Effects of Vanadium Microalloying and Intercritical Annealing on Yield Strength–Ductility Trade-Offs of Medium-Manganese Steels
Abstract
:1. Introduction
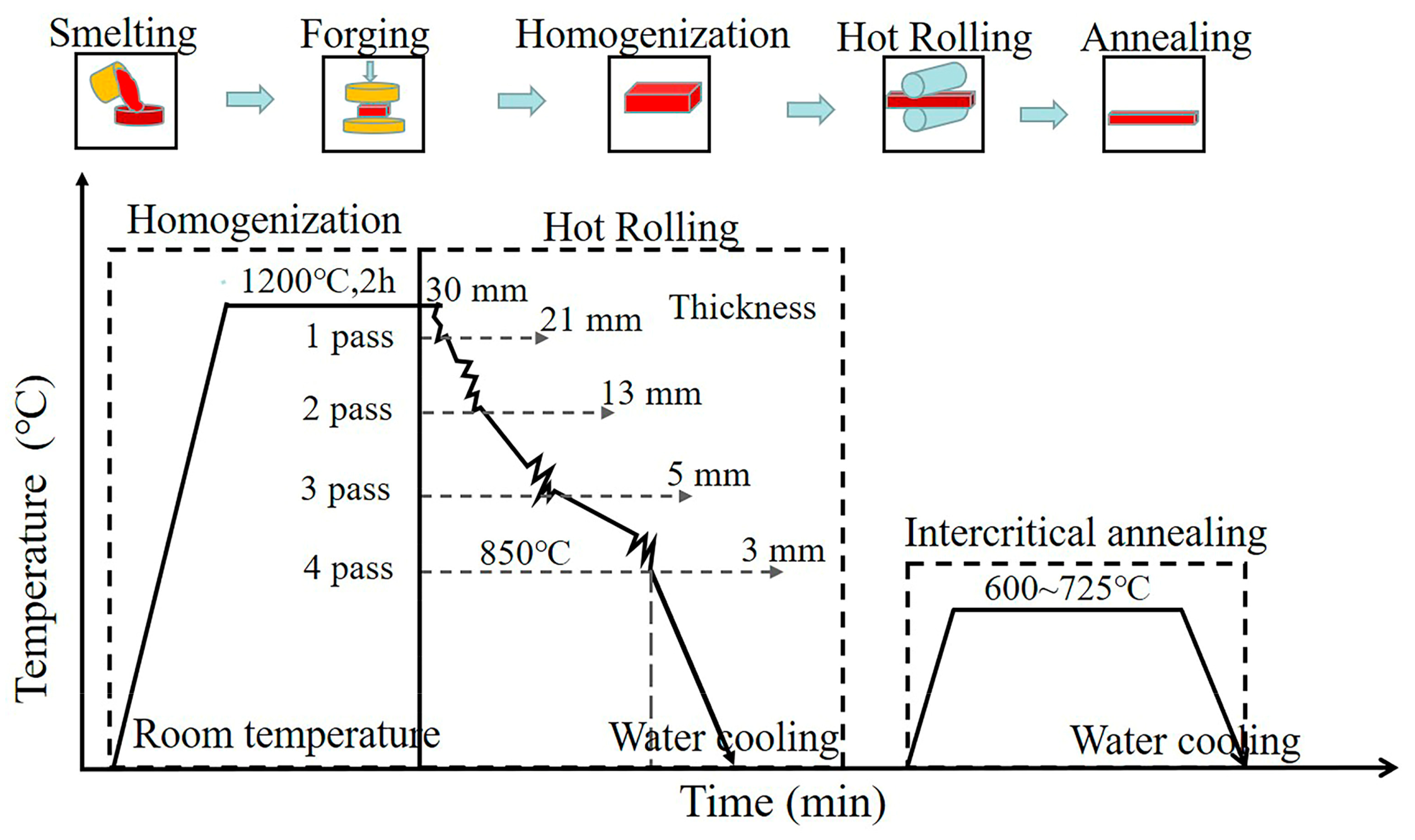
2. Experimental Procedure
3. Results
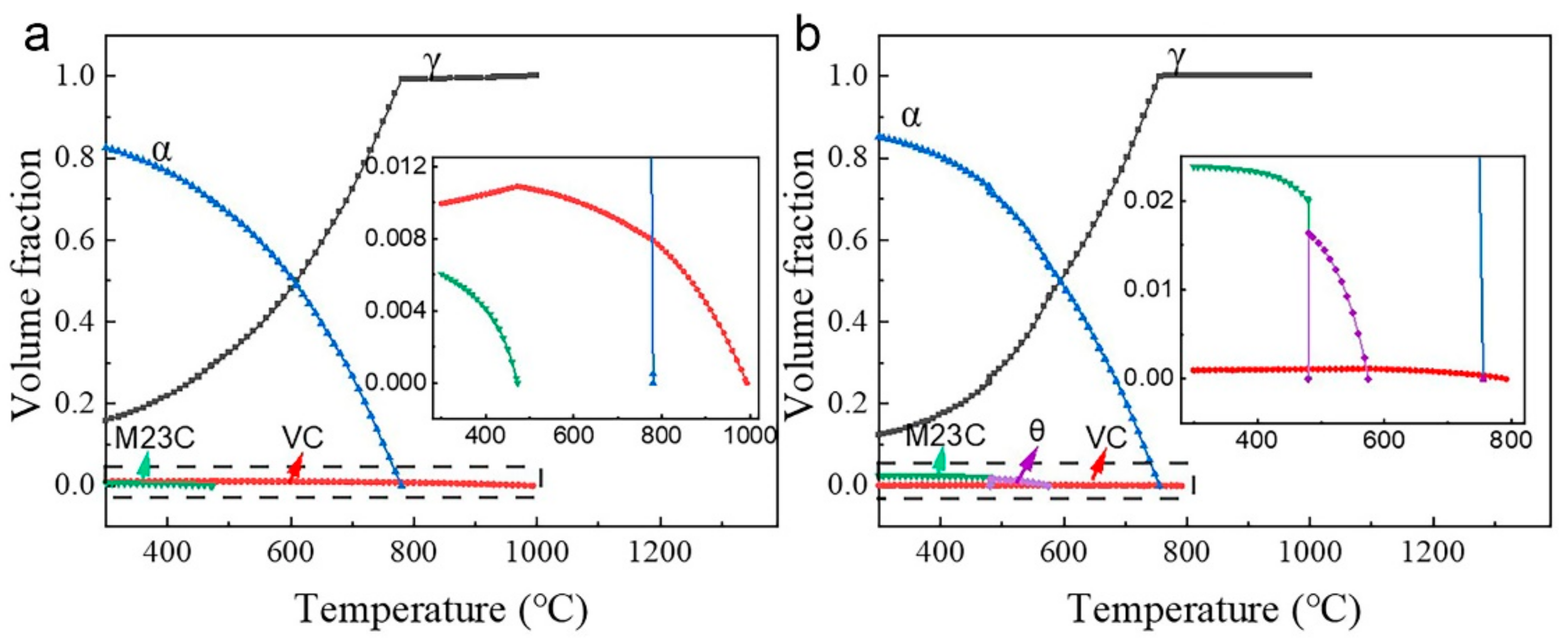
3.1. Thermo-Calc Analysis
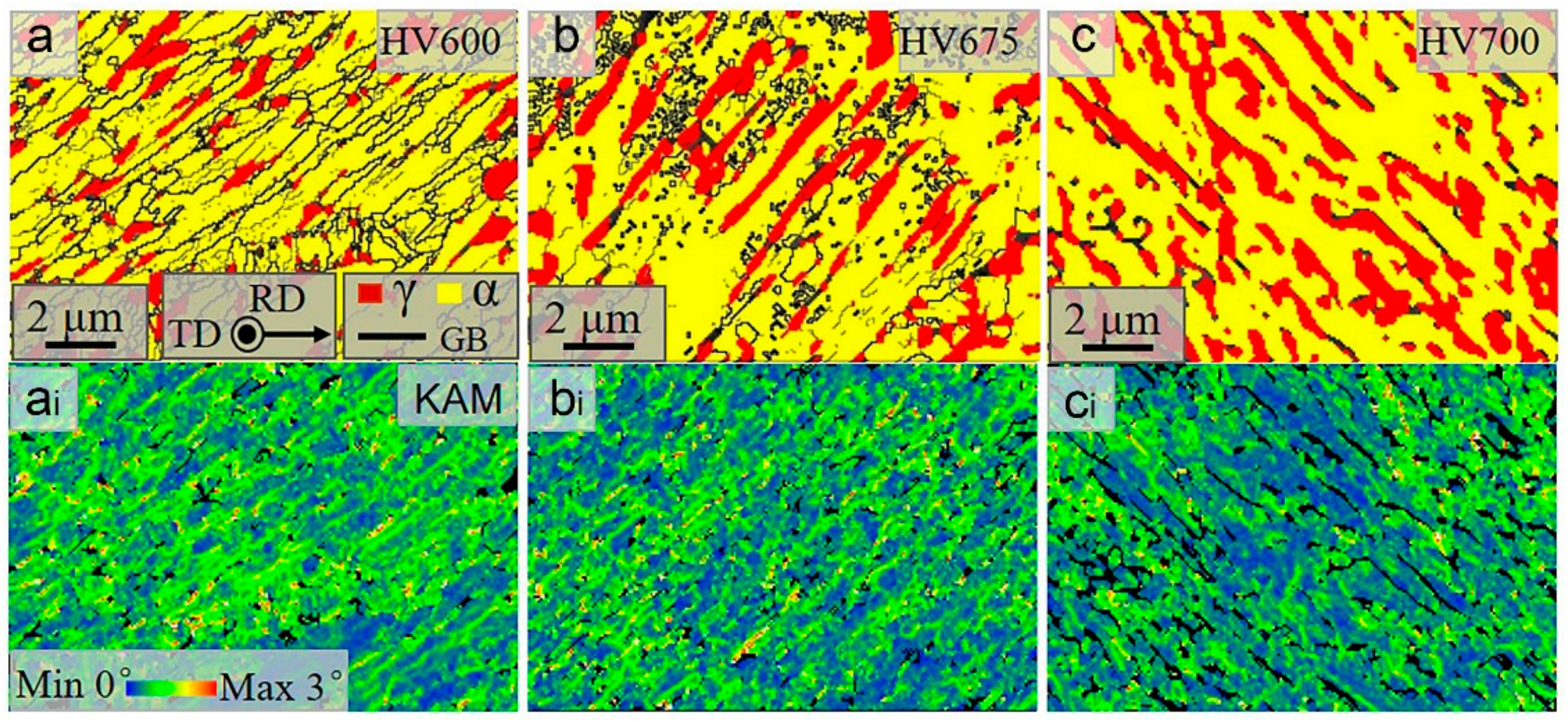
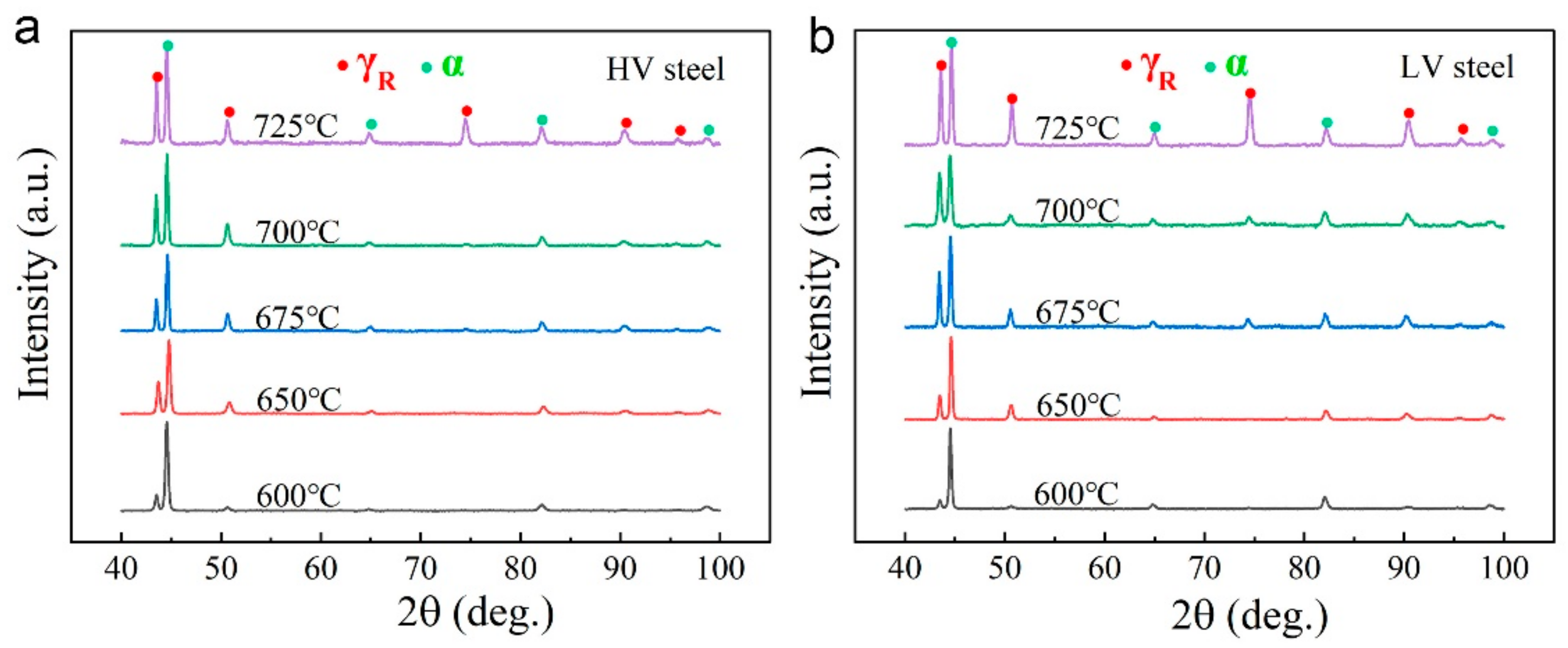
3.2. Microstructures
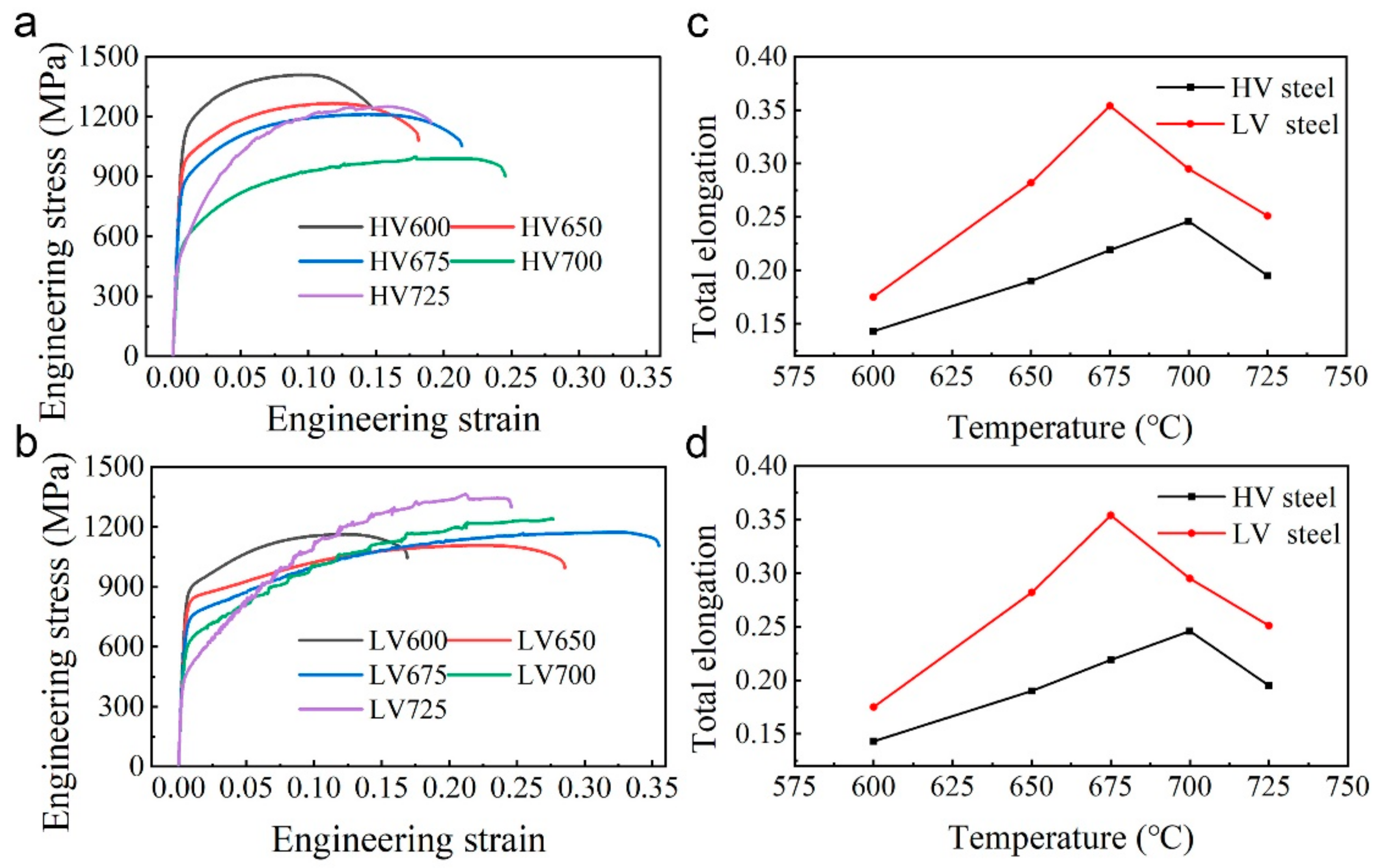
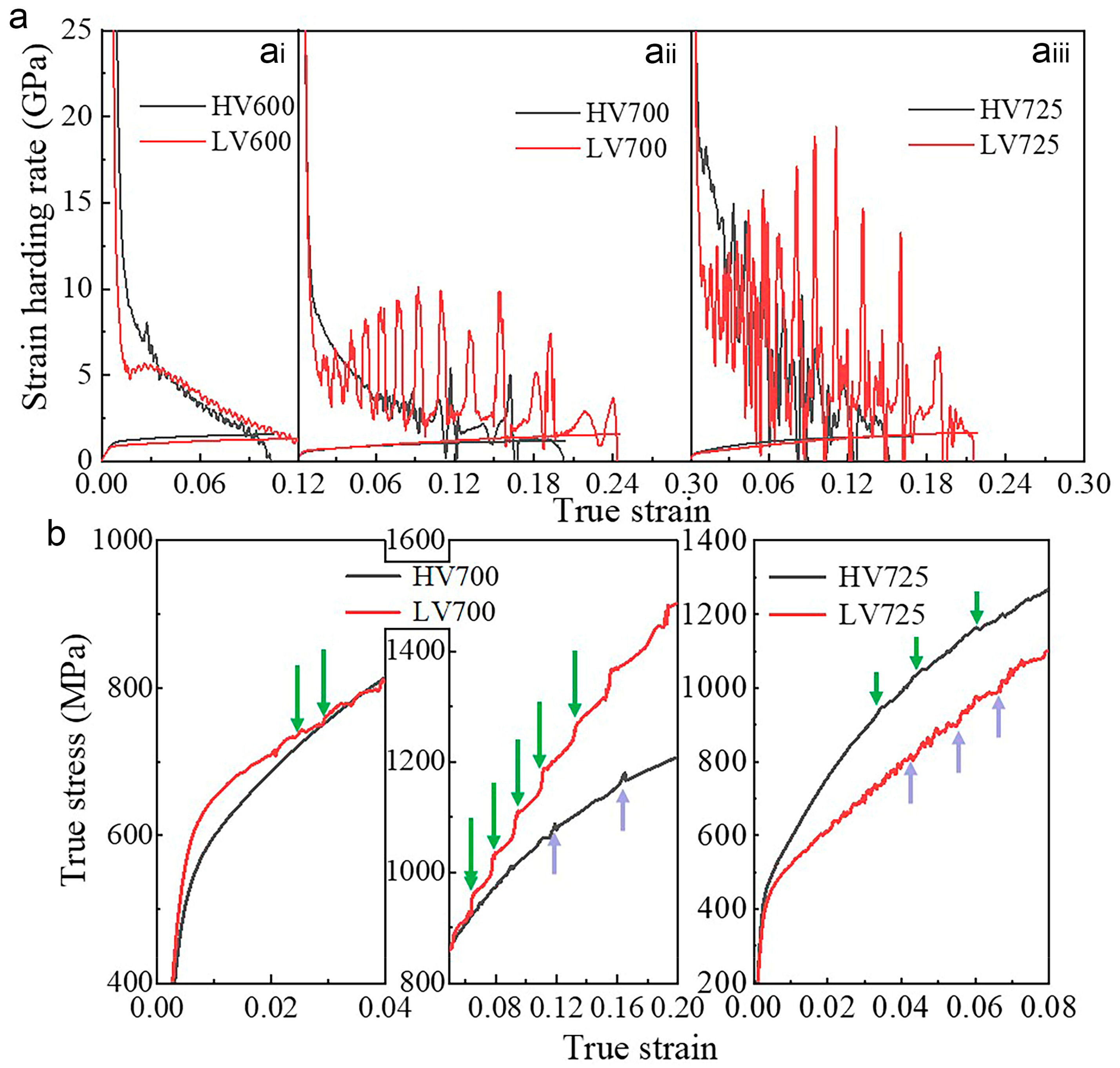
3.3. Mechanical Properties
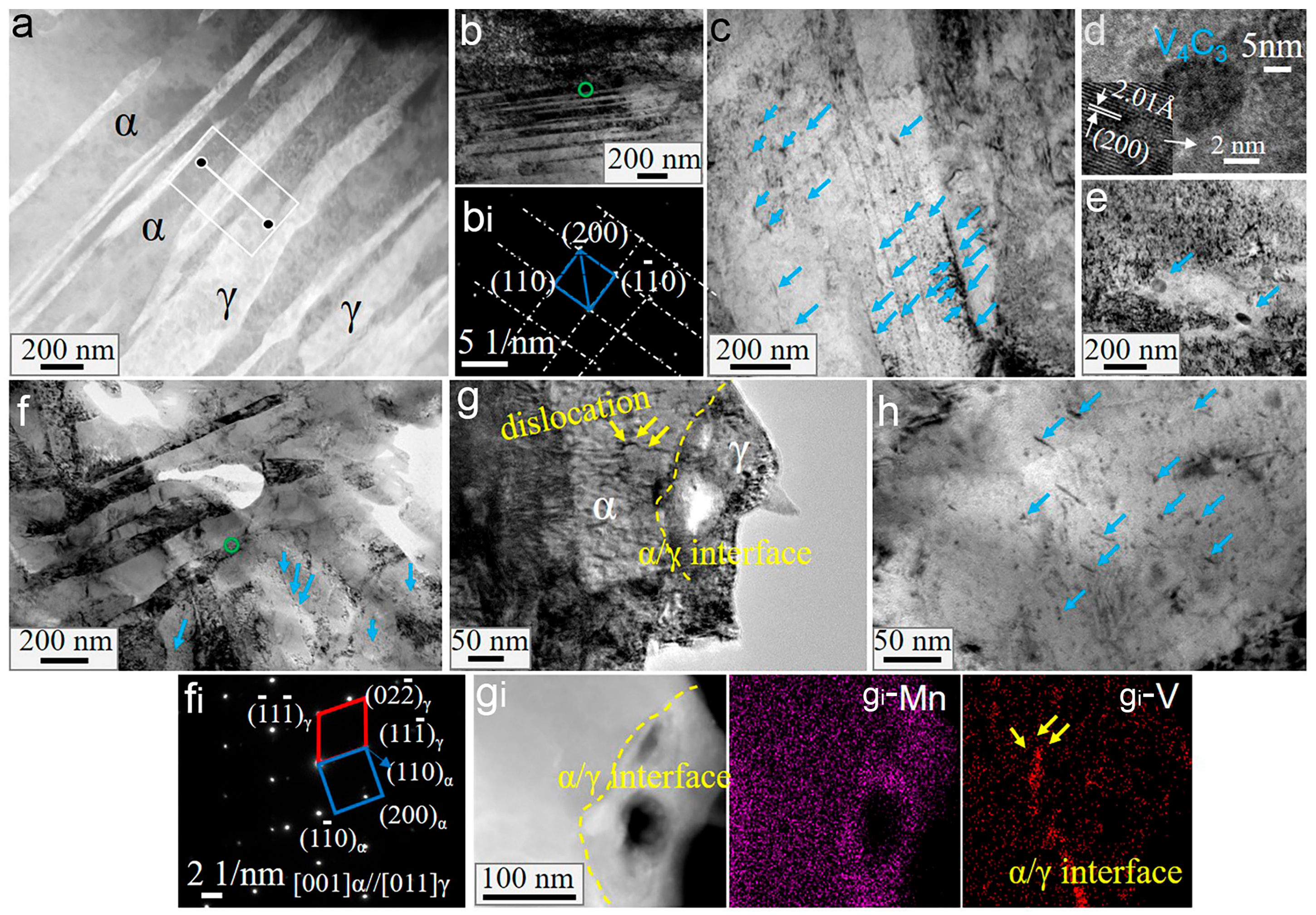
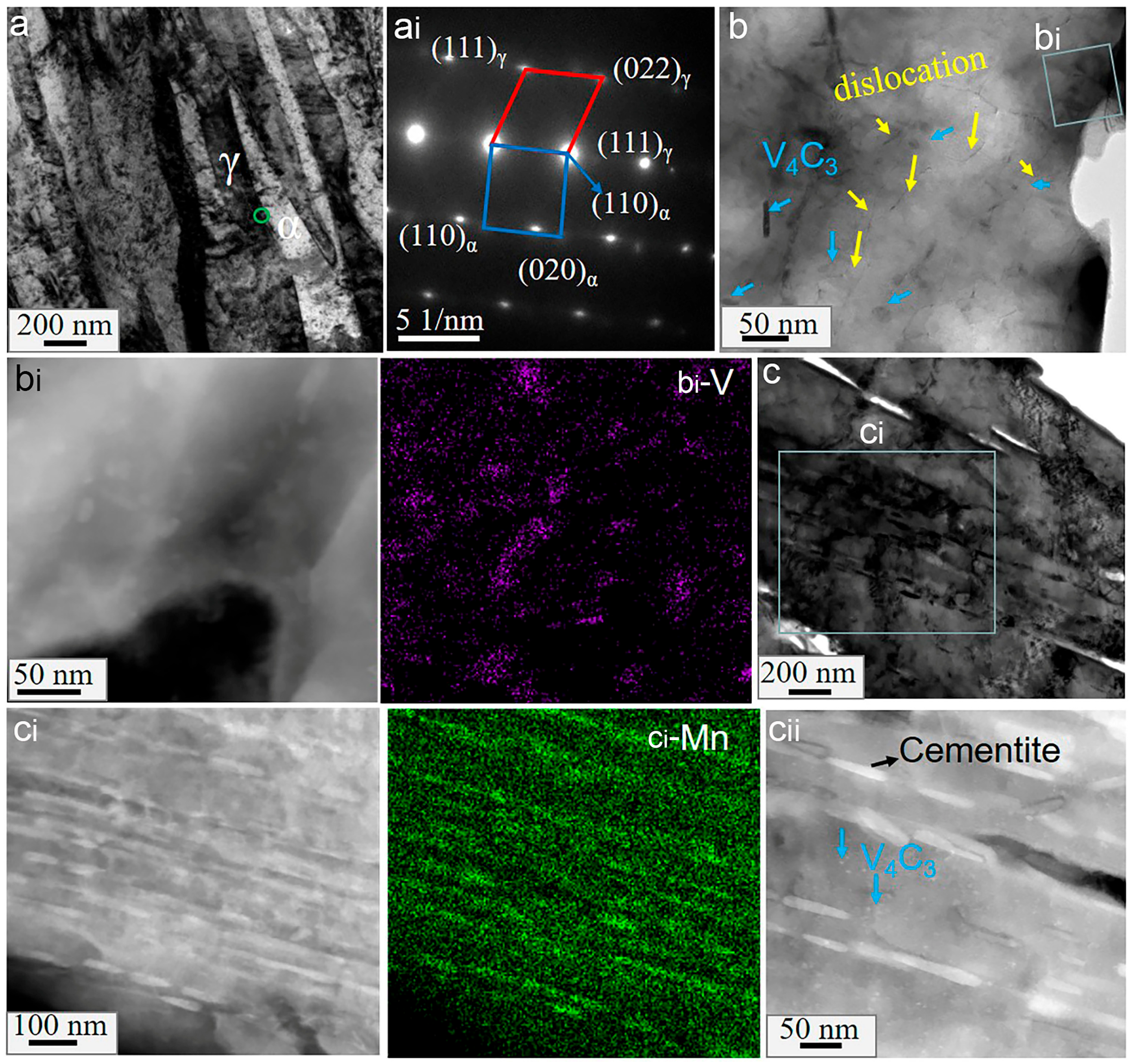
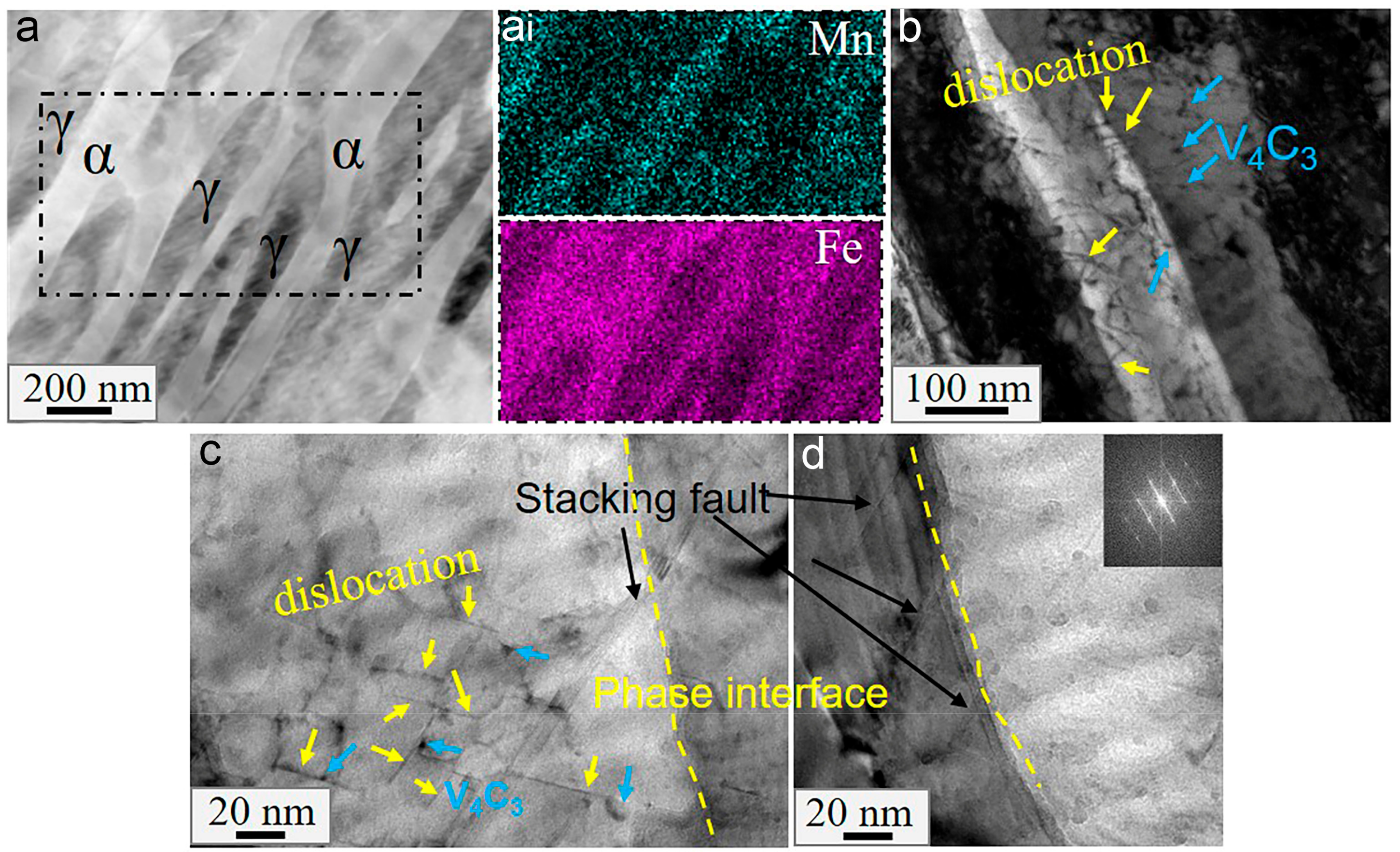
3.4. Nanoscale Morphology
4. Discussion
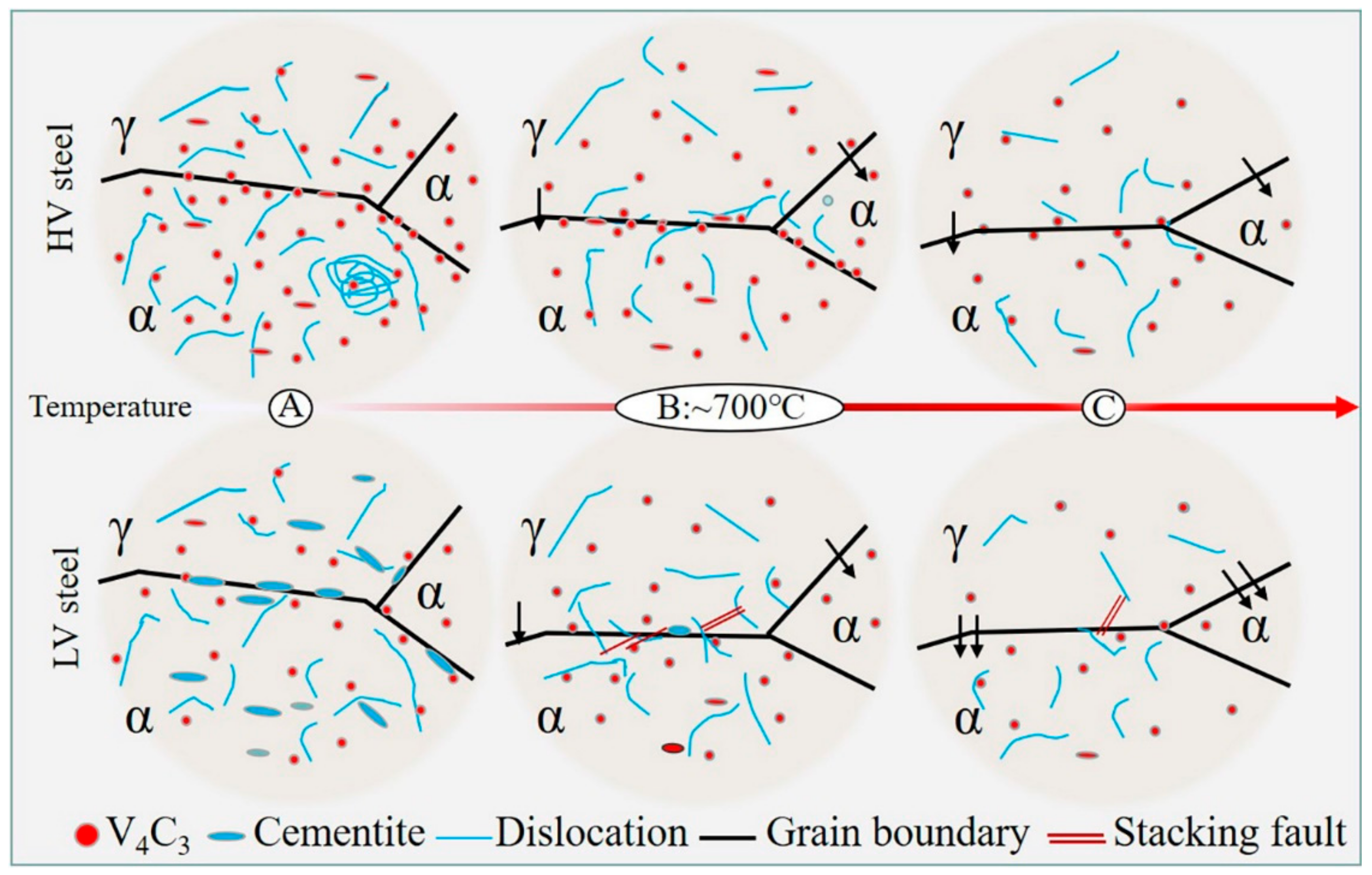
4.1. Effect of Vanadium on the Microstructures
4.2. Effects of Vanadium on the Strain Hardening Rate and Dynamic Strain Ageing
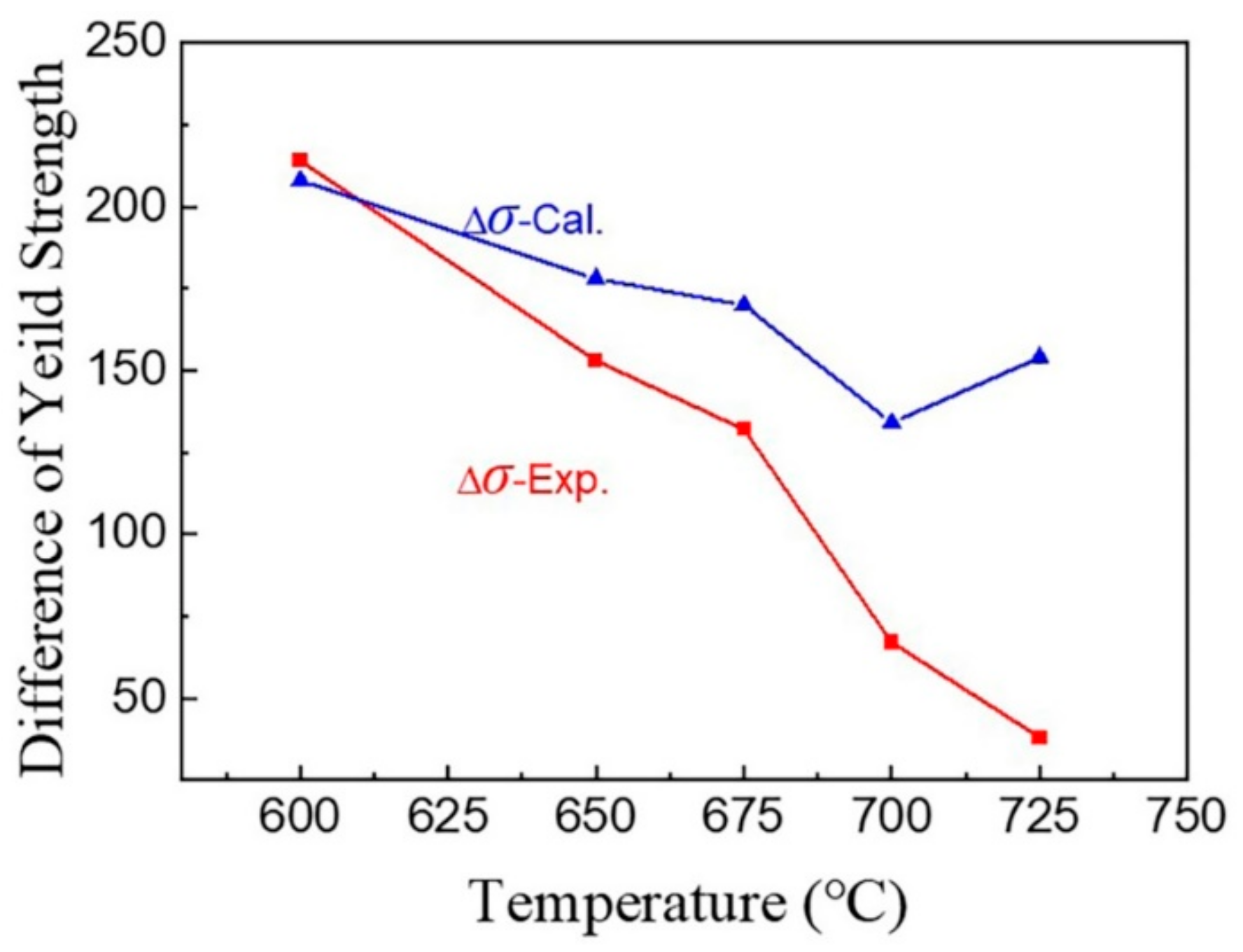
4.3. Effect of Vanadium on the Yield Strength
5. Conclusions
- A high vanadium content leads to a high density of vanadium carbides, which strongly pins the dislocations and grain boundaries in the matrix. The dragging effect caused by the pinning slows down the growth of austenite during intercritical annealing, and the reduction in the solid carbon content caused by high vanadium alloying causes the austenite fraction of HV steel to always be smaller than that of LV steel at the same temperature.
- The formation of vanadium carbide is preferential to that of cementite. During low-temperature intercritical annealing, the addition of high vanadium prevents the formation of brittle cementite and thus favors the mechanical properties. At 600 °C, HV steels have an obvious yield strength increment of almost 200 MPa, which is mainly due to high-density vanadium carbide precipitation and pinned dislocation. However, both the yield strength and its increment clearly decrease with increasing temperature.
- Vanadium carbide has a tendency to segregate at the phase interface in HV steels at 700 °C. This complex interface is not conducive to the redistribution of strain within the grain, meaning that it is easy to produce stress concentration and delay the occurrence of the TRIP effect. Therefore, excessive vanadium addiction is harmful to strain coordination during the plastic deformation of medium-manganese steel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, Y.K.; Han, J. Current opinion in medium manganese steel. Mater. Sci. Technol. 2015, 31, 843–856. [Google Scholar] [CrossRef]
- Bartlett, L.; Van Aken, D. High Manganese and Aluminum Steels for the Military and Transportation Industry. JOM 2014, 66, 1770–1784. [Google Scholar] [CrossRef]
- Ma, Y. Medium-manganese steels processed by austenite-reverted-transformation annealing for automotive applications. Mater. Sci. Technol. 2017, 33, 1713–1727. [Google Scholar] [CrossRef]
- Wei, Y.J.; Li, Y.Q.; Zhu, L.C.; Liu, Y.; Lei, X.Q.; Wang, G.; Wu, Y.X.; Mi, Z.L.; Liu, J.B.; Wang, H.T.; et al. Evading the strength- ductility trade-off dilemma in steel through gradient hierarchical nanotwins. Nat. Commun. 2014, 5, 3580. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; He, B.B.; Cheng, G.J.; Yen, H.W.; Huang, M.X.; Luo, H.W. Super-high-strength and formable medium Mn steel manufactured by warm rolling process. Acta Mater. 2019, 174, 131–141. [Google Scholar] [CrossRef]
- Yong, G.; Han, D. Review of applications of vanadium in steels. In Proceedings of the International Seminar on Production and Application of High Strength Seismic Grade Rebar Containing Vanadium, Beijing, China, 23 June 2010; pp. 1–11. [Google Scholar]
- Li, Z.C.; Zhang, X.T.; Mou, Y.J.; Misra, R.D.K.; He, L.F.; Li, H.P. The impact of intercritical annealing in conjunction with warm deformation process on microstructure, mechanical properties and TRIP effect in medium-Mn TRIP steels. Mater. Sci. Eng. A 2019, 746, 363–371. [Google Scholar] [CrossRef]
- He, B.B.; Hu, B.; Yen, H.W.; Cheng, G.J.; Wang, Z.K.; Luo, H.W.; Huang, M.X. High dislocation density-induced large ductility in deformed and partitioned steels. Science 2017, 357, 1029–1032. [Google Scholar] [CrossRef] [Green Version]
- Niu, G.; Wu, H.B.; Zhang, D.; Gong, N.; Tang, D. Heterogeneous nano/ultrafine-grained medium Mn austenitic stainless steel with high strength and ductility. Mater. Sci. Eng. A 2018, 725, 187–195. [Google Scholar] [CrossRef]
- Bi, N.N.; Liang, J.H.; Wang, X.F.; Kang, T.; Han, F.S. Microstructure evolution and yield strength improvement of a low carbon medium manganese steel experienced intercritical annealing, pre-straining and tempering. Mater. Charact. 2022, 194, 112439. [Google Scholar] [CrossRef]
- Han, Y.; Shi, J.; Xu, L.; Cao, W.Q.; Dong, H. TiC precipitation induced effect on microstructure and mechanical properties in low carbon medium manganese steel. Mater. Sci. Eng. A 2011, 530, 643–651. [Google Scholar] [CrossRef]
- Lee, S.; Estrin, Y.; De Cooman, B.C. Constitutive Modeling of the Mechanical Properties of V-added Medium Manganese TRIP Steel. Metall. Mater. Trans. A 2013, 44, 3136–3146. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.J.; Ding, H.; Cai, M.H. Microstructural evolution and precipitation behavior of the warm-rolled medium Mn steels containing Nb or Nb-Mo during intercritical annealing. Mater. Sci. Eng. A 2018, 736, 375–382. [Google Scholar] [CrossRef]
- Hamada, A.; Kömi, J. Effect of microstructure on mechanical properties of a novel high-Mn TWIP stainless steel bearing vanadium. Mater. Sci. Eng. A 2018, 718, 301–304. [Google Scholar] [CrossRef]
- Kisko, A.; Hamada, A.; Talonen, J.; Porter, D.; Karjalainen, L. Effects of reversion and recrystallization on microstructure and mechanical properties of Nb-alloyed low-Ni high-Mn austenitic stainless steels. Mater. Sci. Eng. A 2016, 657, 359–370. [Google Scholar] [CrossRef]
- Allam, T.; Guo, X.; Sevsek, S.; Lipińska-Chwałek, M.; Hamada, A.; Ahmed, E.; Bleck, W. Development of a Cr-Ni-VN medium manganese steel with balanced mechanical and corrosion properties. Metals 2019, 9, 705. [Google Scholar] [CrossRef] [Green Version]
- Park, T.M.; Kim, H.-J.; Um, H.Y.; Goo, N.H.; Han, J. The possibility of enhanced hydrogen embrittlement resistance of medium-Mn steels by addition of micro-alloying elements. Mater. Charact. 2020, 165, 110386. [Google Scholar] [CrossRef]
- Zhang, G.-T.; Zhu, N.-Q.; Sun, B.-W.; Zhao, Z.-Z.; Zheng, Z.-W.; Tang, D.; Li, L. Effect of V Addition on Microstructure and Mechanical Properties in C–Mn–Si Steels after Quenching and Partitioning Processes. Metals 2021, 11, 1306. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, P.; Li, W.; Guo, Z.H.; Rong, Y.H. High Strength-Ductility Nb-microalloyed Low Martensitic Carbon Steel: Novel Process and Mechanism. Acta. Metall. Sin.-Engl. 2015, 28, 1264–1271. [Google Scholar] [CrossRef] [Green Version]
- Park, T.M.; Jeong, M.S.; Jung, C.; Choi, W.S.; Choi, P.-P.; Han, J. Improved strength of a medium-Mn steel by V addition without sacrificing ductility. Mater. Sci. Eng. A 2021, 802, 140681. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Rong, L.; Li, Y. The influence of tempering temperature on the reversed austenite formation and tensile properties in Fe–13% Cr–4% Ni–Mo low carbon martensite stainless steels. Mater. Sci. Eng. A 2011, 528, 4075–4079. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Zhao, L.; Sietsma, J. Model for the interaction between interface migration and carbon diffusion during annealing of martensite–austenite microstructures in steels. Scripta Mater. 2008, 59, 159–162. [Google Scholar] [CrossRef]
- Varanasi, R.S.; Lipinska-Chwalek, M.; Mayer, J.; Gault, B.; Ponge, D. Mechanisms of austenite growth during intercritical annealing in medium manganese steels. Scripta Mater. 2022, 206, 114228. [Google Scholar] [CrossRef]
- Andrade, H.; Akben, M.; Jonas, J. Effect of molybdenum, niobium, and vanadium on static recovery and recrystallization and on solute strengthening in microalloyed steels. Metall. Trans. A 1983, 14, 1967–1977. [Google Scholar] [CrossRef]
- Ollilainen, V.; Kasprzak, W.; Holappa, L. The effect of silicon, vanadium and nitrogen on the microstructure and hardness of air cooled medium carbon low alloy steels. J. Mater. Process. Technol. 2003, 134, 405–412. [Google Scholar] [CrossRef]
- Perrard, F.; Scott, C. Vanadium precipitation during intercritical annealing in cold rolled TRIP steels. ISIJ Int. 2007, 47, 1168–1177. [Google Scholar] [CrossRef] [Green Version]
- Zackay, V.F. Thermomechanical processing. Mater. Sci. Eng. 1976, 25, 247–261. [Google Scholar] [CrossRef]
- Speich, G.R.; Demarest, V.A.; Miller, R.L. Formation of Austenite during Intercritical Annealing of Dual-Phase Steels. Metall. Trans. A-Phys. Metall. Mater. Sci. 1981, 12, 1419–1428. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Chandiran, E.; Dong, H.K.; Kamikawa, N.; Miyamoto, G.; Furuhara, T. Current Understanding of Microstructure and Properties of Micro-Alloyed Low Carbon Steels Strengthened by Interphase Precipitation of Nano-Sized Alloy Carbides: A Review. JOM 2021, 73, 3214–3227. [Google Scholar] [CrossRef]
- Lai, Q.; Gouné, M.; Perlade, A.; Pardoen, T.; Jacques, P.; Bouaziz, O.; Bréchet, Y. Mechanism of austenite formation from spheroidized microstructure in an intermediate Fe-0.1 C-3.5 Mn steel. Metall. Mater. Trans. A 2016, 47, 3375–3386. [Google Scholar] [CrossRef]
- Murakami, T.; Hatano, H.; Miyamoto, G.; Furuhara, T. Effects of Ferrite Growth Rate on Interphase Boundary Precipitation in V Microalloyed Steels. ISIJ Int. 2012, 52, 616–625. [Google Scholar] [CrossRef] [Green Version]
- Aristeidakis, J.S.S.; Haidemenopoulos, G.N.N. Constitutive and transformation kinetics modeling of epsilon-, alpha′-Martensite and mechanical twinning in steels containing austenite. Acta Mater. 2022, 228, 117757. [Google Scholar] [CrossRef]
- Haghdadi, N.; Cizek, P.; Beladi, H.; Hodgson, P.D. A novel high-strain-rate ferrite dynamic softening mechanism facilitated by the interphase in the austenite/ferrite microstructure. Acta Mater. 2017, 126, 44–57. [Google Scholar] [CrossRef]
- Haghdadi, N.; Cizek, P.; Beladi, H.; Hodgson, P. Dynamic restoration processes in a 23Cr-6Ni-3Mo duplex stainless steel: Effect of austenite morphology and interface characteristics. Metall. Mater. Trans. A 2017, 48, 4803–4820. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.C.; Cheng, X.W.; Liang, J.X.; Li, S.K. Compressive behavior of a Fe-Mn-Al-C lightweight steel at different strain rates. Mater. Sci. Eng. A 2020, 772, 138700. [Google Scholar] [CrossRef]
- Sun, B.H.; Fazeli, F.; Scott, C.; Brodusch, N.; Gauvin, R.; Yue, S. The influence of silicon additions on the deformation behavior of austenite-ferrite duplex medium manganese steels. Acta Mater. 2018, 148, 249–262. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, J.; Kane, S.N.; De Cooman, B.C. On the origin of dynamic strain aging in twinning-induced plasticity steels. Acta Mater. 2011, 59, 6809–6819. [Google Scholar] [CrossRef]
- Koyama, M.; Sawaguchi, T.; Tsuzaki, K. Overview of Dynamic Strain Aging and Associated Phenomena in Fe-Mn-C Austenitic Steels. ISIJ Int. 2018, 58, 1383–1395. [Google Scholar] [CrossRef] [Green Version]
- Magagnosc, D.J.; Field, D.M.; Meredith, C.S.; An, K.; Walter, T.R.; Limmer, K.R.; Lloyd, J.T. Temperature and stress dependent twinning behavior in a fully austenitic medium-Mn steel. Acta Mater. 2022, 231, 117864. [Google Scholar] [CrossRef]
- Park, D.B.; Huh, M.Y.; Shim, J.H.; Suh, J.Y.; Lee, K.H.; Jung, W.S. Strengthening mechanism of hot rolled Ti and Nb microalloyed HSLA steels containing Mo and W with various coiling temperature. Mater. Sci. Eng. A 2013, 560, 528–534. [Google Scholar] [CrossRef]
- Yong, Q.L. The Second Phase of Steel and Iron Material; Metallurgical Industry Press: Beijing, China, 2006. [Google Scholar]
- Bailey, J.; Hirsch, P. The dislocation distribution, flow stress, and stored energy in cold-worked polycrystalline silver. Philos. Mag. 1960, 5, 485–497. [Google Scholar] [CrossRef]

| Steels | Element and Content (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | Al | V | P | S | Fe | |
| HV | 0.13 | 1.26 | 9.70 | 1.58 | 0.481 | 0.029 | 0.006 | Bal. |
| LV | 0.12 | 1.26 | 9.90 | 1.65 | 0.051 | 0.021 | 0.003 | Bal. |
| Samples | Volume Fraction (vol.%) | Dislocation Density in α (m−2) | |
|---|---|---|---|
| γR | α | ||
| HV600 | 28.6 | 70.4 | 3.621 × 1014 |
| HV650 | 34.6 | 64.6 | 3.094 × 1014 |
| HV675 | 42.4 | 56.6 | 2.620 × 1014 |
| HV700 | 47.0 | 52.2 | 2.356 × 1014 |
| HV725 | 56.1 | 42.1 | 2.240 × 1014 |
| LV600 | 36.0 | 64.0 | 2.632 × 1014 |
| LV650 | 39.8 | 60.2 | 2.595 × 1014 |
| LV675 | 52.3 | 47.4 | 2.287 × 1014 |
| LV700 | 58.3 | 41.7 | 2.073 × 1014 |
| LV725 | 69.0 | 31.0 | 1.853 × 1014 |
| Temperature (°C) | (MPa) | (MPa) |
|---|---|---|
| 600 | 60 | 148 |
| 650 | 32 | 146 |
| 675 | 23 | 147 |
| 700 | 20 | 114 |
| 725 | 37 | 117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, N.; Tang, H.; Shi, Z.; Wang, X.; Han, F.; Liang, J. Effects of Vanadium Microalloying and Intercritical Annealing on Yield Strength–Ductility Trade-Offs of Medium-Manganese Steels. Materials 2023, 16, 2220. https://doi.org/10.3390/ma16062220
Bi N, Tang H, Shi Z, Wang X, Han F, Liang J. Effects of Vanadium Microalloying and Intercritical Annealing on Yield Strength–Ductility Trade-Offs of Medium-Manganese Steels. Materials. 2023; 16(6):2220. https://doi.org/10.3390/ma16062220
Chicago/Turabian StyleBi, Nannan, Huaiguang Tang, Zimu Shi, Xingfu Wang, Fusheng Han, and Juhua Liang. 2023. "Effects of Vanadium Microalloying and Intercritical Annealing on Yield Strength–Ductility Trade-Offs of Medium-Manganese Steels" Materials 16, no. 6: 2220. https://doi.org/10.3390/ma16062220





