An Overview of Recent Developments in Improving the Photocatalytic Activity of TiO2-Based Materials for the Treatment of Indoor Air and Bacterial Inactivation
Abstract
1. Introduction
2. Photocatalysis and Mass Transfer
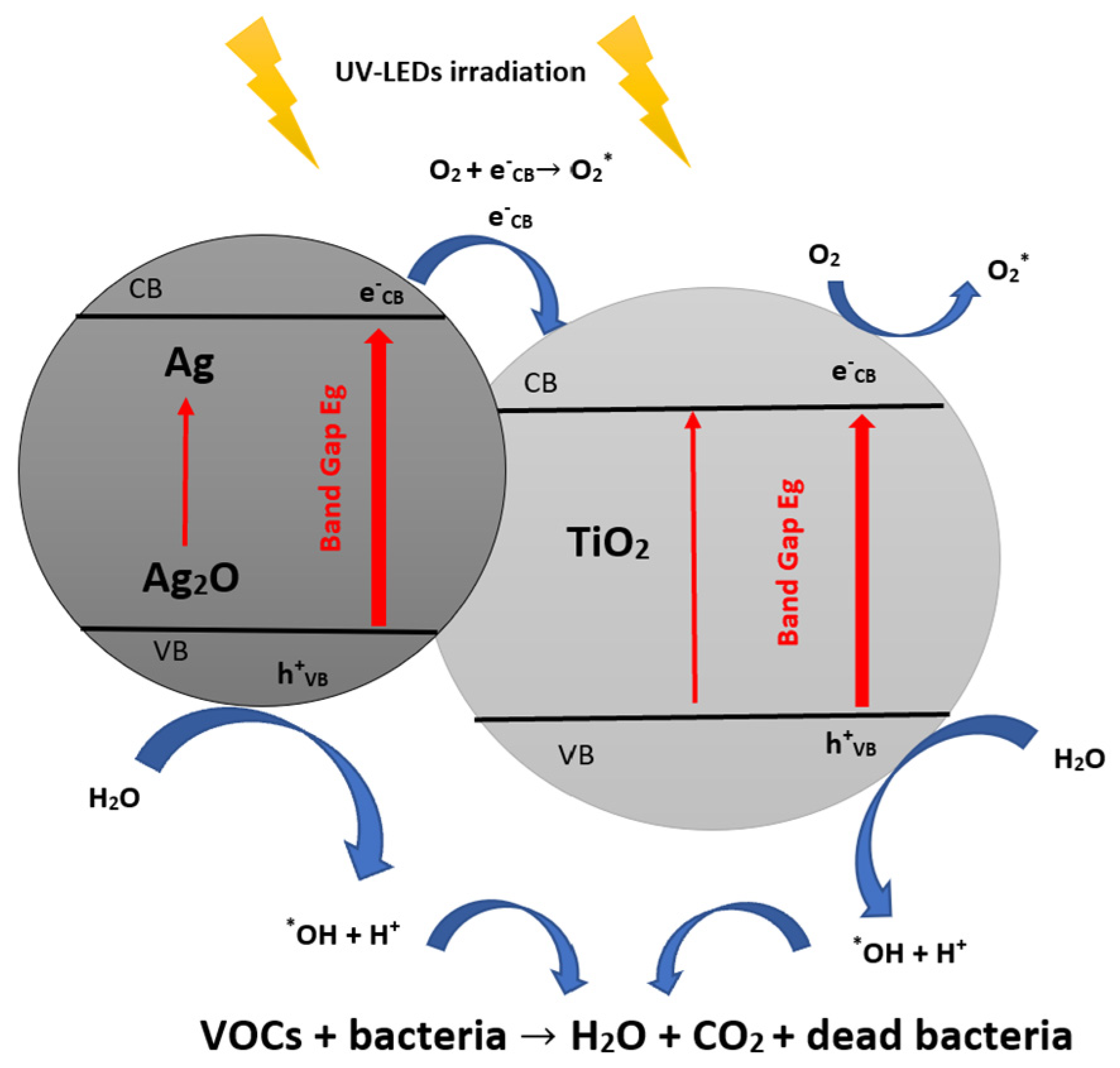
2.1. Principle of Photocatalysis
- (1)
- Transfer the reactants to the air phase.
- (2)
- Adsorption of the reactants on the surface of the catalyst.
- (3)
- Reaction in the adsorbed phase.
- (3.1)
- Absorption of a photon by the catalyst.
- (3.2)
- Generation of the electron-hole pairs.
- (3.3)
- Separation of the pair.
- (4)
- The oxidation and reduction with the adsorbed substrate.
- (5)
- Desorption of the intermediate product.
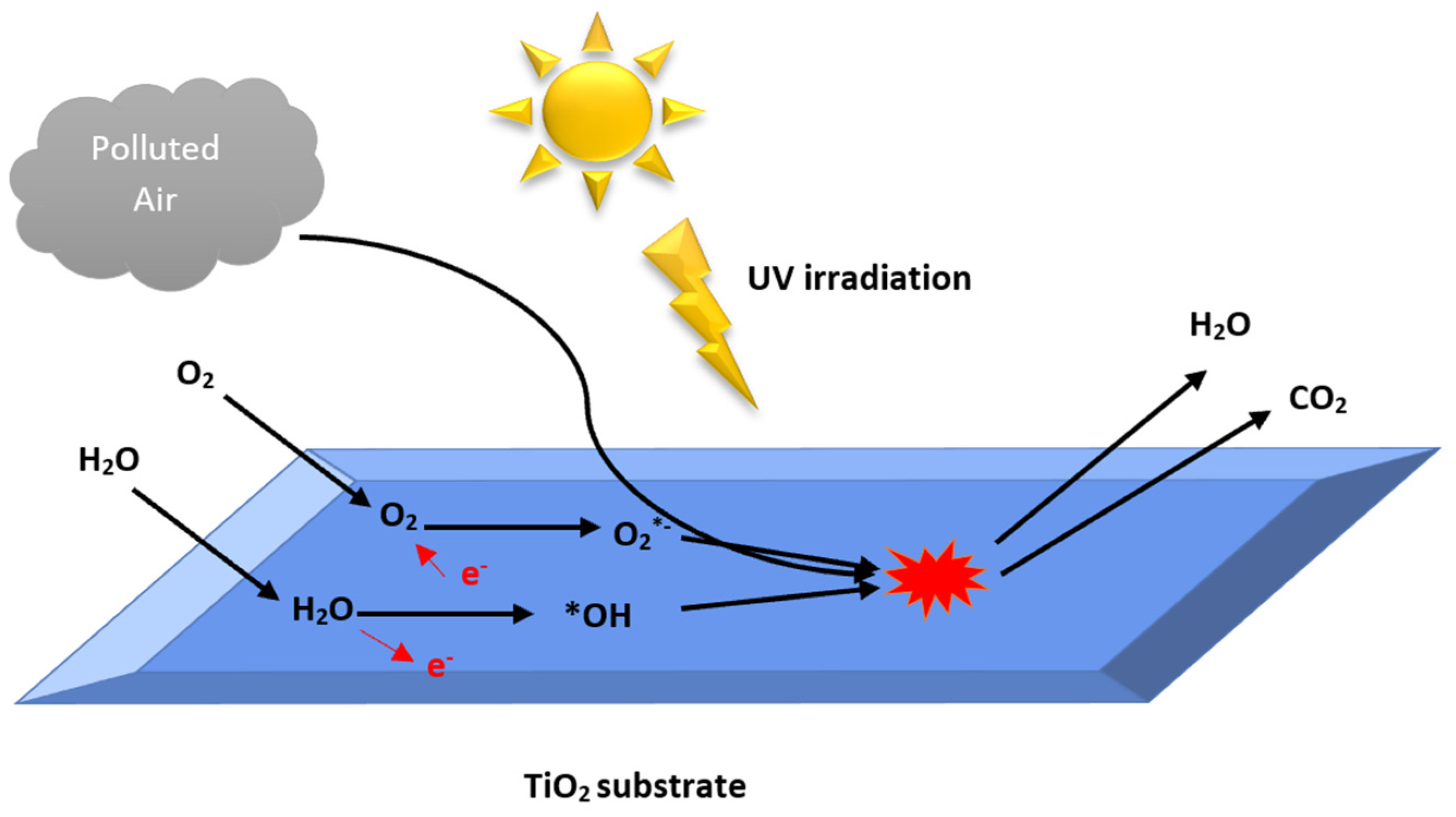
2.2. Development of Heterogeneous Photocatalytic Oxidation
2.3. Reactors and Configurations
3. Volatile Organic Compounds (VOCs)
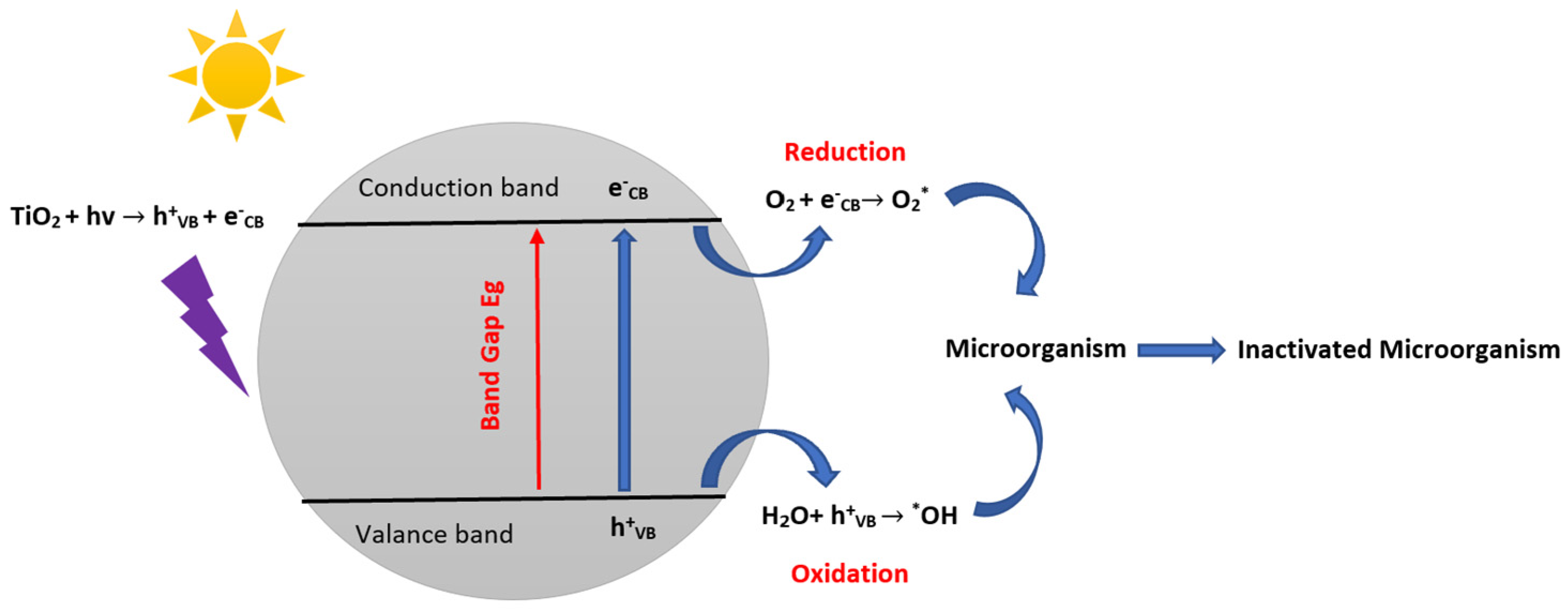
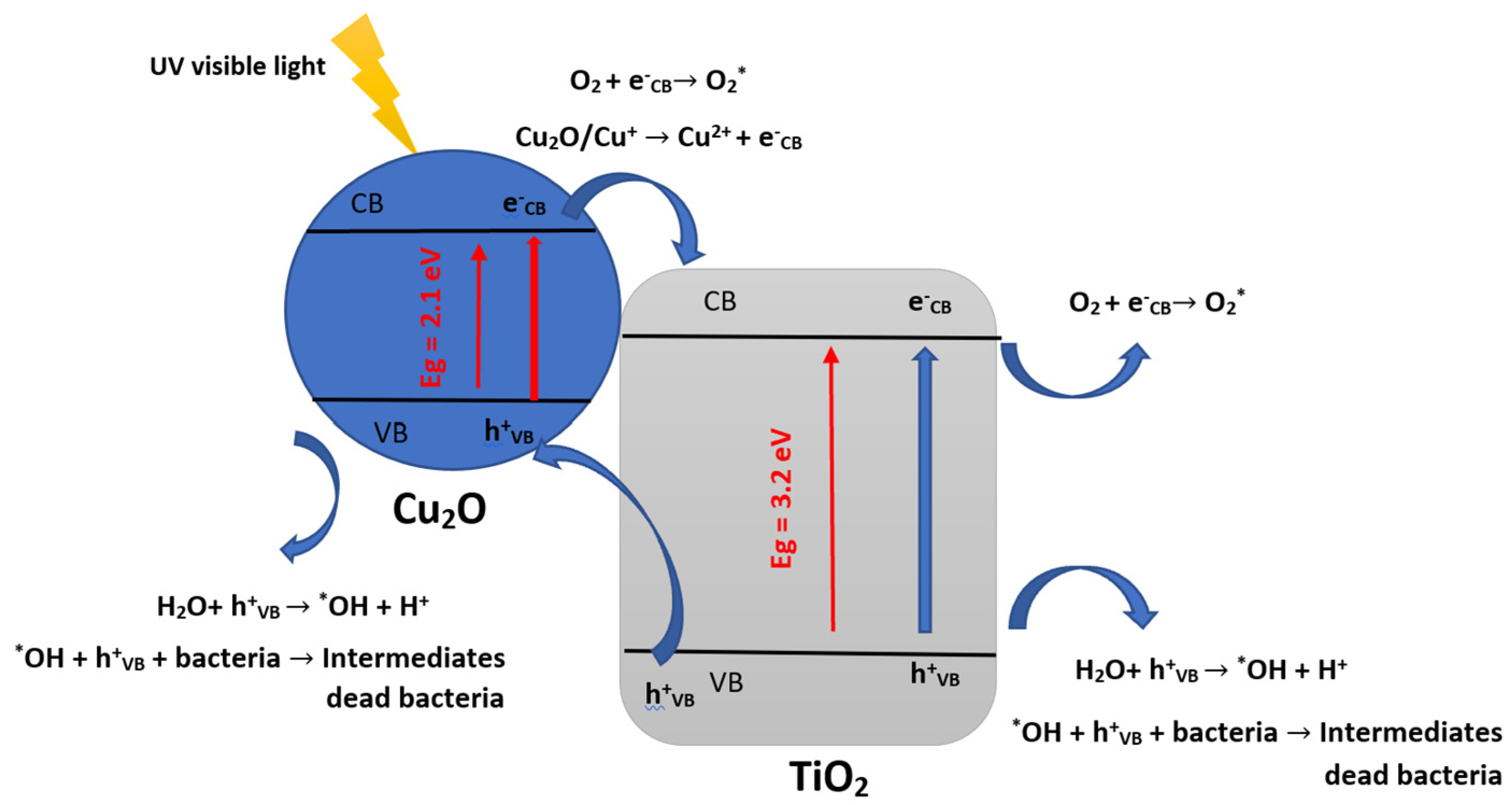
4. Microorganism Inactivation and Reactional Mechanisms
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cincinelli, A.; Martellini, T. Indoor air quality and health. Int. J. Environ. Res. Public Health 2017, 14, 1286. [Google Scholar] [CrossRef]
- Capolongo, S.; Settimo, G.; Gola, M. (Eds.) Indoor Air Quality (IAQ) in Healthcare Facilities; Springer: Berlin/Heidelberg, Germany, 2017; Volume 4386. [Google Scholar]
- Assadi, I.; Guesmi, A.; Baaloudj, O.; Zeghioud, H.; Elfalleh, W.; Benhammadi, N. Review on inactivation of airborne viruses using non-thermal plasma technologies: From MS2 to coronavirus. Environ. Sci. Pollut. Res. 2021, 29, 4880–4892. [Google Scholar] [CrossRef]
- Heinsohn, R.J.; John, M.C. (Eds.) Indoor Air Quality Engineering: Enviromental Health and Control Indoor; CRC Press: Boca Raton, FL, USA, 2003; ISBN 0-8247-4061-0. [Google Scholar]
- Abidi, M.; Hajjaji, A.; Bouzaza, A.; Lamaa, L.; Peruchon, L.; Brochier, C.; Rtimi, S.; Wolbert, D.; Bessais, B.; Assadi, A.A. Modeling of indoor air treatment using an innovative photocatalytic luminous textile: Reactor compactness and mass transfer enhancement. Chem. Eng. J. 2022, 430, 132636. [Google Scholar] [CrossRef]
- Salmon, D.G. Annual Exporter Guide France. Available online: https://www.google.com.hk/url?sa=i&rct=j&q=&esrc=s&source=web&cd=&ved=0CAQQw7AJahcKEwjw49TzpND9AhUAAAAAHQAAAAAQAg&url=https%3A%2F%2Fapps.fas.usda.gov%2Fnewgainapi%2Fapi%2Freport%2Fdownloadreportbyfilename%3Ffilename%3DExporter%2520Guide_Paris_France_12-8-2014.pdf&psig=AOvVaw0bBETSpKjn6_G28dOcdT4j&ust=1678500724709337 (accessed on 25 December 2022).
- Erisman, J.W. Air Pollution Science for the 21st Century. Environ. Sci. Policy 2003, 6, 396. [Google Scholar] [CrossRef]
- Quyen, N.T.; Traikool, T.; Nitisoravut, R.; Onjun, T. Improvement of water quality using dielectric barrier discharge plasma. J. Phys. Conf. Ser. 2017, 860, 12031. [Google Scholar] [CrossRef]
- Pichat, P. Some views about indoor air photocatalytic treatment using TiO2: Conceptualization of humidity effects, active oxygen species, problem of C1-C3 carbonyl pollutants. Appl. Catal. B Environ. 2010, 99, 428–434. [Google Scholar] [CrossRef]
- Ghezzi, S.; Pagani, I.; Poli, G.; Perboni, S.; Vicenzi, E. Rapid Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by Tungsten Trioxide-Based (WO3) Photocatalysis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Assadi, A.A.; Karoui, S.; Trabelsi, K.; Hajjaji, A.; Elfalleh, W.; Ghorbal, A.; Maghzaoui, M.; Assadi, A.A. Synthesis and Characterization of TiO2 Nanotubes (TiO2-NTs) with Ag Silver Nanoparticles (Ag-NPs): Photocatalytic Performance for Wastewater Treatment under Visible Light. Materials 2022, 15, 1463. [Google Scholar] [CrossRef]
- Malayeri, M.; Haghighat, F.; Lee, C.S. Kinetic modeling of the photocatalytic degradation of methyl ethyl ketone in air for a continuous-flow reactor. Chem. Eng. J. 2021, 404, 126602. [Google Scholar] [CrossRef]
- Zhang, Z.; Gamage, J. Applications of photocatalytic disinfection. Int. J. Photoenergy 2010, 2010, 764870. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Punta, C.; Candiani, G. Effect of UV irradiation and TiO2-photocatalysis on airborne bacteria and viruses: An overview. Materials 2021, 14, 1075. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Al-hammadi, S.A.; Saleh, T.A. Simultaneous sorption ofdyes and toxic metals from waters using synthesized titania-incorporated polyamide. J. Mol. Liq. 2018, 269, 564–571. [Google Scholar] [CrossRef]
- Ali, T.; Ahmed, A.; Alam, U.; Uddin, I.; Tripathi, P.; Muneer, M. Enhanced photocatalytic and antibacterial activities of Ag-doped TiO2 nanoparticles under visible light. Mater. Chem. Phys. 2018, 212, 325–335. [Google Scholar] [CrossRef]
- Abidi, M.; Assadi, A.A.; Bouzaza, A.; Hajjaji, A.; Bessais, B.; Rtimi, S. Photocatalytic indoor/outdoor air treatment and bacterial inactivation on CuxO/TiO2 prepared by HiPIMS on polyester cloth under low intensity visible light. Appl. Catal. B Environ. 2019, 259, 118074. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Fu, S.; Lv, X.; He, Q.; Li, Y.; Ji, F.; Xu, X. Preparation and antibacterial activity of Ag/TiO2-functionalized ceramic tiles. Ceram. Int. 2022, 48, 4897–4903. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El, A.; Khezami, L. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, X.; Cao, J.; Zhu, Y.; Yuan, W.; Hu, Z.; Ao, Z.; Brudvig, G.W.; Tian, F.; Yu, J.C.; et al. Photocatalytically recovering hydrogen energy from wastewater treatment using MoS2@TiO2 with sulfur/oxygen dual-defect. Appl. Catal. B Environ. 2022, 303, 120878. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Wolbert, D. Study of synergetic effect by surface discharge plasma/TiO2 combination for indoor air treatment: Sequential and continuous configurations at pilot scale. J. Photochem. Photobiol. A Chem. 2015, 310, 148–154. [Google Scholar] [CrossRef]
- Karoui, S.; Ben Arfi, R.; Mougin, K.; Ghorbal, A.; Assadi, A.A.; Amrane, A. Synthesis of novel biocomposite powder for simultaneous removal of hazardous ciprofloxacin and methylene blue: Central composite design, kinetic and isotherm studies using Brouers-Sotolongo family models. J. Hazard. Mater. 2020, 387, 121675. [Google Scholar] [CrossRef]
- Zeghioud, H.; Khellaf, N.; Amrane, A.; Djelal, H.; Elfalleh, W.; Assadi, A.A.; Rtimi, S. Photocatalytic performance of TiO2 impregnated polyester for the degradation of Reactive Green 12: Implications of the surface pretreatment and the microstructure. J. Photochem. Photobiol. A Chem. 2017, 346, 493–501. [Google Scholar] [CrossRef]
- Baaloudj, O.; Kenfoud, H.; Badawi, A.K.; Assadi, A.A.; El Jery, A.; Assadi, A.A.; Amrane, A. Bismuth Sillenite Crystals as Recent Photocatalysts for Water Treatment and Energy Generation: A Critical Review. Catalysts 2022, 12, 500. [Google Scholar] [CrossRef]
- Kappadan, S.; Gebreab, T.W.; Thomas, S.; Kalarikkal, N. Tetragonal BaTiO3 nanoparticles: An efficient photocatalyst for the degradation of organic pollutants. Mater. Sci. Semicond. Process. 2016, 51, 42–47. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Akerdi, A.G.; Bahrami, S.H. Application of heterogeneous nano-semiconductors for photocatalytic advanced oxidation of organic compounds: A review. J. Environ. Chem. Eng. 2019, 7, 103283. [Google Scholar] [CrossRef]
- Almomani, F.; Rene, E.R.; Veiga, M.C.; Bhosale, R.R.; Kennes, C. Treatment of waste gas contaminated with dichloromethane using photocatalytic oxidation, biodegradation and their combinations. J. Hazard. Mater. 2021, 405, 123735. [Google Scholar] [CrossRef]
- Mohseni, M.; Prieto, L. Biofiltration of hydrophobic VOCs pretreated with UV photolysis and photocatalysis. Int. J. Environ. Technol. Manag. 2008, 9, 47–58. [Google Scholar] [CrossRef]
- Khezami, L.; Nguyen-Tri, P.; Saoud, W.A.; Bouzaza, A.; El Jery, A.; Duc Nguyen, D.; Gupta, V.K.; Assadi, A.A. Recent progress in air treatment with combined photocatalytic/plasma processes: A review. J. Environ. Manag. 2021, 299, 113588. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Yamashita, H. Efficient photocatalytic degradation of organics diluted in water and air using TiO2 designed with zeolites and mesoporous silica materials. J. Mater. Chem. 2011, 21, 2407–2416. [Google Scholar] [CrossRef]
- Baaloudj, O.; Nasrallah, N.; Bouallouche, R.; Kenfoud, H.; Khezami, L.; Assadi, A.A. High efficient Cefixime removal from water by the sillenite Bi12TiO20: Photocatalytic mechanism and degradation pathway. J. Clean. Prod. 2022, 330, 129934. [Google Scholar] [CrossRef]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems. Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Serhane, Y.; Belkessa, N.; Bouzaza, A.; Wolbert, D.; Assadi, A.A. Continuous air purification by front flow photocatalytic reactor: Modelling of the influence of mass transfer step under simulated real conditions. Chemosphere 2022, 295, 133809. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium dioxide: From engineering to applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Hodgson, A.T.; Destaillats, H.; Sullivan, D.P.; Fisk, W.J. Performance of ultraviolet photocatalytic oxidation for indoor air cleaning applications. Indoor Air 2007, 17, 305–316. [Google Scholar] [CrossRef]
- Muscetta, M.; Russo, D. Photocatalytic applications in wastewater and air treatment: A patent review (2010–2020). Catalysts 2021, 11, 834. [Google Scholar] [CrossRef]
- Riaz, N.; Fen, D.A.C.S.; Khan, M.S.; Naz, S.; Sarwar, R.; Farooq, U.; Bustam, M.A.; Batiha, G.E.S.; El Azab, I.H.; Uddin, J.; et al. Iron-zinc co-doped titania nanocomposite: Photocatalytic and photobiocidal potential in combination with molecular docking studies. Catalysts 2021, 11, 1112. [Google Scholar] [CrossRef]
- Malliga, P.; Pandiarajan, J.; Prithivikumaran, N.; Neyvasagam, K. Effect of film thickness on structural and optical properties of TiO2 thin films. In Proceedings of the International Conference on Advanced Nanomaterials & Emerging Engineering Technologies, Chennai, India, 24–26 July 2013; Volume 2, pp. 488–491. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Rabhi, S.; Belkacemi, H.; Bououdina, M.; Kerrami, A.; Ait Brahem, L.; Sakher, E. Effect of Ag doping of TiO2 nanoparticles on anataserutile phase transformation and excellent photodegradation of amlodipine besylate. Mater. Lett. 2019, 236, 640–643. [Google Scholar] [CrossRef]
- Yi, J.; Huang, L.; Wang, H.; Yu, H.; Peng, F. AgI/TiO2 nanobelts monolithic catalyst with enhanced visible light photocatalytic activity. J. Hazard. Mater. 2015, 284, 207–214. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; He, D.; Situ, Y.; Huang, H. Construction of heterostructured g-C3N4/Ag/TiO2 microspheres with enhanced photocatalysis performance under visible-light irradiation. ACS Appl. Mater. Interfaces 2014, 6, 14405–14414. [Google Scholar] [CrossRef]
- Monga, D.; Basu, S. Single-crystalline 2D BiOCl nanorods decorated with 2D MoS2 nanosheets for visible light-driven photocatalytic detoxification of organic and inorganic pollutants. FlatChem 2021, 28, 100267. [Google Scholar] [CrossRef]
- Guan, Z.; Li, Q.; Shen, B.; Bao, S.; Zhang, J.; Tian, B. Fabrication of Co3O4 and Au co-modified BiOBr flower-like microspheres with high photocatalytic efficiency for sulfadiazine degradation. Sep. Purif. Technol. 2020, 234, 116100. [Google Scholar] [CrossRef]
- Raizada, P.; Thakur, P.; Sudhaik, A.; Singh, P.; Thakur, V.K.; Hosseini-Bandegharaei, A. Fabrication of dual Z-scheme photocatalyst via coupling of BiOBr/Ag/AgCl heterojunction with P and S co-doped g-C3N4 for efficient phenol degradation. Arab. J. Chem. 2020, 13, 4538–4552. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Dong, F. Visible-light photocatalytic removal of NO in air over BiOX (X = Cl, Br, I) single-crystal nanoplates prepared at room temperature. Ind. Eng. Chem. Res. 2013, 52, 6740–6746. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Y.; Wang, T. Preparation of porous TiO2/Ag heterostructure films with enhanced photocatalytic activity. Chem. Eng. J. 2015, 270, 418–427. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, J.; Wang, K.; Meng, Q.; Tang, Y.; Zhao, K. Facile fabrication of TiO2-SiO2-C composite with anatase/rutile heterostructure via sol-gel process and its enhanced photocatalytic activity in the presence of H2O2. Ceram. Int. 2022, 48, 9114–9123. [Google Scholar] [CrossRef]
- Eskandari, P.; Farhadian, M.; Solaimany Nazar, A.R.; Jeon, B.H. Adsorption and Photodegradation Efficiency of TiO2/Fe2O3/PAC and TiO2/Fe2O3/Zeolite Nanophotocatalysts for the Removal of Cyanide. Ind. Eng. Chem. Res. 2019, 58, 2099–2112. [Google Scholar] [CrossRef]
- Pal, B.; Sharon, M.; Nogami, G. Preparation and characterization of TiO2/Fe2O3 binary mixed oxides and its photocatalytic properties. Mater. Chem. Phys. 1999, 59, 254–261. [Google Scholar] [CrossRef]
- Hou, F.; Lu, K.; Liu, F.; Xue, F.; Liu, M. Manipulating a TiO2-graphene-Ta3N5 heterojunction for efficient Z-scheme photocatalytic pure water splitting. Mater. Res. Bull. 2022, 150, 111782. [Google Scholar] [CrossRef]
- Li, H.; Hong, W.; Cui, Y.; Hu, X.; Fan, S.; Zhu, L. Enhancement of the visible light photocatalytic activity of Cu2O/BiVO4 catalysts synthesized by ultrasonic dispersion method at room temperature. Mater. Sci. Eng. B 2014, 181, 1–8. [Google Scholar] [CrossRef]
- Aguilera-Ruiz, E.; García-Pérez, U.M.; De La Garza-Galván, M.; Zambrano-Robledo, P.; Bermúdez-Reyes, B.; Peral, J. Efficiency of Cu2O/BiVO4 particles prepared with a new soft procedure on the degradation of dyes under visible-light irradiation. Appl. Surf. Sci. 2015, 328, 361–367. [Google Scholar] [CrossRef]
- Sun, S.; Ding, J.; Bao, J.; Gao, C.; Qi, Z.; Yang, X.; He, B.; Li, C. Photocatalytic degradation of gaseous toluene on Fe-TiO2 under visible light irradiation: A study on the structure, activity and deactivation mechanism. Appl. Surf. Sci. 2012, 258, 5031–5037. [Google Scholar] [CrossRef]
- Burda, C.; Lou, Y.; Chen, X.; Samia, A.C.S.; Stout, J.; Gole, J.L. Enhanced nitrogen doping in TiO2 nanoparticles. Nano Lett. 2003, 3, 1049–1051. [Google Scholar] [CrossRef]
- Hua, L.; Yin, Z.; Cao, S. Recent advances in synthesis and applications of carbon-doped TiO2 nanomaterials. Catalysts 2020, 10, 1431. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Ghumro, S.S.; Lal, B.; Pirzada, T. Visible-Light-Driven Carbon-Doped TiO2-Based Nanocatalysts for Enhanced Activity toward Microbes and Removal of Dye. ACS Omega 2022, 7, 4333–4341. [Google Scholar] [CrossRef]
- Akhter, P.; Arshad, A.; Saleem, A.; Hussain, M. Recent Development in Non-Metal-Doped Titanium Dioxide Photocatalysts for Different Dyes Degradation and the Study of Their Strategic Factors: A Review. Catalysts 2022, 12, 1331. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, N.; Huang, S.; Wu, N.L.; Wei, M. Sulfur-Doped Anatase TiO2 as an Anode for High-Performance Sodium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 3791–3797. [Google Scholar] [CrossRef]
- Niu, P.; Wu, G.; Chen, P.; Zheng, H.; Cao, Q.; Jiang, H. Optimization of Boron Doped TiO2 as an Efficient Visible Light-Driven Photocatalyst for Organic Dye Degradation With High Reusability. Front. Chem. 2020, 8, 172. [Google Scholar] [CrossRef]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-, N- and S-doped TiO2 photocatalysts: A review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Jones, A.P. Indoor air quality and health. Atmos. Environ. 1999, 33, 4535–4564. [Google Scholar] [CrossRef]
- Magureanu, M.; Bogdan, N.; Hu, J.; Richards, R.; Florea, M.; Parvulescu, M. Plasma-assisted catalysis total oxidation of trichloroethylene over gold nano-particles embedded in SBA-15 catalysts. Catal. B Environ. 2007, 76, 275–281. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, L.; Liu, Y.; Yang, T.; Deng, J.; Dai, H. Concurrent catalytic removal of typical volatile organic compound mixtures over Au-Pd/α-MnO2 nanotubes. J. Environ. Sci. 2018, 64, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Bahri, M.; Haghighat, F. Plasma-based indoor air cleaning technologies: The state of the art-review. Clean Soil Air Water 2014, 42, 1667–1680. [Google Scholar] [CrossRef]
- Shah, K.W.; Li, W. A review on catalytic nanomaterials for volatile organic compounds VOC removal and their applications for healthy buildings. Nanomaterials 2019, 9, 910. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Dvoranová, D.; Lajaunie, L.; Rozman, N.; Figueiredo, B.; Seabra, M.P.; Škapin, A.S.; Calvino, J.J.; Brezová, V.; Labrincha, J.A. Graphene-TiO2 hybrids for photocatalytic aided removal of VOCs and nitrogen oxides from outdoor environment. Chem. Eng. J. 2021, 405. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Photocatalytic TiO2/carbon nanotube nanocomposites for environmental applications: An overview and recent developments. Fuller. Nanotub. Carbon Nanostruct. 2014, 22, 471–509. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Zadi, T.; Azizi, M.; Nasrallah, N.; Bouzaza, A.; Zadi, T.; Azizi, M.; Nasrallah, N.; Bouzaza, A.; Maachi, R. Indoor air treatment of refrigerated food chambers with synergetic association between cold plasma and photocatalysis: Process performance and photocatalytic poisoning. Chem. Eng. J. 2020, 382, 122951. [Google Scholar] [CrossRef]
- Abou Saoud, W.; Kane, A.; Le Cann, P.; Gerard, A.; Lamaa, L.; Peruchon, L.; Brochier, C.; Bouzaza, A.; Wolbert, D.; Assadi, A.A. Innovative photocatalytic reactor for the degradation of VOCs and microorganism under simulated indoor air conditions: Cu-Ag/TiO2-based optical fibers at a pilot scale. Chem. Eng. J. 2021, 411, 128622. [Google Scholar] [CrossRef]
- Jia, Z.; Barakat, C.; Dong, B.; Rousseau, A. VOCs Destruction by Plasma Catalyst Coupling Using AL-KO PURE Air Purifier on Industrial Scale. J. Mater. Sci. Chem. Eng. 2015, 3, 19–26. [Google Scholar] [CrossRef]
- Abidi, M.; Hajjaji, A.; Bouzaza, A.; Trablesi, K.; Makhlouf, H.; Rtimi, S.; Assadi, A.A.; Bessais, B. Simultaneous removal of bacteria and volatile organic compounds on Cu2O-NPs decorated TiO2 nanotubes: Competition effect and kinetic studies. J. Photochem. Photobiol. A Chem. 2020, 400, 112722. [Google Scholar] [CrossRef]
- Vincent, G.; Queffeulou, A.; Marquaire, P.M.; Zahraa, O. Remediation of olfactory pollution by photocatalytic degradation process: Study of methyl ethyl ketone (MEK). J. Photochem. Photobiol. A Chem. 2007, 191, 42–50. [Google Scholar] [CrossRef]
- Vincent, G.; Schaer, E.; Marquaire, P.M.; Zahraa, O. CFD modelling of an annular reactor, application to the photocatalytic degradation of acetone. Process Saf. Environ. Prot. 2011, 89, 35–40. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Ren, X.; Liu, K.; Yang, J. Fine platinum nanoparticles supported on a porous ceramic membrane as efficient catalysts for the removal of benzene. Sci. Rep. 2017, 7, 16589. [Google Scholar] [CrossRef]
- Sedjame, H.J.; Fontaine, C.; Lafaye, G.; Barbier, J. On the promoting effect of the addition of ceria to platinum based alumina catalysts for VOCs oxidation. Appl. Catal. B Environ. 2014, 144, 233–242. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, J.; Jaroniec, M. Efficient catalytic removal of formaldehyde at room temperature using AlOOH nanoflakes with deposited Pt. Appl. Catal. B Environ. 2015, 163, 306–312. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wang, L.; Zhang, C.; He, H. Catalytic oxidation of formaldehyde over manganese oxides with different crystal structures. Catal. Sci. Technol. 2015, 5, 2305–2313. [Google Scholar] [CrossRef]
- Zhang, C.; He, H.; Tanaka, K. ichi Catalytic performance and mechanism of a Pt/TiO2 catalyst for the oxidation of formaldehyde at room temperature. Appl. Catal. B Environ. 2006, 65, 37–43. [Google Scholar] [CrossRef]
- Kucherov, A.V.; Tkachenko, O.P.; Kirichenko, O.A.; Kapustin, G.I.; Mishin, I.V.; Klementiev, K.V.; Ojala, S.; Kustov, L.M.; Keiski, R. Nanogold-containing catalysts for low-temperature removal of S-VOC from air. Top. Catal. 2009, 52, 351–358. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Vallet, C.; Wolbert, D. Use of DBD plasma, photocatalysis, and combined DBD plasma/photocatalysis in a continuous annular reactor for isovaleraldehyde elimination—Synergetic effect and byproducts identification. Chem. Eng. J. 2014, 254, 124–132. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wu, L.C.; Chen, H.Y.; Chung, Y.C. Inactivation of Staphylococcus aureus and Escherichia coli in water using photocatalysis with fixed TiO2. Water. Air Soil Pollut. 2010, 212, 231–238. [Google Scholar] [CrossRef]
- Blanco-Galvez, J.; Fernández-Ibáñez, P.; Malato-Rodríguez, S. Solar photo catalytic detoxification and disinfection of water: Recent overview. J. Sol. Energy Eng. Trans. ASME 2007, 129, 4–15. [Google Scholar] [CrossRef]
- Magaña-López, R.; Zaragoza-Sánchez, P.I.; Jiménez-Cisneros, B.E.; Chávez-Mejía, A.C. The use of TiO2 as a disinfectant in water sanitation applications. Water 2021, 13, 1641. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium dioxide (TiO2)-based photocatalyst materials activity enhancement for contaminants of emerging concern (CECs) degradation: In the light of modification strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Rtimi, S.; Dionysiou, D.D.; Pillai, S.C.; Kiwi, J. Advances in catalytic/photocatalytic bacterial inactivation by nano Ag and Cu coated surfaces and medical devices. Appl. Catal. B Environ. 2019, 240, 291–318. [Google Scholar] [CrossRef]
- Saoud, W.A.; Assadi, A.A.; Kane, A.; Jung, A.; Cann, P.L.; Bazantay, F.; Bouzaza, A.; Wolbert, D. Integrated process for the removal of indoor VOCs from food industry manufacturing Elimination of Butane-2,3-dione and Heptan-2-one by cold plasma-photocatalysis combination. Photochem. Photobiol. A Chem. 2020, 386, 112071. [Google Scholar] [CrossRef]
- Venieri, D.; Gounaki, I.; Bikouvaraki, M.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Mantzavinos, D. Solar photocatalysis as disinfection technique: Inactivation of Klebsiella pneumoniae in sewage and investigation of changes in antibiotic resistance profile. J. Environ. Manag. 2017, 195, 140–147. [Google Scholar] [CrossRef]
- Ray, S.K.; Dhakal, D.; Regmi, C.; Yamaguchui, T.; Lee, S.W. Inactivation of Staphylococcus aureus in visible light by morphology tuned α-NiMoO4. J. Photochem. Photobiol. A Chem. 2018, 350, 59–68. [Google Scholar] [CrossRef]
- Kőrösi, L.; Pertics, B.; Schneider, G.; Bognár, B.; Kovács, J.; Meynen, V.; Scarpellini, A.; Pasquale, L.; Prato, M. Photocatalytic inactivation of plant pathogenic bacteria using TiO2 nanoparticles prepared hydrothermally. Nanomaterials 2020, 10, 1730. [Google Scholar] [CrossRef] [PubMed]
- Salavati-Niasari, M.; Davar, F. Synthesis of copper and copper(I) oxide nanoparticles by thermal decomposition of a new precursor. Mater. Lett. 2009, 63, 441–443. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, P.; Wang, W.; Cai, Y.; Tan, F.; Wang, X.; Qiao, X.; Wong, P.K. Enhanced dark adsorption and visible-light-driven photocatalytic properties of narrower-band-gap Cu2S decorated Cu2O nanocomposites for efficient removal of organic pollutants. J. Hazard. Mater. 2020, 384, 121302. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Song, J.; Kang, Y.; Chai, D.; Zhao, R.; Lei, Z. Sm2O3 embedded in nitrogen doped carbon with mosaic structure: An effective catalyst for oxygen reduction reaction. Energy 2017, 133, 115–120. [Google Scholar] [CrossRef]
- Gupta, S.B.; Bluhm, H. The potential of pulsed underwater streamer discharges as a disinfection technique. IEEE Trans. Plasma Sci. 2008, 36, 1621–1632. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Madzivire, G.; Petrik, L.F. A review of combined advanced oxidation technologies for the removal of organic pollutants from water. Water Air Soil Pollut. 2014, 225, 2102. [Google Scholar] [CrossRef]
- Ado, A.; Tukur, A.I.; Ladan, M.; Gumel, S.M.; Muhammad, A.A.; Habibu, S.; Koki, I.B. A Review on Industrial Effluents as Major Sources of Water Pollution in Nigeria. Chem. J. 2015, 1, 159–164. [Google Scholar]
- Weiss, C.; Carriere, M.; Fusco, L.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Pasquali, M.; Pasquali, M.; Scott, J.A.; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef] [PubMed]
- Karbasi, M.; Karimzadeh, F.; Raeissi, K.; Rtimi, S.; Kiwi, J.; Giannakis, S.; Pulgarin, C. Insights into the photocatalytic bacterial inactivation by flower-like Bi2WO6 under solar or visible light, through in situ monitoring and determination of reactive oxygen species (ROS). Water 2020, 12, 1099. [Google Scholar] [CrossRef]
- Singh, J.; Juneja, S.; Palsaniya, S.; Manna, A.K.; Soni, R.K.; Bhattacharya, J. Evidence of oxygen defects mediated enhanced photocatalytic and antibacterial performance of ZnO nanorods. Colloids Surf. B Biointerfaces 2019, 184, 110541. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Li, G.; An, T.; Zhao, H.; Wong, P.K. Catalyst-free activation of persulfate by visible light for water disinfection: Efficiency and mechanisms. Water Res. 2019, 157, 106–118. [Google Scholar] [CrossRef]
- Hajjaji, A.; Elabidi, M.; Trabelsi, K.; Assadi, A.A.; Bessais, B.; Rtimi, S. Bacterial adhesion and inactivation on Ag decorated TiO2-nanotubes under visible light: Effect of the nanotubes geometry on the photocatalytic activity. Colloids Surf. B Biointerfaces 2018, 170, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Ubonchonlakate, K.; Sikong, L.; Saito, F. Photocatalytic disinfection of P. aeruginosa bacterial Ag-doped TiO2 film. Procedia Eng. 2012, 32, 656–662. [Google Scholar] [CrossRef]
- Lee, M.; Shahbaz, H.M.; Kim, J.U.; Lee, H.; Lee, D.U.; Park, J. Efficacy of UV-TiO2 photocatalysis technology for inactivation of Escherichia coli K12 on the surface of blueberries and a model agar matrix and the influence of surface characteristics. Food Microbiol. 2018, 76, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Singh, R.P.; Pandey, A.; Pandey, A. Photocatalytic antibacterial performance of TiO2 and Ag-doped TiO2 against S. aureus, P. aeruginosa and E. coli. Beilstein J. Nanotechnol. 2013, 4, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef] [PubMed]

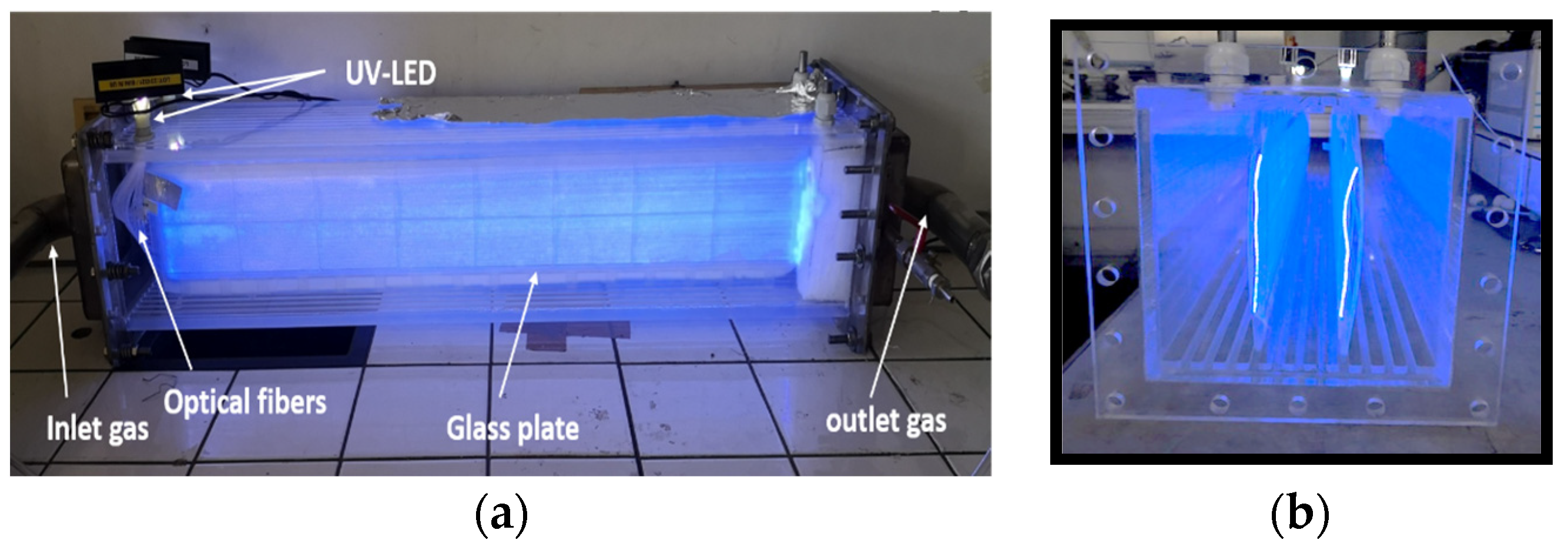
| Target Pollutants | Reactors | Catalyst | Radical Species | Operating Conditions | Degradation Performance | Formed Products (Intermediate and Final) | Ref. |
|---|---|---|---|---|---|---|---|
| Propionic acid (PPA) and benzene (BENZ) | annular reactor + dielectric barrier discharge (DBD) | SiO2-TiO2 + UV | °OH, CH3 CH2° | SiO2 = 6.5 g m−2 et TiO2 = 6.5 g m−2 performance lamp UV-A (80 W/10) output intensity (25 W/m2) Odor inlet concentrations 0.068 to 0.405 mmol m−3, Q = 2 at 6 m3 h−1 relative Humidity: 5 to 90%, T = 20 °C | RE tested alone: 55% (APP) et 40% (BENZ) RE of mixture: 50% for APP and 30% for BENZ RE combined process: 60% for a voltage equal to 9 kV RE of mixture gaseous effluent (5% HR): 50% APP et 50% BENZ | BENZ: CO2 dominating CO weak, O3, CH3CH2OOH instable → Alcool + Aldéhyde → CO2 PPA: CO2, ethanoic acid (CH3CH2OOH), ethanol (CH3CH2OH), aldehyde (CH3CHO), H2O, O2 | [75] |
| Butane-2,3-dione and Heptane-2-one | Continuous Planar Reactor | TiO2, TiO2-Cu et TiO2-Ag | •OH, O2°− | Q = 1–12 m3 h−1 concentration of COV= 5–20 mg.m−3 Humidity level = 5–70%, under UV-A light oxidation. | RE of TiO2 alone: 63% RE of TiO2-Ag: 46% RE of TiO2-Cu: 52% | acetone (C3H5O) propionic acid (C3H6O2) butanoic acid (C4H8O2) pentanoic acid (C5H10O2) acetic acid (C2H4O2) acetaldehyde (C2H4O) formic acid (HCOH) carbon dioxide (CO2) and H2O | [76] |
| Acetone and toluene | Surface DBD discharge | Pt/TiO2 and MnO2/CuO2/Al2O3 | NS | Concentration: 0.2 ppm flow rate: 38.42 m3/h | 100% toluene destruction of toluene at 0.2 ppm and 100% acetone destruction at 0.46 ppm | NS | [77] |
| Butane-2,3-dione (BUT) + E. coli | spherical batch reactor | Cu2O/TiO2 and TiO2-Ag | •OH, HO2° and O2°− | Concentration: 4.4 g/m3 T = 50 at 100 °C λ = 380–420 nm, under UV–vis light irradiation. | 99.7% E. coli inactivation and 100% VOC degradation within 60 min and 25 min with TiO2-Ag for simultaneous treatment | CO2, H2O | [78] |
| methyl ethyl ketone (MEK) or 2-butanone | annular reactor | TiO2 (fiberglass + Ahlström support) | •OH, O2−°, °H2C-CH3, °CH3, H3C-C°=O, °H2C-CO-CH2-CH3 | MEK concentration on glass fibers: 1.51 mg/L MEK concentration on Ahlström: 1.75 mg/L HR glass fibers: 0.11–3.94 mW/cm2 HR Ahlström: 0.12–2.53 mW/cm2 T = 30 °C and 20 vol.% O2, under UV light source. | Deposition of TiO2 on glass fibers leads to 10% degradation of MEK for 1.5 mg/L. TiO2 Ahlström leads to the elimination of 40% of MEK for 1.5 mg/L. | acetaldehyde (C2H4O) ethane (C2H6) methane (CH4) methanol (CH3OH) acetone (C3H6O) methyl formate (C2H4O2) carbon dioxide (CO2) and H2O | [79] |
| Acetone | annular reactor | TiO2 (fiberglass + Ahlström support) | °CH3, •OH, H2C°-COOH, H3C-°C=O | Concentration: 14.9 ng/L and 66.0 ng/L light power: 0.21 to 3.94 mW/cm2 T = 30 °C, 20 vol.% O2 Volume flow: 150 to 300 mL/min, under UV light. | 90% of Acetone conversion has been obtained for low initial concentrations with TiO2 photocatalyst deposited on fiberglass for simultaneous treatment | acetaldehyde (C2H4O) methyl alcohol (CH3OH) isopropyl alcohol (C3H8O) methyl ethyl ketone (C4H8O) acetic acid (CH3 COOH) mesityl oxide (C6H10O) diacetone-alcohol (C6H12O2) | [80] |
| Benzene | the outer surface of the rectangular SiC ceramic membrane | Pt/SiC@Al2O3 | NS | 0.176% by mass of Pt | 90% reduction at 215 °C with a space velocity of 6000 mg−1 h−1 | CO2, H2O | [81] |
| n-butanol and acetic acid | fixed-bed tubular reactor | Pt/CeO2-AlO3 | NS | 1000 ppm of COV T = 50–350 °C 0, 7, 15, 23 et 51% by weight of CeO2 | 100% reduction for n-butanol at T < 250 °C 50 or 90% reduction for a reduction of 80 or 20 °C. | Butanal (C4H8O) methanol (CH4OH) propanol (C3H8O) isopropanol (C3H8O) formaldehyde (HCOH) propanal (C3H6O) carbon dioxide (CO2) | [82] |
| Formaldehyde | organic glass reactor | Pt/AlOOH/, Pt/AlOOH-c, Pt/c-Al2O3 and Pt/TiO2 | NS | HCHO concentration: 127 ppm for adsorption and 139 ppm for catalytic oxidation, fan: 5 W T: 35 °C HR: 25% oxidation time: 51 min. | Pt/AlOOH > Pt/AlOOH-c > Pt/c-Al2O3 > Pt/TiO2 | surface formate carbon dioxide (CO2) water (H2O) | [83] |
| Formaldehyde | fixed-bed quartz flow reactor | Ag/TiO2, Ag/Al2O3 et Ag/CeO2 | NS | Concentration: 110 ppm T = 35 to 125 °C Debit: 100 mL min−1, under light containing ultraviolet. | Ag/TiO2 > Ag/Al2O3 > Ag/CeO2 100% HCHO conversion with Ag/TiO2 at T = 95°C | carbon dioxide (CO2) another carbon-containing compound | [84] |
| Formaldehyde | NS | Pt/TiO2, Rh/TiO2, Pd/TiO2, Au/TiO2 (noble metals/TiO2) | NS | Concentration: 100 ppm 1% noble metals/TiO2 O2 20 vol.% Debit: 50 cm3 min−1 T: 20 °C GHSV: 5000 h−1 | Pt/TiO2 ≫ Rh/TiO2 > Pd/TiO2 > Au/TiO2 | carbon dioxide (CO2)carbon monoxyde (CO); water (H2O) | [85] |
| Dimethyl disulfide (DMDS) | Continuous Flow Quartz Tubular Reactor | (Au + Pd)/TiO2, Au/MCM-41, (AU + Rh)/MCM and Au/TiO2, Pd/TiO2 | NS | 3%Pd/TiO2 and 1%Au/TiO2(1%Au + 3%Pd)/TiO2 gas flow: 42,000 h−1 Temperature: 20–320 °C | Au/TiO2 and Au-Pd/TiO2 effectively remove DMDS for T < 155 °C Au/MCM-41 less effective in DMDS eliminating | methanol (CH3OH) ethanol (C2H6O) methyl mercaptan (CH3SH) ethyl mercaptan (CH3SCH3) hydrogen sulfur (H2S) carbon dioxide (CO2) carbon monoxide (CO) sulfur dioxide (SO2) water (H2O) | [86] |
| toluene + m-xylene + ethyl acetate or acetone | fixed-bed Quartz Continuous Flow Microreactor (ICP-AES) | 0.91 wt.% Au0.48 Pd/α-MnO2et α-MnO2 | α-, β- et γ-oxygène | 1% (Au-Pd) Mixing flow: 17 mL/min concentration: 1000 ppm + O2 + N2 (solid) molar ratio COV/O2 = 1/400 SV (space velocity) = 40,000 mL (g h) T = 320 °C | 0.91 wt.% Au 0.48 Pd/α-MnO2 > α-MnO2 | carbon dioxide (CO2)water (H2O) | [69] |
| Isovaleraldehyde | continuous annular plasma reactor DBD combined photocatalysis | TiO2 | •OH, O2•− | concentration: 75 to 200 mg m−3 Debit: 2 m3 h−1 HR: 5% T: 20 °C I: 20 W m−2 SE: 17 J L−1, under UV light. | NS | propanoic acid (CH3CH2COOH) propanone (CH3COCH3) ethanoic acid (CH3COOH) carbon dioxide (CO2) carbon monoxide (CO) ozone (O3) | [87] |
| Benzene | New UV-LED frontal flow photocatalytic reactor | TiO2 deposed on luminous textiles | OH°, O2°− | concentration: 100 to 200 mg m−3 Debit: 1 m3 h−1 HR: 5 to 80% T: 20 °C | CO2 and H2O | [72] |
| Bio Contaminants | Reactor | Catalyst | Operations Parameters | Performance | Ref. |
|---|---|---|---|---|---|
| E. coli | Petri dishes | TiO2-NT and Ag-TiO2-NTs | Concentration: 4 × 106 UFC/mL volume: 100 mL diameter TiO2: 100 nm at 70V diameter Ag: 8 nm | TiO2: reduction of 1.6 log with 180 min Ag/TiO2: reduction of 99.99% after 90 min | [107] |
| P. aeruginosa | Glass fiber tissue (GFT) | Poroux TiO2TiO2 pur (TiO2-PEG) and TiO2-Ag | Concentration: 103 UFC/mL TiO2 pur: 14.7 nm TiO2-Ag-PEG:16.6 nm TiO2-Ag: 25.3 nm, under UV light. | TiO2-1Ag: 100% of inactivation after 10 min TiO2 poroux: 57% TiO2-PEG: 93% | [108] |
| E. coli K12 | Agar matrix surface + blueberry skin + calyx | UV-TiO2& UV alone | Initial bacterial populations: 7 log CFU/g UV-Photocatalysis (4.5 mW/cm2) UV alone (6.0 mW/cm2). TiO2-coated quartz tubes (38 cm length, 24.5 mm outer diameter, thickness 0.7–0.9 mm. | 4.5 log CFU/g for UV alone and 5.3 log CFU/g for UV-TiO2 in 30 s. 3.4 log and 4.6 log CFU/g, respectively, UV alone and UV-TiO2 for the first 30 s. 4.0 log and 5.2 log CFU/g, respectively, UV alone and photocatalysis. | [109] |
| S. aureus. P. aeruginosa and E. coli | LB agar plates | TiO2-Ag (TiO2 (calcinated at 300 °C) (CB300) at (500 °C) (CB500) et TiO2 (not calcinated) (CB)) | Concentration: 10 µL with 109 UFC/mL 5%w of TiO2 | TiO2 (calcined 300 °C)-Ag: reduces bacterial growth by 95%, i.e., 1.05 × 108 CFU/mL with UV. TiO2 (calcined 500 °C) without Ag: reduces bacterial growth by 30% with UV. TiO2 (calcined at 300 °C) without Ag: reduces growth by 75%. | [110] |
| E. coli | Planar reactor | TiO2, TiO2-Ag and TiO2-Cu deposed on optical fibers | Initial bacterial populations: 2.4 × 107 UFC/mL. The core of optical fibers is constructed of polymethyl methacrylate resin with a mean diameter of 480 m and coated with 10 m of a thick fluorinated polymer, under UVA-LEDs (365 nm, UVA-LED intensity = 1.5 W m−2). | 3 log of removal with TiO2/Ag and TiO2/Cu | [76] |
| S. aureus CCM 3955 & S. aureus CCM 3953 (Gram+) E. coli & P. aeruginosa (Gram−) | Disposable plates | Ag NPs | Initial bacterial populations: from 105 to 106 UFC/mL, Particle size from 40 to 60 nm, Temperature 35 °C. | Higher activity at 7 ppm against P. aeruginosa. NP Ag synthesized based on AgNO3: considerable antibacterial activity at 14 and 29 ppm (82.49% inactivation). NP Ag synthesized based on AgNO3 and citrate: 88.56 inactivations. | [111] |
| E. coli | Batch reactor | Cu2O-NPs/TiO2-NTs catalyst | Initial bacterial populations: from 106 to 107 UFC/mL. Under visible light irradiation (380–720) nm. Temperature 37 °C. | Bacterial inactivation rate of 98% and a concomitant 99.7% VOC removal within 60 min and 25 min | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assadi, A.A.; Baaloudj, O.; Khezami, L.; Ben Hamadi, N.; Mouni, L.; Assadi, A.A.; Ghorbal, A. An Overview of Recent Developments in Improving the Photocatalytic Activity of TiO2-Based Materials for the Treatment of Indoor Air and Bacterial Inactivation. Materials 2023, 16, 2246. https://doi.org/10.3390/ma16062246
Assadi AA, Baaloudj O, Khezami L, Ben Hamadi N, Mouni L, Assadi AA, Ghorbal A. An Overview of Recent Developments in Improving the Photocatalytic Activity of TiO2-Based Materials for the Treatment of Indoor Air and Bacterial Inactivation. Materials. 2023; 16(6):2246. https://doi.org/10.3390/ma16062246
Chicago/Turabian StyleAssadi, Achraf Amir, Oussama Baaloudj, Lotfi Khezami, Naoufel Ben Hamadi, Lotfi Mouni, Aymen Amine Assadi, and Achraf Ghorbal. 2023. "An Overview of Recent Developments in Improving the Photocatalytic Activity of TiO2-Based Materials for the Treatment of Indoor Air and Bacterial Inactivation" Materials 16, no. 6: 2246. https://doi.org/10.3390/ma16062246
APA StyleAssadi, A. A., Baaloudj, O., Khezami, L., Ben Hamadi, N., Mouni, L., Assadi, A. A., & Ghorbal, A. (2023). An Overview of Recent Developments in Improving the Photocatalytic Activity of TiO2-Based Materials for the Treatment of Indoor Air and Bacterial Inactivation. Materials, 16(6), 2246. https://doi.org/10.3390/ma16062246












