as a surrogate of blood pressure. Sci. Rep. 2021, 11, 22767. [Google Scholar] [CrossRef] [PubMed]
- Sola, J.; Proenca, M.; Chetelat, O. Wearable PWV technologies to measure Blood Pressure: Eliminating brachial cuffs. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2013, 2013, 4098–4101. [Google Scholar]
- Fung, P.; Dumont, G.; Ries, C.; Mott, C.; Ansermino, M. Continuous Noninvasive blood pressure measurement by pulse transit time. In Proceedings of the 26th Annual International Conference of the IEEE-Engineering-in-Medicine-and-Biology-Society, San Francisco, CA, USA, 1–5 September 2004; pp. 738–741. [Google Scholar]
- Chen, Y.; Wen, C.Y.; Tao, G.C.; Bi, M. Continuous and Noninvasive Measurement of Systolic and Diastolic Blood Pressure by One Mathematical Model with the Same Model Parameters and Two Separate Pulse Wave Velocities. Ann. Biomed. Eng. 2012, 40, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Atef, M.; Li, X.; Guoxing, W.; Yong, L. PTT based continuous time non-invasive blood pressure system. In Proceedings of the IEEE 59th International Midwest Symposium on Circuits and System, Dhabi, United Arab Emirates, 16–19 October 2016. [Google Scholar] [CrossRef]
- Solà, J.; Vetter, R.; Renevey, P.; Chételat, O.; Sartori, C.; Rimoldi, S.F. Parametric estimation of pulse arrival time: A robust approach to pulse wave velocity. Physiol. Meas. 2009, 30, 603. [Google Scholar] [CrossRef]
- Sola, J.; Chetelat, O.; Luprano, J. Continuous Monitoring of Coordinated Cardiovascular Responses. In Proceedings of the 30th Annual International Conference of the IEEE-Engineering-in-Medicine-and-Biology-Society, Vancouver, BC, Canada, 20–24 August 2008; pp. 1423–1426. [Google Scholar]
- Lee, J.; Matsumura, K.; Yamakoshi, K.; Rolfe, P.; Tanaka, S.; Yamakoshi, T. Comparison Between Red, Green and Blue Light Reflection Photoplethysmography for Heart Rate Monitoring During Motion. In Proceedings of the 35th Annual International Conference of the IEEE-Engineering-in-Medicine-and-Biology-Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 1724–1727. [Google Scholar]
- Renevey, P.; Vetter, R.; Krauss, J.; Celka, P.; Depeursinge, Y. Wrist-located pulse detection using IR signals, activity and nonlinear artifact cancellation. In Proceedings of the 23rd Annual International Conference of the IEEE-Engineering-in-Medicine-and-Biology-Society, Istanbul, Turkey, 25–28 October 2001; pp. 3030–3033. [Google Scholar]
- Kim, K.K.; Chee, Y.J.; Lim, Y.G.; Choi, J.W.; Park, K.S. A New Method for Unconstrained Pulse Arrival Time (PAT) Measurement on a Chair. J. Biomed. Eng. Res. 2006, 27, 83–88. [Google Scholar]
- Jung, D.K.; Ha, J.S.; Kang, K.T.; Kim, K.N.; Kim, K.R.; Ye, S.Y.; Ro, J.H.; Jeon, G.R. Development of a Wearable Pulse Transit Time Monitoring Device. Healthc. Inform. Res. 2008, 14, 295–302. [Google Scholar] [CrossRef]
- Espina, J.; Falck, T.; Muehlsteff, J.; Yilin, J.; Adan, M.A.; Aubert, X. Wearable body sensor network towards continuous cuff-less blood pressure monitoring. In Proceedings of the 5th International Summer School and Symposium on Medical Devices and Biosensor, Hong Kong, China, 1–3 June 2008; pp. 28–32. [Google Scholar] [CrossRef]
- Espina, J.; Falck, T.; Muehlsteff, J.; Aubert, X. Wireless body sensor network for continuous cuff-less blood pressure monitoring. In Proceedings of the IEEE/EMBS International Summer School on Medical Devices and Biosensors, Cambridge, MA, USA, 4–6 September 2006. [Google Scholar]
- Lin, X.; Dagang, G.; Tay, F.E.H.; Shusong, X. A wearable vital signs monitoring system for pervasive healthcare. In Proceedings of the IEEE Conference on Sustainable Utilization and Development in Engineering and Technology, Kuala Lumpur, Malaysia, 20–21 November 2010; pp. 86–89. [Google Scholar] [CrossRef]
- Franco, J.; Aedo, J.; Rivera, F. Continuous, Non-invasive and Cuff-free Blood Pressure Monitoring System. 2012. In Proceedings of the VI Andean Region International Conference, Cuenca, Ecuador, 7–9 November 2012; pp. 31–34. [Google Scholar] [CrossRef]
- Winokur, E.S.; Da He, D.; Sodini, C.G. A Wearable Vital Signs Monitor at the Ear for Continuous Heart Rate and Pulse Transit Time Measurements. In Proceedings of the 34th Annual International Conference of the IEEE Engineering-in-Medicine-and-Biology-Society (EMBS), San Diego, CA, USA, 28 August–1 September 2012; pp. 2724–2727. [Google Scholar]
- Zheng, Y.; Leung, B.; Sy, S.; Zhang, Y.; Poon, C.C.Y. A Clip-free Eyeglasses-based Wearable Monitoring Device for Measuring Photoplethysmograhic Signals. In Proceedings of the 34th Annual International Conference of the IEEE Engineering-in-Medicine-and-Biology-Society (EMBS), San Diego, CA, USA, 28 August–1 September 2012; pp. 5022–5025. [Google Scholar]
- Fuke, S.; Suzuki, T.; Nakayama, K.; Tanaka, H.; Minami, S. Blood pressure estimation from pulse wave velocity measured on the chest. In Proceedings of the 35th Annual International Conference of the IEEE-Engineering-in-Medicine-and-Biology-Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 6107–6110. [Google Scholar]
- Ruedi, P.F.; Bishof, A.; Augustyniak, M.K.; Persechini, P.; Nagel, J.L.; Pons, M.; Emery, S.; Chetelat, O. Ultra low power microelectronics for wearable and medical devices. In Proceedings of the 20th Conference and Exhibition on Design, Automation and Test in Europe (DATE), EPFL Campus, Lausanne, Switzerland, 27–31 March 2017; pp. 1426–1431. [Google Scholar]
- Kim, H.; Park, Y.; Ko, Y.; Mun, Y.; Lee, S.; Ko, H. Biosignal integrated circuit with simultaneous acquisition of ECG and PPG for wearable healthcare applications. Technol. Health Care 2018, 26, 3–9. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Ko, H. Low-Power Photoplethysmogram Acquisition Integrated Circuit with Robust Light Interference Compensation. Sensors 2016, 16, 46. [Google Scholar] [CrossRef]
- Qian, Z.Y.; Takezawa, Y.; Shimokawa, K.; Kino, H.; Fukushima, T.; Kiyoyama, K.; Tanaka, T. Development of integrated photoplethysmographic recording circuit for trans-nail pulse-wave monitoring system. Jpn. J. Appl. Phys. 2018, 57, 04FM11. [Google Scholar] [CrossRef]
- Zaki, W.S.W.; Correia, R.; Korposh, S.; Hayes-Gill, B.R.; Morgan, S.P. Development of Wearable Optical-based Fibre Sensor System for Pulse Transit Time Measurement. In Proceedings of the 2nd International Conference on Applied Photonics and Electronics (InCape), Putrajaya, Malaysia, 22 August 2020. [Google Scholar]
- Vilarinho, D.; Theodosiou, A.; Leitao, C.; Leal, A.G.; Domingues, M.D.; Kalli, K.; Andre, P.; Antunes, P.; Marques, C. POFBG-Embedded Cork Insole for Plantar Pressure Monitoring. Sensors 2017, 17, 2924. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Wu, F.; Qiu, C.; Redoute, J.-M.; Yuce, M.R. A Rigid-Flex Wearable Health Monitoring Sensor Patch for IoT-Connected Healthcare Applications. IEEE Internet Things J. 2020, 7, 6932–6945. [Google Scholar] [CrossRef]
- Bhagat, Y.A.; Das, K.; Bui, T. Where the silicone meets the wrist: Direct over molding of biometric sensors on a wearable band. In Smart Biomedical and Physiological Sensor Technology XVIII; SPIE: Bellingham, WA, USA, 2021; p. 117570D. [Google Scholar] [CrossRef]
- Ganti, V.G.; Carek, A.M.; Nevius, B.N.; Heller, J.A.; Etemadi, M.; Inan, O.T. Wearable Cuff-Less Blood Pressure Estimation at Home via Pulse Transit Time. IEEE J. Biomed. Health Inform. 2021, 25, 1926–1937. [Google Scholar] [CrossRef] [PubMed]
- Pardoe, D.; Stone, P. Boosting for Regression Transfer. In Proceedings of the International Conference on Machine Learning. Haifa, Israel, 21–24 June 2010. [Google Scholar]
- Buxi, D.; Redoute, J.M.; Yuce, M.R. A survey on signals and systems in ambulatory blood pressure monitoring using pulse transit time. Physiol. Meas. 2015, 36, R1–R26. [Google Scholar] [CrossRef]
- Yoon, Y.; Cho, J.H.; Yoon, G. Non-constrained Blood Pressure Monitoring Using ECG and PPG for Personal Healthcare. J. Med. Syst. 2009, 33, 261–266. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, D.; Zeng, X. Highly wearable cuff-less blood pressure and heart rate monitoring with single-arm electrocardiogram and photoplethysmogram signals. Biomed. Eng. Online 2017, 16, 23. [Google Scholar] [CrossRef] [Green Version]
- Mishra, B.; Arora, N.; Vora, Y. An ECG-PPG Wearable Device for Real Time Detection of Various Arrhythmic Cardiovascular Diseases. In Proceedings of the 9th IEEE International Symposium on Embedded Computing and System Design (ISED), Amrita Vishwa Vidyapeetham, Kollam, India, 13–14 December 2019; pp. 23–27. [Google Scholar]
- Rachim, V.P.; Chung, W.-Y. Multimodal Wrist Biosensor for Wearable Cuff-less Blood Pressure Monitoring System. Sci. Rep. 2019, 9, 7947. [Google Scholar] [CrossRef] [Green Version]
- Bote, J.M.; Recas, J.; Hermida, R. Evaluation of blood pressure estimation models based on pulse arrival time. Comput. Electr. Eng. 2020, 84, 106616. [Google Scholar] [CrossRef]
- Kim, T.; Park, J.; Sohn, J.; Cho, D.; Jeon, S. Bioinspired, Highly Stretchable, and Conductive Dry Adhesives Based on 1D-2D Hybrid Carbon Nanocomposites for All-in-One ECG Electrodes. Acs Nano 2016, 10, 4770–4778. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Luo, M.; He, P.; Yang, J. Washable and flexible screen printed graphene electrode on textiles for wearable healthcare monitoring. J. Phys. D-Appl. Phys. 2020, 53, 125402. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suvarnaphaet, P.; Sasivimolkul, S.; Sukkasem, C.; Pukesamsombut, D.; Tanadchangsaeng, N.; Boonyagul, S.; Pechprasarn, S. Biodegradable Electrode patch made of Graphene/PHA for ECG detecting Applications. In Proceedings of the 2019 12th Biomedical Engineering International Conference, Ubon Ratchathani, Thailand, 19–22 November 2019. [Google Scholar] [CrossRef]
- Hsiao, C.-C.; Kuo, H.-C.; Lee, R.-G.; Lin, R. Effects of Pulse Transit Time and Physiological Differences on Wearable Device based Blood Pressure Estimation. In Proceedings of the International Automatic Control Conference (CACS), Taichung, Taiwan, 9–11 November 2016; pp. 242–246. [Google Scholar]
- Aboughaly, A.A.; Iqbal, D.; Abd El Ghany, M.A.; Hofmann, K. NICBPM: Non-invasive cuff-less blood pressure monitor. In Proceedings of the 29th International Conference on Microelectronics 2017; IEEE: Piscataway, NJ, USA, 2017; 4p. [Google Scholar]
- Lu, Y.; Peng, H.; Zhao, J.; Deng, Z.; Huang, Z.; Zhang, J.; Deng, J.; Wang, Z.; Wei, C. Ubiquitous Blood Pressure Monitoring Using EEG and PPG Signals. In Proceedings of the ACM International Joint Conference on Pervasive and Ubiquitous Computing (UBICOMP)/ACM International Symposium on Wearable Computers (ISWC), Maui, HI, USA, 11–15 September 2017; pp. 257–260. [Google Scholar]
- Ebrahim, M.P.; Heydari, F.; Wu, T.; Walker, K.; Joe, K.; Redoute, J.-M.; Yuce, M.R. Blood Pressure Estimation Using On-body Continuous Wave Radar and Photoplethysmogram in Various Posture and Exercise Conditions. Sci. Rep. 2019, 9, 16346. [Google Scholar] [CrossRef] [Green Version]
- Landry, C.; Hedge, E.T.; Hughson, R.L.; Peterson, S.D.; Arami, A. Accurate Blood Pressure Estimation During Activities of Daily Living: A Wearable Cuffless Solution. IEEE J. Biomed. Health Inform. 2021, 25, 2510–2520. [Google Scholar] [CrossRef] [PubMed]
- Mukkamala, R.; Hahn, J.-O.; Inan, O.T.; Mestha, L.K.; Kim, C.-S.; Toreyin, H.; Kyal, S. Toward Ubiquitous Blood Pressure Monitoring via Pulse Transit Time: Theory and Practice. IEEE Trans. Biomed. Eng. 2015, 62, 1879–1901. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.-R.; Zhao, N.; Yang, G.-Z.; Pettigrew, R.I.; Lo, B.; Miao, F.; Li, Y.; Liu, J.; Zhang, Y.-T. Continuous Blood Pressure Measurement From Invasive to Unobtrusive: Celebration of 200th Birth Anniversary of Carl Ludwig. IEEE J. Biomed. Health Inform. 2016, 20, 1455–1465. [Google Scholar] [CrossRef]
- Sharma, M.; Barbosa, K.; Ho, V.; Griggs, D.; Ghirmai, T.; Krishnan, S.K.; Hsiai, T.K.; Chiao, J.C.; Cao, H. Cuff-Less and Continuous Blood Pressure Monitoring: A Methodological Review. Technologies 2017, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Liu, J.; Zhang, J.; Zhou, G.; Luo, N.; Zhao, N. Flexible Organic/Inorganic Hybrid Near-Infrared Photoplethysmogram Sensor for Cardiovascular Monitoring. Adv. Mater. 2017, 29, 1700975. [Google Scholar] [CrossRef] [PubMed]
- Cano, J.; Hornero, F.; Quesada, A.; Martinez-Rodrigo, A.; Alcaraz, R.; Rieta, J.J. Improved Discrimination Between Healthy and Hypertensive Individuals Combining Photoplethysmography and Electrocardiography. In Proceedings of the Conference on Computing in Cardiology (CinC), Brno, Czech Republic, 12–15 September 2021. [Google Scholar]
- Dunn, J.; Runge, R.; Snyder, M. Wearables and the medical revolution. Pers. Med. 2018, 15, 429–448. [Google Scholar] [CrossRef] [Green Version]
- Jonathan, E.; Leahy, M. Investigating a smartphone imaging unit for photoplethysmography. Physiol. Meas. 2010, 31, N79–N83. [Google Scholar] [CrossRef]
- Landreani, F.; Caiani, E.G. Smartphone accelerometers for the detection of heart rate. Expert Rev. Med. Devices 2017, 14, 935–948. [Google Scholar] [CrossRef]
- Yousefian, P.; Shin, S.; Mousavi, A.S.; Tivay, A.; Kim, C.-S.; Mukkamala, R.; Jang, D.-G.; Ko, B.H.; Lee, J.; Kwon, U.-K.; et al. Pulse Transit Time-Pulse Wave Analysis Fusion Based on Wearable Wrist Ballistocardiogram for Cuff-Less Blood Pressure Trend Tracking. IEEE Access 2020, 8, 138077–138087. [Google Scholar] [CrossRef]
- Pandian, P.S.; Mohanavelu, K.; Safeer, K.P.; Kotresh, T.M.; Shakunthala, D.T.; Gopal, P.; Padaki, V.C. Smart Vest: Wearable multi-parameter remote physiological monitoring system. Med. Eng. Phys. 2008, 30, 466–477. [Google Scholar] [CrossRef] [PubMed]

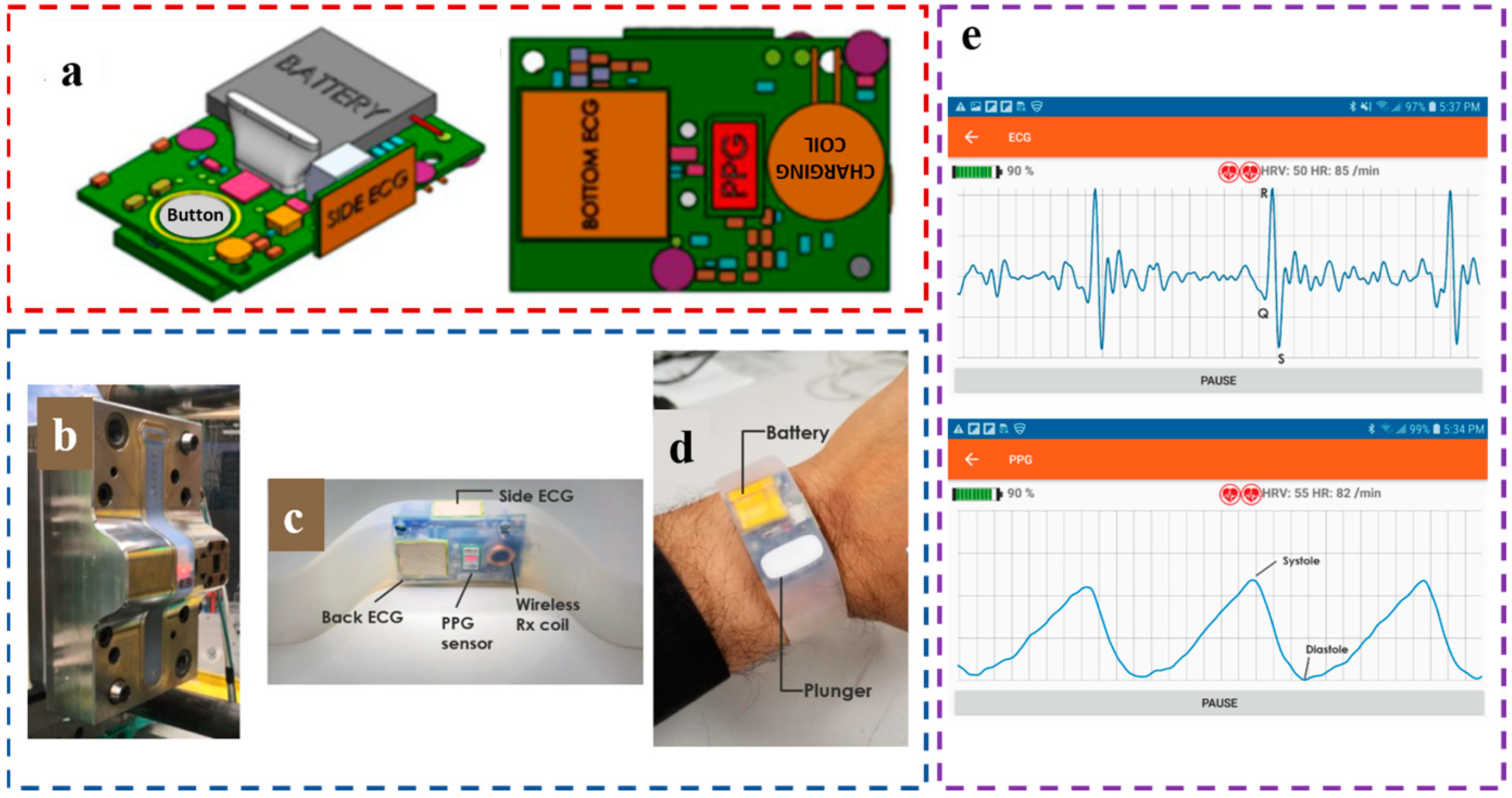
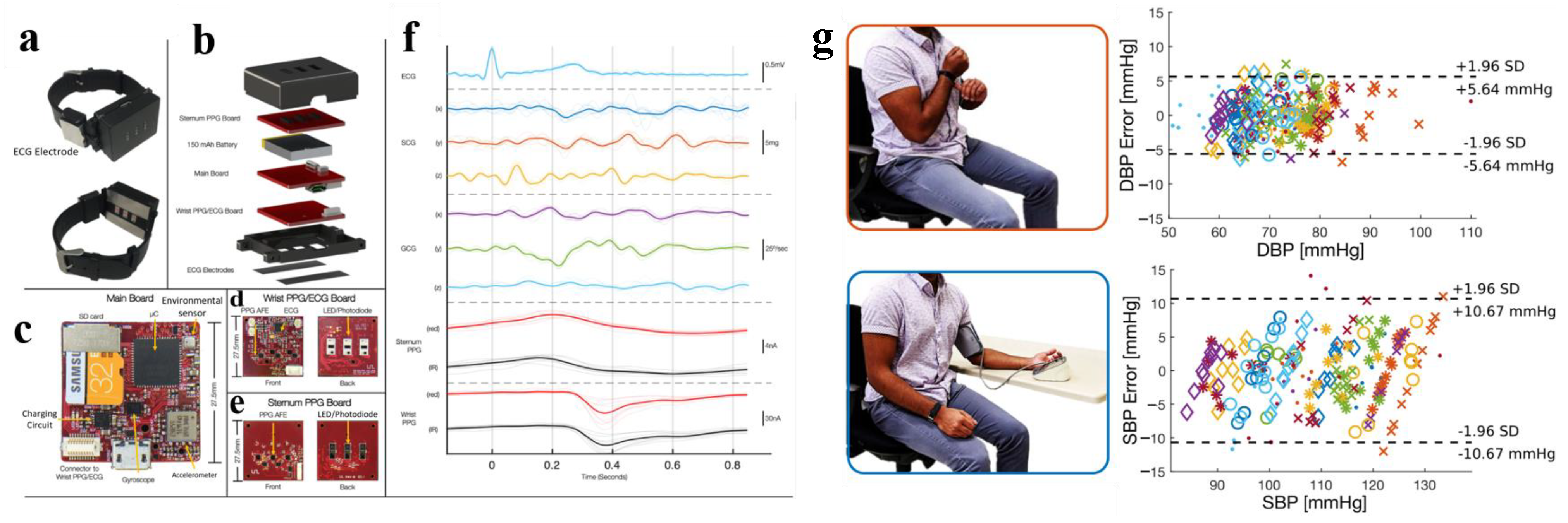

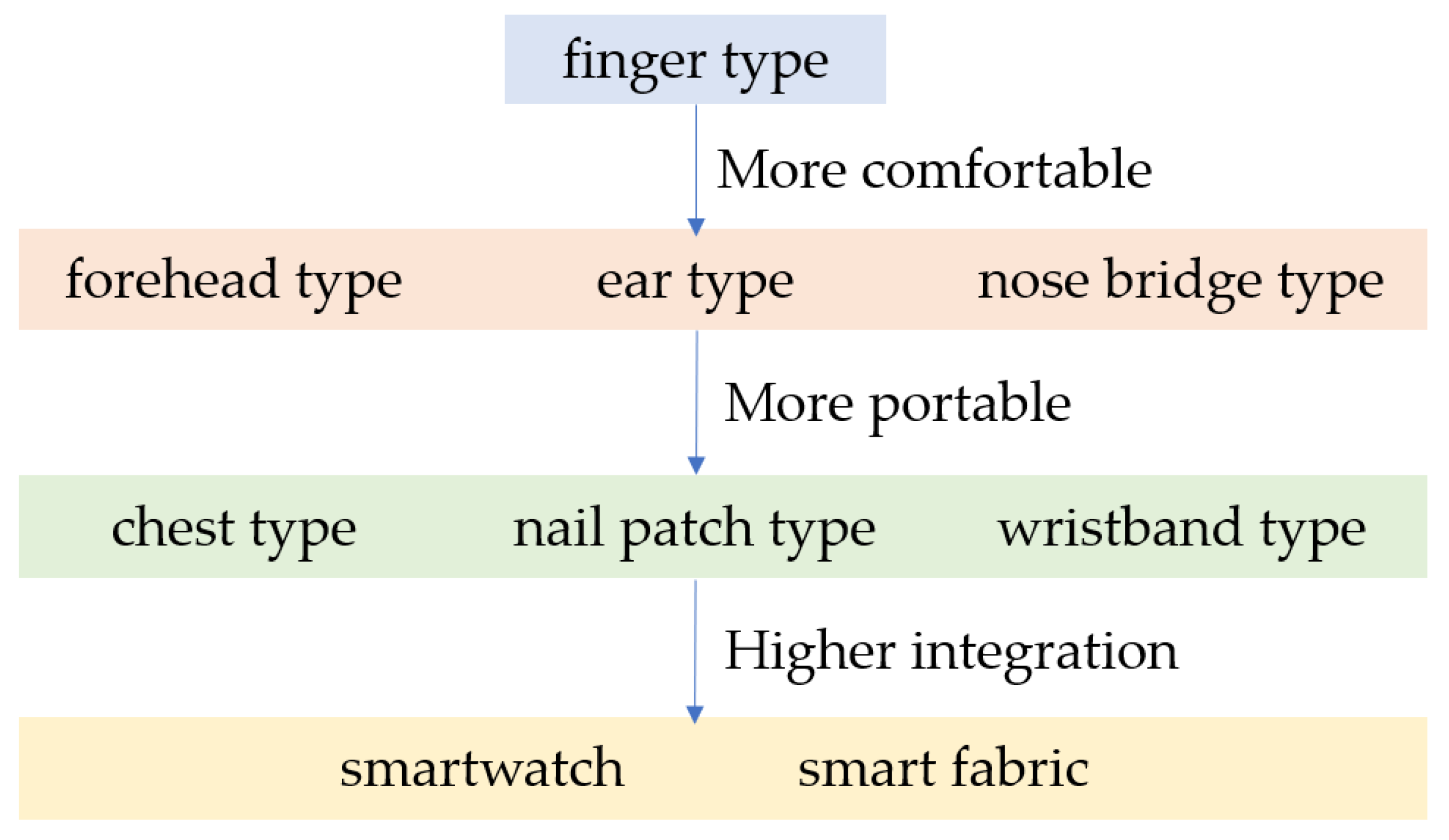
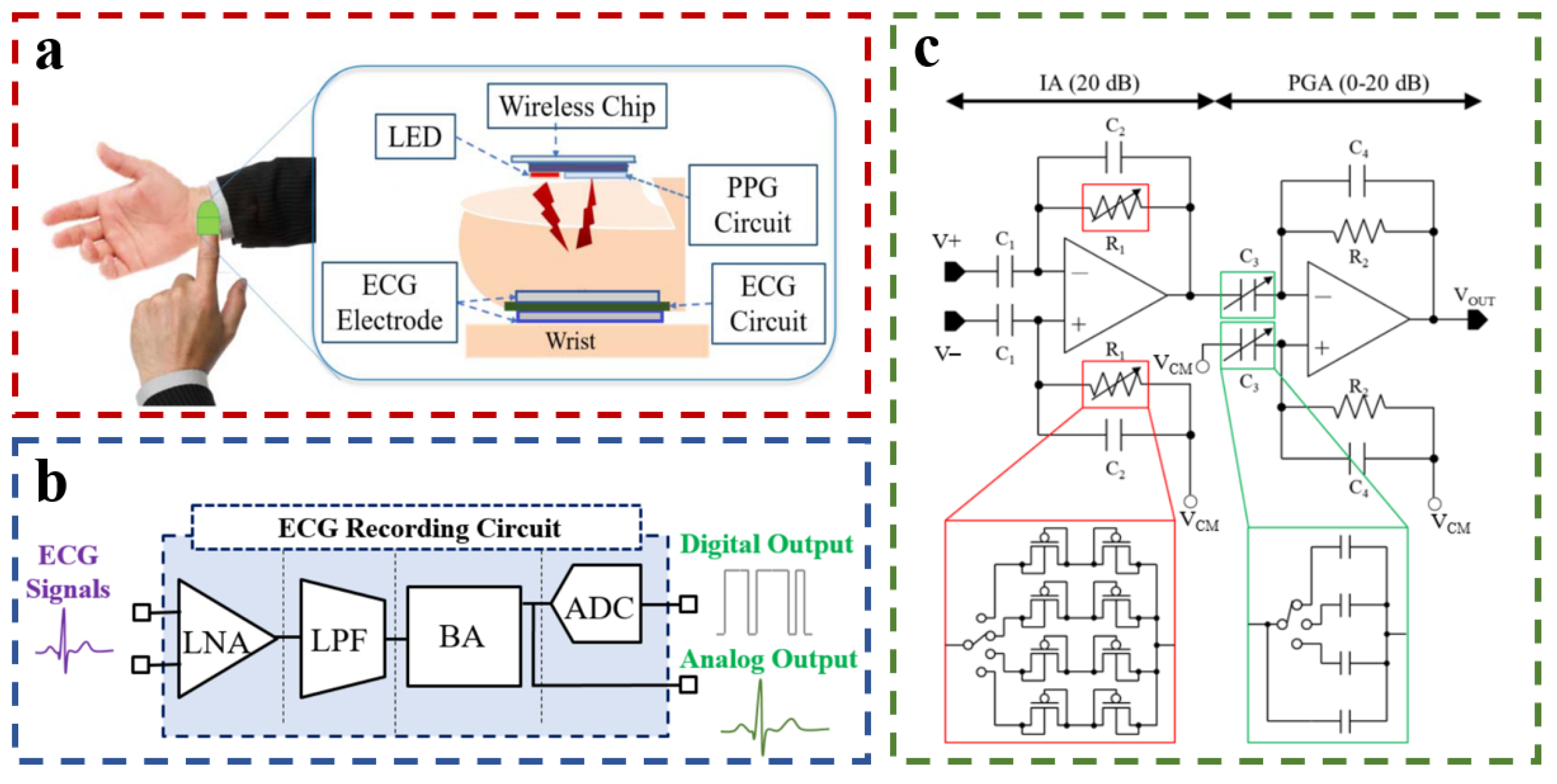


| Equation | D ± Std Dev (mmHg) |
|---|---|
| SBP = a × PTT + b × HR + k | 7.33 ± 9.02 |
| SBP = a × PTT + b × HR + c × BMI + k | 6.96 ± 8.78 |
| SBP = a × PTT + b × HR + c × AGE + k | 6.82 ± 8.60 |
| SBP = a × PTT + b × HR + c × BMI + d × AGE + k | 6.72 ± 8.50 |
| Ref | Measuring Position | ME ± SD (mmHg) | BP Estimation Model | AAMI | Gold Standard |
|---|---|---|---|---|---|
| Jung et al. (2008) [50] | PPG: finger ECG: chest | N/R | N/R | N/R | N/R |
| Espina et al. (2008) [51] | PPG: finger ECG: chest | N/R | N/R | N/R | |
| Xu et al. (2010) [53] | PPG: finger ECG: wrist | N/R | N/R | CBP | |
| Franco et al. (2012) [54] | PPG: forehead ECG: chest | SBP = 0.79 ± 5.50 DBP = −3.59 ± 3.29 | Y | CBP | |
| Winokur et al. (2012) [55] | PPG: mastoid bone ECG: mastoid bone, neck | MAP = −0.07 ± 3.64 | N/R | FABP | |
| Zheng et al. (2012) [56] | PPG&ECG: finger, earlobe, and nose bridge | N/R | N/R | N/R | N/R |
| Puke et al. (2013) [57] | PPG: chest ECG: chest | SBP = 6.91 ± 4.23 | N | CBP | |
| Wang et al. (2015) [19] | PPG: eyebrow ECG: earlobe | N/R error is within 5% | N/R | Y | CBP |
| Mohamed et al. (2016) [44] | PPG: finger ECG: chest | SBP = −1.36 ± 7.51 DBP = −2.44 ± 3.49 | Y | CBP | |
| Hsiao et al. (2016) [78] | PPG&ECG: wrist | The same as Table 1 | The same as Table 1 | N | CBP |
| Zhang et al. (2017) [70] | PPG&ECG: upper arm | SBP = 1.63 ± 4.44 | N | CBP | |
| Kim et al. (2018) [59] | PPG: finger ECG: wrist | N/R | N/R | N/R | N/R |
| Lee et al. (2018) [61] | PPG: fingernail | N/R | N/R | N/R | N/R |
| Zaki et al. (2019) [62] | PPG: finger, wrist, and ankle ECG: chest | N/R | N/R | N/R | CBP |
| Mishra et al. (2019) [71] | PPG: wrist ECG: chest | SBP = 4.20 ± 1.66 DBP = 2.90 ± 0.90 | Y | CBP | |
| Ebrahim et al. (2019) [81] | PPG: earlobe ECG: chest | N/R | N/R | CBP | |
| Wu et al. (2020) [64] | PPG&ECG: chest | N/R | N/R | CBP | |
| Bhagat et al. (2021) [65] | PPG&ECG: wrist | N/R | N/R | N/R | N/R |
| Ganti et al. (2021) [66] | PPG&ECG: wrist | SBP = 4.75 ± 2.29 DBP = 2.72 ± 0.75 | Y | CBP | |
| Landry et al. (2021) [82] | PPG: forehead ECG: wearable body metrics vest | N/R | N/R | Y | FABP |
| He et al. (2022) [8] | PPG&ECG: wrist | SBP = 0.30 ± 7.69 DBP = 0.02 ± 5.94 | N/R | Y | CBP |
| Wang et al. (2022) [22] | PPG&ECG: wrist | SBP = −2.10 ± 7.07 DBP = 0.04 ± 7.34 | N/R | Y | CBP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.-B.; Cui, T.-R.; Li, D.; Jian, J.-M.; Li, Z.; Ji, S.-R.; Li, X.; Xu, J.-D.; Liu, H.-F.; Yang, Y.; et al. Wearable Continuous Blood Pressure Monitoring Devices Based on Pulse Wave Transit Time and Pulse Arrival Time: A Review. Materials 2023, 16, 2133. https://doi.org/10.3390/ma16062133
Zhou Z-B, Cui T-R, Li D, Jian J-M, Li Z, Ji S-R, Li X, Xu J-D, Liu H-F, Yang Y, et al. Wearable Continuous Blood Pressure Monitoring Devices Based on Pulse Wave Transit Time and Pulse Arrival Time: A Review. Materials. 2023; 16(6):2133. https://doi.org/10.3390/ma16062133
Chicago/Turabian StyleZhou, Zi-Bo, Tian-Rui Cui, Ding Li, Jin-Ming Jian, Zhen Li, Shou-Rui Ji, Xin Li, Jian-Dong Xu, Hou-Fang Liu, Yi Yang, and et al. 2023. "Wearable Continuous Blood Pressure Monitoring Devices Based on Pulse Wave Transit Time and Pulse Arrival Time: A Review" Materials 16, no. 6: 2133. https://doi.org/10.3390/ma16062133
APA StyleZhou, Z.-B., Cui, T.-R., Li, D., Jian, J.-M., Li, Z., Ji, S.-R., Li, X., Xu, J.-D., Liu, H.-F., Yang, Y., & Ren, T.-L. (2023). Wearable Continuous Blood Pressure Monitoring Devices Based on Pulse Wave Transit Time and Pulse Arrival Time: A Review. Materials, 16(6), 2133. https://doi.org/10.3390/ma16062133






