Microstructure and Corrosion Behavior of ZnAl12Mg3Si0.3 Double-Batch Hot-Dip Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hot-Dip Procedure
2.3. Characterization Methods
2.4. Corrosion Testing Method
3. Results and Discussion
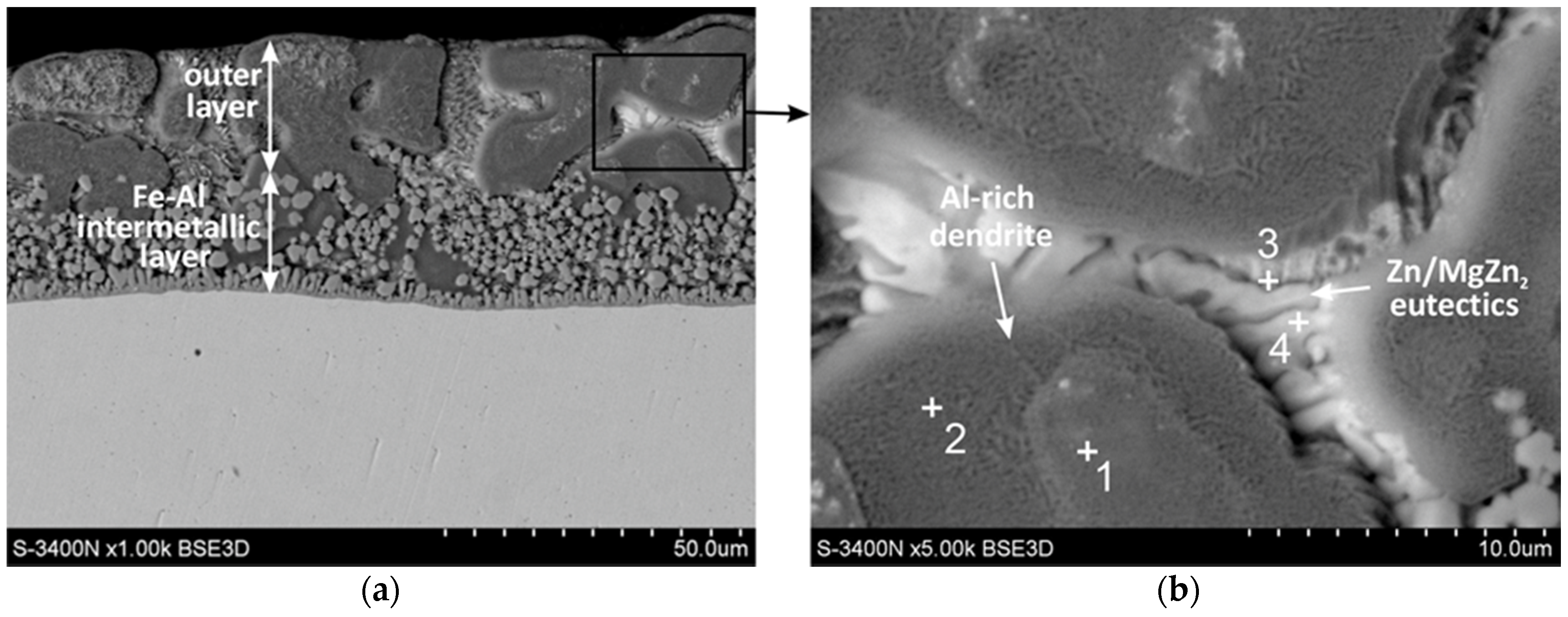
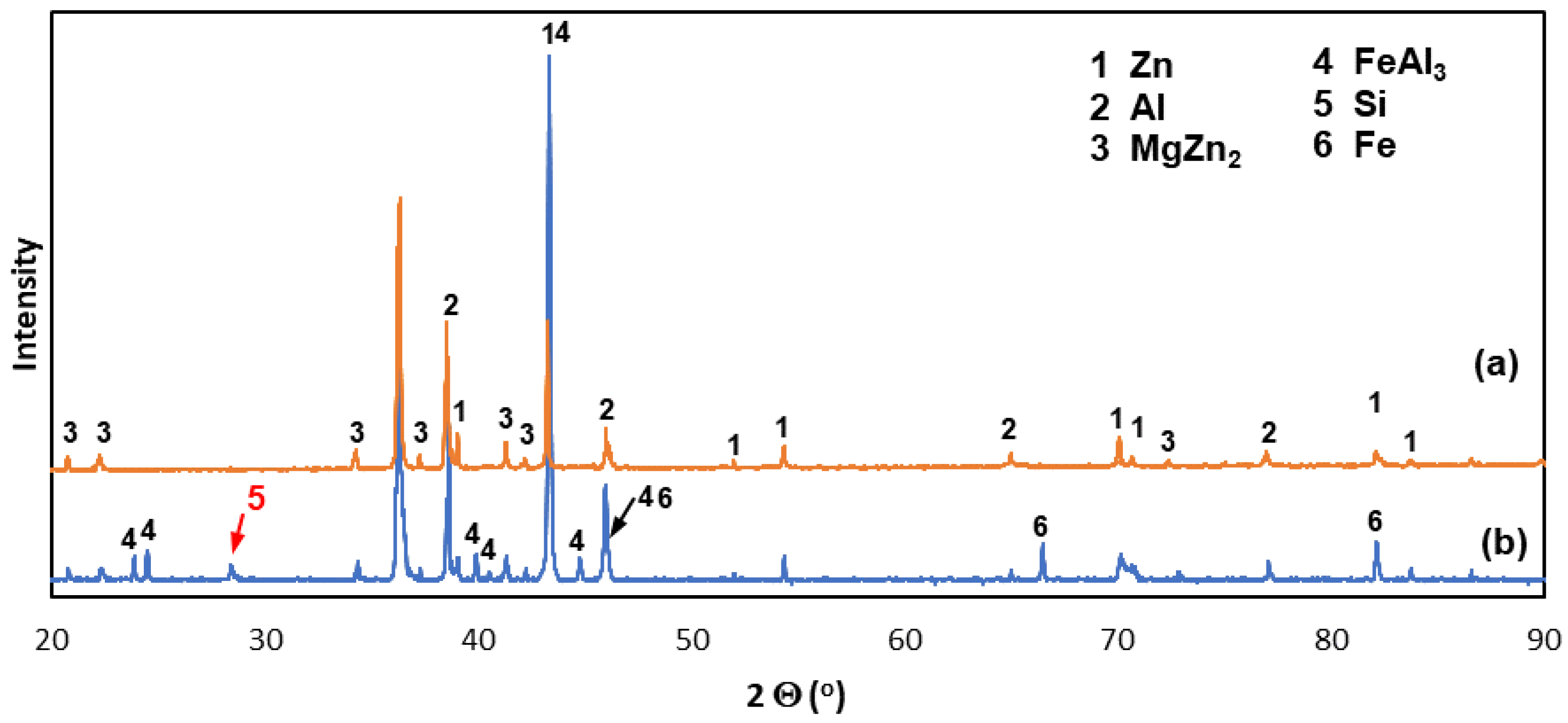
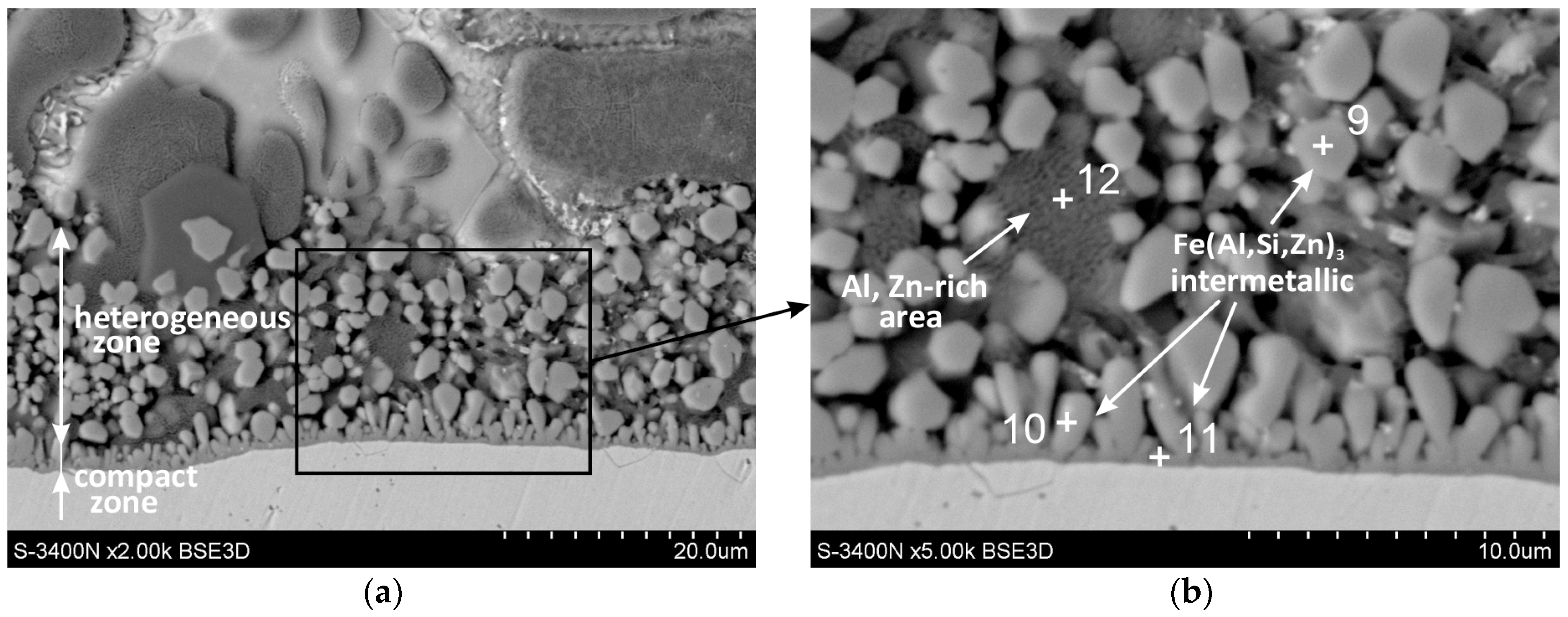
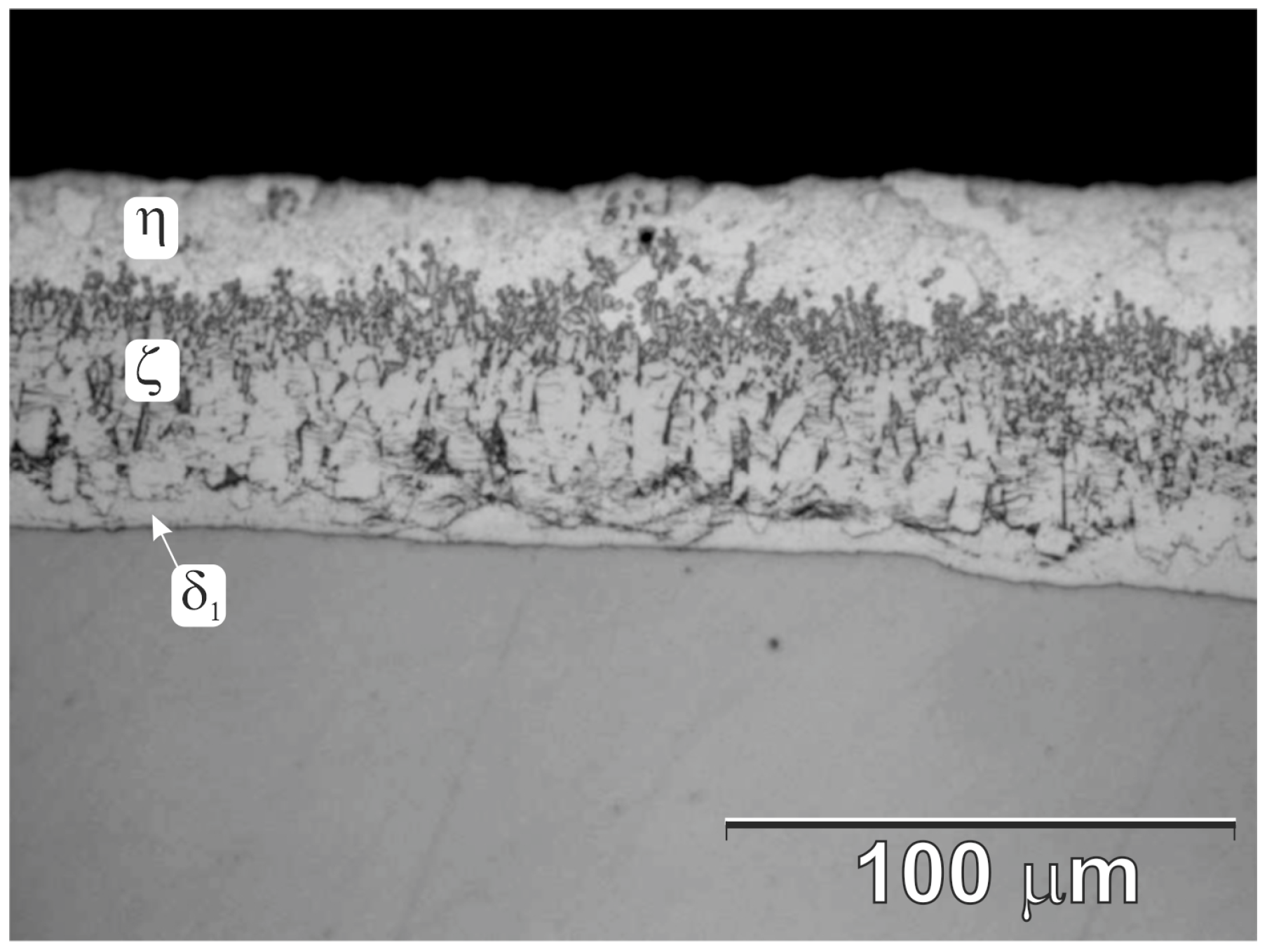
3.1. Cross-Section Microstructure of Coatings
3.2. Corrosion Resistance Determined via NSS Test
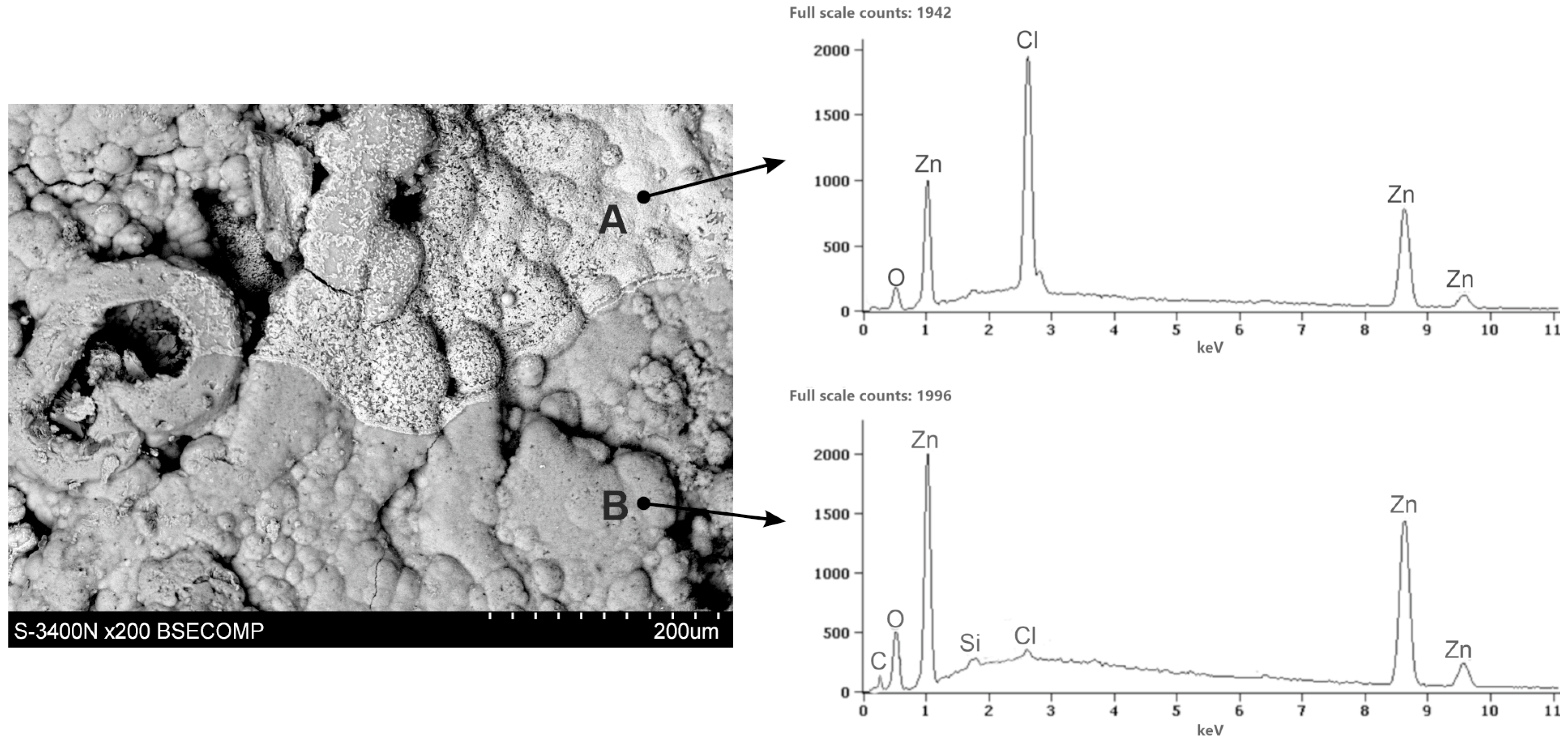
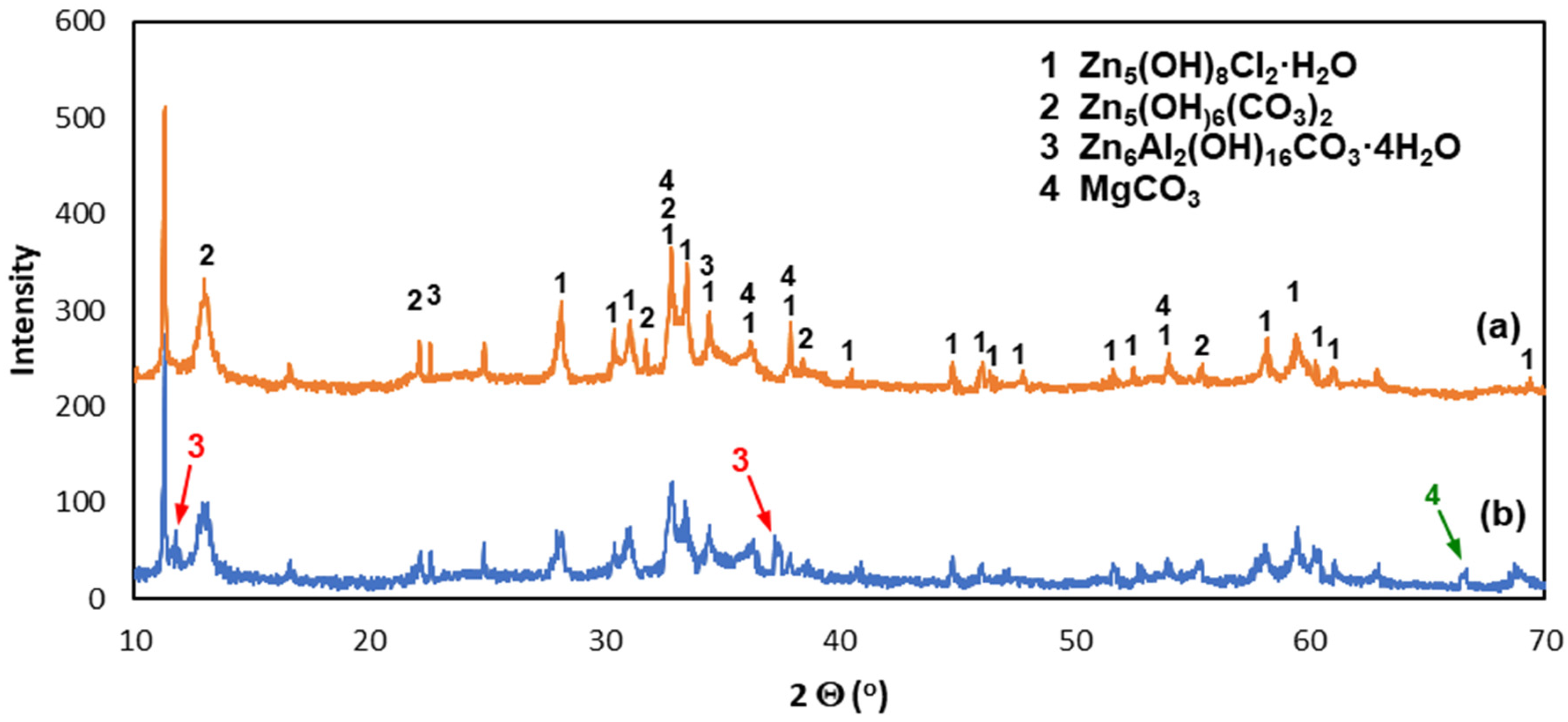
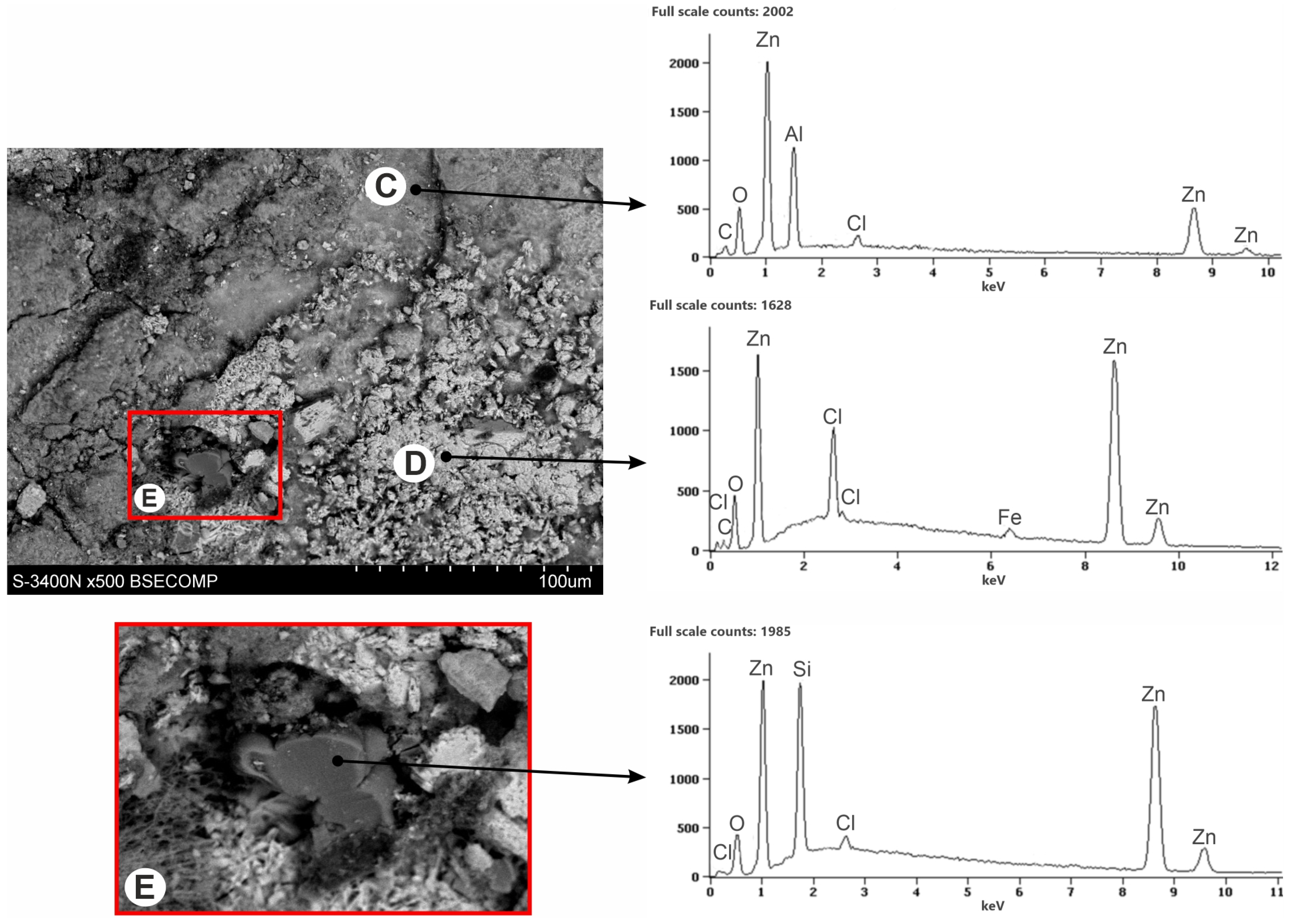
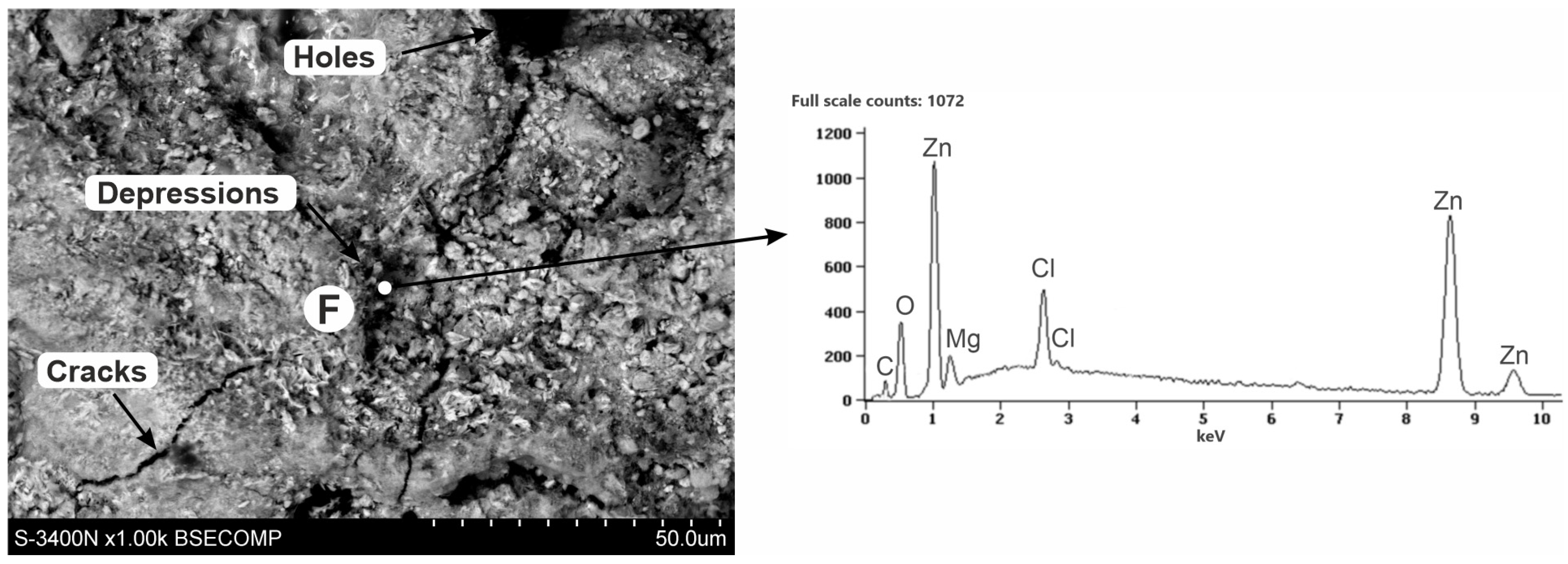
3.3. Corrosion Products’ Characterization
3.4. Corrosion Behavior of Coating
4. Conclusions
- The ZAMS coatings obtained by the double hot-dip method on Sebisty steel with increased strength had a duplex structure. The diffusion layer of the coating was made of the Fe(Al,Si,Zn)3 intermetallic phase, and the outer layer was Al-rich dendrites and Zn-rich inter-dendrites, in which Zn/MgZn2 eutectic and MgZn2 intermetallic precipitates were located. Si precipitates were also locally formed in the inter-dendritic areas.
- The ZAMS coatings showed better corrosion resistance than conventional HDG coatings. In the NSS test, the ZAMS coatings showed lower mass gains of corrosion products and did not penetrate the substrate, despite the lower thickness compared to HDG coatings.
- The ZAMS coatings had high corrosion resistance due to the formation of protective corrosion products. In the NSS test, the coating was covered with a layer of simonkolleite—Zn5(OH)8Cl2·H2O, which was transformed into hydrozincite—Zn5(OH)6(CO3)2. On the surface of Al-rich dendrites, a Zn6Al2(OH)16CO3·4H2O layer was formed, which may passivate this phase. The presence of Mg in the inter-dendritic areas probably caused the formation of MgCO3, which may be the reason for limiting the transformation of the simonkolleite, which protects against further corrosion, into porous hydrozincite.
- The presence of MgZn2 intermetallics in the coating can provide sacrificial protection for both Zn-rich inter-dendrites as well as Al-rich dendrites.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Suliga, M.; Wartacz, R.; Hawryluk, M.; Kostrzewa, J. The Effect of Drawing in Conventional and Hydrodynamic Dies on Structure and Corrosion Resistance of Hot-Dip Galvanized Zinc Coatings on Medium-Carbon Steel Wire. Materials 2022, 15, 6728. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejczyk, D. Effect of High Temperature Oxidation on Structure and Corrosion Resistance of the Zinc Coating Deposited on Cast Iron. Arch. Metall. Mater. 2012, 57, 145–154. [Google Scholar] [CrossRef]
- Šmak, M.; Kubíček, J.; Kala, J.; Podaný, K.; Vaněrek, J. The Influence of Hot-Dip Galvanizing on the Mechanical Properties of High-Strength Steels. Materials 2021, 14, 5219. [Google Scholar] [CrossRef] [PubMed]
- Kania, H.; Saternus, M. Evaluation and Current State of Primary and Secondary Zinc Production—A Review. Appl. Sci. 2023, 13, 2003. [Google Scholar] [CrossRef]
- Kuklik, V.; Kudláček, J. Hot-Dip Galvanizing of Steel Structures; Elsevier Ltd.: Boston, MA, USA, 2016. [Google Scholar]
- Shimoda, N.; Ueda, K.; Kubo, Y. Corrosion Resistance of Several Zn-Al-Mg Alloy Coated Steels. Nippon Steel & Sumitomo Metal; Technical Report No. 108. March 2015, pp. 60–63. Available online: https://www.nipponsteel.com/en/tech/report/nssmc/pdf/108-14.pdf (accessed on 17 January 2023).
- Tanaka, S.; Honda, K.; Takahashi, A.; Morimoto, Y.; Kurosaki, M.; Shindo, H.; Nishimura, K.; Sugiyama, M. The performance of Zn-Al-Mg-Si hot-dip galvanized steel sheet. In Proceedings of the 5th International Conference on Zinc and Zinc Alloy Coated Steel, GALVATECH’ 2001, Brussels, Belgium, 26‒28 June 2001; pp. 153–160. [Google Scholar]
- Tsujimura, T.; Komatsu, A.; Andoh, A. Influence of Mg content in coating layer and coating structure on corrosion resistance of hot-dip Zn-Al-Mg alloy coated steel sheet. In Proceedings of the 5th International Conference on Zinc and Zinc Alloy Coated Steel, GALVATECH’2001, Brussels, Belgium, 26‒28 June 2001; pp. 145–152. [Google Scholar]
- Volt, M.; Bleeker, R.; Maalman, T.; van Perlstein, E. MagiZincTM: A new generation of hot-dip galvanized products. In Proceedings of the Galvanized Steel Sheet Forum, ILZRO and IZA, Duesseldorf, Germany, 30–31 May 2006; pp. 13–24. [Google Scholar]
- Magnelis, A.M. Innowacyjna powłoka metaliczna gwarantująca ochronę w najsurowszych warunkach. Available online: http://industry.arcelormittal.com/repository/fce/Brochures/Magnelis_brochure_PL.pdf (accessed on 19 February 2015).
- Du, A.; Huo, Y.; Hu, J. Study of Fluxing Process for Hot Dipping Galfan Alloy on Steel Wire. Adv. Mater. Res. 2012, 433–440, 111–115. [Google Scholar] [CrossRef]
- Manna, M. Effect of Fluxing Chemical: An Option for Zn-5wt.%Al Alloy Coating on Wire Surface by Single Hot Dip Process. Surf. Coat. Technol. 2011, 205, 3716–3721. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Grandhi, S.; Oh, M.-S. Improving the Coatability of Zn–Mg–Al Alloy on Steel Substrate by the Surface Pretreatment of SnCl2-Added Zinc Ammonium Chloride. Appl. Sci. 2023, 13, 950. [Google Scholar] [CrossRef]
- Kania, H. Structure Shaping and Corrosion Resistance of Zn-Al Coatings Obtained in Hot Dip Metalization; Silesian University of Technology: Gliwice, Poland, 2017. [Google Scholar]
- Gao, L.; Li, Z.; Kuang, X.; Yin, F.; Ji, H. Formation of periodic layered structure during hot-dip galvanizing in Al-Zn-Mg bath. Surf. Coat. Technol. 2016, 304, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Porter, F.C. Zinc Handbook: Properties Processing and Use in Design; Marcel Dekker: New York, NY, USA, 1991. [Google Scholar]
- Massalski, T.B.; Okamoto, H. Binary alloy phase diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Nowacki, K.; Kania, H.; Wieczorek, J.; Smalcerz, A. The Properties of ZnAlMg Alloys for Batch Hot Dip Metallization. Solid State Phenom. 2016, 24, 143–148. [Google Scholar] [CrossRef]
- Skupińska, A. Fundamentals of the Technology of Producing Zn-Al-Mg Coatings with Increased Corrosion Resistance by Double Dip Batch Method. Ph.D. Thesis, Silesian University of Technology, Gliwice, Poland, 2020. [Google Scholar]
- Mendala, J. The influence of Si addition in 55AlZn bath on the coating structures obtained in the batch hot dip metallization. IOP Conf. Ser. Mater. Sci. Eng. 2011, 22, 012005. [Google Scholar] [CrossRef]
- Marek, A. Hot dip Zn-5Al coatings with improved corrosion resistance of reinforcement steel. Metalurgija 2022, 61, 389–391. [Google Scholar]
- ISO 9227:2017; Corrosion Tests In Artificial Atmospheres—Salt Spray Tests. Polish Committee for Standardization: Warszawa, Poland, 2017.
- Honda, K.; Ushioda, K.; Yamada, W. Influence of Si Addition to the Coating Bath on the Growth of the Al-Fe Alloy Layer in Hot-dip Zn-Al-Mg Alloy-coated Steel Sheets. ISIJ Int. 2011, 51, 1895. [Google Scholar] [CrossRef] [Green Version]
- Ranjan, M.; Tewari, R.; van Ooij, W.J.; Vasudevan, V.K. Effect of Ternary Additions on the Structure and Properties of Coatings Produced by a High Aluminum Galvanizing Bath. Metall. Trans. A 2004, 35, 3707–3720. [Google Scholar] [CrossRef]
- Perrot, P.; Tissier, J.C.; Dauphin, J.Y. Stable and Metastable Equilibria in the Fe-Zn-Al System at 450 °C. Int. J. Mater. Res. 1992, 83, 786–790. [Google Scholar] [CrossRef]
- Peng, H.P.; Su, X.P.; Wang, J.H.; Li, Z. Interface reaction mechanism for galvanizing in Zn-Al baths. Chin. J. Nonferrous. Met. 2012, 22, 3168–3175. [Google Scholar]
- McDevitt, E.; Morimoto, Y.; Meshii, M. Characterization of the Fe-Al interfacial layer in a commercial hot-dip galvanized coating. ISIJ Int. 1997, 37, 776–782. [Google Scholar] [CrossRef]
- Horstmann, D. The Course of Reactions between Iron and Molten Zinc Containing Aluminum; Zinc Development Association: London, UK, 1976. [Google Scholar]
- Zamanzadea, M.; Hasemannb, G.; Motza, C.; Krügerb, M.; Barnoushac, A. Vacancy effects on the mechanical behaviour of B2-FeAl intermetallics. Mater. Sci. Eng. A 2018, 712, 88–96. [Google Scholar] [CrossRef]
- Zamanzade, M.; Barnoush, A.; Motz, C. A Review on the Properties of Iron Aluminide Intermetallics. Crystals 2016, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Qian, W.J.; Gu, W.G. Inhibitory action of Si on growth of interfacial compound layer during hot-dip aluminizing. Acta Metall. Sin. 1994, 30, 404–408. [Google Scholar]
- Li, K.; Liu, Y.; Tu, H.; Su, X.; Wang, J. Effect of Si on the growth of Fe-Al intermetallic layer in Zn-11%Al-3%Mg coating. Surf. Coat. Technol. 2016, 306, 390–396. [Google Scholar] [CrossRef]
- Maitra, T.; Gupta, S.P. Intermetallic compound formation in Fe–Al–Si ternary system: Part II. Mater. Charact. 2002, 49, 293–311. [Google Scholar] [CrossRef]
- Du, Y.; Schuster, J.; Liu, Z.; Hu, R.; Nash, P.; Sun, W.; Zhang, W.; Wang, J.; Zhang, L.; Tang, C. A thermodynamic description of the Al–Fe–Si system over the whole composition and temperature ranges via a hybrid approach of CALPHAD and key experiments. Intermetallics 2008, 16, 554–570. [Google Scholar] [CrossRef]
- Liberski, P. Physicochemical Basic of Rational Shaping of Aluminium Dip Coatings on Iron; Silesian University of Technology: Gliwice, Poland, 2002. [Google Scholar]
- Riabov, W.R. Aluminizing of Steel; Oxonian Press: New Delhi, India, 1985. [Google Scholar]
- Pokorny, P.; Kolisko, J.; Balik, L.; Novak, P. Description of structure of Fe-Zn intermetallic compounds present in hot-dip galvanized coatings on steel. Metalurgija 2015, 54, 707–710. [Google Scholar]
- Zhang, X.G. Corrosion and Electrochemistry of Zinc; Springer: New York, NY, USA, 2013. [Google Scholar]
- Kania, H.; Sipa, J. Microstructure characterization and corrosion resistance of zinc coating obtained on high-strength grade 10.9 bolts using a new thermal diffusion process. Materials 2019, 12, 1400. [Google Scholar] [CrossRef] [Green Version]
- Prosek, T.; Thierry, D.; Taxen, C.; Maixner, J. Effect of cations on corrosion of zincand carbon steel covered with chloride deposits under atmosphericconditions. Corros. Sci. 2007, 49, 2676–2693. [Google Scholar] [CrossRef]
- Volovitch, P.; Allely, C.; Ogle, K. Understanding corrosion via corrosion productcharacterization: I. Case study of the role of Mg alloying in Zn–Mg coating on steel. Corros. Sci. 2009, 51, 1251–1262. [Google Scholar] [CrossRef]
- Komatsu, A.; Izutani, H.; Tsujimura, T.; Andoh, A.; Kittaka, T. Corrosion resistanceand protection mechanism of hot-dip Zn–Al–Mg alloy coated steel sheet under accelerated corrosion environment. Tetsu-to-Hagane 2000, 86, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Hosking, N.C.; Ström, M.A.; Shipway, P.H.; Rudd, C.D. Corrosion resistance of zinc–magnesium coated steel. Corros. Sci. 2007, 49, 3669–3695. [Google Scholar] [CrossRef]
- Yang, D.; Chen, J.; Han, Q.; Liu, K.R. Effects of lanthanum addition on corrosion resistance of hot-dipped galvalume coating. J. Rare Earths 2009, 27, 114–118. [Google Scholar] [CrossRef]
- Baszkiewicz, J.; Kamiński, N. Korozja Materiałów; Oficyna Wydawnicza Politechniki Warszawskiej: Warszawa, Poland, 2006. [Google Scholar]
- Birbilis, N.; Buchheit, R.G. Electrochemical Characteristics of Intermetallic Phases in Aluminum Alloys. J. Electrochem. Soc. 2005, 152, B140–B151. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Ogle, K. Dealloying of MgZn2 Intermetallic in Slightly Alkaline Chloride Electrolyte and Its Significance in Corrosion Resistance. J. Electrochem. Soc. 2017, 164, C952–C961. [Google Scholar] [CrossRef]
- Jönsson, M.; Persson, D.; Thierry, D. Corrosion product formation during NaCl induced atmospheric corrosion of magnesium alloy AZ91D. Corros. Sci. 2007, 49, 1540–1558. [Google Scholar] [CrossRef]
- Li, S.; Gao, B.; Tu, G.; Hu, L.; Sun, S.; Zhu, G.; Yin, S. Effects of magnesium on the microstructure and corrosion resistance of Zn-55Al-1.6Si coating. Constr. Build. Mater. 2014, 71, 124–131. [Google Scholar] [CrossRef]
- Vu, A.Q.; Vuillemin, B.; Oltra, R.; Allély, C. In Situ Investigation of Sacrificial Behaviour of Hot Dipped AlSi Coating in Sulphate and Chloride Solutions. Corros. Sci. 2013, 70, 112–118. [Google Scholar] [CrossRef]



| Content (wt.%) | Strength Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | S | P | Al | Fe and Others | Re (N/mm2) | Rm (N/mm2) | A (%) |
| 0.06 | 0.20 | 0.80 | 0.003 | 0.009 | 0.035 | rest | 465 | 528 | 23 |
| Bath | Bath Designation | Content (wt.%) | |||||
|---|---|---|---|---|---|---|---|
| Al | Fe | Si | Mg | Bi | Zn and Others | ||
| Zn | HDG (Hot-Dip Galvanizing) | 0.0059 | 0.031 | 0.001 | 0.002 | 0.061 | rest |
| ZnAl12Mg3Si0.3 | ZAMS (Zinc, Aluminum, Magnesium, Silicon) | 11.86 | 0.024 | 0.32 | 3.15 | 0.0001 | rest |
| Point No. | Mg (at.%) | Al (at.%) | Fe (at.%) | Zn (at.%) | Phase |
|---|---|---|---|---|---|
| 1 | - | 82.5 | - | 17.5 | Al-rich phase |
| 2 | - | 81.7 | - | 18.3 | Al-rich phase |
| 3 | 7.8 | 8.7 | 0.9 | 82.5 | MgZn2 intermetallic |
| 4 | 0.7 | 6.0 | 0.6 | 92.8 | Zn-rich phase |
| Point No. | Mg (at. %) | Al (at. %) | Si (at. %) | Fe (at. %) | Zn (at. %) | Atom Ratio Zn/Mg | Phase |
|---|---|---|---|---|---|---|---|
| 5 | - | 82.1 | - | - | 17.9 | - | Al-rich phase |
| 6 | - | 7.1 | - | 0.4 | 92.5 | - | Zn-rich phase |
| 7 | 31.77 | - | - | - | 68.23 | 2.14 | MgZn2 intermetallic |
| 8 | - | 2.0 | 98.0 | - | - | - | Si particles |
| Point No. | Al (at.%) | Si (at.%) | Fe (at.%) | Zn (at.%) | (Al + Si + Zn) (at. %) | Atom Ratio (Al + Si)/Fe | Atom Ratio (Al + Si + Zn)/Fe | Phase |
|---|---|---|---|---|---|---|---|---|
| 9 | 55.6 | 8.5 | 25.2 | 10.7 | 74.8 | 2.54 | 2.96 | Fe (Al, Si, Zn)3 |
| 10 | 56.2 | 8.3 | 24.6 | 10.9 | 75.4 | 2.62 | 3.06 | Fe (Al, Si, Zn)3 |
| 11 | 52.6 | 7.7 | 25.5 | 14.2 | 74.5 | 2.36 | 2.92 | Fe (Al, Si, Zn)3 |
| 12 | 73.8 | 1.4 | 4.9 | 19.9 | - | - | - | Al-rich phase |
| Phase | Ecorr vs. SCE * (mV) |
|---|---|
| Al | −849 |
| Zn | −1028 |
| MgZn2 | −1095 |
| Si | −452 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kania, H.; Marek, A.; Zoran, M.; Spławski, M.; Kupczyk, P.; Wiewióra, M.; Kupczyk, A. Microstructure and Corrosion Behavior of ZnAl12Mg3Si0.3 Double-Batch Hot-Dip Coatings. Materials 2023, 16, 2162. https://doi.org/10.3390/ma16062162
Kania H, Marek A, Zoran M, Spławski M, Kupczyk P, Wiewióra M, Kupczyk A. Microstructure and Corrosion Behavior of ZnAl12Mg3Si0.3 Double-Batch Hot-Dip Coatings. Materials. 2023; 16(6):2162. https://doi.org/10.3390/ma16062162
Chicago/Turabian StyleKania, Henryk, Anżelina Marek, Michał Zoran, Marcin Spławski, Przemysław Kupczyk, Mateusz Wiewióra, and Aleksandra Kupczyk. 2023. "Microstructure and Corrosion Behavior of ZnAl12Mg3Si0.3 Double-Batch Hot-Dip Coatings" Materials 16, no. 6: 2162. https://doi.org/10.3390/ma16062162





