Study on the Effect of Temperature on the Crystal Transformation of Microporous Calcium Silicate Synthesized of Extraction Silicon Solution from Fly Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
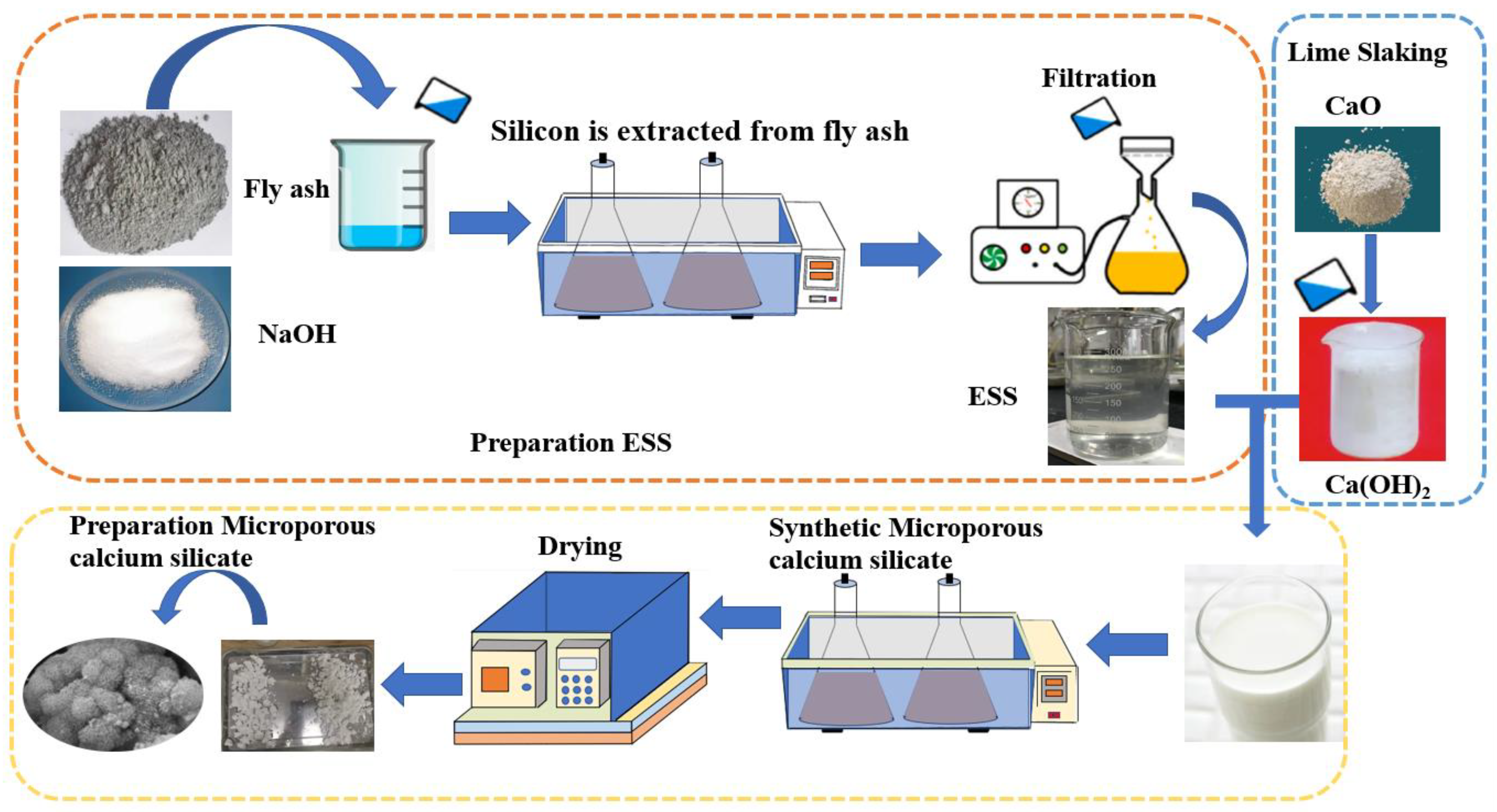
2.2.1. Preparation of ESS
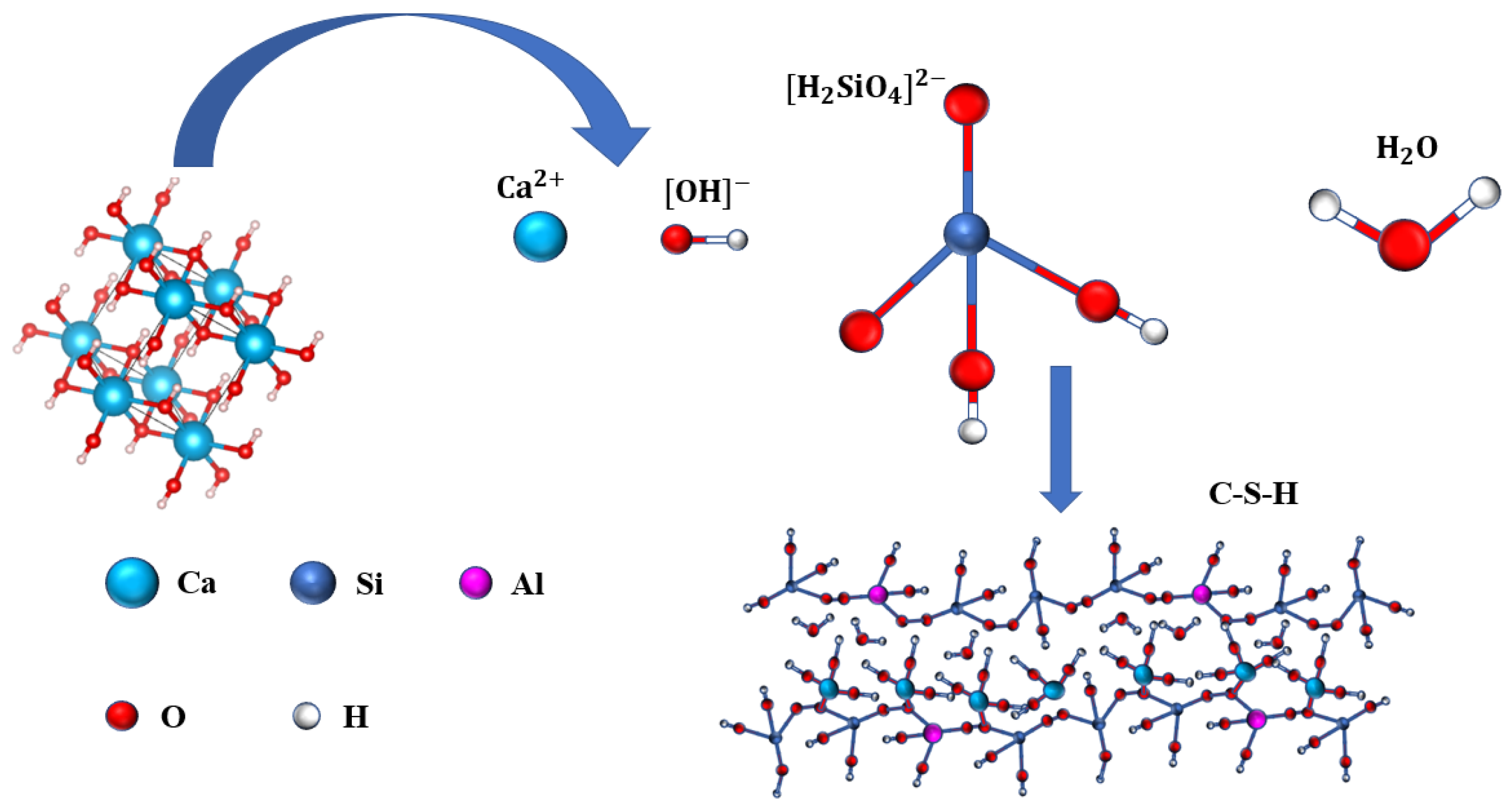
2.2.2. Preparation of Microporous Calcium Silicate
3. Results and Discussion
3.1. Thermodynamic Analysis
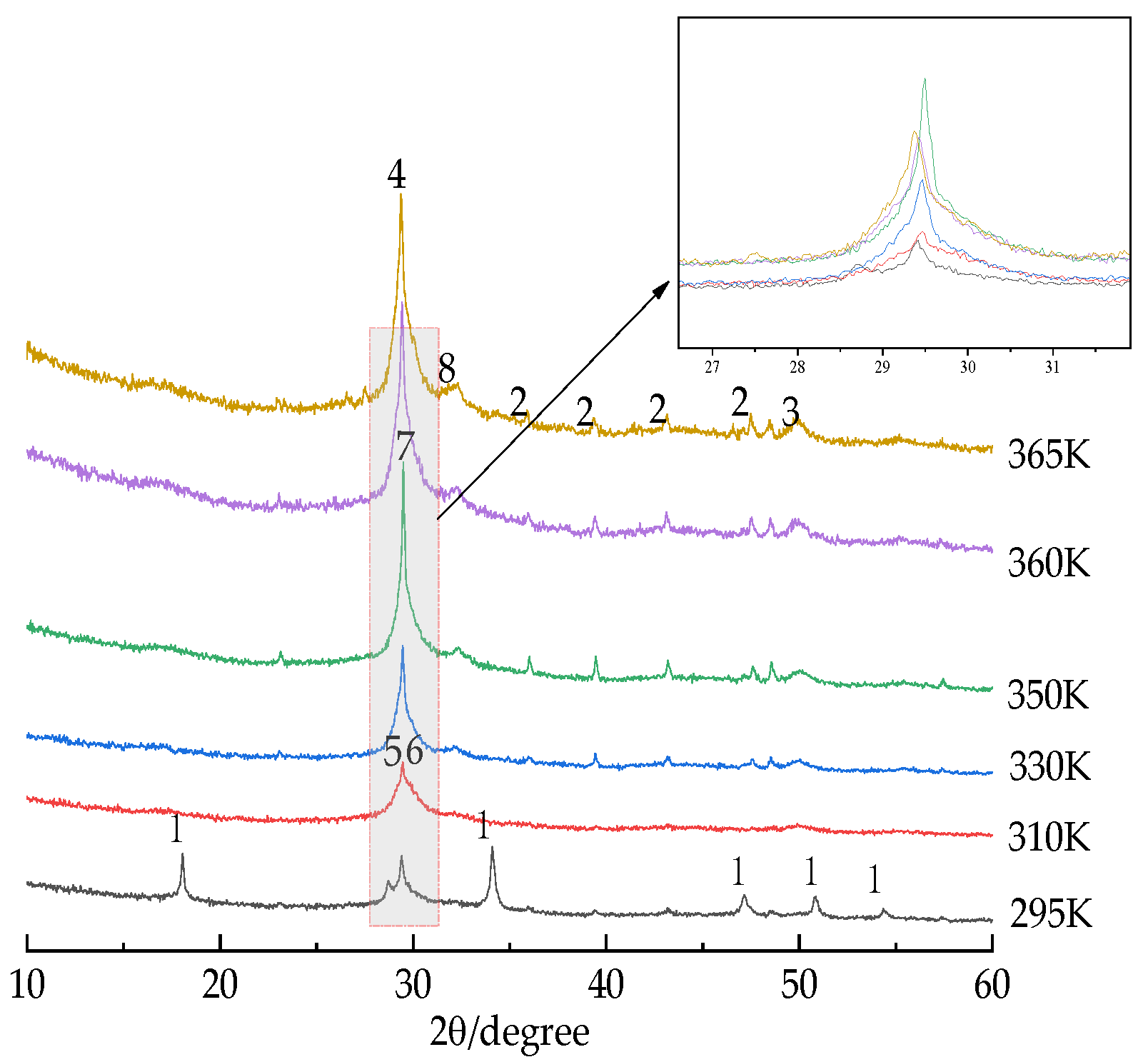
3.2. Phase Analysis
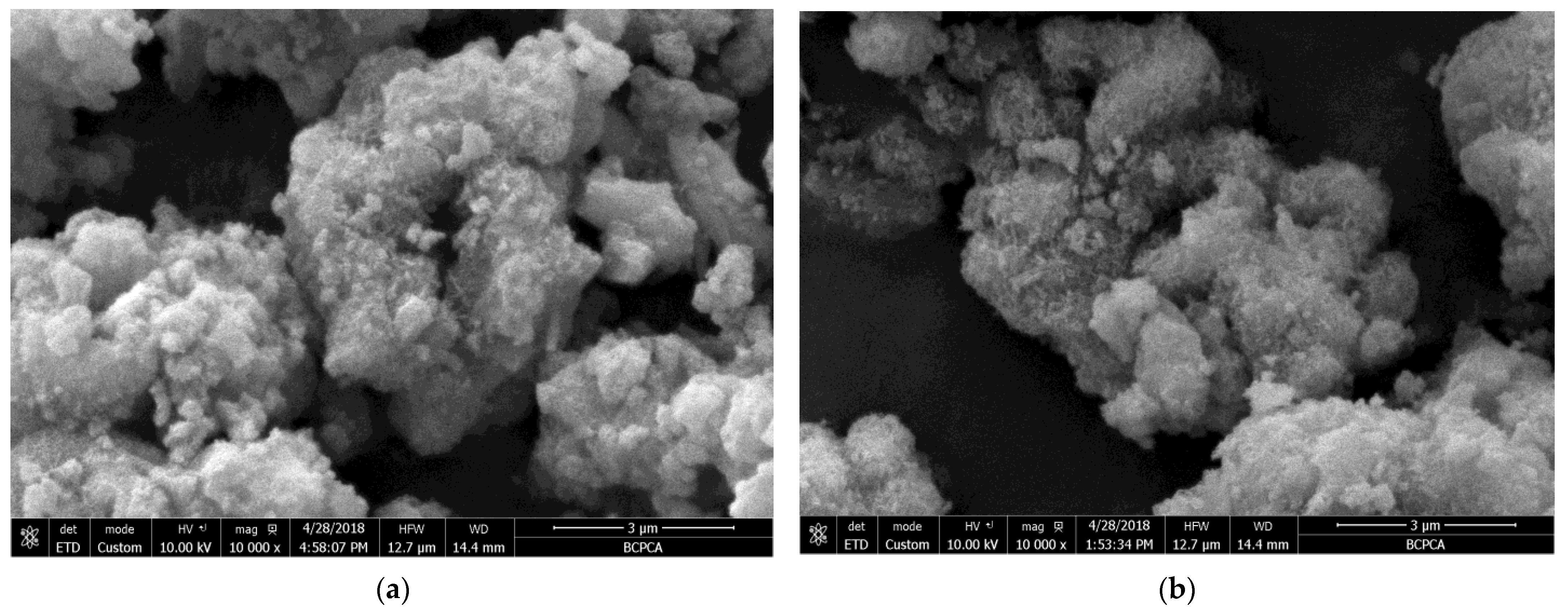
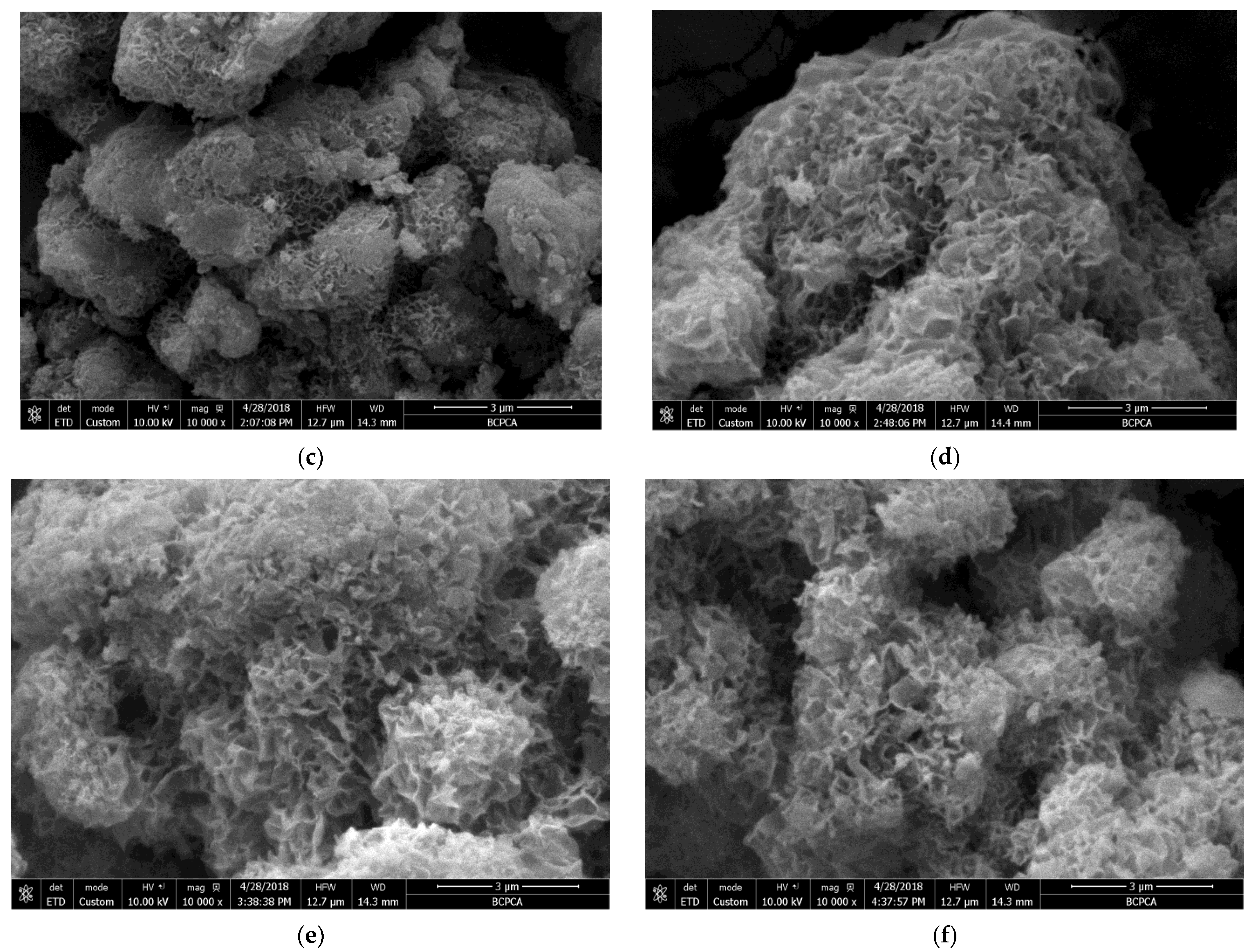
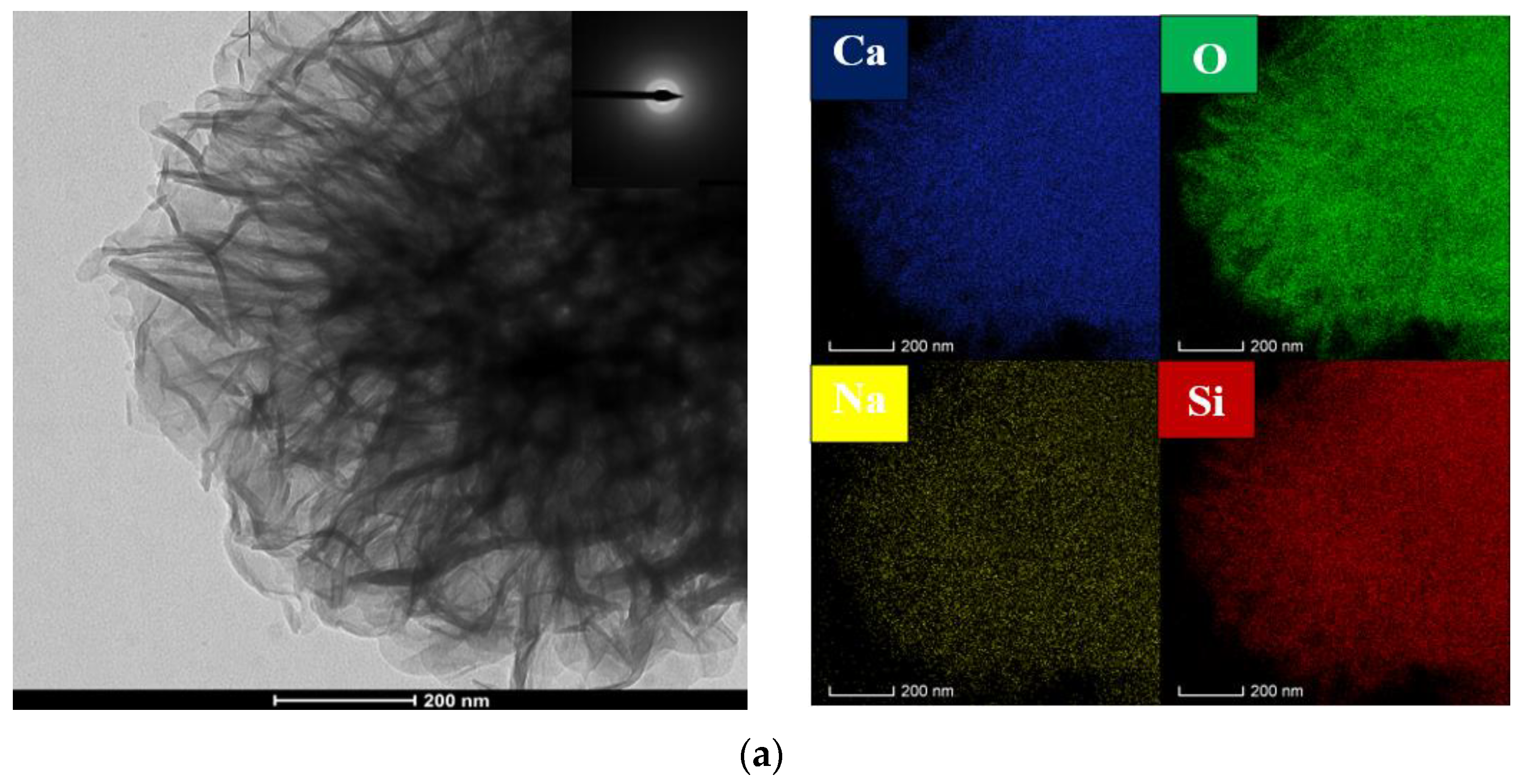
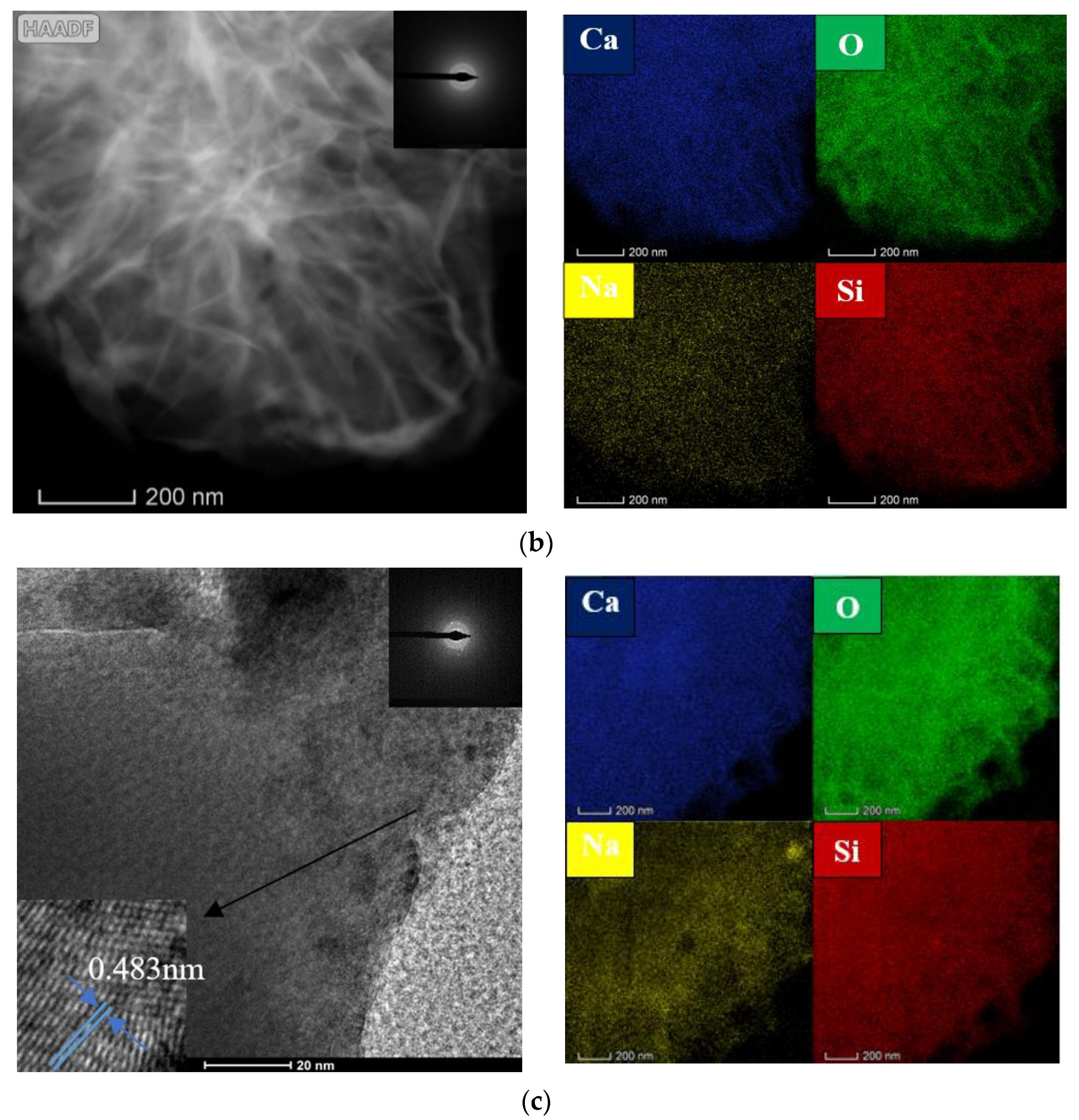
3.3. Microstructure Analysis
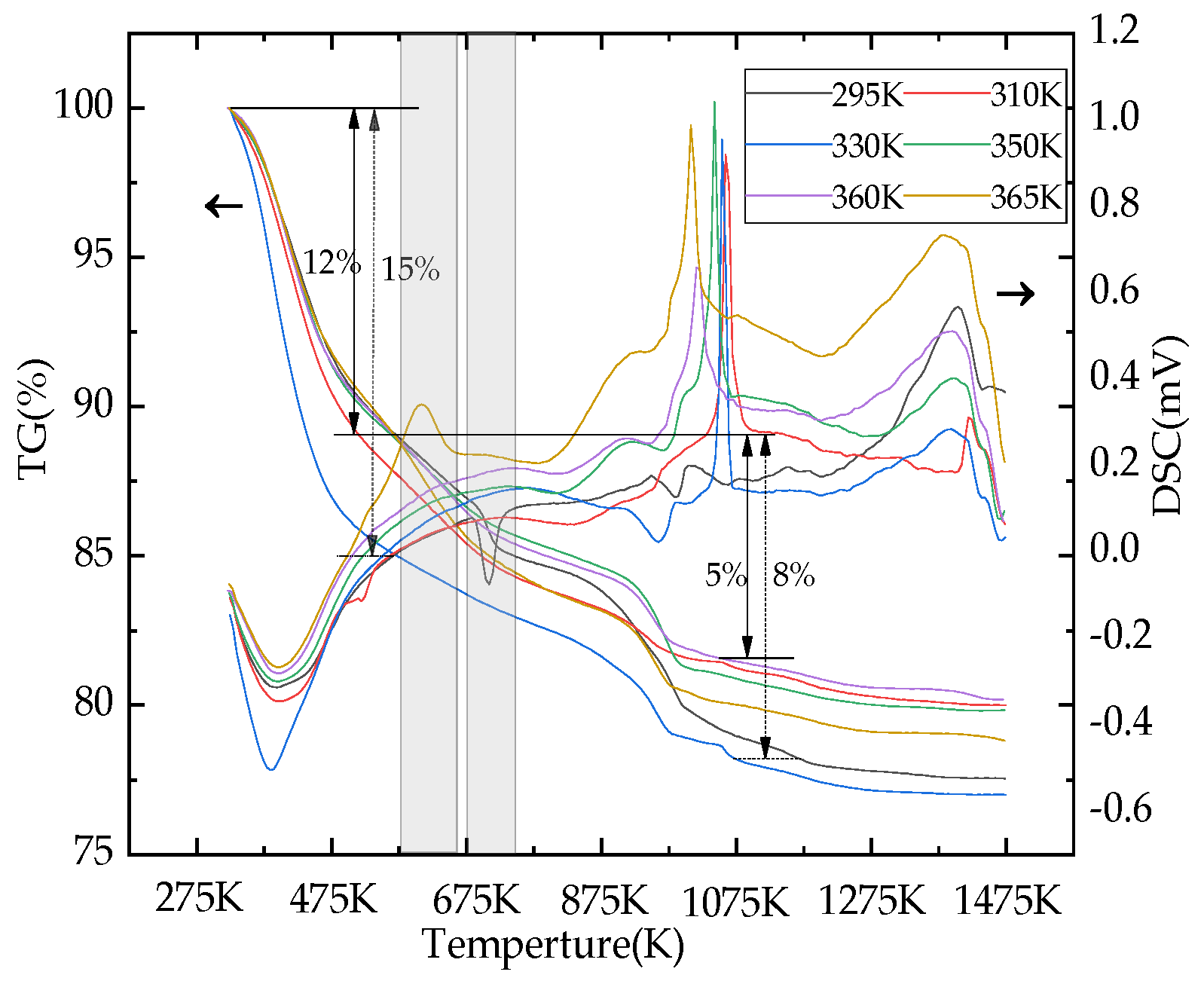
3.4. FTIR and TG–DSC Analysis
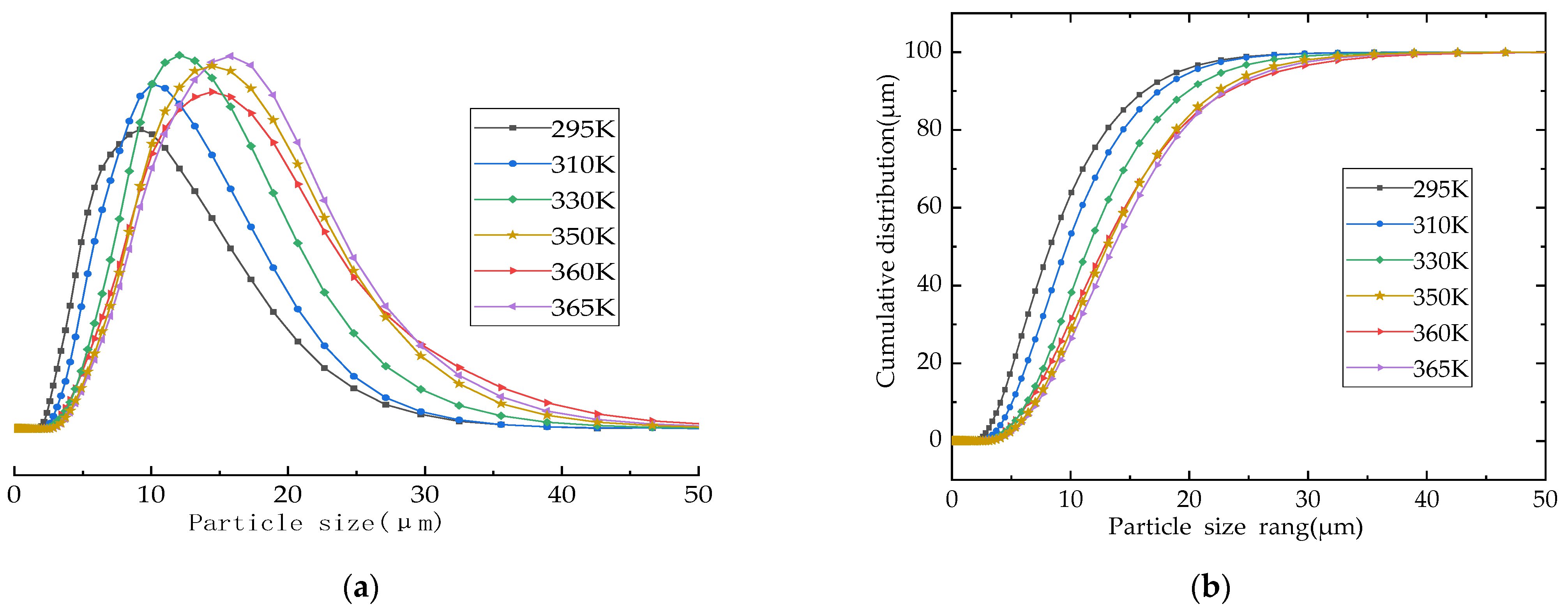
3.5. Chemical Composition and Particle Size Analysis
3.6. Synthesis Process Analysis
4. Conclusions
- (1)
- The XRD test results combined with thermodynamic calculation show that when ESS is used to synthesize microporous calcium silicate, calcium silicate products with different Si/Ca ratios are formed continuously with the progress of synthesis reaction. The crystalline forms of calcium silicate minerals synthesized in the early stage are also transformed, and 5CaO·6SiO2·5.5H2O and 5CaO·6SiO2·3H2O are more easily generated. When the synthesis temperature is 350 K (77 °C), 2CaO·3SiO2·2.5H2O will be generated. As the synthesis temperature continues to rise, 2CaO·SiO2·1.17H2O will be generated when it reaches 330 K (57 °C), and CaO·SiO2·H2O will be generated, but will eventually transform into the xonotlite-type calcium silicate crystal with a silicon to calcium ratio of 6:6, which is consistent with the thermodynamic calculation results.
- (2)
- The formation rate, crystallization degree, and particle size of the calcium silicate hydrate are positively correlated with temperature. The particle size of the calcium silicate hydrate was about 8 μm when the temperature was 295 K (22 °C). When the synthesis temperature was 330 K (57 °C), the particle size increased to about 14 μm. When the temperature reached above 350 K (77 °C), the average particle size reached about 17 μm, and the synthesis temperature was 360 K (87 °C). Therefore, the synthesized calcium silicate showed obvious crystal characteristics, which marks the transition from irregular amorphous to ordered crystalline.
- (3)
- When using ESS to synthesize calcium silicate minerals, Na and Al are the main components of the impurities. The XRF test results showed that the proportion of Ca and Na in the sample showed an obvious inverse relationship. Na+ will replace a small part of Ca2+ into the calcium silicate compound, forming a competitive relationship in the microporous calcium silicate structure. It is speculated that the reason is that both Na+ and Ca2+ play a role in balancing charge, while the electrostatic effect of Na+ is weaker than Ca2+.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohenoja, K.; Rissanen, J.; Kinnunen, P. Direct carbonation of peat-wood fly ash for carbon capture and utilization in construction application. J. CO2 Util. 2020, 40, 101203. [Google Scholar] [CrossRef]
- Xiaofeng, H.; Tova, J.; Andrey, K. Utilization of fly ash and waste lime from pulp and paper mills in the Argon Oxygen Decarburization process. J. Clean. Prod. 2020, 261, 121182. [Google Scholar]
- Guojing, W.; Xiao, F.; Min, G. Resource utilization of municipal solid waste incineration fly ash in iron ore sintering process: A novel thermal treatment. J. Clean. Prod. 2020, 263, 121400. [Google Scholar]
- Zhijie, Y.; De, Z.; Chenyang, F. Hydration Mechanisms of Alkali-Activated Cementitious Materials with Ternary Solid Waste Composition. Materials 2022, 15, 3616. [Google Scholar]
- Kaushal, K.; Rishabh, A.; Sarah, K. Characterization of fly ash for potential utilization in green concrete. Mater. Today: Proc. 2022, 56, 1886–1890. [Google Scholar]
- Rui, X.; Tao, L.; Lijing, W. Utilization of coal fly ash waste for effective recapture of phosphorus from waters. Chemosphere 2022, 287, 132431. [Google Scholar]
- Jinliang, L.; Yao, W. Predicting the chloride diffusion in concrete incorporating fly ash by a multi-scale model. J. Clean. Prod. 2022, 330, 129767. [Google Scholar]
- Zihao, L.; Koji, T.; Hidehiro, K. A study on engineering properties and environmental impact of sustainable concrete with fly ash or GGBS. Constr. Build. Mater. 2022, 316, 125776. [Google Scholar]
- Cheng, F.; Baomin, W.; Hongmei, A. A comparative study on characteristics and leaching toxicity of fluidized bed and grate furnace MSWI fly ash. J. Environ. Manag. 2022, 305, 114345. [Google Scholar]
- Teixeira, E.R.; Camões, A.; Branco, F.G.; Camões, A.; Branco, F.G. Synergetic effect of biomass fly ash on improvement of high-volume coal fly ash concrete properties. Constr. Build. Mater. 2022, 314, 125680. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, G.; Qiu, L.; Ye, X. Facile synthesis of Mg-doped calcium silicate porous nanoparticles for targeted drug delivery and ovarian cancer treatment. Ceram. Int. 2021, 47, 24942–24948. [Google Scholar] [CrossRef]
- Yayoi, K.; Shiyang, C.; Takehisa, H. Adsorption of a poorly water-soluble drug onto porous calcium silicate by the sealed heating method. Int. J. Pharm. 2020, 587, 119637. [Google Scholar]
- Yangyu, L.; Hongwei, J.; Guangxin, Z. Synthesis and humidity control performances of natural opoka based porous calcium silicate hydrate. Adv. Powder Technol. 2019, 30, 2733–2741. [Google Scholar]
- Sonjia, H.; Mikkel, S.B.; Søren, R.H. Impact of amorphous micro silica on the C-S-H phase formation in porous calcium silicates. J. Non-Cryst. Solids 2018, 481, 556–561. [Google Scholar]
- Kaihui, Z.; Lei, M.; Wei, M. Preparation of high strength lamellar porous calcium silicate ceramics by directional freeze casting. Ceram. Int. 2021, 47, 31187–31193. [Google Scholar]
- Lin, L.; Tao, J.; Bojian, C. Recycling of Ti-extraction blast furnace slag: Preparation of calcium silicate board with high slag content by steam pressure curing. Process Saf. Environ. Prot. 2022, 158, 432–444. [Google Scholar]
- Congqi, L.; Yong, Z.; Yongyi, L. Effects of nano-SiO2, nano-CaCO3 and nano-TiO2 on properties and microstructure of the high content calcium silicate phase cement (HCSC). Constr. Build. Mater. 2022, 314, 125377. [Google Scholar]
- Liu, Y.; Xie, M.; Xu, E.; Gao, X.; Yang, Y.; Deng, H. Development of calcium silicate-coated expanded clay based form-stable phase change materials for enhancing thermal and mechanical properties of cement-based composite. Sol. Energy 2018, 174, 24–34. [Google Scholar] [CrossRef]
- Boissonnet, G.; Chalk, C.; Nicholls, J. Thermal insulation of CMAS (Calcium-Magnesium-Alumino-Silicates)-attacked plasma-sprayed thermal barrier coatings. J. Eur. Ceram. Soc. 2020, 40, 2042–2049. [Google Scholar] [CrossRef]
- Leite, F.H.G.; Almeida, T.F.; FariaJr, R.T. Synthesis and characterization of calcium silicate insulating material using avian eggshell waste. Ceram. Int. 2017, 43, 4674–4679. [Google Scholar] [CrossRef]
- Fang, Q.; Junya, C.; Ganyu, Z. Crystallization behavior of calcium silicate hydrate in highly alkaline system: Structure and kinetics. J. Cryst. Growth 2022, 584, 126578. [Google Scholar]
- Zhijie, Y.; Dong, K.; De, Z. Crystal transformation of calcium silicate minerals synthesized by calcium silicate slag and silica fume with increase of C/S molar ratio. J. Mater. Res. Technol. 2021, 15, 4185–4192. [Google Scholar]
- Sylvain, G.; Francies, C.; Yannick, L. X-ray diffraction: A powerful tool to probe and understand the structure of nanocrystalline calcium silicate hydrates. Struct. Sci. Cryst. Eng. Mater. 2013, 69, 465–473. [Google Scholar]
- Shaw, S.; Clark, S.M.; Henderson, C.M.B. Hydrothermal formation of the calcium silicate hydrates, tobermorite (Ca5Si6O16(OH)2⋅4H2O) and xonotlite (Ca6Si6O17(OH)2): An in situ synchrotron study. Chem. Geol. 2000, 167, 129–140. [Google Scholar] [CrossRef]


| Constituents | Content (g/L) |
|---|---|
| SiO2 | 47.9 |
| Al2O3 | 40.1 |
| CaO | 4.09 |
| MgO | 0.65 |
| Fe2O3 | 3.43 |
| K2O | 0.84 |
| Na2O | 0.32 |
| SO3 | 0.52 |
| Constituents | Content (g/L) |
|---|---|
| SiO2 | 40~60 |
| NaOH | 60~80 |
| Al3+ | 0.5~2 |
| Fe3+ | 0.01~0.05 |
| Number | Temperature | Proportion (%) | |||||
|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | Na2O | MgO | Others | ||
| 1 | 295 K | 55.14 | 37.31 | 3.35 | 0.71 | 0.70 | 2.79 |
| 2 | 310 K | 42.26 | 50.65 | 2.44 | 2.27 | 0.10 | 2.28 |
| 3 | 330 K | 42.14 | 50.52 | 2.38 | 2.44 | 0.17 | 2.35 |
| 4 | 350 K | 41.30 | 50.41 | 2.40 | 3.20 | 0.29 | 2.40 |
| 5 | 360 K | 39.70 | 52.21 | 2.10 | 3.95 | 0.21 | 1.83 |
| 6 | 365 K | 40.04 | 50.04 | 2.34 | 3.97 | 0.20 | 3.05 |
| Chemical Formula | a + b × 10−3 + c × 10−5 T−2 | ||||
|---|---|---|---|---|---|
| a | b | c | |||
| 3CaO·2SiO2·3H2O | −4782.312 | 312.126 | 341.163 | 188.698 | −61.3790 |
| CaO·2SiO2·2H2O | −3138.837 | 171.126 | 187.485 | 78.241 | −43.304 |
| 2CaO·SiO2·1.17H2O | −2665.208 | 160.666 | 171.711 | 93.722 | −30.962 |
| 5CaO·6SiO2·3H2O | −9935.745 | 513.168 | 600.613 | 312.545 | −87.111 |
| 5CaO·6SiO2·5.5H2O | −10,686.770 | 611.492 | 462.750 | 791.194 | 0.000 |
| 2CaO·3SiO2·2.5H2O | −4920.384 | 271.542 | 332.503 | 151.879 | −73.429 |
| CaO·SiO2·H2O | −1917.845 | 112.707 | 263.112 | 64.952 | −36.401 |
| 6CaO·6SiO2·H2O | −10,024.864 | 507.519 | 553.334 | 272.797 | −76.776 |
| Ca(OH)2 | −1002.947 | −74.517 | 89.248 | 33.150 | −10.348 |
| Na2SiO3 | −1561.427 | 113.847 | 112.789 | 76.665 | −19.708 |
| H2O | −285.830 | 69.950 | 186.884 | −464.247 | −19.565 |
| NaOH | −469.863 | 44.769 | 12,683.218 | −52,451.899 | −2228.366 |
| Number | Chemical Formula | The Equation for T(K) and ΔG(kJ/mol) |
|---|---|---|
| 1 | 3CaO·2SiO2·3H2O | ΔG = −0.3455T + 41.1508 |
| 2 | CaO·2SiO2·2H2O | ΔG =0.0257T − 38.66 |
| 3 | 2CaO·SiO2·1.17H2O | ΔG = −0.2754T + 10.6294 |
| 4 | 5CaO·6SiO2·3H2O | ΔG = −0.4426T − 52.4165 |
| 5 | 5CaO·6SiO2·5.5H2O | ΔG = −0.3622T − 89.9723 |
| 6 | 2CaO·3SiO2·2.5H2O | ΔG = −0.0891T − 52.8534 |
| 7 | CaO·SiO2·H2O | ΔG = −0.0953T − 6.6816 |
| 8 | 6CaO·6SiO2·H2O | ΔG = −0.7291T + 6.1358 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.; Yang, Z.; Zhang, D.; Jiao, Y.; Fang, C.; Wang, K. Study on the Effect of Temperature on the Crystal Transformation of Microporous Calcium Silicate Synthesized of Extraction Silicon Solution from Fly Ash. Materials 2023, 16, 2154. https://doi.org/10.3390/ma16062154
Kang D, Yang Z, Zhang D, Jiao Y, Fang C, Wang K. Study on the Effect of Temperature on the Crystal Transformation of Microporous Calcium Silicate Synthesized of Extraction Silicon Solution from Fly Ash. Materials. 2023; 16(6):2154. https://doi.org/10.3390/ma16062154
Chicago/Turabian StyleKang, Dong, Zhijie Yang, De Zhang, Yang Jiao, Chenyang Fang, and Kaiyue Wang. 2023. "Study on the Effect of Temperature on the Crystal Transformation of Microporous Calcium Silicate Synthesized of Extraction Silicon Solution from Fly Ash" Materials 16, no. 6: 2154. https://doi.org/10.3390/ma16062154





