Physico-Chemical Properties of Lithium Silicates Related to Their Utilization for Concrete Densifiers
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Lithium Silicate Densifiers
2.2. Preparation and Surface Treatment of Cement Mortar Samples
2.3. Chemical Characterization
2.3.1. FT-IR Measurement
2.3.2. NMR Measurement
2.4. Rheological Investigation
2.5. Water Permeability Measurement
3. Results and Discussion
3.1. Characterization of Lithium Silicate Densifiers
3.2. Monitoring of Gelation Process by FT-IR
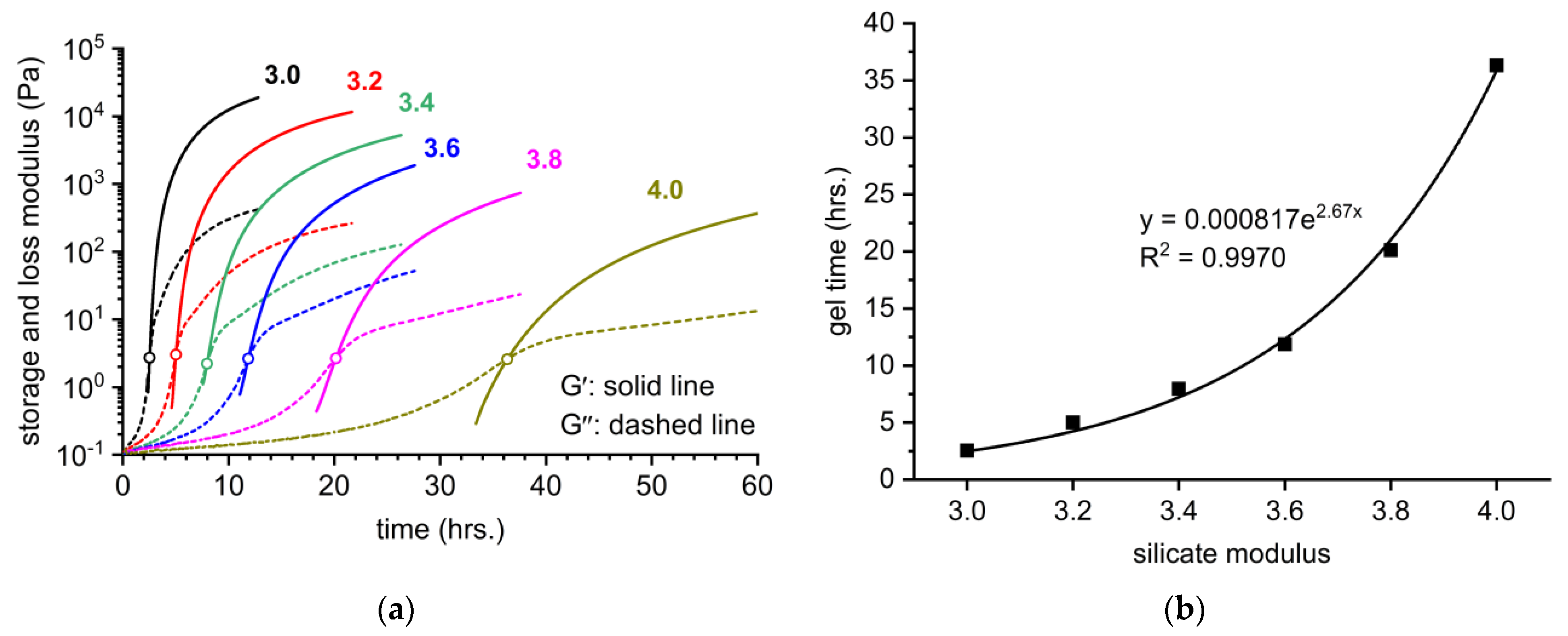
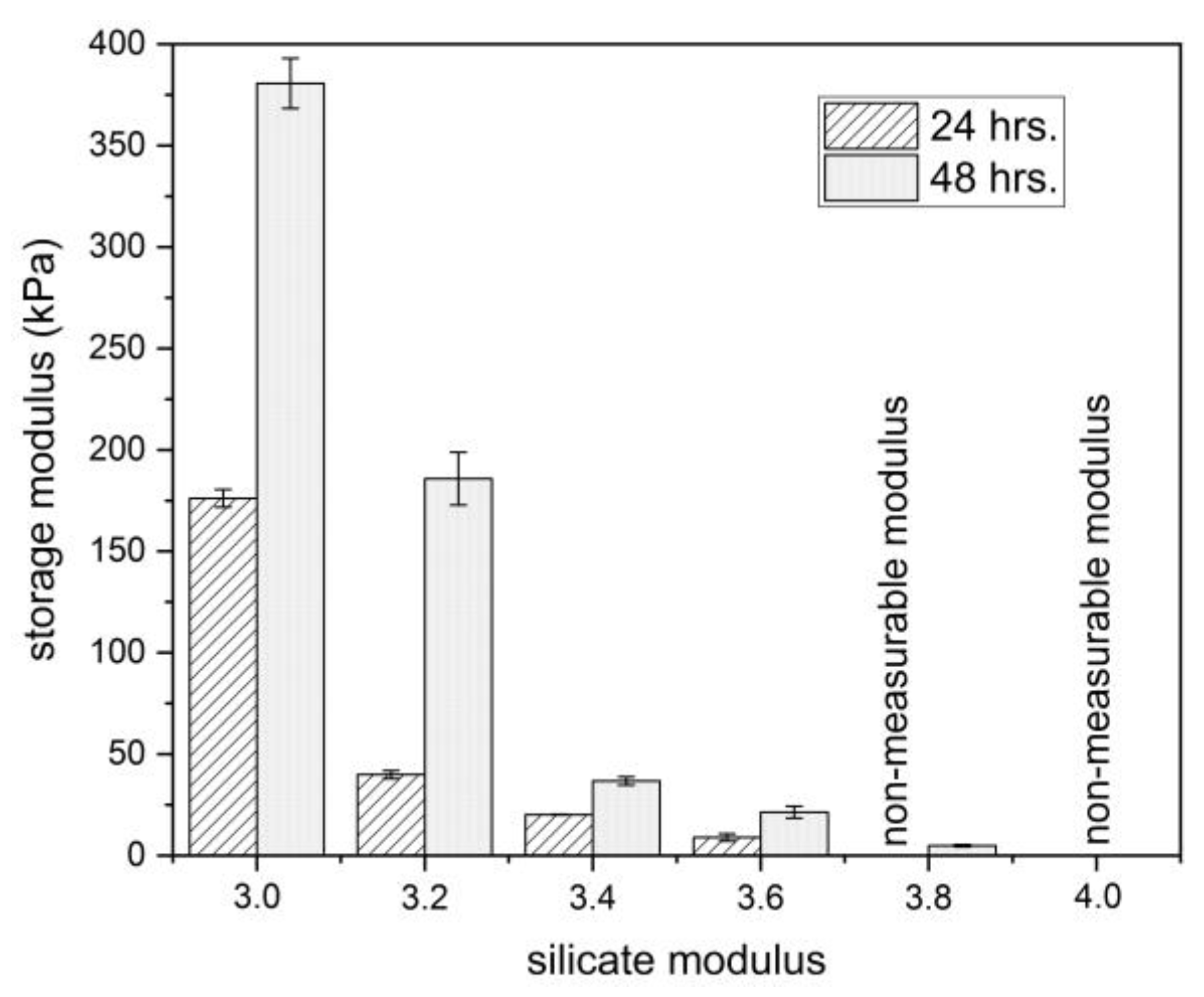
3.3. Monitoring of Gelation Process by Rheological Approach
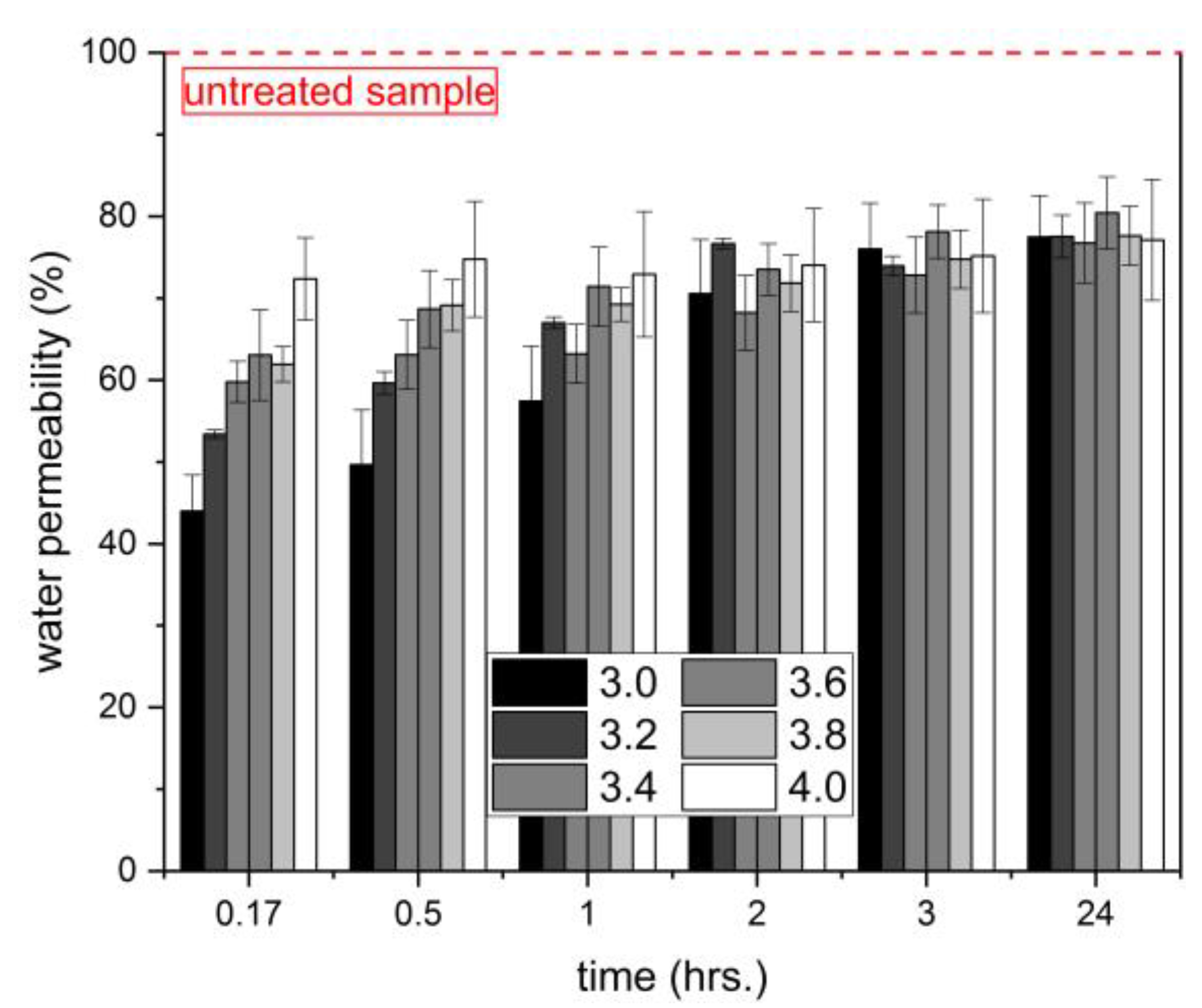
3.4. Water Permeability
4. Conclusions
- For the constant content of SiO2 (18 wt%), the gelation time of the lithium silicate densifiers exponentially increased as the molar SiO2 to Li2O ratio decreased from 4.0 to 3.0. It follows that the lithium silicate solution with a silicate modulus of 4.0 shows a delay of up to 14.5 times in gelation time compared to a solution with MS = 3.0.
- The storage modulus of the obtained gels also considerably increased with the decrease in the silicate modulus. This trend was observed for both continuous rheological measurements of the gelation process in situ and the measurements of the samples taken from externally prepared gel after 24 and 48 h.
- The gelation process was accompanied by the shift in the FT-IR band related to Q2 units from the wavenumbers of 1010–1020 cm−1 to the wavenumbers of 960–980 cm−1. The magnitude of this change increased with the decrease in silicate modulus, which correlates with the viscoelastic properties of the gels.
- The surface treatment of the hardened cementitious mortars with the investigated densifiers led to a decrease in water permeability by 20% after 24 h of the test duration. During the first 60 min, the amount of absorbed water considerably decreased with decreasing silicate modulus, and thus with the gelation time of the used densifiers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, X.Y.; Shi, Z.G.; Shi, C.J.; Ling, T.C.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. Constr. Build Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Berube, M.A.; Tremblay, C.; Fournier, B.; Thomas, M.D.; Stokes, D.B. Influence of lithium-based products proposed for counteracting ASR on the chemistry of pore solution and cement hydrates. Cement Concrete Res. 2004, 34, 1645–1660. [Google Scholar] [CrossRef]
- Wang, W.C. Effects of fly ash and lithium compounds on the water-soluble alkali and lithium content of cement specimens. Constr. Build Mater. 2014, 50, 727–735. [Google Scholar] [CrossRef]
- Souza, L.M.S.; Polder, R.B.; Copuroglu, O. Lithium migration in a two-chamber set-up as treatment against expansion due to alkali-silica reaction. Constr. Build Mater. 2017, 134, 324–335. [Google Scholar] [CrossRef]
- Veinot, D.E.; Langille, K.B.; Nguyen, D.T.; Bernt, J.O. Efflorescence of soluble silicate coatings. J. Non-Cryst. Solids 1991, 127, 221–226. [Google Scholar] [CrossRef]
- Moon, H.Y.; Shin, D.G.; Choi, D.S. Evaluation of the durability of mortar and concrete applied with inorganic coating material and surface treatment system. Constr. Build Mater. 2007, 21, 362–369. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, Y.Y.; Lee, B.J.; Kwon, S.J. Evaluation of concrete durability performance with sodium silicate impregnants. Adv. Mater. Sci. Eng. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Jiang, L.H.; Xue, X.; Zhang, W.D.; Yang, J.N.; Zhang, H.Q.; Li, Y.W.; Zhang, R.P.; Zhang, Z.Y.; Xu, L.J.; Qu, J.; et al. The investigation of factors affecting the water impermeability of inorganic sodium silicate-based concrete sealers. Constr. Build Mater. 2015, 93, 729–736. [Google Scholar] [CrossRef]
- Medeiros, M.H.F.; Castro-Borges, P.; Aleixo, D.M.; Quarcioni, V.A.; Marcondes, C.G.N.; Helene, P. Reducing water and chloride penetration through silicate treatments for concrete as a mean to control corrosion kinetics. Int. J. Electrochem. Sci. 2012, 7, 9682–9696. [Google Scholar]
- Ibrahim, M.; Al-Gahtani, A.S.; Maslehuddin, M.; Dakhil, F.H. Use of surface treatment materials to improve concrete durability. J. Mater. Civil. Eng. 1999, 11, 36–40. [Google Scholar] [CrossRef]
- Baltazar, L.; Santana, J.; Lopes, B.; Rodrigues, M.P.; Correia, J.R. Surface skin protection of concrete with silicate-based impregnations: Influence of the substrate roughness and moisture. Constr. Build Mater. 2014, 70, 191–200. [Google Scholar] [CrossRef]
- Thompson, J.L.; Silsbee, M.R.; Gill, P.M.; Scheetz, B.E. Characterization of silicate sealers on concrete. Cement Concrete Res. 1997, 27, 1561–1567. [Google Scholar] [CrossRef]
- Tognonvi, M.T.; Rossignol, S.; Bonnet, J.P. Effect of alkali cation on irreversible gel formation in basic medium. J. Non-Cryst. Solids 2011, 357, 43–49. [Google Scholar] [CrossRef]
- Visser, J.H.M. Fundamentals of alkali-silica gel formation and swelling: Condensation under influence of dissolved salts. Cement Concrete Res. 2018, 105, 18–30. [Google Scholar] [CrossRef]
- MunozAguado, M.J.; Gregorkiewitz, M. Sol-gel synthesis of microporous amorphous silica from purely inorganic precursors. J. Colloid Interfaces Sci. 1997, 185, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Sogaard, C.; Funehag, J.; Abbas, Z. Silica sol as grouting material: A physio-chemical analysis. Nano Converg. 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Tognonvi, M.T.; Soro, J.; Rossignol, S. Physical-chemistry of silica/alkaline silicate interactions during consolidation. Part 1: Effect of cation size. J. Non-Cryst. Solids 2012, 358, 81–87. [Google Scholar] [CrossRef]
- Tamar, Y.; Sasson, Y. Examination of the regime controlling sol-gel based colloidal silica aggregation. J. Non-Cryst. Solids 2013, 380, 35–41. [Google Scholar] [CrossRef]
- British Standards, I. Methods of Testing Cement; British Standards Institution: London, UK, 2010. [Google Scholar]
- Schraml, J.; Sandor, P.; Korec, S.; Krump, M.; Foller, B. Improved baseline in 29si nmr spectra of water glasses. Magn. Reson. Chem. 2013, 51, 403–406. [Google Scholar] [CrossRef]
- British Standards, I. Paints and Varnishes. Coating Materials and Coating Systems for Exterior Masonry and Concrete; British Standards Institution: London, UK, 1997. [Google Scholar]
- Innocenzi, P. Infrared spectroscopy of sol-gel derived silica-based films: A spectra-microstructure overview. J. Non-Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Mojet, B.L.; Ebbesen, S.D.; Lefferts, L. Light at the interface: The potential of attenuated total reflection infrared spectroscopy for understanding heterogeneous catalysis in water. Chem. Soc. Rev. 2010, 39, 4643–4655. [Google Scholar] [CrossRef]
- MacDonald, S.A.; Schardt, C.R.; Masiello, D.J.; Simmons, J.H. Dispersion analysis of ftir reflection measurements in silicate glasses. J. Non-Cryst. Solids 2000, 275, 72–82. [Google Scholar] [CrossRef]
- Brew, D.R.M.; Glasser, F.P. Synthesis and characterisation of magnesium silicate hydrate gels. Cement Concrete Res. 2005, 35, 85–98. [Google Scholar] [CrossRef]
- Aouissi, A.; Aldhayan, D.; Abduh, N.A.Y.; Al Kahtani, A. Propylene carbonate synthesis from propylene oxide and CO2 over Ga-Silicate-1 catalyst. Green Proc. Synth. 2020, 9, 440–450. [Google Scholar] [CrossRef]
- Armelao, L.; Bassan, A.; Bertoncello, R.; Biscontin, G.; Daolio, S.; Glisenti, A. Silica glass interaction with calcium hydroxide: A surface chemistry approach. J. Cult. Herit. 2000, 1, 375–384. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Fernandez-Jimenez, A.; Blanco, M.T.; Palomo, A. FTIR study of the sol-gel synthesis of cementitious gels: C-S-H and N-A-S-H. J. Sol. Gel. Sci. Technol. 2008, 45, 63–72. [Google Scholar] [CrossRef]
- Schneider, J.F.; Hasparyk, N.P.; Silva, D.A.; Monteiro, P.J.M. Effect of lithium nitrate on the alkali-silica reaction gel. J. Am. Ceram. Soc. 2008, 91, 3370–3374. [Google Scholar] [CrossRef]
- Gaboriaud, F.; Chaumont, D.; Nonat, A.; Hanquet, B.; Craeivich, A. Study of the influence of alkaline ions (Li, Na and K) on the structure of the silicate entities in silico-alkaline sol and on the formation of the silico-calco-alkaline gel. J. Sol. Gel. Sci. Technol. 1998, 13, 353–358. [Google Scholar] [CrossRef]
- Pan, X.Y.; Shi, Z.G.; Shi, C.J.; Ling, T.C.; Li, N. A review on surface treatment for concrete—Part 2: Performance. Constr. Build Mater. 2017, 133, 81–90. [Google Scholar] [CrossRef]
- Song, Z.N.; Xue, X.; Li, Y.W.; Yang, J.N.; He, Z.Y.; Shen, S.Z.; Jiang, L.H.; Zhang, W.D.; Xu, L.J.; Zhang, H.Q.; et al. Experimental exploration of the waterproofing mechanism of inorganic sodium silicate-based concrete sealers. Constr. Build Mater. 2016, 104, 276–283. [Google Scholar] [CrossRef]


| Quantity (g) | |||
|---|---|---|---|
| Commercial Lithium Waterglass (Li2O = 2.1 wt%; SiO2 = 19.04 wt%) | LiOH·H2O (Li2O = 35.61 wt%) | H2O | |
| LiSD–3.0 | 111.55 | 3.31 | 3.14 |
| LiSD–3.2 | 111.55 | 2.69 | 3.75 |
| LiSD–3.4 | 111.55 | 2.15 | 4.30 |
| LiSD–3.6 | 111.55 | 1.66 | 4.78 |
| LiSD–3.8 | 111.55 | 1.23 | 5.22 |
| LiSD–4.0 | 111.55 | 0.84 | 5.61 |
| Sample/(mol%) | Q0 | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|---|
| LiSD–3.0 | 0.90 | 6.10 | 28.44 | 52.74 | 11.81 |
| LiSD–3.2 | 0.87 | 5.77 | 27.96 | 53.84 | 11.56 |
| LiSD–3.4 | 0.83 | 5.45 | 26.37 | 54.70 | 12.66 |
| LiSD–3.6 | 0.83 | 4.90 | 25.13 | 54.98 | 14.16 |
| LiSD–3.8 | 0.82 | 4.70 | 24.72 | 55.58 | 14.17 |
| LiSD–4.0 | 0.80 | 4.53 | 24.23 | 55.33 | 15.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalina, L.; Bílek, V.; Sedlačík, M.; Cába, V.; Smilek, J.; Švec, J.; Bartoníčková, E.; Rovnaník, P.; Fládr, J. Physico-Chemical Properties of Lithium Silicates Related to Their Utilization for Concrete Densifiers. Materials 2023, 16, 2173. https://doi.org/10.3390/ma16062173
Kalina L, Bílek V, Sedlačík M, Cába V, Smilek J, Švec J, Bartoníčková E, Rovnaník P, Fládr J. Physico-Chemical Properties of Lithium Silicates Related to Their Utilization for Concrete Densifiers. Materials. 2023; 16(6):2173. https://doi.org/10.3390/ma16062173
Chicago/Turabian StyleKalina, Lukáš, Vlastimil Bílek, Martin Sedlačík, Vladislav Cába, Jiří Smilek, Jiří Švec, Eva Bartoníčková, Pavel Rovnaník, and Josef Fládr. 2023. "Physico-Chemical Properties of Lithium Silicates Related to Their Utilization for Concrete Densifiers" Materials 16, no. 6: 2173. https://doi.org/10.3390/ma16062173
APA StyleKalina, L., Bílek, V., Sedlačík, M., Cába, V., Smilek, J., Švec, J., Bartoníčková, E., Rovnaník, P., & Fládr, J. (2023). Physico-Chemical Properties of Lithium Silicates Related to Their Utilization for Concrete Densifiers. Materials, 16(6), 2173. https://doi.org/10.3390/ma16062173





