Effect of Sintering Temperature and Solution Treatment on Phase Changes and Mechanical Properties of High-Nitrogen Stainless Steel Prepared by MIM
Abstract
:1. Introduction
2. Materials and Methods
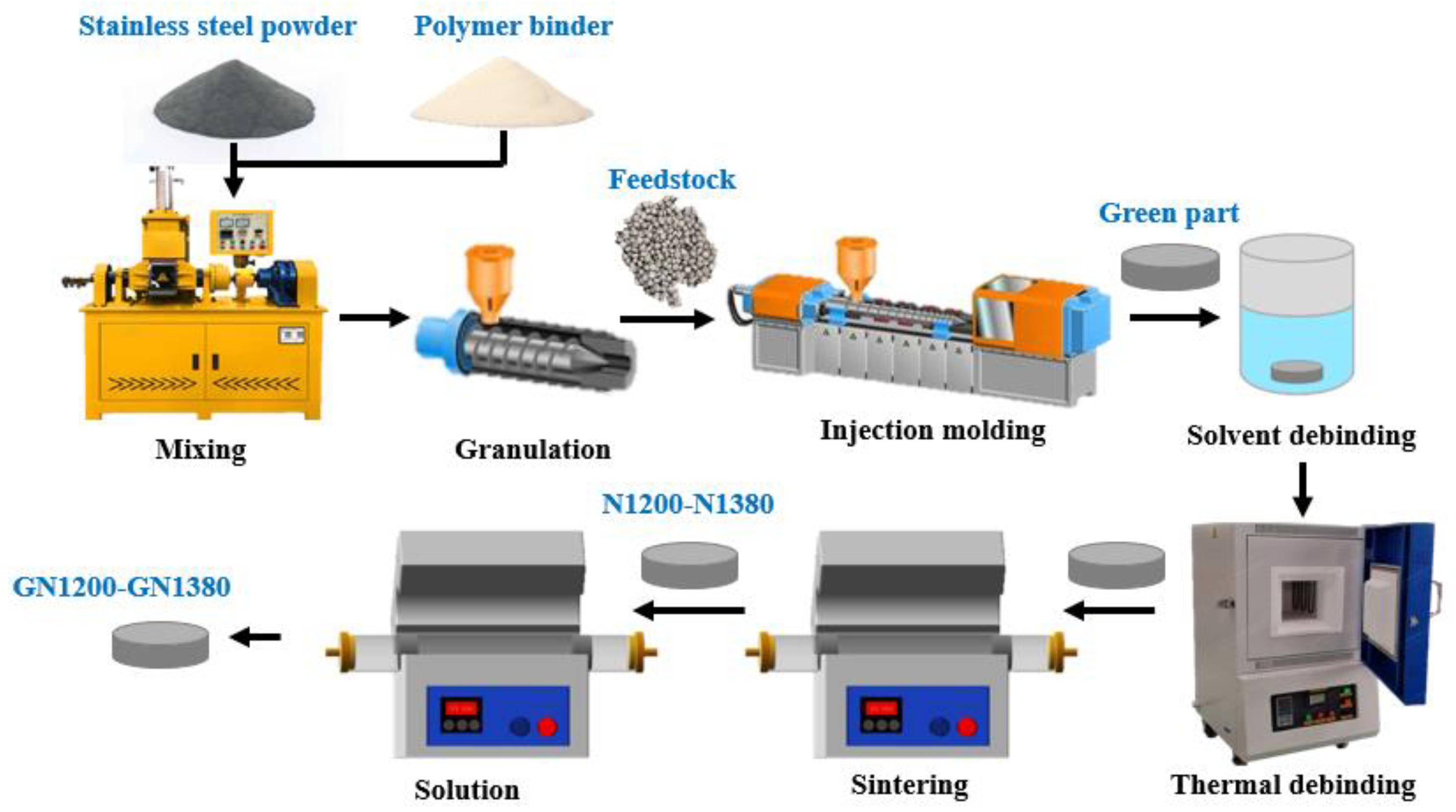
2.1. Specimen Preparation
2.2. Characterization of Sintered Specimens
3. Results
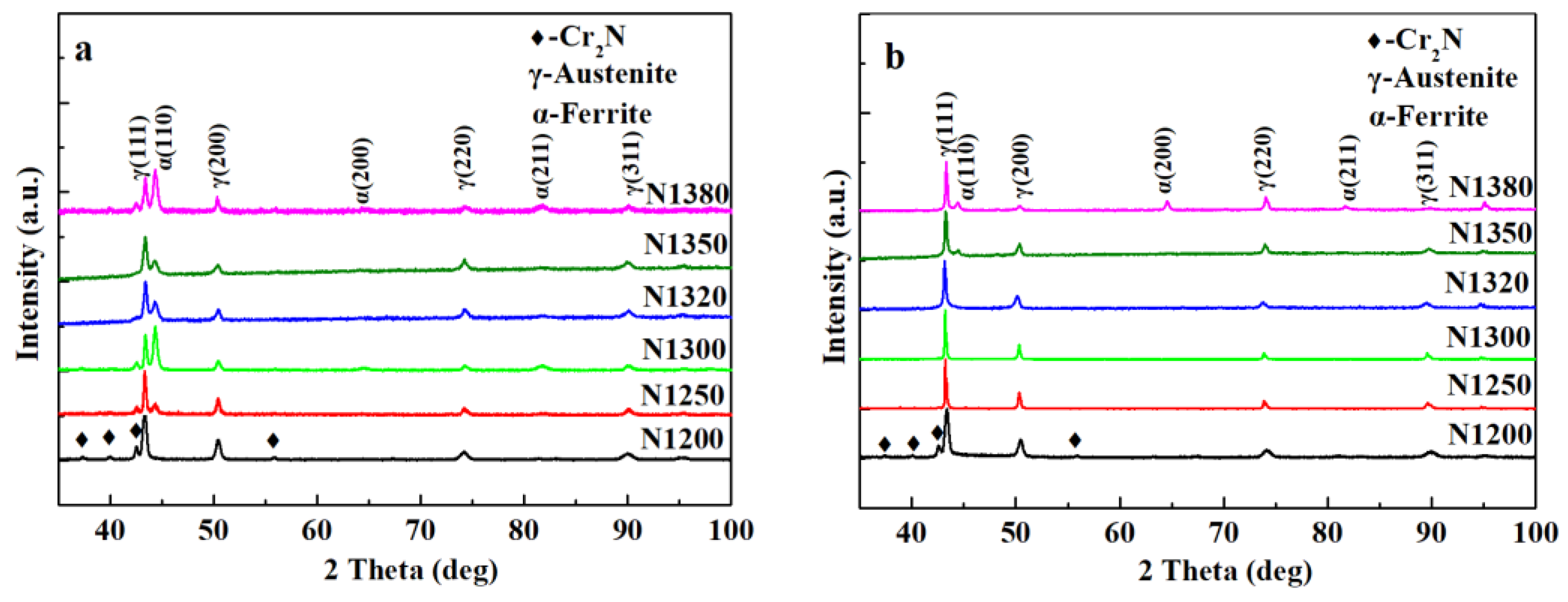
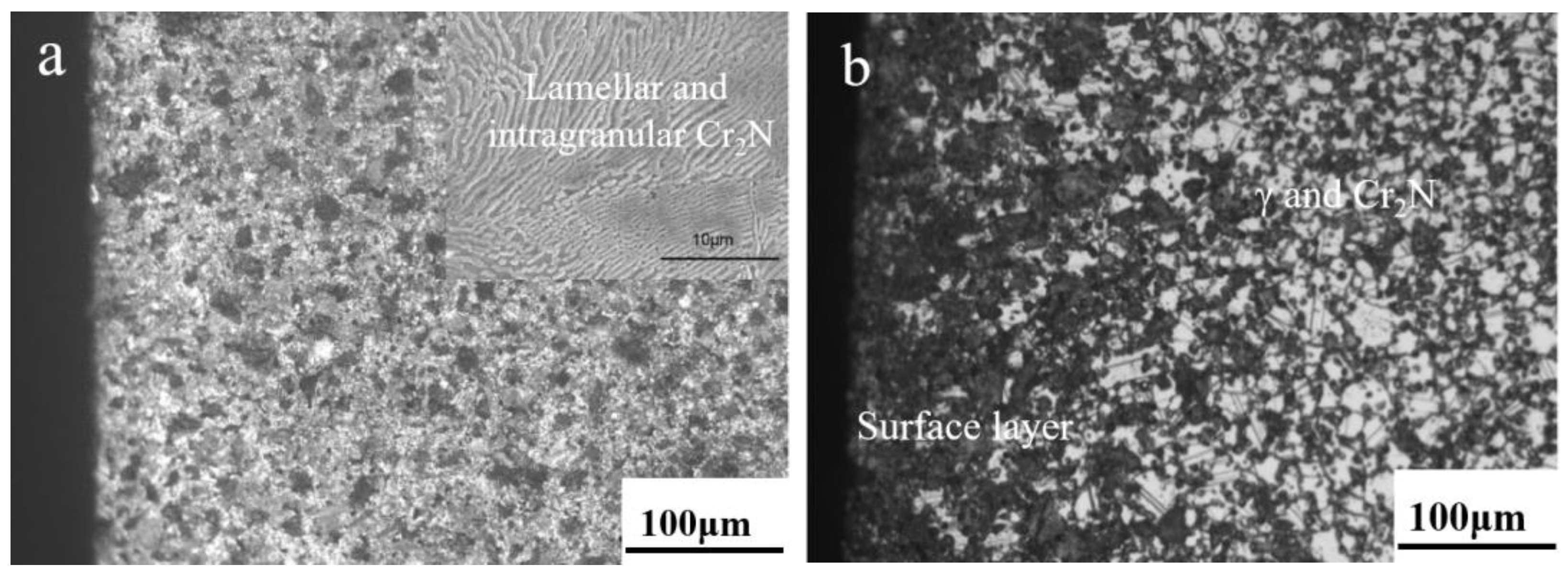
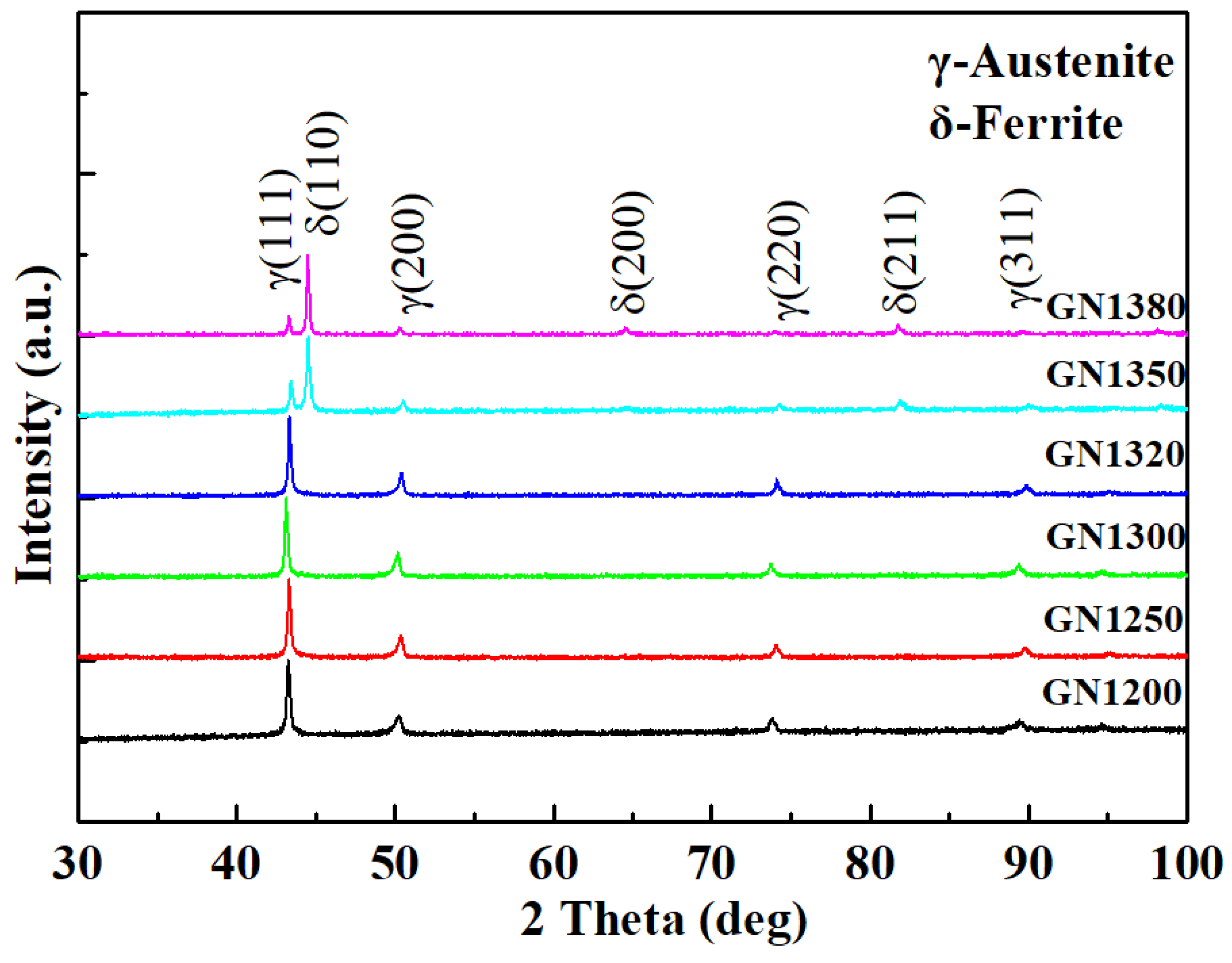
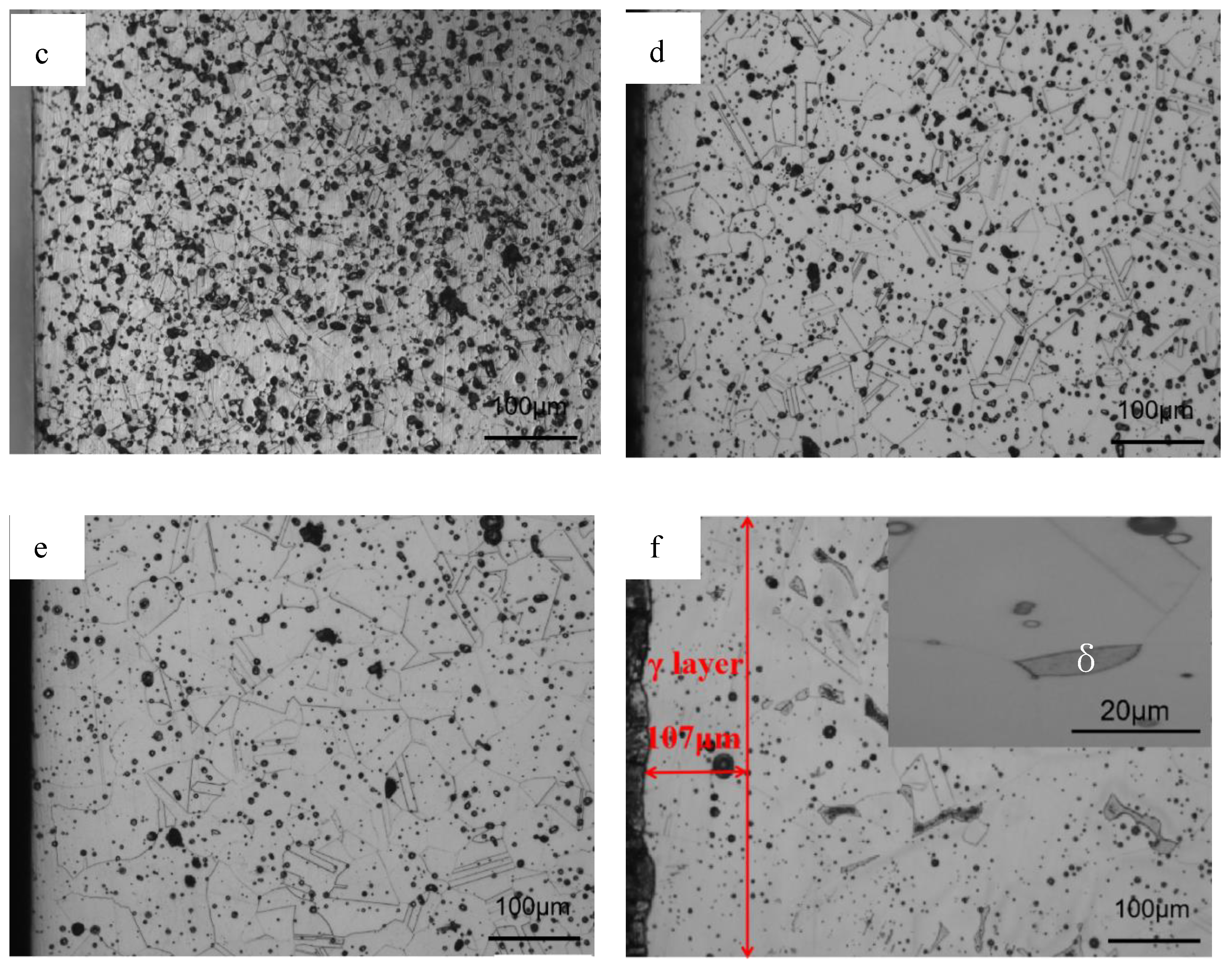
3.1. Phase Constituent and Microstructure of the Sintered Samples
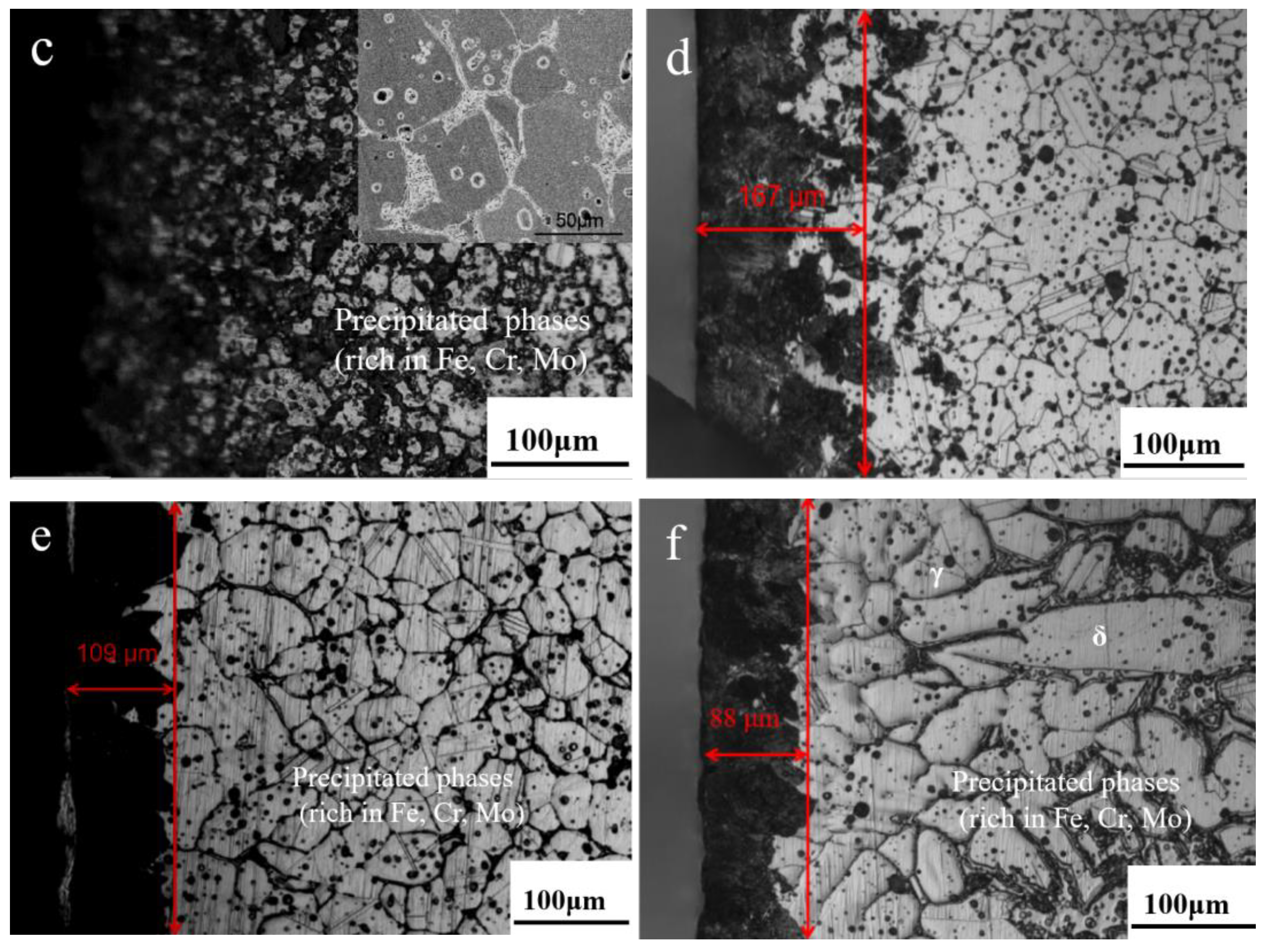
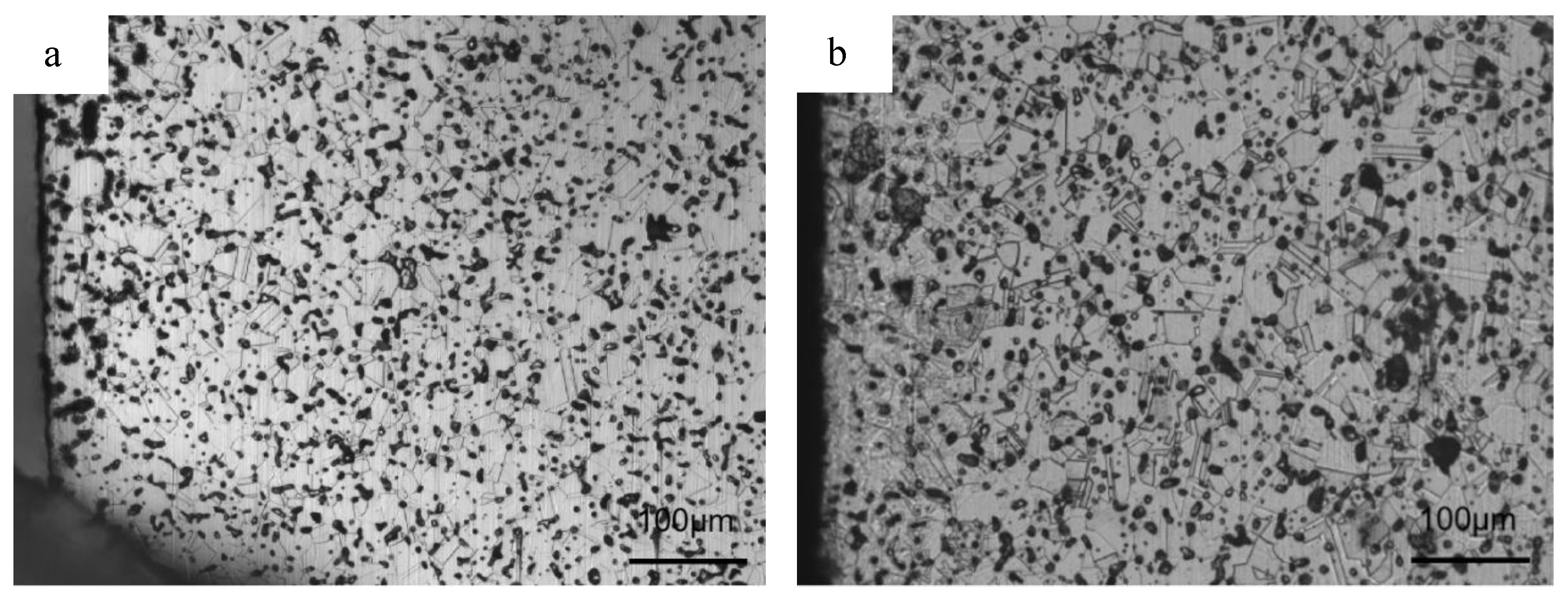
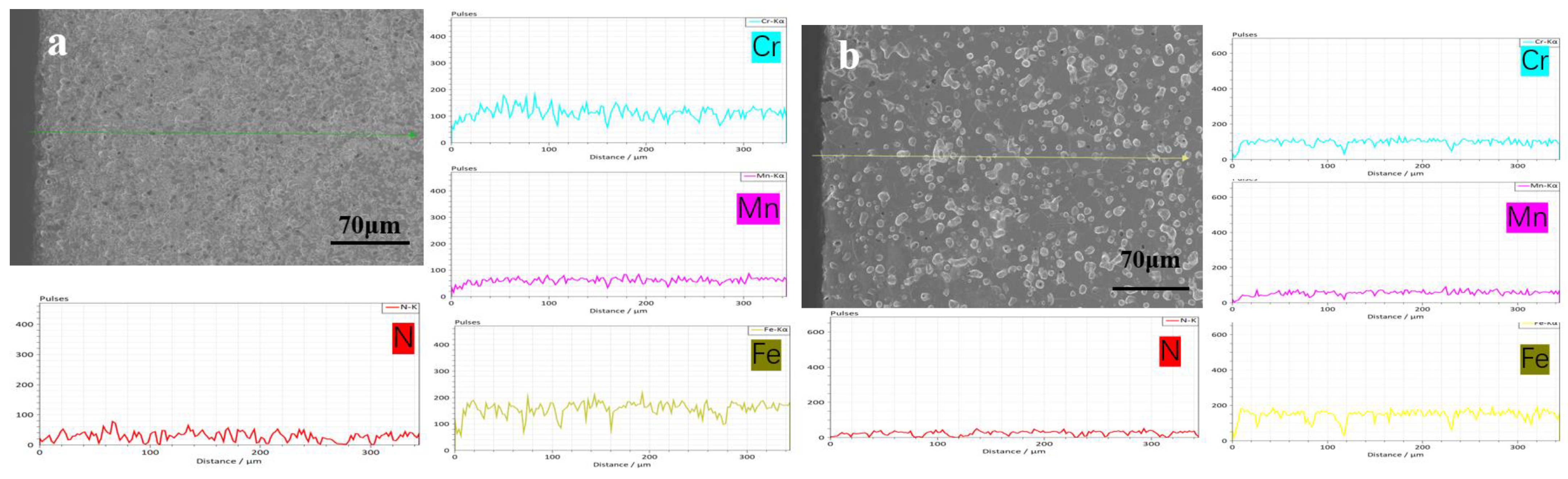
3.2. N Concentration Gradient and Microstructure Evolution of Sintered Samples
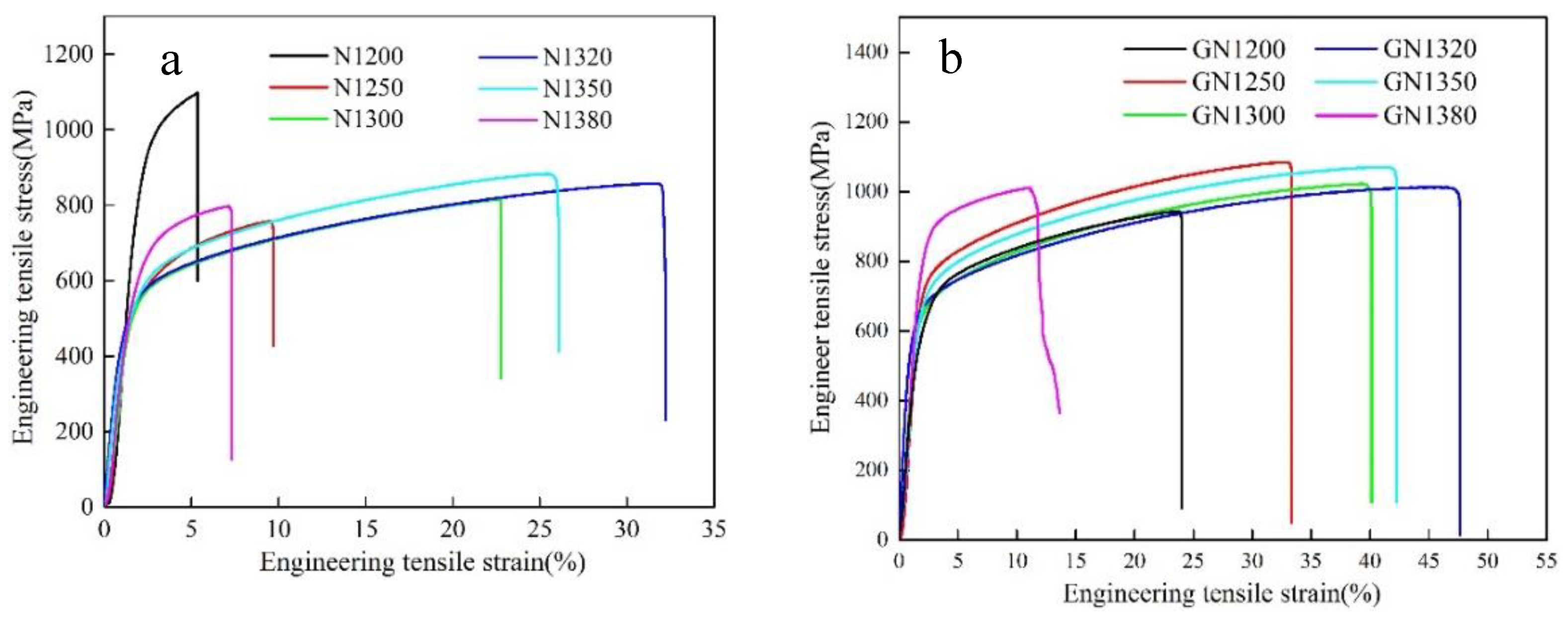
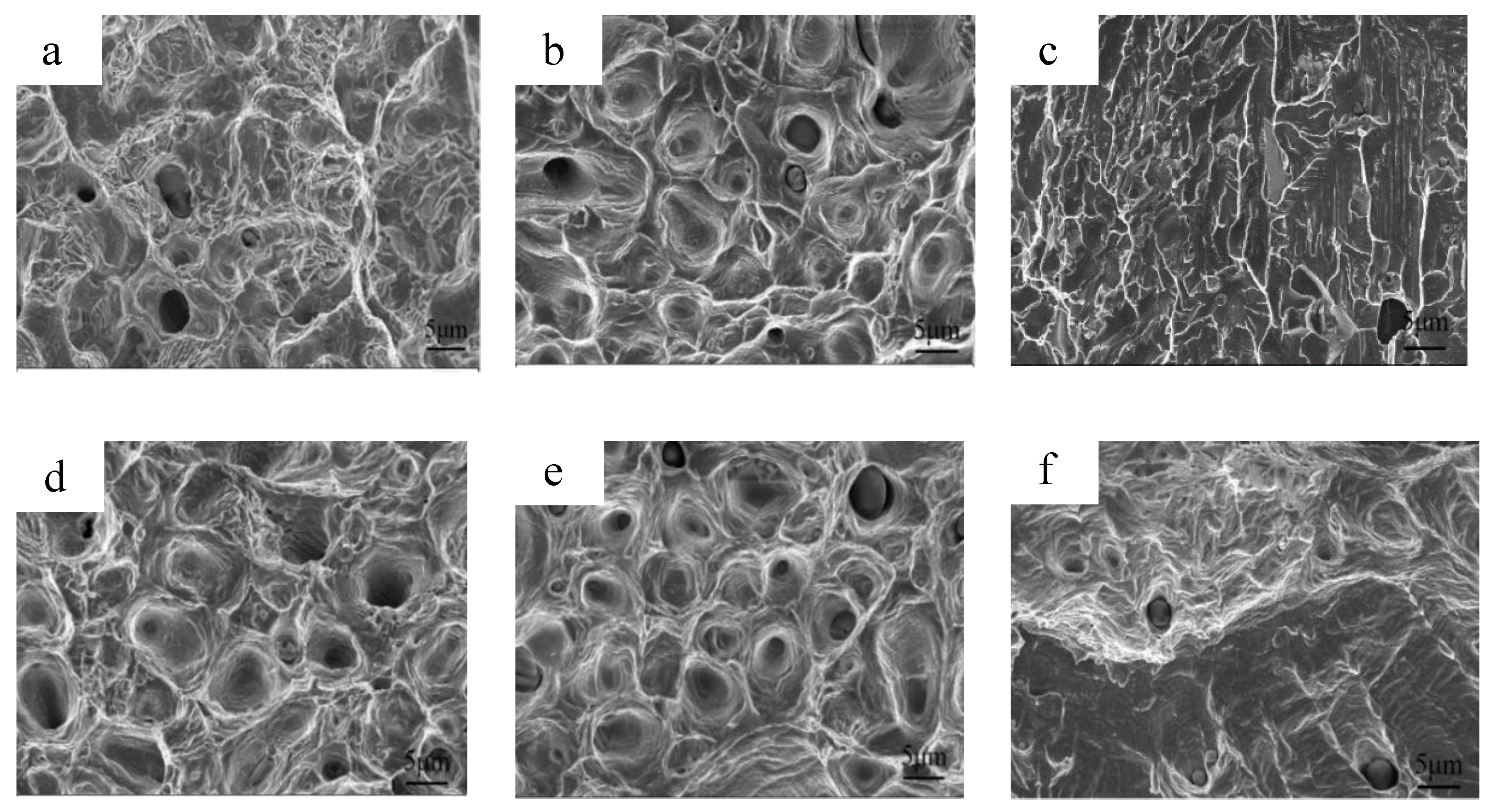
3.3. Mechanical Properties after Sintering
3.4. Microstructure after Solution Treatment
3.5. Mechanical Properties of Solution-Treated Samples
4. Discussion
5. Conclusions
- With increasing sintering temperature from 1200 °C to 1380 °C, the sample density increases, but the nitrogen content gradually decreases.
- Nitrogen content and distribution along the cross-section of as-sintered samples are not homogeneous. After sintering under the N2 atmosphere, the subsequent furnace cooling causes continuous nitriding and Cr2N precipitation, resulting in a sample surface layer rich in N and Cr elements.
- High N content promotes austenite decomposition: γsaturated translated to γ and Cr2N. Meanwhile, Mn is easily effused on the surface of the sample surface and is oxidized, resulting in the decomposition of γ: γ translated to α and Cr2N.
- Samples with a homogeneous γ microstructure can be obtained after solution treatment at 1150 °C for 1.5 h under an N2 atmosphere for samples sintered at 1320 °C or lower. For solution-treated austenitic samples sintered at 1320 °C, its tensile strength is 988.76 MPa; yield strength is 615.61 MPa, and elongation is 42.58%.
- Solution-treated samples with a dual-phase structure (γ and δ) that sintered at 1350 °C have a tensile strength of 1036.12 MPa, a yield strength of 636.14 MPa, and an elongation of 40.08%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, P.; Ren, Y.; Zhang, B.; Yang, K. Analysis of Magnetism in High Nitrogen Austenitic Stainless Steel and Its Elimination by High Temperature Gas Nitriding. J. Mater. Sci. Technol. 2011, 27, 1139–1142. [Google Scholar] [CrossRef]
- Lang, Y.; Qu, H.; Chen, H.; Weng, Y. Research Progress and Development Tendency of Nitrogen-alloyed Austenitic Stainless Steels. J. Iron Steel Res. Int. 2015, 22, 91–98. [Google Scholar] [CrossRef]
- Mohammadzadeh, R.; Akbari, A.; Mohammadzadeh, M. Impact Toughness Properties of Nickel- and Manganese-Free High Nitrogen Austenitic Stainless Steels. Metall. Mater. Trans. A 2016, 47, 6032–6041. [Google Scholar] [CrossRef]
- Speidel, M.O. New Nitrogen-Bearing Austenitic Stainless Steels with High Strength and Ductility. Met. Sci. Heat Treat. 2005, 47, 489–493. [Google Scholar] [CrossRef]
- Kuroda, D.; Hiromoto, S.; Hanawa, T.; Katada, Y. Corrosion Behavior of Nickel-Free High Nitrogen Austenitic Stainless Steel in Simulated Biological Environments. Mater. Trans. 2002, 43, 3100–3104. [Google Scholar] [CrossRef] [Green Version]
- Talha, M.; Behera, C.K.; Sinha, O.P. A Review on Nickel-Free Nitrogen Containing Austenitic Stainless Steels for Biomedical Applications. Mater. Sci. Eng. C 2013, 33, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.W. Overview: High-Nitrogen Alloying of Stainless Steels. Mater. Sci. Eng. A 1996, 207, 159–169. [Google Scholar] [CrossRef]
- Yuan, Z.Z.; Dai, Q.X.; Cheng, X.N.; Chen, K.M. Microstructural Thermostability of High Nitrogen Austenitic Stainless Steel. Mater. Charact. 2007, 58, 87–91. [Google Scholar] [CrossRef]
- Li, H.; Jiang, Z.; Zhang, Z.; Xu, B.; Liu, F. Mechanical Properties of Nickel Free High Nitrogen Austenitic Stainless Steels. J. Iron Steel Res. Int. 2007, 14, 330–334. [Google Scholar] [CrossRef]
- Pujar, M.G.; Kamachi Mudali, U.; Singh, S.S. Electrochemical Noise Studies of the Effect of Nitrogen on Pitting Corrosion Resistance of High Nitrogen Austenitic Stainless Steels. Corros. Sci. 2011, 53, 4178–4186. [Google Scholar] [CrossRef]
- Berns, H.; Bouwman, J.W.; Eul, U.; Izagirre, J.; Juse, R.L.; Niederau, H.J.; Tavernier, G.; Zieschang, R. Solution Nitriding of Stainless Steels for Process Engineering. Materwiss. Werksttech. 2000, 31, 152–161. [Google Scholar] [CrossRef]
- Ye, H.; Liu, X.Y.; Hong, H. Fabrication of Metal Matrix Composites by Metal Injection Molding—A review. J. Mater. Process. Technol. 2008, 200, 12–24. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, L.; Khalil, K.A. Effect of Powder Loading on Metal Injection Molding Stainless Steels. J. Mater. Process. Technol. 2007, 183, 432–439. [Google Scholar] [CrossRef]
- Uggowitzer, P.J.; Bähre, W.F.; Wohlfromm, H.; Speidel, M.O. Nickel-Free High Nitrogen Austenitic Stainless Steels Produced by Metal Injection Molding. Mater. Sci. Forum. 1999, 318, 663–672. [Google Scholar] [CrossRef]
- Knutsen, R.D.; Lang, C.I.; Basson, J.A. Discontinuous cellular precipitation in a Cr-Mn-N steel with niobium and vanadium additions. Acta Mater. 2004, 52, 2407–2417. [Google Scholar] [CrossRef]
- Simmons, J.W.; Atteridge, D.G.; Rawers, J.C. Sensitization of high-nitrogen austenitic stainless steels by dichromium nitride precipitation. Corrosion 1994, 50, 491–501. [Google Scholar] [CrossRef]
- Chao, K.L.; Liao, H.Y.; Shyue, J.J.; Lian, S.S. Corrosion behavior of high nitrogen nickel-free Fe-16Cr-Mn-Mo-N stainless steels. Metall. Mater. Trans. B 2014, 45, 381–391. [Google Scholar] [CrossRef]
- Feng, H.; Jiang, Z.; Li, H.; Lu, P.; Zhang, S.; Zhu, H.; Zhang, B.; Zhang, T.; Xu, D.; Chen, Z. Influence of Nitrogen on Corrosion Behaviour of High Nitrogen Martensitic Stainless Steels Manufactured by Pressurized Metallurgy. Corros. Sci. 2018, 144, 288–300. [Google Scholar] [CrossRef] [Green Version]
- Mohammadzadeh, R.; Akbari, A. Effect of Pressurized Solution Nitriding on Phase Changes and Mechanical Properties of Ferritic Fe-22.7Cr-2.4Mo Stainless Steel. Mater. Sci. Eng. A 2014, 592, 153–163. [Google Scholar] [CrossRef]
- Tsuchiyama, T.; Takaki, S.; Ito, H.; Kataoka, K. Fabrication of ultrahigh nitrogen austenitic steels by nitrogen gas absorption into solid solution. Metall. Mater. Trans. A 2003, 34, 2591–2599. [Google Scholar] [CrossRef]
- Xu, Z.W.; Jia, C.C.; Kuang, C.J.; Qu, X.H. Fabrication and Sintering Behavior of High-Nitrogen Nickel-Free Stainless Steels by Metal Injection Molding. Int. J. Miner. Metall. Mater. 2010, 17, 423–428. [Google Scholar] [CrossRef]
- Kuroda, Y.; Komada, M.; Murakami, R.; Fukumoto, S.; Tsuchida, N.; Harada, Y.; Fukaura, K. Metal Injection Molding of Nickel-Free Austenitic Stainless-Steels I-Manufacturing Process. Adv. Mater. Res. 2007, 26–28, 15–18. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Zhang, Z.R.; Li, H.B.; Li, Z.; Ma, Q.F. Microstructural Evolution and Mechanical Properties of Aging High Nitrogen Austenitic Stainless Steels. Int. J. Miner. Metall. Mater. 2010, 17, 729–736. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Y.; Yao, C.; Yang, K.; Misra, R.D.K. Residual Ferrite and Relationship Between Composition and Microstructure in High-Nitrogen Austenitic Stainless Steels. Metall. Mater. Trans. A 2015, 46, 5537–5545. [Google Scholar] [CrossRef]
- Cui, D.; Jiang, J.; Cao, G.; Xiao, E.; Qu, X. Preparation of High Nitrogen and Nickel-Free Austenitic Stainless Steel by Powder Injection Molding. J. Univ. Sci. Technol. Beijing Miner. Metall. Mater. 2008, 15, 150–154. [Google Scholar] [CrossRef]
- Cui, D.; Qu, X.; Guo, P.; Li, K. Sintering Optimization and Solution Annealing of High Nitrogen Nickel Free Austenitic Stainless Steels Prepared by PIM. Powder Metall. 2010, 53, 91–95. [Google Scholar] [CrossRef]
- NIST Data Base. Available online: https://srdata.nist.gov/xps/ (accessed on 3 January 2023).
- Major, G.H.; Farley, N.; Sherwood, P.M.A.; Linford, M.R.; Terry, J.; Fernandez, V.; Artyushkova, K. Practical guide for curve fitting in X-ray photoelectron spectroscopy. J. Vac. Sci. Technol. A 2020, 38, 061203. [Google Scholar] [CrossRef]
- Cojocaru, V.D.; Şerban, N.; Angelescu, M.L.; Cojocaru, E.M.; Cinca, I.; Răducanu, D.; Vintilă, A.N. Effect of Short Duration Solution Treatment at Temperatures Below 1000 °C on σ-Phase Precipitation in a Super Duplex Stainless Steel Alloy. Materwiss. Werksttech. 2018, 49, 530–537. [Google Scholar] [CrossRef]
- Fukumoto, S.; Okane, T.; Umeda, T.; Kurz, W. Crystallographic Relationships between δ-Ferrite and γ-Austenite during Unidirectional Solidification of Fe-Cr-Ni Alloys. ISIJ Int. 2000, 40, 677–684. [Google Scholar] [CrossRef]
- Salahinejad, E.; Amini, R.; Marasi, M.; Hadianfard, M.J. The Effect of Sintering Time on the Densification and Mechanical Properties of a Mechanically Alloyed Cr-Mn-N Stainless Steel. Mater. Des. 2010, 31, 527–532. [Google Scholar] [CrossRef]
- Ali, S.; Abdul Rani, A.M.; Mufti, R.A.; Hastuty, S.; Hussain, M.; Shehzad, N.; Baig, Z.; Abdu Aliyu, A.A. An Efficient Approach for Nitrogen Diffusion and Surface Nitriding of Boron-Titanium Modified Stainless Steel Alloy for Biomedical Applications. Metals 2019, 9, 755. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Baker, I.; Yi, Y.; Hu, L.; Fang, W.; Li, L.; Luo, B.; Luo, Z. Dissimilar electron beam welding of the medium-entropy alloy (NiCoCr)94Al3Ti3 to 304 stainless steel. Scr. Mater. 2022, 214, 114659. [Google Scholar] [CrossRef]
- Wang, L.J.; Sheng, L.Y.; Hong, C.M. Influence of Grain Boundary Carbides on Mechanical Properties of High Nitrogen Austenitic Stainless Steel. Mater. Des. 2012, 37, 349–355. [Google Scholar] [CrossRef]
- Lefor, K.; Walter, M.; Weddeling, A.; Hryha, E.; Huth, S.; Weber, S.; Nyborg, L.; Theisen, W. Influence of the PM-Processing Route and Nitrogen Content on the Properties of Ni-Free Austenitic Stainless Steel, Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2015, 46, 1154–1167. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Peng, H.; Baker, I.; Li, L.; Zhang, W.; Ngai, T. Characterization of High-Strength High Nitrogen Austenitic Stainless Steel Synthesized from Nitrided Powders by Spark Plasma Sintering. Mater. Charact. 2019, 152, 76–84. [Google Scholar] [CrossRef]

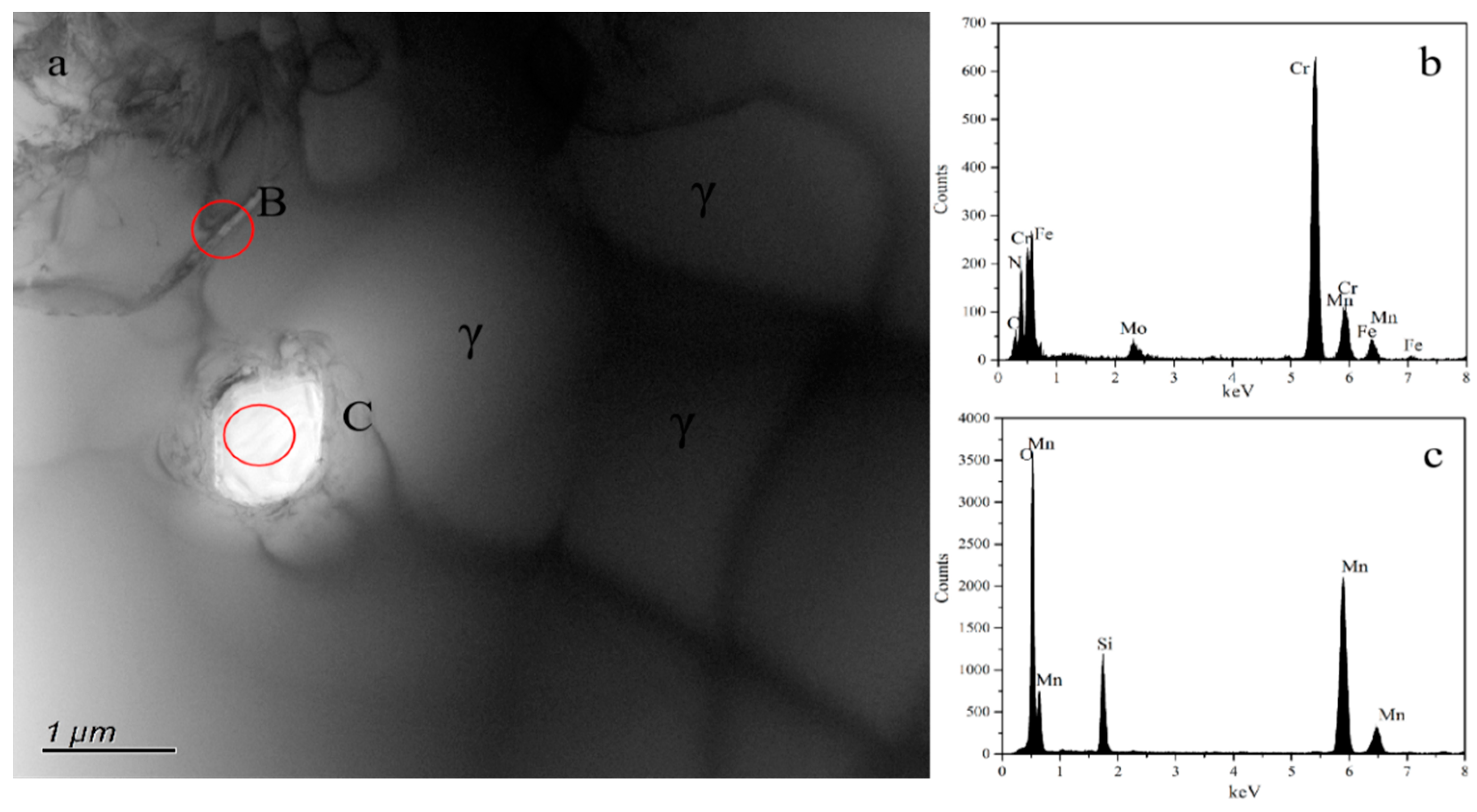
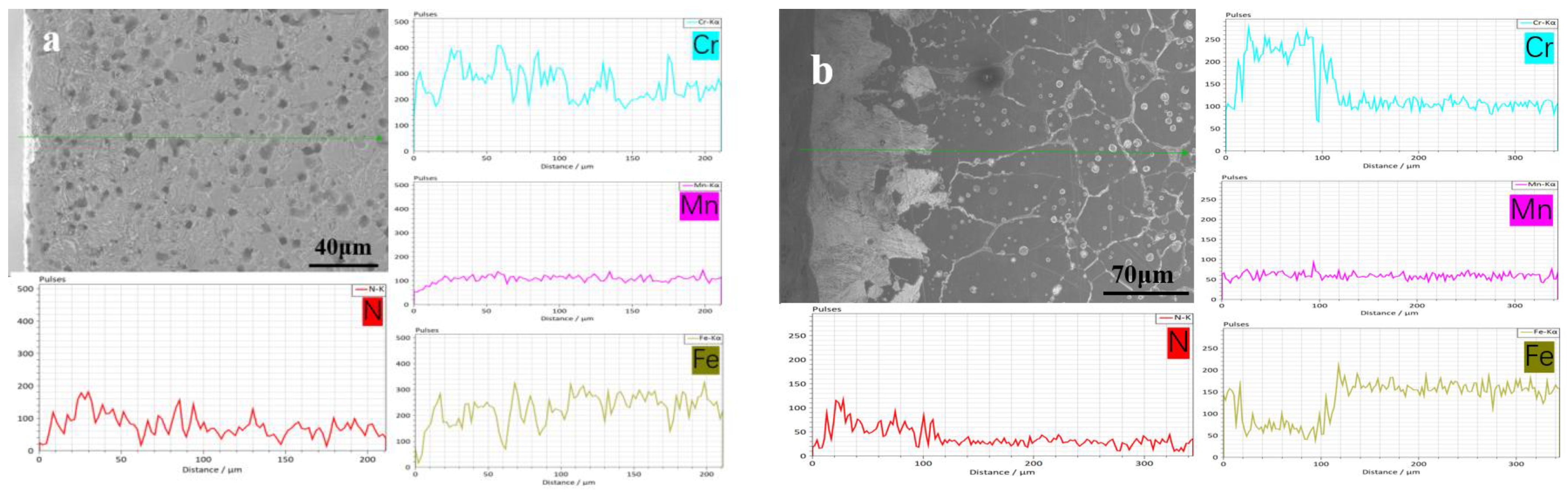
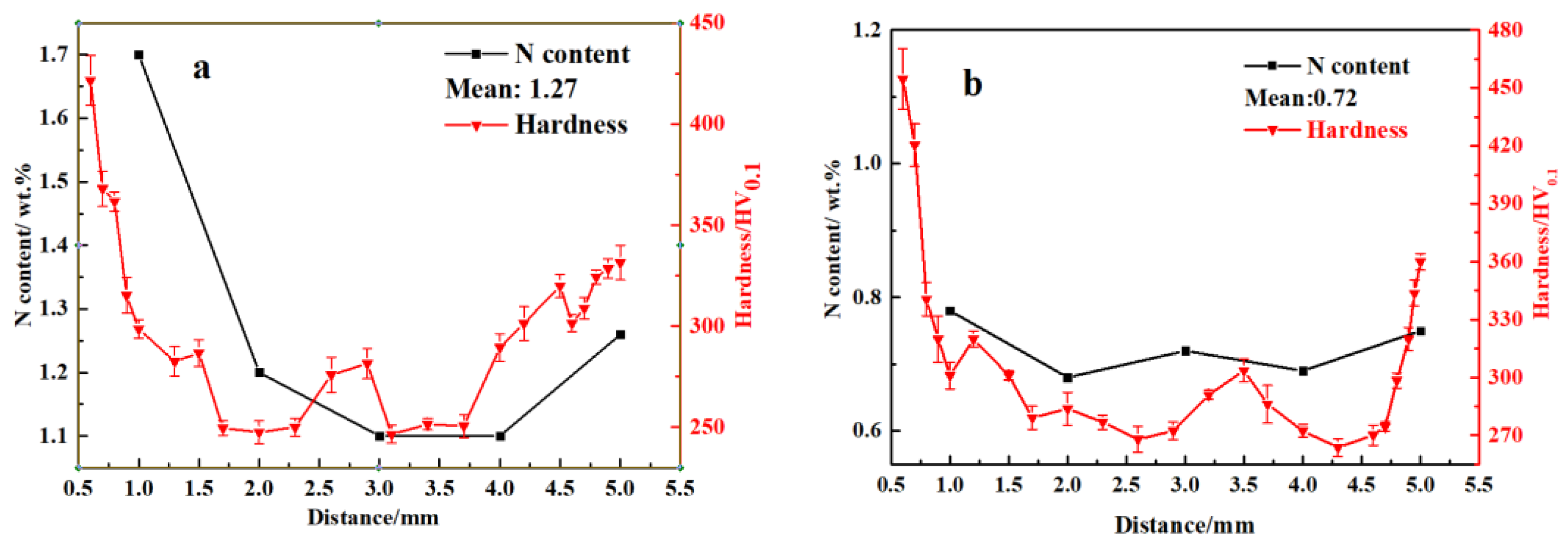
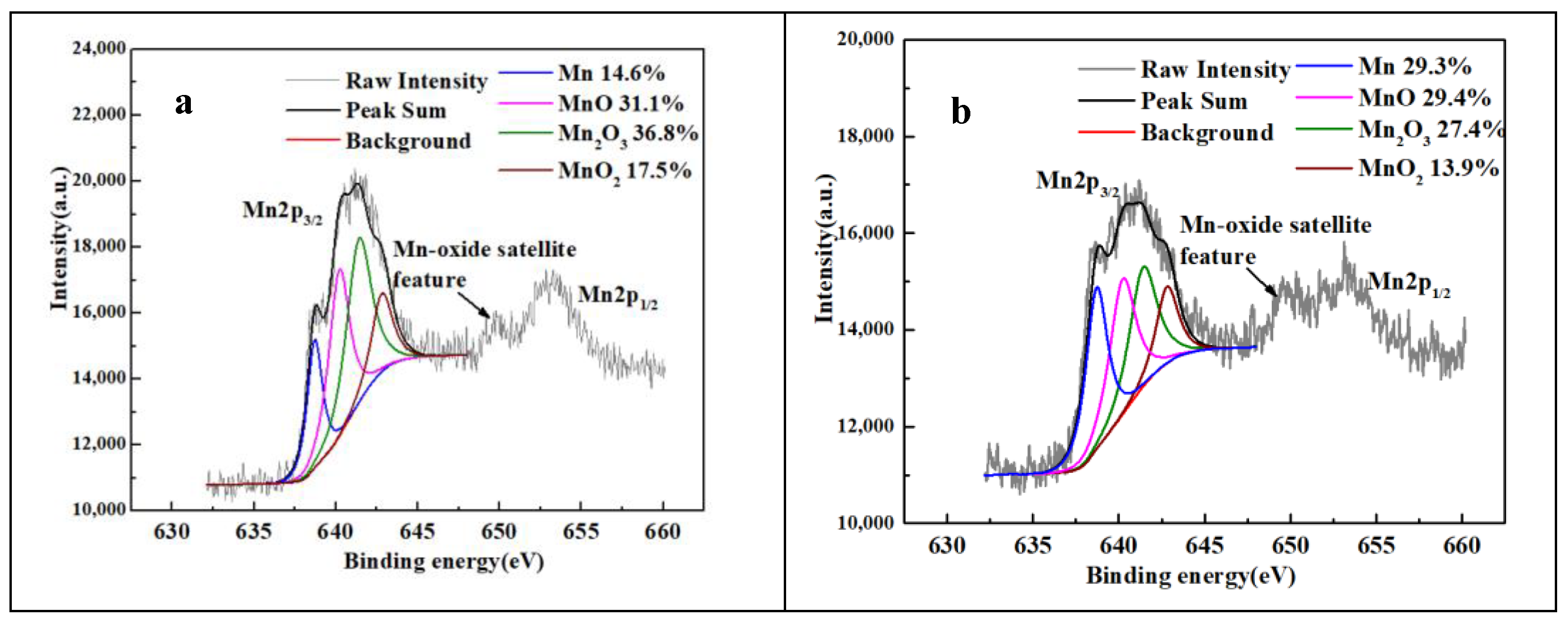
| Sample | N1200 | N1250 | N1300 | N1320 | N1350 | N1380 |
|---|---|---|---|---|---|---|
| Surface/wt.% | 0.68 | 0.72 | 0.67 | 0.62 | 0.57 | 0.43 |
| Interior/wt.% | 0.83 | 1.01 | 1.05 | 1.11 | 0.92 | 0.83 |
| Sample Code | Relative Density | Hardness | σb | σ0.2 | Elongation |
|---|---|---|---|---|---|
| (%) | (HV) | (MPa) | (MPa) | (%) | |
| N1200 | 88.71 ± 0.8 | 271.76 ± 30 | 1113.8 ± 30 | 1087.3 ± 50 | 3.4 ± 1 |
| N1250 | 92.82 ± 0.5 | 296.23 ± 35 | 804.7 ± 50 | 689.5 ± 30 | 9.5 ± 2.3 |
| N1300 | 94.41 ± 0.3 | 272.38 ± 28 | 835.6 ± 42 | 613.8 ± 25 | 18.4 ± 1.6 |
| N1320 | 95.87 ± 0.5 | 239.54 ± 32 | 842.5 ± 28 | 588.1 ± 26 | 25.8 ± 3.2 |
| N1350 | 97.61 ± 0.6 | 291.39 ± 38 | 916.3 ± 38 | 690.7 ± 23 | 19.4 ± 1.8 |
| N1380 | 98.18 ± 0.9 | 309.26 ± 40 | 804.7 ± 46 | 709.2 ± 18 | 5.1 ± 1 |
| GN1200 | --- | --- | 958.1 ± 50 | 671.5 ± 30 | 17.5 ± 1 |
| GN1250 | --- | --- | 1006.9 ± 80 | 635.4 ± 40 | 25.5 ± 1 |
| GN1300 | --- | --- | 1033.7 ± 30 | 615.5 ± 10 | 37.1 ± 2 |
| GN1320 | --- | --- | 988.8 ± 30 | 615.6 ± 40 | 42.6 ± 3.5 |
| GN1350 | --- | --- | 1036.1 ± 30 | 636.2 ± 60 | 40.1 ± 3.2 |
| GN1380 | --- | --- | 1021.2 ± 50 | 810.1 ± 10 | 12.1 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Li, L.; Huang, C.; Ngai, T.; Hu, L. Effect of Sintering Temperature and Solution Treatment on Phase Changes and Mechanical Properties of High-Nitrogen Stainless Steel Prepared by MIM. Materials 2023, 16, 2135. https://doi.org/10.3390/ma16062135
Zhang W, Li L, Huang C, Ngai T, Hu L. Effect of Sintering Temperature and Solution Treatment on Phase Changes and Mechanical Properties of High-Nitrogen Stainless Steel Prepared by MIM. Materials. 2023; 16(6):2135. https://doi.org/10.3390/ma16062135
Chicago/Turabian StyleZhang, Weipeng, Liejun Li, Chengcheng Huang, Tungwai Ngai, and Ling Hu. 2023. "Effect of Sintering Temperature and Solution Treatment on Phase Changes and Mechanical Properties of High-Nitrogen Stainless Steel Prepared by MIM" Materials 16, no. 6: 2135. https://doi.org/10.3390/ma16062135






